Abstract
Ericameria nauseosa (Pall. ex Pursh) G.L. Nesom & G.I. Baird) is used in traditional medicine to treat various diseases; however, little is known about the immunomodulatory activity of essential oil from this plant. Thus, we isolated essential oil from the aerial parts of E. nauseosa and evaluated their chemical composition and biological activity. Compositional analysis of E. nauseosa essential oil revealed that the main (>2%) components were γ-decalactone (13.3%), cryptone (9.4%), terpinen-4-ol (9.3%), (E)-methyl cinnamate (6.0%), T-cadinol (4.7%), spathulenol (3.6%), 8Z-2,3-dihydromatricaria ester (3.1%), β-phellandrene (3.0%), p-cymen-8-ol (2.2%), 3-ethoxy-2-cycloocten-1-one (2.2%), and trans-p-menth-2-en-1-ol (2.1%). Distinctive features were the lactones (up to 15%) and polyacetylenes (up to 3.1%), including (2Z,8Z)-matricaria ester and 8Z-2,3-dihydromatricaria ester. A comparison with other reported E. nauseosa essential oil samples showed that our samples were distinct from those collected in other areas of the country; however, they did have the most similarity to one sample collected in North Central Utah. Pharmacological studies showed that E. nauseosa essential oil activated human neutrophil Ca2+ influx, which desensitized these cells to subsequent agonist-induced functional responses. Based on our previously reported data that nerolidol, β-pinene, spathulenol, sabinene, and γ-terpinene were active in human neutrophils, these compounds are the most likely constituents contributing to this immunomodulatory activity. However, the relatively high amount of polyacetylenes may also contribute, as these compounds have been characterized as potent immunomodulators.
1. Introduction
The Asteraceae (Compositae), commonly known as the sunflower or daisy family, is one of the most species-diverse families of all extant angiosperm families. With an estimated 25,000–35,000 species, this family comprises 10% of all flowering plant species and is distributed worldwide [1,2]. Ericameria nauseosa (Pall. ex Pursh) G.L. Nesom & G.I. Baird (also called rubber rabbitbrush) is one of the more common Asteraceae members of the western North American flora. This perennial shrub is characterized by narrow resin-coated greenish-gray leaves, and the leaves and rubbery stems have a soft felt-like covering [3]. E. nauseosa yellow flowers bloom from late July to October.
The genus Ericameria Nutt. is taxonomically complex and has been historically of interest because of the close phylogenetic relationship between Ericameria and the genus Chrysothamnus Nutt. and the occurrence of intergeneric hybrids [4]. Several different classifications have been proposed for Ericameria, with the most inclusive taxonomical evaluation of the species being published by Hall in 1928 [5]. Since that time, the genus Ericameria has been greatly expanded by transfer of species from the genus Chrysothamnus and now contains four sections: sect. Asiris, sect. Ericameria, sect. Macronema, and sect. Stenotopsis [6]. One of the more recent transfers was Chrysothamnus nauseosus (Pall. ex Pursh) Britton, which is now known as E. nauseosa (Pall. ex Pursh) G.L. Nesom & G.I. Baird from the section Macronema [3,7].
Ericameria species have been used ethnopharmacologically throughout history. In reviewing the literature, one must consider reports regarding the use of different Ericameria species as well as Chrysothamnus species due to their phylogenetic relationship [8]. These plant species produce a considerable variety of secondary metabolites, including grindelane diterpenoids [9], labdane-type acids [10], flavonoids [11], coumarins, phenolic acids, and cinnamic acid derivatives [12], chromanones and acetophenones [13], polyacetylenes [14], and mono- and sesquiterpenes [15]. Although there is little information regarding the biological activity and pharmacological properties of E. nauseosa, this species has been used historically by American Indians for a number of medical treatments. For example, the Shoshone Indians used steeped E. nauseosa leaves as a tea for colds, coughs, and stomach disorders, and also steeped dried E. nauseosa flowers and/or leaves as a general tonic [16]. Likewise, the Northern Cheyenne Indians of Montana used E. nauseosa as a remedy for colds, coughs, and tuberculosis [17], and Indian Tribes of Nevada boiled E. nauseosa roots and tops together for treating hematochezia [18]. More recently, several studies have investigated the pharmacological properties of some components of E. nauseosa. For example, a distillate of E. nauseosa was reported to have significant antimicrobial activity [19]. Similarly, E. nauseosa leaf extracts were found to have anthelmintic activity [20]. Recently, Hell et al. [11] reported that flavonoids from E. nauseosa inhibited the phosphoinositide-3-kinase (PI3K)/protein kinase B (AKT) pathway in human melanoma cells and suggested that they may have potential as anticancer drugs [11].
Among the pharmacologically important bioactive components present in medicinal plant extracts are essential oils, and essential oils have been shown to exhibit immunomodulatory, antimicrobial, antioxidant, and anti-inflammatory effects [21,22,23,24]. Although not much has been reported regarding pharmacological properties of essential oils extracted from Ericameria and Chrysothamnus species, the sesquiterpene chrysothol isolated from the essential oil of C. viscidiflorus Nutt. var. viscidiflorus was found to exhibit anti-cancer activity against human breast cancer cells [12]. Essential oil from C. nauseosus (Pall.) Britt. var. nauseosus has been reported to have antifungal activity against the plant pathogens Colletotrichum acutatum, C. fragariae, and C. gloeosporioides [25]. However, there is no reported information regarding the pharmacological activity of essential oil from E. nauseosa.
We isolated essential oil from the aerial parts of E. nauseosa collected in Southwestern Montana and analyzed its chemical composition and biological activity. A comparison with previous reports on E. nauseosa essential oils collected in other parts of the country suggested similar and unique components, indicating that the location where these plants are collected affects their essential oil composition. Analysis of its pharmacological properties showed that essential oil isolated from E. nauseosa aerial parts was immunomodulatory and activated human neutrophils, leading to downregulated responses to subsequent activation by an inflammatory stimulus. Thus, these studies suggest that essential oils may contribute to the beneficial medicinal properties reported for extracts from E. nauseosa.
2. Results and Discussion
2.1. Composition of Essential Oil from E. nauseosa
Hydrodistillation of E. nauseosa aerial parts resulted in a yield of 1.3% (w/v) essential oil. Gas-chromatography (GC-FID and GC/MS) was used to investigate the essential oil chemical composition, and a total of 74 compounds representing 91.9% of the essential oil were identified and quantified (Table 1). E. nauseosa essential oil was predominantly enriched in monoterpenes (oxygenated monoterpenes 37.7%; hydrocarbons 10.0%), with the irregular monoterpene cryptone (9.4%) as the main constituent (Table 2). Among the other important compounds were terpinen-4-ol (9.3%), β-phellandrene (3.0%), p-cymen-8-ol (2.2%), trans-p-menth-2-en-1-ol (2.1%), and cis-p-menth-2-en-1-ol (2.0%). This composition was distinguished from those reported previously for E. nauseosa/C. nauseousus, especially with respect to their high cryptone and terpinen-4-ol contents. Specifically, the major constituents of C. nauseousus var. albicaulis were β-pinene (16.8%), limonene (18.6%), and β-phellandrene (26.0%); the main constituents of C. nauseousus var. consimilis were limonene (33.2%), β-phellandrene (18.0%), and (Z)-β-ocimene (14.6%); and the major constituents of C. nauseousus var. glabratus were β-pinene (30.3%), myrcene (10.5%), limonene (16.5%), and β-phellandrene (10.9%) [26]. Finally, essential oils extracted from E. nauseosa collected in North Central Utah and Southwestern Idaho were recently reported to contain high percentages of monoterpenes, including β-phellandrene (1.8–56.5%), β-pinene (0.3–23.3%), limonene (0.7–22.3%), and (Z)-β-ocimene (0.0–29.3%) [27].

Table 1.
Chemical composition of essential oil from E. nauseosa of Southwestern Montana.

Table 2.
Distribution of major compound classes in E. nauseosa essential oil.
It should be noted that the sesquiterpenes of E. nauseosa essential oil were represented only by oxygenated compounds (14.7%), with T-cadinol (4.7%), spathulenol (3.6%), and α-cadinol (1.6%) as the main representatives. In comparison, the sesquiterpene-rich essential oil of C. nauseosus ssp. hololeucus (A. Gray) H.M.Hall & Clem. collected from a site in Provo, Utah contained (E)-β-farnesene (3.4–23.7%), α-muurolene (1.2–7.1%), γ-muurolene (0.9–9.8%), and β-humulene (2.0–3.9%) as the major constituents [48]. Likewise, essential oil of C. nauseosus (Pall.) Britt. var. nauseosus collected from Blaine County, Idaho (Crockett NW16) contained mono- and sesquiterpenes as the main constituents: β-phellandrene (22.8%), β-pinene (19.8%), and β-eudesmol (7.7%) [25].
A distinctive feature of E. nauseosa essential oil was the presence of high concentrations of lactones (15.0%), including γ-decalactone (13.3%) and γ-dodecalactone (1.7%). This is the first time such high amounts of lactones were detected in Ericameria or Chrysothamnus essential oil. Southwestern Montana E. nauseosa essential oil also contained significant amounts of polyacetylenes, including (2Z,8Z)-matricaria ester and 8Z-2,3-dihydromatricaria ester (together 3.1%). These findings are consistent with a previous report by Rose [14], who found that the volatiles of C. nauseosus contained methyl Z,Z-10-acetoxymatricariate, methyl Z,Z-10-hydroxymatricariate, methyl 2(Z)-10-acetoxy-8,9-epoxydecen-4,6-diynoate, and methyl 2(Z)-10-hydroxy-8,9-epoxydecen-4,6-diynoate and Stirling et al. [27], who reported (E,Z)- and (Z,E)-matricaria esters (up to 2.6%) in most essential oils from samples of E. nauseosa collected in North Central Utah, but not in Southwestern Idaho.
Another distinguishing property of Southwestern Montana E. nauseosa essential oil was the significant content of cis- and trans-cinnamic acid methyl esters (6.0%). In comparison, essential oils of E. nauseosa from Utah [27] contained scarce amounts of cinnamic acid methyl and ethyl esters.
