Competition in the Periphytic Algal Community during the Colonization Process: Evidence from the World’s Largest Water Diversion Project
Abstract
:1. Introduction
2. Results
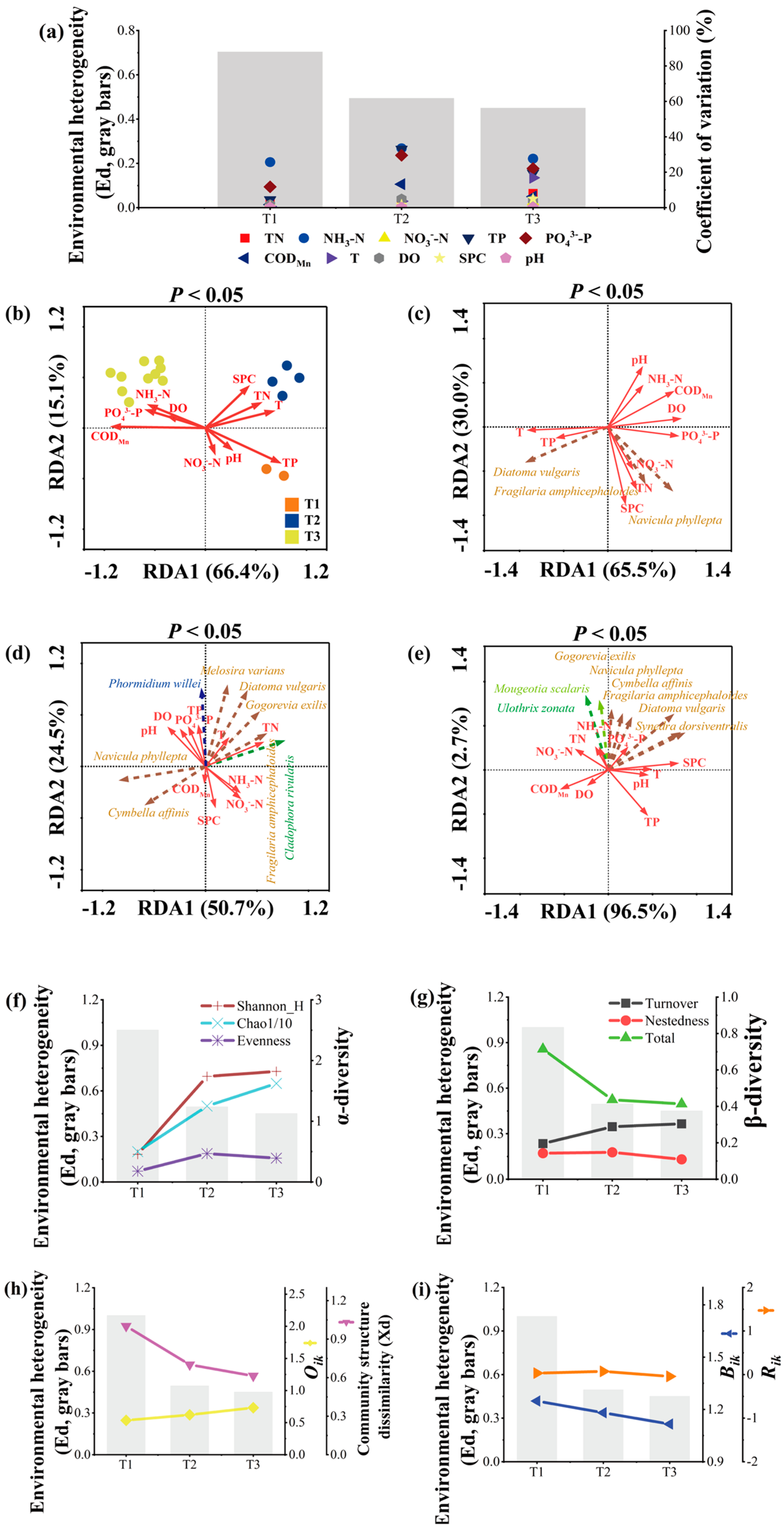
2.1. The Colonization Process
2.2. Niche Analysis
3. Discussion
3.1. Colonization Process and the Influencing Factors
3.2. The Interspecific Competition Driving the Colonization Process
4. Materials and Methods
4.1. Sample Station and Sample Collection
4.2. Periphytic Algal Identification and Quantity
4.3. Environmental Parameters
4.4. Niche Analysis
4.5. Environmental Heterogeneity
4.6. Periphytic Algal Community Structure
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gerson, J.R.; Topp, S.N.; Vega, C.M.; Gardner, J.R.; Yang, X.; Fernandez, L.E.; Bernhardt, E.S.; Pavelsky, T.M. Artificial lake expansion amplifies mercury pollution from gold mining. Sci. Adv. 2020, 6, eabd4953. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.; Xi, C.; Raskin, L. Microbial ecology of drinking water distribution systems. Curr. Opin. Biotechnol. 2006, 17, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yin, W.; Wang, C.; Zhang, A.; Zhang, H.; Zhang, T.; Ju, F. Untangling microbiota diversity and assembly patterns in the world’s largest water diversion canal. Water Res. 2021, 204, 117617. [Google Scholar] [CrossRef] [PubMed]
- de Vega, G.B.; Ceballos, J.A.; Anzalone, A.; Digman, M.A.; Gratton, E. A laser-scanning confocal microscopy study of carrageenan in red algae from seaweed farms near the Caribbean entrance of the Panama Canal. J. Appl. Phycol. 2017, 29, 495–508. [Google Scholar] [CrossRef]
- Bru, D.; Ramette, A.; Saby, N.P.; Dequiedt, S.; Ranjard, L.; Jolivet, C.; Arrouays, D.; Philippot, L. Determinants of the distribution of nitrogen-cycling microbial communities at the landscape scale. ISME 2011, 5, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.S.; Zalmon, I.R.; Love, M. Overview and trends of ecological and socioeconomic research on artificial reefs. Mar. Environ. Res. 2019, 145, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Munoz, I.; Real, M.; Guasch, H.; Navarro, E.; Sabater, S. Effects of atrazine on periphyton under grazing pressure. Aquat. Toxicol. 