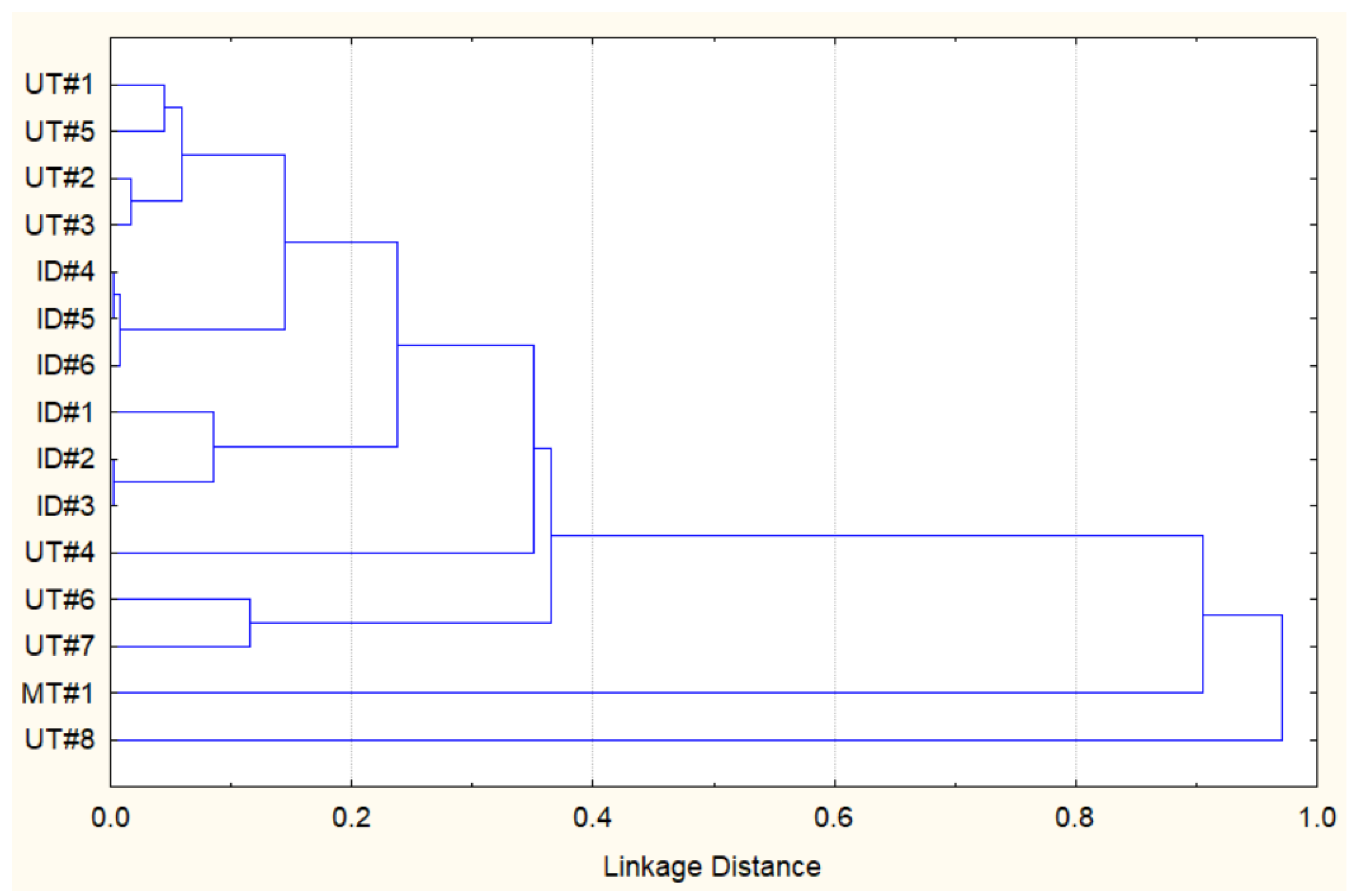
Considering the data above, it appears that the essential oil composition of Southwestern Montana E. nauseosa is qualitatively different from those reported previously. However, to further evaluate relationships between the Southwestern Montana E. nauseosa essential oil and those previously reported from plants collected in Utah (UT#1…8) and Idaho (ID#1…6) [27], we performed a similar hierarchical cluster analysis (HCA) based on the concentration data for 68 different components (Supplemental Table S1). Figure 1 shows that MT#1 from plants collected in Southwestern Montana and UT#8 from plants collected in North Central Utah seem to be quite similar to each other, whereas they both had low similarities to all of the other essential oil samples.
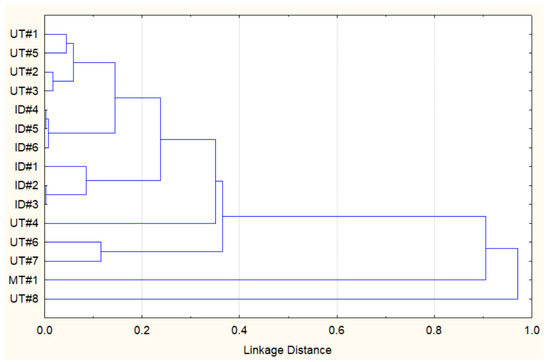
Figure 1.
HCA dendrogram representing the similarities of the essential oil compositions of E. nauseosa collected in Southwestern Montana (MT#1), North Central Utah (UT#1…8), and Southwestern Idaho (ID#1…6). Pearson correlation was used to measure the linkage distance.
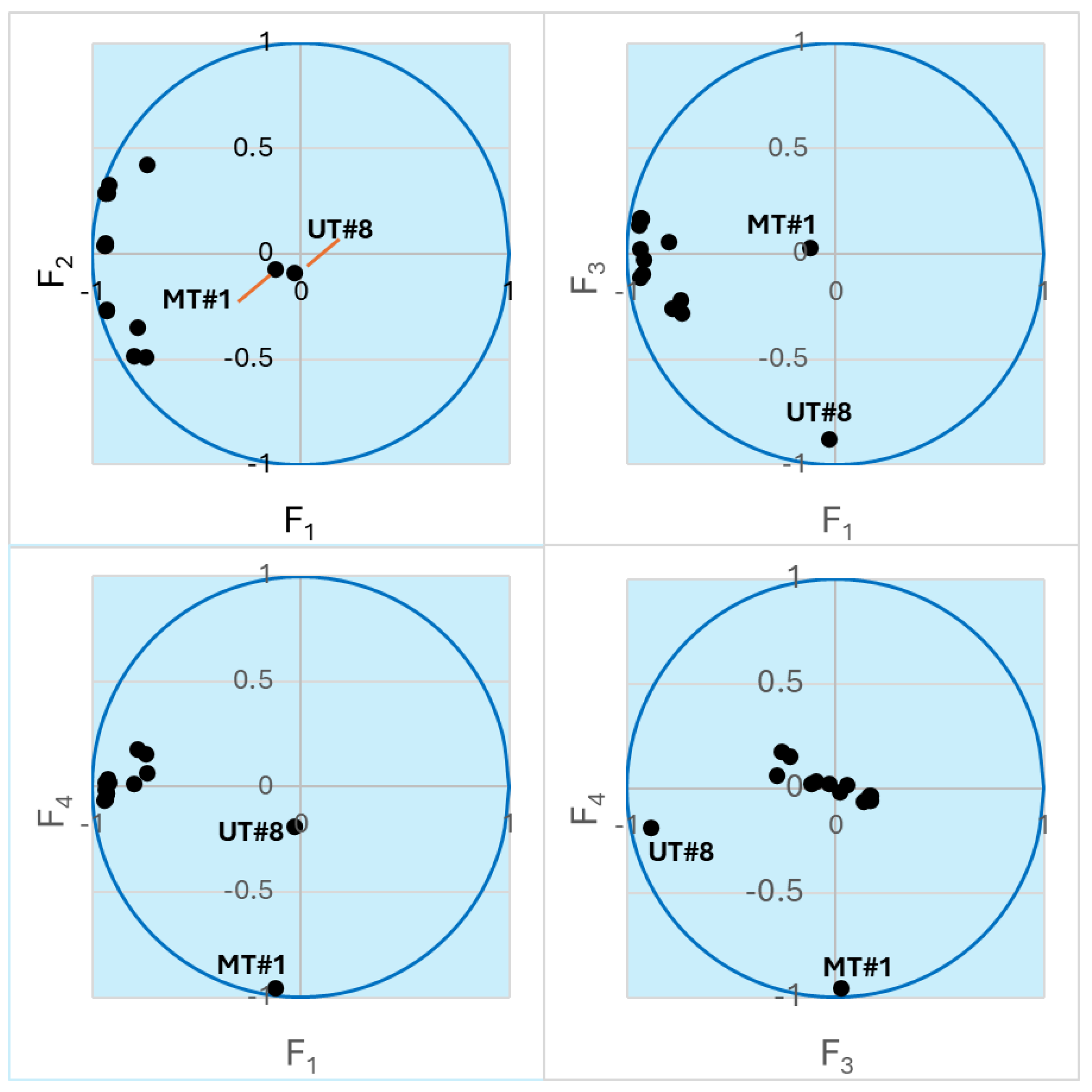
To better explore the compound concentration information obtained for the 15 samples (Supplemental Table S1), we performed principal component analysis (PCA), which allows embedding multidimensional data into a subspace of reduced dimensionality defined by mutually orthogonal principal components (PCs). Directions of the principal axes were chosen in order to explain much of the variance in the initial massif of data [49]. Thus, instead of 68 concentration values, each sample can be represented by just a few coordinates in the subspace of the principal components F1, F2, …, Fn, which are linear combinations of the actual concentrations. We found that the four most important PCs (F1–F4) captured 67.92, 8.66, 7.56, and 6.85% of the variance, respectively. In total, 91% of the initial variance was accounted for by these PCs. Thus, the multidimensional initial data can be reduced to four-dimensional subspace without significant loss of information. Note that components F2–F4 are also of almost equal importance for the data analysis. Figure 2 shows a representation of the samples in biplots of the PCs. The biplots show that the compositions of UT#1…UT#8 and ID#1…ID#6 are well described by components F1 and F2, while the points for UT#8 and MT#1 lie along the F3 and F4 axes, respectively, which is indicative of unique compositions of these two essential oils and is in accordance with the HCA results. Nevertheless, the UT#8 datapoint has a noticeable projection along the F4 axis towards the MT#1 point (Figure 2). Hence, there may be some similarity between these two samples.
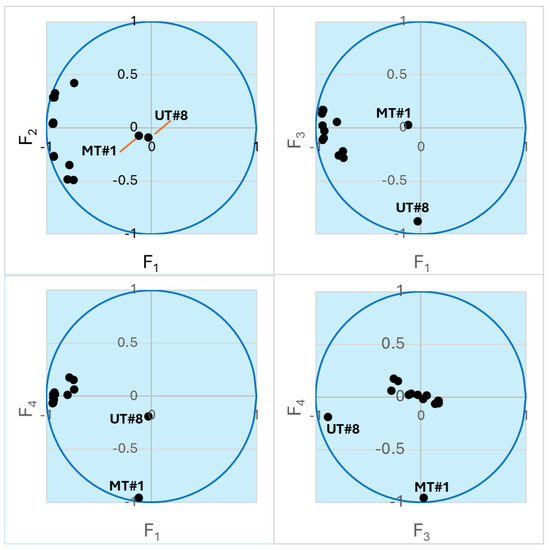
Figure 2.
Biplots of the essential oil samples in the axes of the principal components. The points lying close to the unit circle are best described by the corresponding pair of variables. Points for UT#8 and MT#1 are indicated. The other clustered points refer to UT#1…7 and ID#1…6.
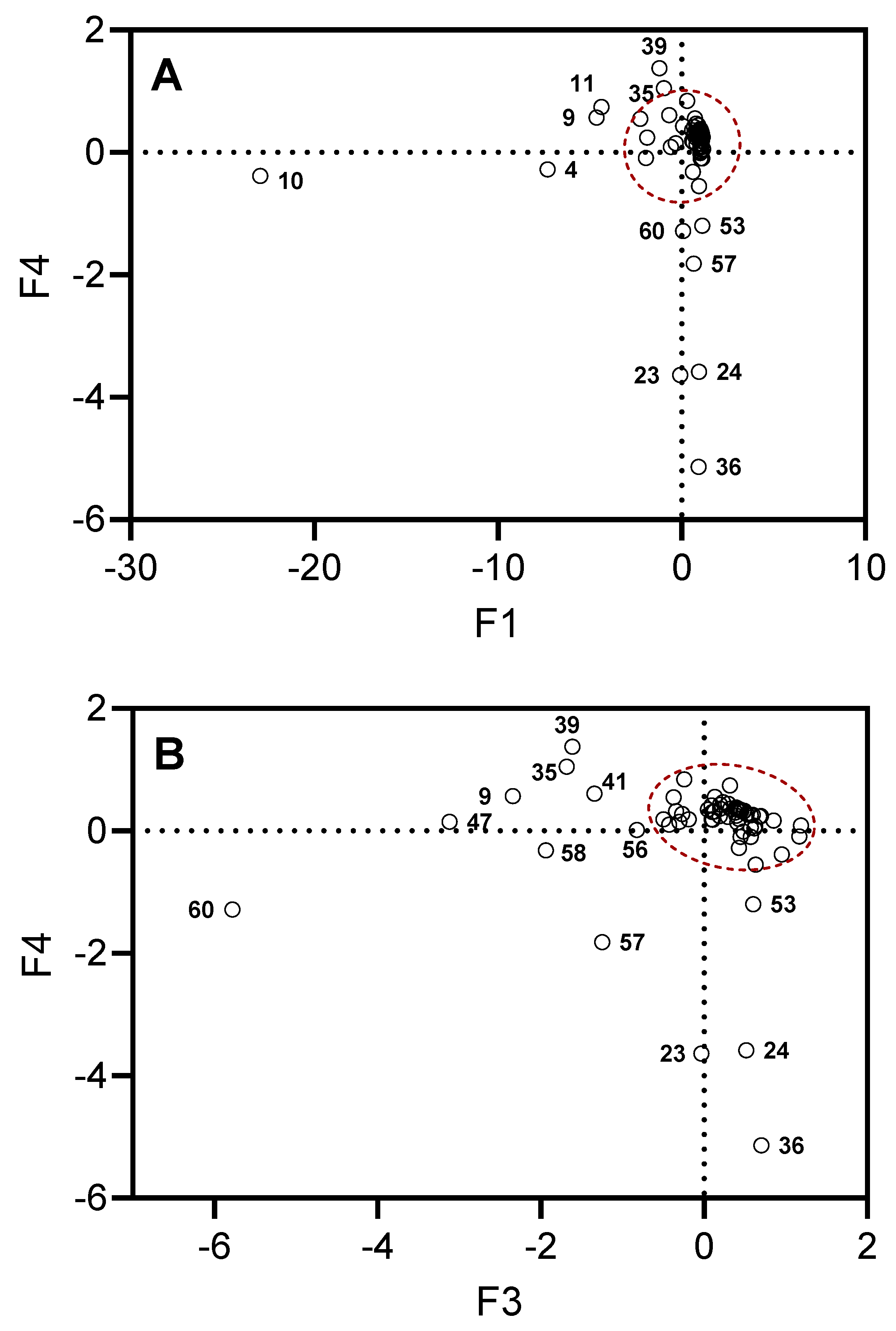
Figure 3 shows projections of the selected compounds contained in the essential oils on the planes defined by F1, F4 and F3, F4. The projections stretched along the principal axes correspond to the compounds responsible for the key differences between the investigated types of essential oil samples. For example, there is high content of β-phellandrene (compound 10) in all samples (14.3–56.5%), with the exception of UT#8 (1.8%) and MT#1 (3.0%), and a high content of β-pinene (compound 4) in all samples (2.2–23.3%), with the exception of UT#8 (0.3%) and MT#1 (1.7%). Although all samples contained γ-curcumene (from traces to 8.3%) and σ-cadinene (from traces to 10.5%), these compounds (39 and 47, respectively) were not detected in MT#1. A low amount or even absence (0–3.3%) of terpinen-4-ol (compound 23), cryptone (compound 24), and γ-decalactone (compound 36) was found in all samples, with the exception of MT#1 (9.3%, 9.4%, 13.3%, and 4.7%, respectively). Finally, a low amount or even absence (0–1.2%) of T-cadinol (compound 57) was found in all samples, with the exception of UT#8 and MT#1. Thus, we conclude that UT#8 and MT#1 are similar but still distinct in their essential oil compositions.
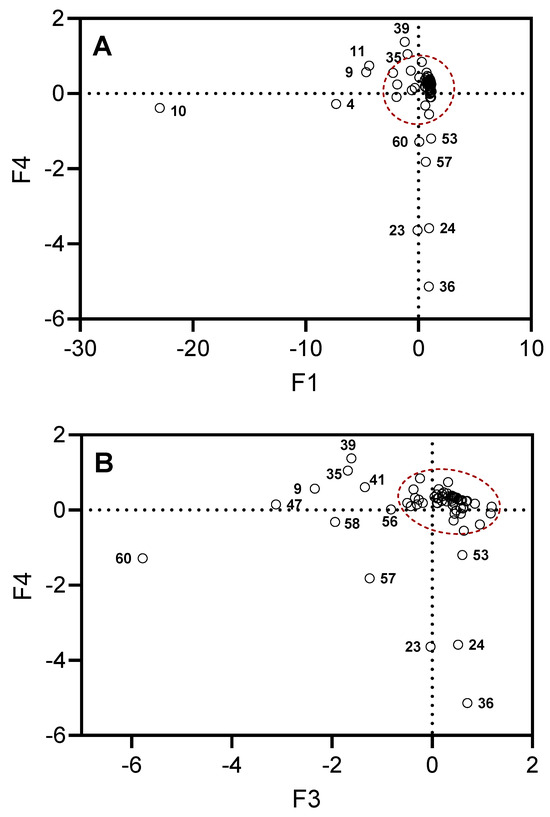
Figure 3.
Projections of the selected compounds contained in the essential oils on the planes defined by F1, F4 (Panel A) and F3, F4 (Panel B). The dots outside of the red dashed areas correspond to the compounds most responsible for the differences in MT#1 and UT#8 from the other essential oil samples. Compound numbers correspond to Supplementary Table S1.
2.2. Effect of E. nauseosa Essential Oil on Neutrophil Ca2+ Influx and Chemotaxis
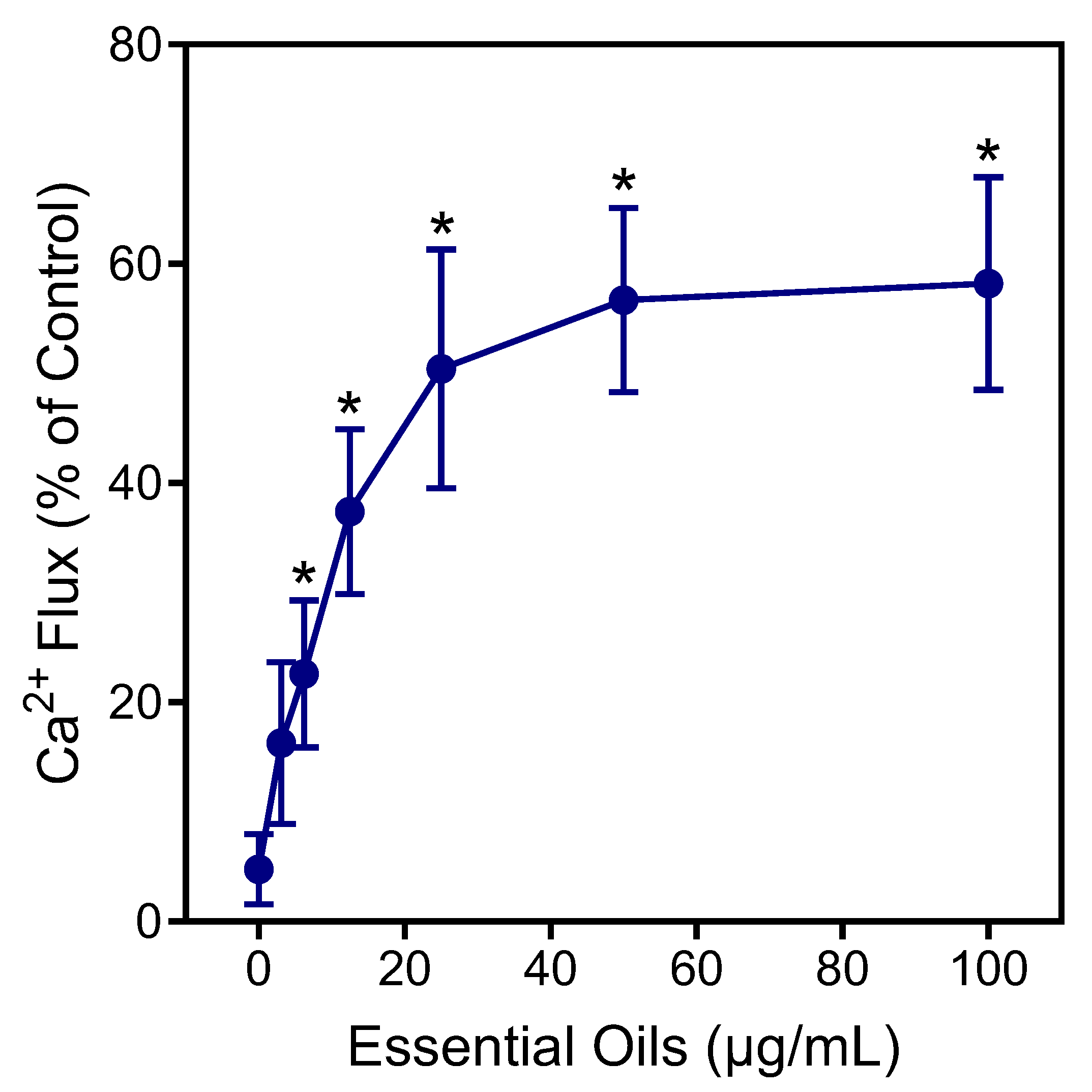
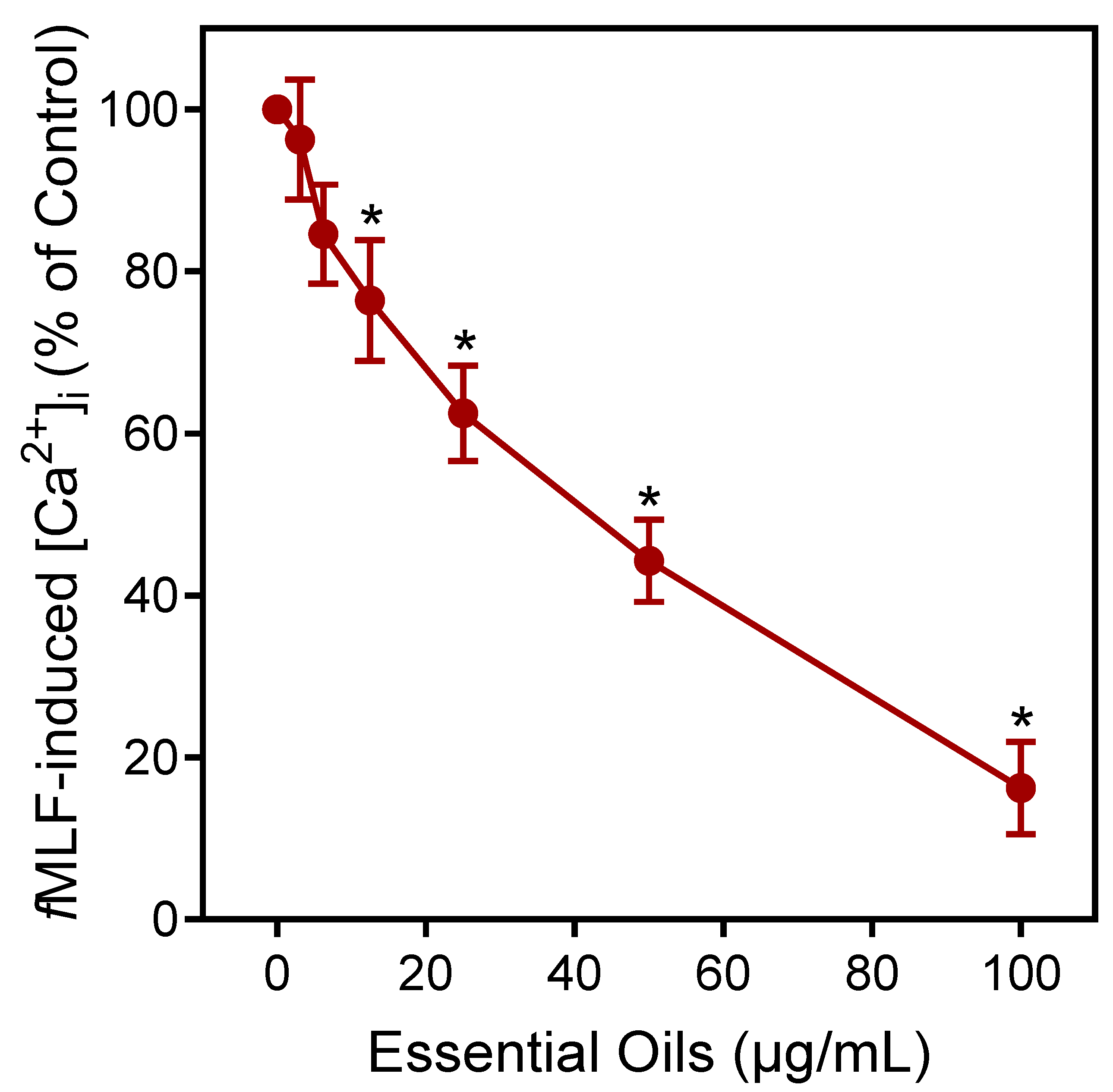
Neutrophils are essential for host innate immunity and, therefore, represent an ideal pharmacological target for therapeutic development [50]. Thus, we evaluated E. nauseosa essential oil for its immunomodulatory effects on human neutrophils. In particular, effects on neutrophil intracellular Ca2+ flux ([Ca2+]i) were evaluated, since [Ca2+]i is essential during neutrophil activation [51]. As shown in Figure 4, treatment of neutrophils with E. nauseosa essential oil activated these phagocytes, resulting in increased [Ca2+]i, with an EC50 of 27.2 ± 1.4 µg/mL.
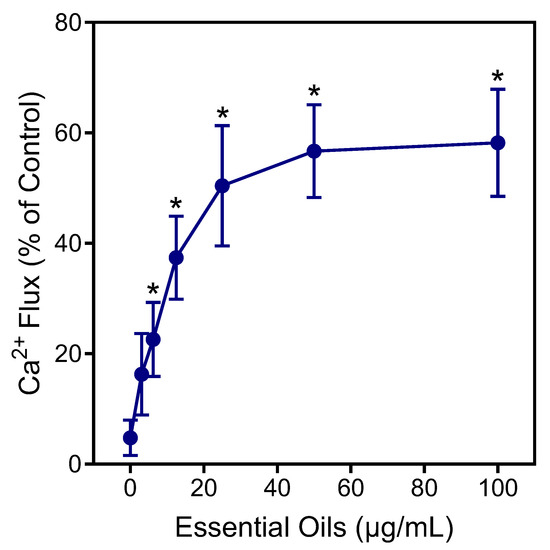
Figure 4.
Effect of E. nauseosa essential oil on human neutrophil Ca2+ mobilization. Human neutrophils were treated with the indicated concentrations of E. nauseosa essential oil, and [Ca2+]i was measured, as described. The data are expressed as the change in [Ca2+]i and compared to control [Ca2+]i induced by 5 nM fMLF (100%) in neutrophils and plotted as the mean ± SD. The data presented are from one experiment that is representative of three independent experiments with similar results. * p < 0.01 compared to dimethyl sulfoxide (DMSO) control [Ca2+]i.
Agonists can downregulate or desensitize phagocyte responses to subsequent treatment with homologous or heterologous agonists [52]. Thus, we evaluated whether E. nauseosa essential oil could alter agonist-induced [Ca2+]i in human neutrophils stimulated with the inflammatory chemoattractant N-formyl-methionine-leucine-phenylalanine (fMLF). As shown in Figure 5, pre-incubation of neutrophils with E. nauseosa essential oil inhibited the subsequent neutrophil [Ca2+]i response to fMLF with an IC50 of 42.1 ± 2.8 µg/mL. Thus, these data confirm that one or more E. nauseosa essential oil components can act as human neutrophil agonists and, if briefly encountered by neutrophils, can downregulate neutrophil activation by subsequent agonist treatment.
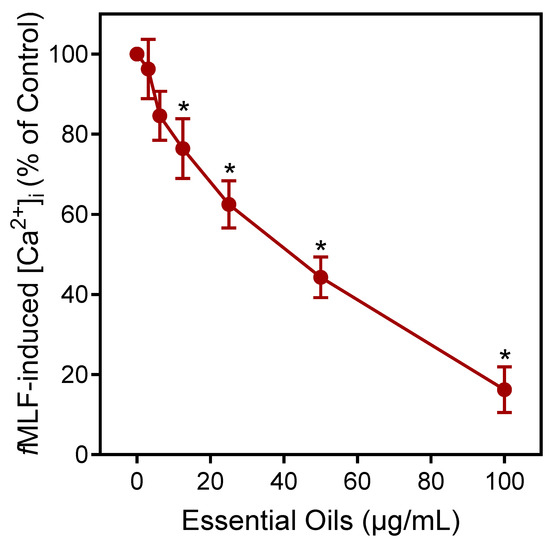
Figure 5.
Downregulation of fMLF-induced neutrophil Ca2+ flux by E. nauseosa essential oil. Human neutrophils were pretreated with E. nauseosa essential oil or control 1% DMSO for 10 min. After pretreatment, the neutrophils were stimulated with 5 nM fMLF, and intracellular Ca2+ flux was assessed. The data shown represent the mean ± SD from one experiment that is representative of three independent experiments with similar results. * p < 0.01 compared to DMSO control [Ca2+]i.
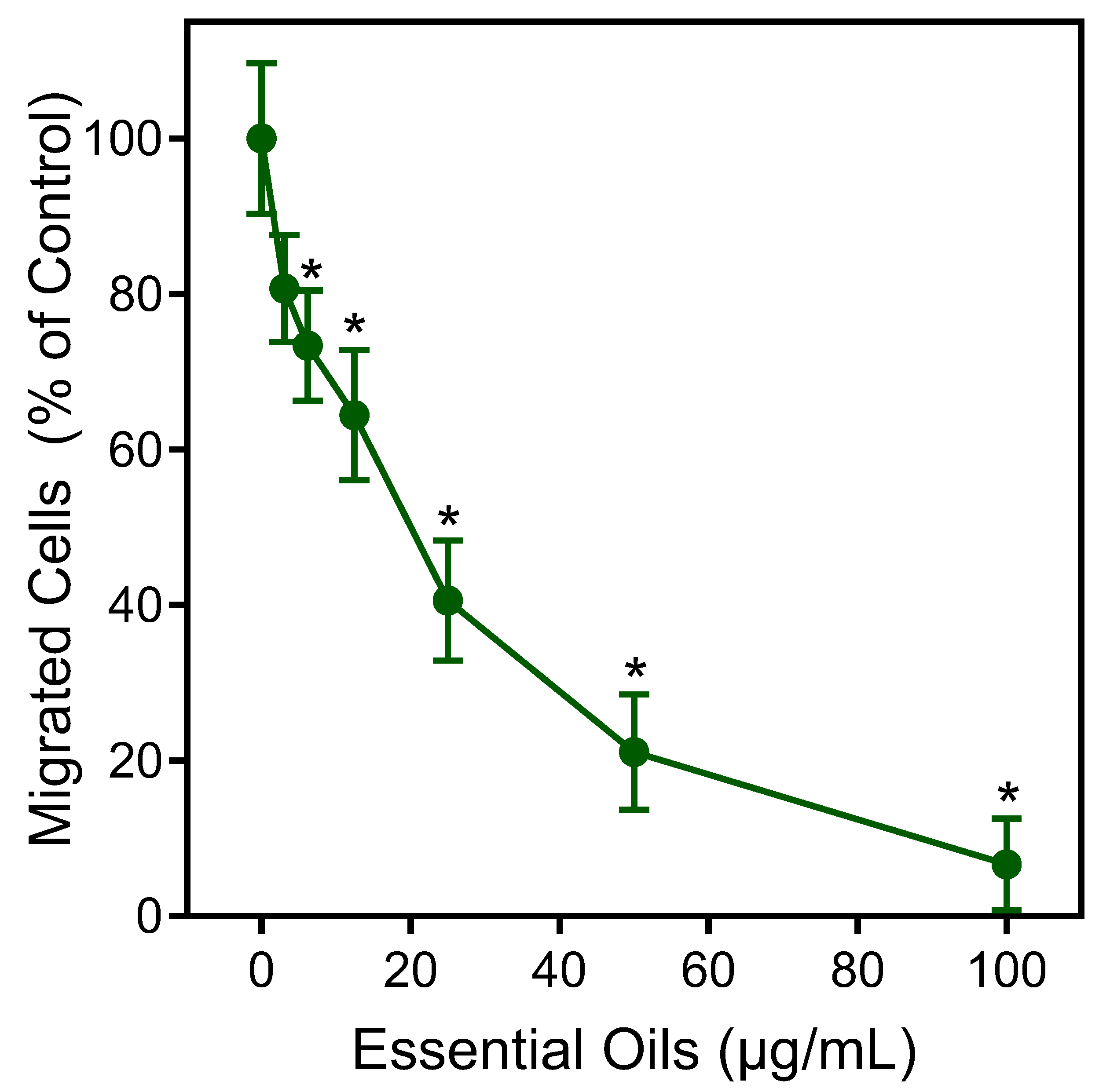
The effects of the E. nauseosa essential oil on human neutrophil chemotaxis were also evaluated. As shown in Figure 6, pretreatment of neutrophils with E. nauseosa essential oil dose-dependently inhibited fMLF-induced chemotaxis with an IC50 of 21.5 ± 3.8 μg/mL, again supporting the conclusion that exposure to E. nauseosa essential oil components can downregulate or desensitize neutrophil activation by subsequent agonist treatment.
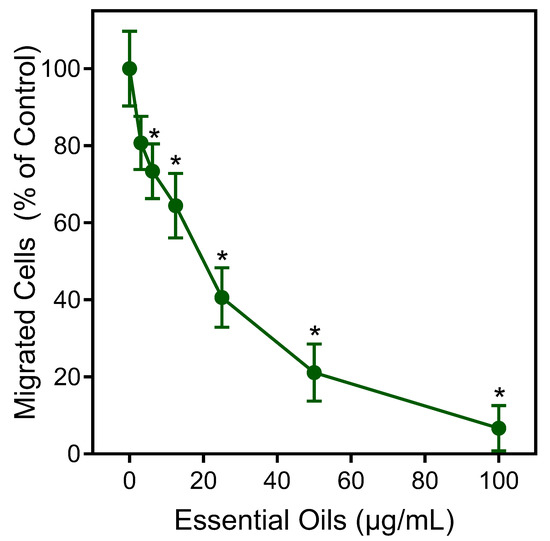
Figure 6.
Inhibition of neutrophil chemotaxis by E. nauseosa essential oil. Human neutrophils were incubated with E. nauseosa essential oil or control 1% DMSO for 30 min. After pretreatment, neutrophil chemotaxis was analyzed using 1 nM fMLF as the chemoattractant. The data shown represent the mean ± SD from one experiment that is representative of two independent experiments. * p < 0.01 compared to DMSO control [Ca2+]i.
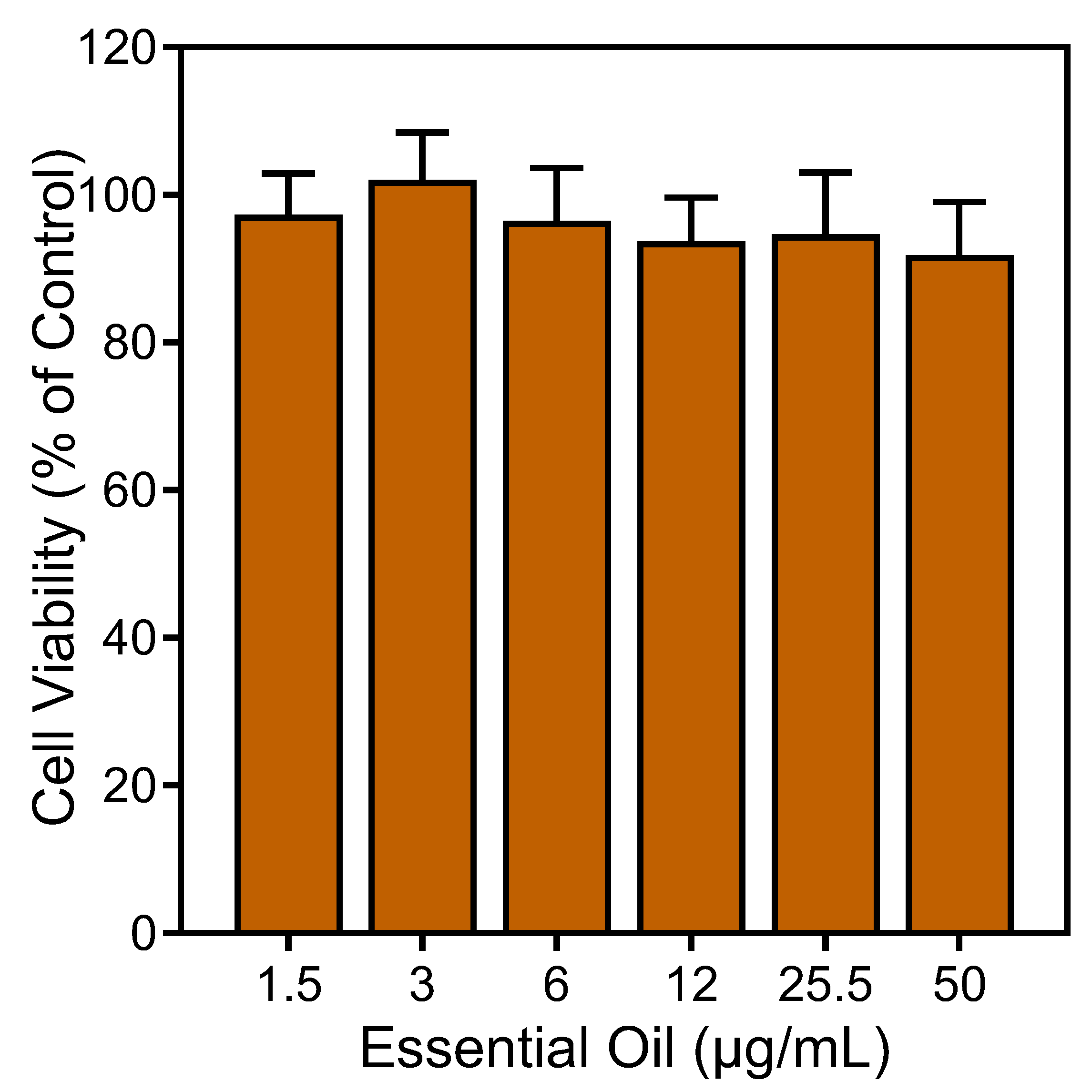
To ensure that the effects of the E. nauseosa essential oil on neutrophil responses were not impacted by possible toxicity, we assessed cytotoxicity of our samples (up to 50 µg/mL) during a 90 min incubation period. This incubation period is comparable to the times used in the Ca2+ mobilization (up to 30 min) and cell migration (up to 90 min) assays. We found that the E. nauseosa essential oil had minimal effects on cell viability during a 90 min incubation, verifying the lack of cytotoxicity during our assays (Figure 7).
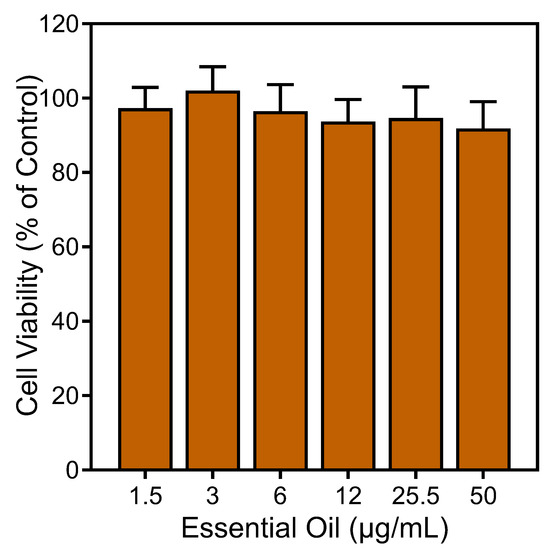
Figure 7.
Analysis of the cytotoxicity of E. nauseosa essential oil. Human neutrophils were treated with the indicated concentrations of E. nauseosa essential oil for 90 min, and cell viability was analyzed, as described. Values are the mean ± SD of triplicate samples from one experiment that is representative of two independent experiments with similar results.
In previous studies, we found that p-cymene, p-cymen-8-ol, limonene, myrcene, (E/Z)-β-ocimene, β-phellandrene, α-pinene, piperitenone, terpinen-4-ol, α-terpineol, α-thujone, terpinolene, and pulegone did not directly activate human neutrophil [Ca2+]i [39,53,54,55,56]. Thus, we can conclude that these compounds, which represent 19.3% of the total composition of E. nauseosa essential oil, are similarly not contributing to the neutrophil immunomodulatory activity of this essential oil. In contrast, we reported previously that nerolidol, β-pinene, spathulenol, sabinene, and γ-terpinene activated human neutrophil [Ca2+]i [53,55,57]. Thus, these compounds, which represent 6.2% of total composition of E. nauseosa essential oil, are likely contributing at least part of its immunomodulatory activity by activating neutrophil Ca2+ flux and desensitizing these cells to subsequent agonist activation. Note that ~60% of the remaining components of the E. nauseosa essential oil, including polyacetylenes, are not commercially available, have not yet been studied for immunomodulatory activity, and thus may also contribute to the effects on neutrophil function.
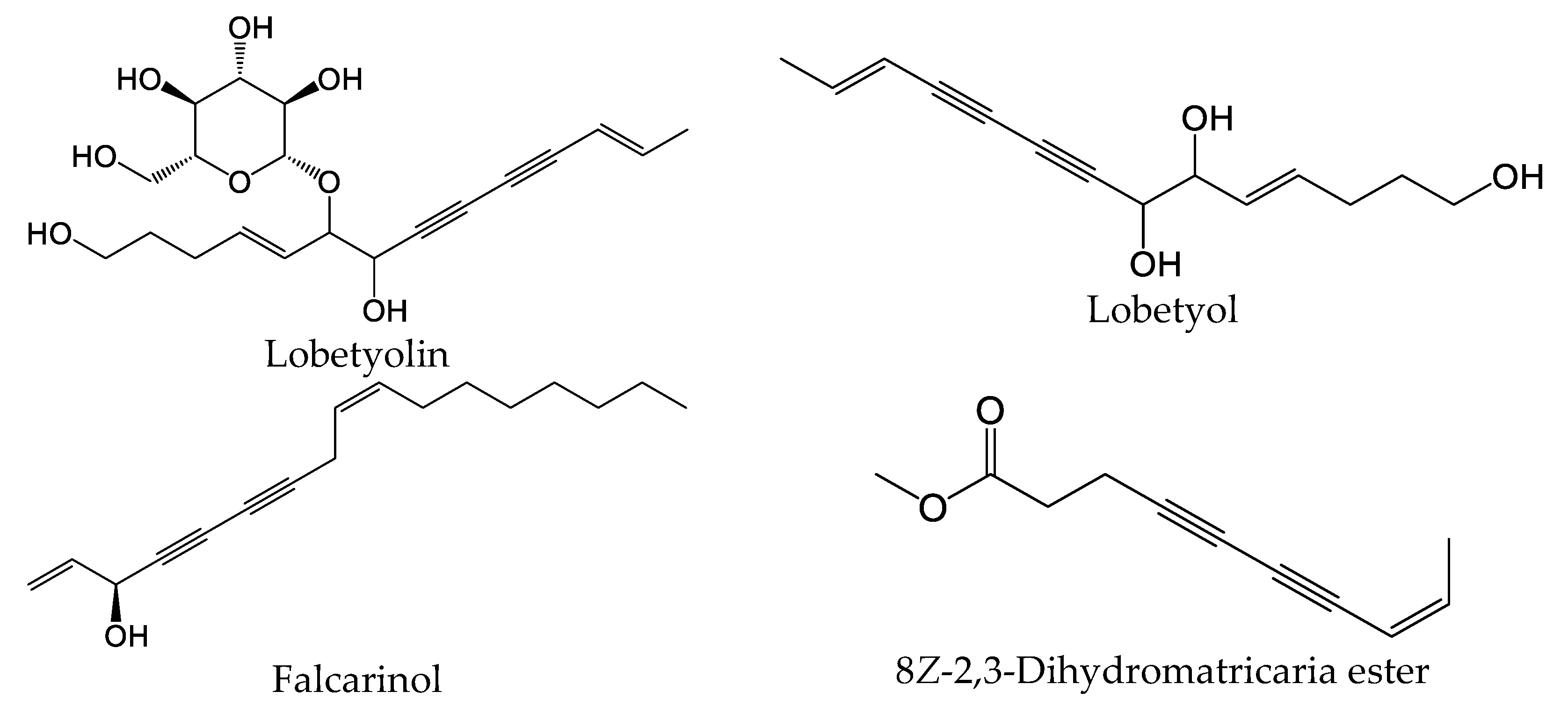
Polyacetylenes are a type of compound with carbon–carbon triple bonds and are found in many plant species [58]. This core structure is commonly substituted with alkyl groups, allylic alcohols, and esters in a variety of alkene isomer combinations. Dehydromatricaria-type esters were previously isolated from various plants, including Artemisia ordosica [59], Solidago altissima [60], and Tanacetum falconeri [61]. Chemical structures of 8Z-2,3-dihydromatricaria ester, which was found in our E. nauseosa essential oil samples, and some selected bioactive polyacetylenes are shown in Figure 8. It should be noted that natural matricaria-type esters, such as the tiglate and hydroxy esters, possess insecticidal activity, as well as activity against Mycobacterium tuberculosis [62]. Importantly, pharmacological studies indicate that polyacetylenes possess multiple biological activities, including immunomodulatory activity [58]. For example, lobetyolin significantly downregulated the expression of interleukin (IL)-6, tumor necrosis factor (TNF), and IL-1β in lipopolysaccharide (LPS)-stimulated peritoneal macrophages [63]. This compound was found to be a potent antagonist of G protein-coupled receptor GPR105, which is highly expressed in human neutrophils and sensitive to monosodium urate crystals [64]. Likewise, falcarinol (also known as carotatoxin or panaxynol) and dendranacetylene A were found to inhibit LPS-induced nitric oxide (NO) production in cultured mouse macrophages [65,66]. Finally, adociacetylene A was found to inhibit endothelial cell–neutrophil leukocyte adhesion in vitro [67]. Thus, it is reasonable to suggest that polyacetylenes may contribute to the biological properties of E. nauseosa essential oil; however, future studies will be necessary to assess this issue.
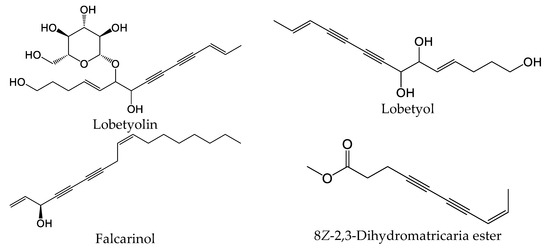
Figure 8.
Chemical structures of 8Z-2,3-dihydromatricaria ester found in Southwestern Montana E. nauseosa essential oil and selected other bioactive polyacetylenes.
3. Materials and Methods
3.1. Plant Material
We collected wild plants in August 2020, approximately 4 miles south of Norris, MT, USA (45.536519° N, 111.698579° E); voucher specimen number EN-SW-1. Botanical identification of the plant material was performed in the Department of Plant Sciences and Plant Pathology, Montana State University, Bozeman, MT, USA. Aerial parts were air-dried for 7–10 days at room temperature in the dark prior to hydrodistillation.
3.2. Chemicals and Reagents
n-Hexane was purchased from Merck (Darmstadt, Germany). A C8–C40 n-alkane standard solution was purchased from Fluka (Buchs, Switzerland). Dimethyl sulfoxide (DMSO), N-formyl-Met-Leu-Phe (fMLF), ethylenediaminetetraacetic acid (EDTA), and Histopaque 1077 were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Fluo-4AM was purchased from Invitrogen (Carlsbad, CA, USA). Hanks’ balanced salt solution (HBSS; 0.137 M NaCl, 5.4 mM KCl, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 4.2 mM NaHCO3, 5.56 mM glucose, and 10 mM HEPES, pH 7.4) was purchased from Life Technologies (Grand Island, NY, USA). HBSS without Ca2+ and Mg2+ was designated as HBSS–; HBSS containing 1.3 mM CaCl2 and 1.0 mM MgSO4 was designated as HBSS+.
3.3. Essential Oil Distillation
Essential oil was extracted by hydrodistillation of air-dried plant material (leaves and flowers) using a Clevenger-type apparatus. We used conditions accepted by the European Pharmacopoeia (European Directorate for the Quality of Medicines, Council of Europe, Strasbourg, France, 2014) to avoid artifacts. Yields of the essential oil were calculated based on the amount of air-dried plant material used. Stock solutions of the essential oil were prepared in DMSO (10 mg/mL) for biological evaluation and in n-hexane (10% w/v) for gas chromatographic analysis.
3.4. Gas Chromatography–Flame Ionization Detector (GC-FID) and Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
GC-MS analysis was performed with an Agilent 5975 GC-MSD system (Agilent Technologies, Santa Clara, CA, USA), as reported previously [68]. An Agilent Innowax FSC column (60 m × 0.25 mm, 0.25 μm film thickness) was used with He as the carrier gas (0.8 mL/min). The GC oven temperature was kept at 60 °C for 10 min, increased to 220 °C at a rate of 4 °C/min, kept constant at 220 °C for 10 min, and then increased to 240 °C at a rate of 1 °C/min. The split ratio was adjusted to 40:1, and the injector temperature was 250 °C. MS spectra were monitored at 70 eV with a mass range of 35 to 450 m/z. GC analysis was carried out using an Agilent 6890N GC system. To obtain the same elution order as with GC-MS, the line was split for FID and MS detectors, and a single injection was performed using the same column and appropriate operational conditions. The FID temperature was 300 °C. The essential oil components were identified by co-injection with standards (whenever possible), which were purchased from commercial sources or isolated from natural sources. In addition, compound identities were confirmed by comparison of their mass spectra with those in the Wiley GC/MS Library (Wiley, NY, USA), MassFinder software 4.0 (Dr. Hochmuth Scientific Consulting, Hamburg, Germany), Adams Library, and NIST Library. Confirmation was also achieved using the in-house “Başer Library of Essential Oil Constituents” database, obtained from chromatographic runs of pure compounds performed with the same equipment and conditions. A C8–C40 n-alkane standard solution (Fluka, Buchs, Switzerland) was used to spike the samples for the determination of relative retention indices (RRIs). Relative percentage amounts of the separated compounds were calculated from the FID chromatograms.
3.5. Isolation of Human Neutrophils
For isolation of human neutrophils, blood was collected from healthy donors in accordance with a protocol approved by the Institutional Review Board at Montana State University (protocol #MQ041017). Neutrophils were purified from the blood using dextran sedimentation, followed by Histopaque 1077 gradient separation and hypotonic lysis of red blood cells, as described previously [57].
3.6. Ca2+ Mobilization Assay
Changes in intracellular Ca2+ concentrations ([Ca2+]i) were measured with a FlexStation 3 scanning fluorometer (Molecular Devices, Sunnyvale, CA, USA), as described previously [57]. To assess the direct effects of pure essential oil on Ca2+ influx, the essential oil was added to the wells (final concentration of DMSO was 1%), and changes in fluorescence were monitored (λex = 485 nm, λem = 538 nm) every 5 s for 240 s at room temperature after addition of the test compound. To evaluate inhibitory effects of the compounds on FPR1-dependent Ca2+ influx, the compound/oil was added to the wells (final concentration of DMSO was 1%) with human neutrophils. The samples were preincubated for 10 min, followed by addition of 5 nM fMLF. The maximum change in fluorescence, expressed in arbitrary units over baseline, was used to determine the agonist response.
3.7. Chemotaxis Assay
To evaluate the effects of the E. nauseosa essential oil and its components on neutrophil migration, we resuspended the neutrophils in chemotaxis media (HBSS+ containing 2% (v/v) heat-inactivated FBS) at 2 × 106 cells/mL. We analyzed chemotaxis using 96-well ChemoTx chambers (Neuroprobe, Gaithersburg, MD, USA), as described previously [57]. Calculation of median effective concentrations (IC50) was performed by nonlinear regression analysis of the dose–response curves.
3.8. Cytotoxicity Assay
Cytotoxicity of the essential oil and pure compounds in human neutrophils was analyzed with a CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol and as previously described [57].
3.9. Statistical Analysis
The concentrations of 68 compounds found in our essential oil samples from plants collected in Southwestern Montana (MT#1) and those reported for essential oil samples from plants collected in North Central Utah and Southwestern Idaho (UT#1…UT#8, ID#1…ID#6) [27] (see Supplemental Table S1) were used for comparison and statistical analyses. Concentrations indicated as “trace” were assigned as 0.01% for statistical analysis. HCA and principal component analysis (PCA) were performed using STATISTICA 6.0 software (StatSoft, Moscow, Russia). For HCA, the unweighted pair group method with arithmetic average was used for cluster definition, while the Pearson correlation was applied to measure linkage distance or similarity. For performing the PCA, components F1–F4 (each accounting for >5% of the initial variance) were retained and used to explore the differences in the sample compositions. One-way analysis of variance (ANOVA) was performed on datasets in the biological experiments, followed by Tukey’s pair-wise comparisons. Pair-wise comparisons with differences at p < 0.05 were considered to be statistically significant.
4. Conclusions
Essential oil isolated from the leaves of E. nauseosa contained relatively high (>2%) amounts of γ-decalactone, cryptone, terpinen-4-ol, (E)-methyl cinnamate, T-cadinol, spathulenol, 8Z-2,3-dihydromatricaria ester, β-phellandrene, p-cymen-8-ol, 3-ethoxy-2-cycloocten-1-one, and trans-p-menth-2-en-1-ol. Overall, E. nauseosa essential oil was predominantly composed of monoterpenes (oxygenated monoterpenes and hydrocarbons), with cryptone as the main representative. The sesquiterpenes were represented only by oxygenated compounds, with T-cadinol, spathulenol, and α-cadinol as the main representatives. Distinctive components were the lactones (up to 15%) and polyacetylenes (up to 3.1%), namely (2Z,8Z)-matricaria ester and 8Z-2,3-dihydromatricaria ester, which are structurally characterized by a conjugated ene–diyne–ene system. A comparison with other reported E. nauseosa essential oil samples showed that our samples from plants collected in Southwestern Montana were quite distinct from those collected in other areas of the country; however, they did have the most similarity to one sample collected in North Central Utah.
Pharmacological studies showed that E. nauseosa essential oil activated human neutrophil Ca2+ influx, which desensitized these cells to subsequent agonist-induced functional responses. Based on previously reported data that nerolidol, β-pinene, spathulenol, sabinene, and γ-terpinene were active in human neutrophils, these compounds are the most likely constituents contributing to this immunomodulatory activity. However, the relatively high amount of polyacetylenes may also contribute, as these compounds have been characterized as potent immunomodulators. Thus, future studies are needed to evaluate the potential of the matricaria esters as modulators of innate immune cells. In addition, it will be interesting to evaluate potential anti-inflammatory properties of polyacetylene compounds if they really do contribute to the therapeutic effects of E. nauseosa essential oil; however, this will require extensive work to isolate these pure compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13152063/s1, Supplementary Table S1. Chemical composition of essential oil from E. nauseosa of North-Central Utah (UT#1-8), Southwestern Idaho (ID#1-6), and Southwestern Montana (MT#1). Supplementary Figure S1. GC Chromatogram of E. nauseosa essential oil.
Author Contributions
Conceptualization, I.A.S., G.Ö. and M.T.Q.; methodology, G.Ö., T.Ö. and L.N.K.; formal analysis, I.A.S., G.Ö., T.Ö., L.N.K., K.A. and M.L.; data curation, T.Ö. and M.T.Q.; statistical analysis, A.I.K.; writing—original draft preparation, I.A.S., G.Ö. and M.T.Q.; writing—review and editing, I.A.S., G.Ö., M.T.Q. and L.N.K.; supervision, T.Ö. and M.T.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by National Institutes of Health IDeA Program Grant GM103474, USDA National Institute of Food and Agriculture Hatch project 1009546, the Montana State University Agricultural Experiment Station, and the Ministry of Science and Higher Education of the Russian Federation (project no. FSWW-2023-0008).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mandel, J.R.; Dikow, R.B.; Siniscalchi, C.M.; Thapa, R.; Watson, L.E.; Funk, V.A. A fully resolved backbone phylogeny reveals numerous dispersals and explosive diversifications throughout the history of Asteraceae. Proc. Natl. Acad. Sci. USA 2019, 116, 14083–14088. [Google Scholar] [CrossRef]
- Bremer, K.; Jansen, R.K.; Karis, P.O.; Källersjö, M.; Keeley, S.C.; Kim, K.J.; Michaels, H.J.; Palmer, J.D.; Wallace, R.S. A review of the phylogeny and classification of the Asteraceae. Nord. J. Bot. 1992, 12, 141–148. [Google Scholar] [CrossRef]
- Roberts, R.P.; Urbatsch, L.E. Molecular phylogeny of Ericameria (Asteraceae, Astereae) based on nuclear ribosomal 3′ ETS and ITS sequence data. Taxon 2003, 52, 209–228. [Google Scholar]
- Anderson, L.C. Unique Chrysothamnus hybridizations in Ash Meadows, Nevada. Bull. Torrey Bot. Club 1973, 100, 171–177. [Google Scholar] [CrossRef]
- Hall, H.M. The Genus Haplopappus—A Phylogenetic Study in the Compositae; Carnegie Institution of Washington: Washington, DC, USA, 1928; pp. 1–391. [Google Scholar]
- Nesom, G.L. Taxonomic summary of Ericameria (Asteraceae, Astereae), with the inclusion of Haplopappus sects. Macronema and Asiris. Phytologia 1990, 68, 144–155. [Google Scholar] [CrossRef]
- Reveal, J.L. Proposal to conserve the name Chrysocoma nauseosa (Chrysothamnus nauseosus, Ericameria nauseosa) with a conserved type (Asteraceae). Taxon 2008, 57, 305–306. [Google Scholar]
- Anderson, L.C. The Chrysothamnus-Ericameria connection (Asteraceae). Great Basin Nat. 1995, 55, 84–88. [Google Scholar]
- Rose, A.F. Grindelane diterpenoids from Chrysothamnus nauseosus. Phytochemistry 1980, 19, 2689–2693. [Google Scholar] [CrossRef]
- Bohlmann, F.; Dutta, L.; Robinson, H.; King, R.M. Neue labdan-derivate aus Chrysothamnus nauseusus. Phytochemistry 1979, 18, 1889–1892. [Google Scholar] [CrossRef]
- Hell, T.; Dobrzyński, M.; Gröflin, F.; Reinhardt, J.K.; Dürr, L.; Pertz, O.; Hamburger, M.; Garo, E. Flavonoids from Ericameria nauseosa inhibiting PI3K/AKT pathway in human melanoma cells. Biomed. Pharm. 2022, 156, 113754. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Hegazy, M.-E.F.; Hassan, N.M.; Wojcinska, M.; Karchesy, J.; Pare, P.W.; Mabry, T.J. Constituents of Chrysothamnus viscidiflorus. Phytochemistry 2006, 67, 1547–1553. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; Mohamed, A.E.-H.H.; El-Razek, M.H.A.; Hammouda, F.M.; Hassan, N.M.; Mahalel, U.A.; El-Halawany, A.M.; Mahmoud, A.A.; Karchesy, J.; Hirata, T. Genus Chrysothamnus: A source of bioactive compounds. Nat. Prod. Commun. 2007, 2, 951–957. [Google Scholar] [CrossRef]
- Rose, A.F.; Butt, B.; Jermy, T. Polyacetylenes from the rabbitbrush, Chrysothamnus nauseosus. Phytochemistry 1980, 19, 563–566. [Google Scholar] [CrossRef]
- Hegerhorst, D.; Weber, D.; McArthur, E.; Khan, A. Chemical analysis and comparison of subspecies of Chrysothamnus nauseosus and other related species. Biochem. Syst. Ecol. 1987, 15, 201–208. [Google Scholar] [CrossRef]
- Moerman, D.E. Native American Medicinal Plants: An Ethnobotanical Dictionary; Timber Press: Portland, OR, USA, 2009; p. 139. [Google Scholar]
- Hart, J.A. The ethnobotany of the Northern Cheyenne Indians of Montana. J. Ethnopharmacol. 1981, 4, 1–55. [Google Scholar] [CrossRef]
- Train, P.; Henrichs, J.R.; Archer, W.A. Medicinal Uses of Plants by Indian Tribes of Nevada. In US Department of Agriculture; Division of Plant Exploration and Introduction, Bureau of Plant Industry, U.S. Department of Agriculture: Washington, DC, USA, 1957; p. 57. [Google Scholar]
- Childress, D.; Sanchez, S.; French, V.; Terry, T.J. Isolation, separation, and identification of antimicrobial compounds from Ericameria nauseosa. N. M. J. Sci. 2018, 52, 45–46. [Google Scholar]
- Acharya, J.; Hildreth, M.B.; Reese, R.N. In vitro screening of forty medicinal plant extracts from the United States Northern Great Plains for anthelmintic activity against Haemonchus contortus. Vet. Parasitol. 2014, 201, 75–81. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [PubMed]
- Sandner, G.; Heckmann, M.; Weghuber, J. Immunomodulatory activities of selected essential oils. Biomolecules 2020, 10, 1139. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Haran, G.H.; Calisto, V.; Jothi, G.; Quintans, J.S.S.; Cuevas, L.E.; Narain, N.; Júnior, L.J.Q.; Cipolotti, R.; et al. Essential oils and its bioactive compounds modulating cytokines: A systematic review on anti-asthmatic and immunomodulatory properties. Phytomedicine 2020, 73, 152854. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Kirpotina, L.N.; Schepetkin, I.A.; Khlebnikov, A.I.; Lisonbee, B.L.; Black, J.L.; Woolf, H.; Thurgood, T.L.; Graf, B.L.; Satyal, P.; et al. Volatile composition, antimicrobial activity, and in vitro innate immunomodulatory activity of Echinacea purpurea (L.) Moench essential oils. Molecules 2023, 28, 7330. [Google Scholar] [CrossRef]
- Tabanca, N.; Demirci, B.; Crockett, S.L.; Başer, K.H.C.; Wedge, D.E. Chemical composition and antifungal activity of Arnica longifolia, Aster hesperius, and Chrysothamnus nauseosus essential oils. J. Agric. Food Chem. 2007, 55, 8430–8435. [Google Scholar] [CrossRef]
- Chao, S.; Young, D.G.; Casabianca, H.; Bertrand, M.-C. Composition of the oils of three Chrysothamnus nauseousus varieties. J. Essent. Oil Res. 2003, 15, 425–427. [Google Scholar] [CrossRef]
- Stirling, J.; Platt, B.G.; Satyal, P.; Swor, K.; Setzer, W.N. The essential oils of rubber rabbitbrush (Ericameria nauseosa) from North-Central Utah and Southwestern Idaho. Nat. Prod. Commun. 2023, 18, 1–12. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Wedge, D.E.; Tabanca, N.; Sampson, B.J.; Werle, C.; Demirci, B.; Baser, K.H.C.; Nan, P.; Duan, J.; Liu, Z. Antifungal and insecticidal activity of two Juniperus essential oils. Nat. Prod. Commun. 2009, 4, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Luo, L.; Zhang, H.; Yang, X.; Lv, Y.; Song, H. Common aroma-active components of propolis from 23 regions of China. J. Sci. Food Agric. 2010, 90, 1268–1282. [Google Scholar] [CrossRef]
- Piasenzotto, L.; Gracco, L.; Conte, L. Solid phase microextraction (SPME) applied to honey quality control. J. Sci. Food Agric. 2003, 83, 1037–1044. [Google Scholar] [CrossRef]
- Chenni, M.; El Abed, D.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Comparative study of essential oils extracted from Egyptian basil leaves (Ocimum basilicum L.) using hydro-distillation and solvent-free microwave extraction. Molecules 2016, 21, 113. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Demirci, B.; Kurkcuoglu, M.; Satin, F.; Tumen, G. Comparative morphological and phytochemical charactertization of Salvia cadmica and S. smyrnaea. Pak. J. Bot. 2009, 41, 1545–1555. [Google Scholar]
- Tunalier, Z.; Kirimer, N.; Baser, K.H.C. The composition of essential oils from various parts of Juniperus foetidissima. Chem. Nat. Compd. 2002, 38, 43–47. [Google Scholar] [CrossRef]
- Wedge, D.E.; Klun, J.A.; Tabanca, N.; Demirci, B.; Ozek, T.; Baser, K.H.C.; Liu, Z.; Zhang, S.; Cantrell, C.L.; Zhang, J. Bioactivity-guided fractionation and GC/MS fingerprinting of Angelica sinensis and Angelica archangelica root components for antifungal and mosquito deterrent activity. J. Agric. Food Chem. 2009, 57, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Hazzit, M.; Baaliouamer, A.; Faleiro, M.L.; Miguel, M.G. Composition of the essential oils of Thymus and Origanum species from Algeria and their antioxidant and antimicrobial activities. J. Agric. Food Chem. 2006, 54, 6314–6321. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Özek, T.; Demirci, B.; Kürkcüoglu, M.; Aytac, Z.; Duman, H. Composition of the essential oils of Zosima absinthifolia (Vent.) Link and Ferula elaeochytris Korovin from Turkey. Flavour Fragr. J. 2000, 15, 371–372. [Google Scholar] [CrossRef]
- Suleimen, Y.; Atazhanova, G.; Ozek, T.; Demirci, B.; Kulyyasov, A.T.; Adekenov, S.M.; Baser, K.H.C. Essential oil composition of three species of Achillea from Kazakhstan. Chem. Nat. Compd. 2006, 37, 447–450. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Özek, G.; Özek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Chemical composition and immunomodulatory activity of Hypericum perforatum essential oils. Biomolecules 2020, 10, 916. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Özek, T.; Dmirci, B.; Duman, H. Composition of the essential oil of Glaucosciadium cordifolium (Boiss.) Burtt et Davis from Turkey. Flavour Fragr. J. 2000, 15, 45–46. [Google Scholar] [CrossRef]
- Sylvestre, M.; Pichette, A.; Longtin, A.; Nagau, F.; Legault, J. Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L. from Guadeloupe. J. Ethnopharmacol. 2006, 103, 99–102. [Google Scholar] [CrossRef]
- Lorenzo, D.; Dellacassa, E.; Atti-Serafini, L.; Santos, A.C.; Frizzo, C.; Paroul, N.; Moyna, P.; Mondello, L.; Dugo, G. Composition and stereoanalysis of Cymbopogon winterianus Jowitt oil from Southern Brazil. Flavour Fragr. J. 2000, 15, 177–181. [Google Scholar] [CrossRef]
- Viljoen, A.M.; Subramoney, S.; van Vuuren, S.F.; Baser, K.H.C.; Demirci, B. The composition, geographical variation and antimicrobial activity of Lippia javanica (Verbenaceae) leaf essential oils. J. Ethnopharmacol. 2005, 96, 271–277. [Google Scholar] [CrossRef]
- Göger, G.; Yavaş, I.; Yur, S.; Köse, Y.B.; Özek, G. Volatiles and fatty acid analyzes of Tripleurospermum decipiens (Fisch & C. A. Mey) Bornm and biological activities of the extracts. J. Res. Pharm. 2021, 25, 429–440. [Google Scholar]
- Vernin, G.; Merad, O.; Vernin, G.; Zamkotsian, R.; Parkanyi, C. GC-MS analysis of Artemisia herba alba Asso essential oils from Algeria. Dev. Food Sci. 1995, 37, 147–205. [Google Scholar]
- Adawe, A.M.; Dari, Y.; Uysal, Ü.D.; Özek, T.; Özek, G.; Güray, T. Determination of biological activity of the of Azadiracta indica A. Juss. grown In Somalia. Int. J. Sci. Res. Chem. 2022, 7, 1–14. [Google Scholar]
- Maggio, A.; Riccobono, L.; Spadaro, V.; Scialabba, A.; Bruno, M.; Senatore, F. Chemical composition of the essential oils of three endemic species of Anthemis Sect. Hiorthia (DC.) R. Fern. growing wild in Sicily and chemotaxonomic volatile markers of the genus Anthemis L.: An update. Chem. Biodiver. 2014, 11, 652–672. [Google Scholar] [CrossRef] [PubMed]
- Halls, S.C.; Gang, D.R.; Weber, D.J. Seasonal variation in volatile secondary compounds of Chrysothamnus nauseosus (Pallas) Britt.; Asteraceae ssp. hololeucus (Gray) Hall. & Clem. influences herbivory. J. Chem. Ecol. 1994, 20, 2055–2063. [Google Scholar]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A-Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M.; Borregaard, N. Neutrophils at work. Nat. Immunol. 2014, 15, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Dixit, N.; Simon, S.I. Chemokines, selectins and intracellular calcium flux: Temporal and spatial cues for leukocyte arrest. Front. Immunol. 2012, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Richardson, R.M.; Haribabu, B.; Snyderman, R. Chemoattractant receptor cross-desensitization. J. Biol. Chem. 1999, 274, 6027–6030. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Ozek, G.; Ozek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Chemical composition and immunomodulatory activity of essential oils from Rhododendron albiflorum. Molecules 2021, 26, 3652. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Ozek, G.; Ozek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Klein, R.A.; Quinn, M.T. Neutrophil immunomodulatory activity of farnesene, a component of Artemisia dracunculus essential oils. Pharmaceuticals 2022, 15, 642. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kushnarenko, S.V.; Ozek, G.; Kirpotina, L.N.; Sinharoy, P.; Utegenova, G.A.; Abidkulova, K.T.; Ozek, T.; Baser, K.H.; Kovrizhina, A.R.; et al. Modulation of human neutrophil responses by the essential oils from Ferula akitschkensis and their constituents. J. Agric. Food Chem. 2016, 64, 7156–7170. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kushnarenko, S.V.; Ozek, G.; Kirpotina, L.N.; Utegenova, G.A.; Kotukhov, Y.A.; Danilova, A.N.; Ozek, T.; Baser, K.H.; Quinn, M.T. Inhibition of human neutrophil responses by the essential oil of Artemisia kotuchovii and its constituents. J. Agric. Food Chem. 2015, 63, 4999–5007. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Ozek, G.; Ozek, T.; Kirpotina, L.N.; Kokorina, P.I.; Khlebnikov, A.I.; Quinn, M.T. Neutrophil immunomodulatory activity of nerolidol, a major component of essential oils from Populus balsamifera Buds and propolis. Plants 2022, 11, 3399. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, C. Polyacetylenes in herbal medicine: A comprehensive review of its occurrence, pharmacology, toxicology, and pharmacokinetics (2014–2021). Phytochemistry 2022, 201, 113288. [Google Scholar] [CrossRef]
- Wang, Q.H.; Hao, J.S.; Gong, J.H.; Bao, W.Q. Isolation and structure elucidation of two new compounds from Artemisia ordosica krasch. Nat. Prod. Res. 2020, 34, 1862–1867. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Allelopathy and Allelochemicals of Solidago canadensis L. and S. altissima L. for Their Naturalization. Plants 2022, 11, 3235. [Google Scholar] [CrossRef]
- Ismail, M.; Kowsar, A.; Javed, S.; Choudhary, M.I.; Khan, S.W.; Abbas, Q.; Tang, Y.R.; Wang, W. The antibacterial, insecticidal and nematocidal activities and toxicity studies of Tanacetum falconeri Hook. f. Turk. J. Pharm. Sci. 2021, 18, 744–751. [Google Scholar] [CrossRef]
- Garrais, S.; Turkington, J.; Goldring, W.P. Synthesis of isomeric polyacetylenes based on natural hydroxy matricaria esters. Tetrahedron 2009, 65, 8418–8427. [Google Scholar] [CrossRef]
- Chen, Z.H.; Su, Y.X.; Ding, J.T.; He, J.; Lai, L.H.; Song, Y.J. Lobetyolin protects mice against LPS-induced sepsis by downregulating the production of inflammatory cytokines in macrophage. Front. Pharmacol. 2024, 15, 1405163. [Google Scholar] [CrossRef]
- Liu, C.X.; Zhou, M.Z.; Jiang, W.J.; Ye, S.M.; Tian, S.; Jiang, C.; Hao, K.; Li, H.Q.; Hu, Q.H. GPR105-targeted therapy promotes gout resolution as a switch between NETosis and apoptosis of neutrophils. Front. Immunol. 2022, 13, 870183. [Google Scholar] [CrossRef]
- Matsuda, H.; Murakami, T.; Kageura, T.; Ninomiya, K.; Toguchida, I.; Nishida, N.; Yoshikawa, M. Hepatoprotective and nitric oxide production inhibitory activities of coumarin and polyacetylene constituents from the roots of Angelica furcijuga. Bioorg. Med. Chem. Lett. 1998, 8, 2191–2196. [Google Scholar] [CrossRef]
- Li, M.; Zeng, M.N.; Zhang, J.K.; Shi, J.Y.; Lv, J.J.; Tang, Y.Y.; Zheng, X.K.; Feng, W.S. Anti-inflammatory Dendranacetylene A, a new polyacetylene glucoside from the flower of Chrysanthemum morifolium Ramat. Nat. Prod. Res. 2021, 35, 5692–5698. [Google Scholar] [CrossRef]
- Kobayashi, M.; Mahmud, T.; Tajima, H.; Wang, W.Q.; Aoki, S.; Nakagawa, S.; Mayumi, T.; Kitagawa, I. Marine natural products. XXXVI. Biologically active polyacetylenes, adociacetylenes A, B, C, and D, from an Okinawan marine sponge of Adocia sp. Chem. Pharmaceut. Bull. 1996, 44, 720–724. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Özek, G.; Özek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Neutrophil immunomodulatory activity of (−)-borneol, a major component of essential oils extracted from Grindelia squarrosa. Molecules 2022, 27, 4897. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).