2001, 55, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Wolfstein, K.; Colijn, F.; Doerffer, R. Seasonal dynamics of microphytobenthos biomass and photosynthetic characteristics in the northern German Wadden Sea, obtained by the photosynthetic light dispensation system. Estuar. Coast. Shelf Sci. 2000, 51, 651–662. [Google Scholar] [CrossRef]
- Battin, T.J.; Besemer, K.; Bengtsson, M.M.; Romani, A.M.; Packmann, A.I. The ecology and biogeochemistry of stream biofilms. Nat. Rev. Microbiol. 2016, 14, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.X.; Li, S.J.; Hou, K.; Ji, G.D. Spatial and seasonal bacterioplankton community dynamics in the main channel of the Middle Route of South-to-North Water Diversion Project. Res. Microbiol. 2019, 170, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, R.G. Limnology: Lake and River Ecosystems; Wetzel, R.G., Ed.; Academic Press: San Diego, CA, USA, 2001. [Google Scholar] [CrossRef]
- dos Santos, T.R.; Ferragut, C. The successional phases of a periphytic algal community in a shallow tropical reservoir during the dry and rainy seasons. Limnetica 2013, 32, 337–351. [Google Scholar] [CrossRef]
- França, R.C.S.; Lopes, M.R.M.; Ferragut, C. Structural and successional variability of periphytic algal community in a Amazonian lake during the dry and rainy season (Rio Branco, Acre). Acta Amaz. 2011, 41, 257–266. [Google Scholar] [CrossRef]
- Cross, W.F.; Benstead, J.P.; Frost, P.C.; Thomas, S.A. Ecological stoichiometry in freshwater benthic systems: Recent progress and perspectives. Freshw. Biol. 2005, 50, 1895–1912. [Google Scholar] [CrossRef]
- Montalvo, A.M.; Williams, S.L.; Rice, K.J.; Buchmann, S.L.; Cory, C.; Handel, S.N.; Nabhan, G.P.; Primack, R.; Robichaux, R.H. Restoration biology: A population biology perspective. Restor. Ecol. 1997, 5, 277–290. [Google Scholar] [CrossRef]
- Barabas, G.; D’Andrea, R.; Stump, S.M. Chesson’s coexistence theory. Ecol. Monogr. 2018, 88, 277–303. [Google Scholar] [CrossRef]
- Pastore, A.I.; Barabas, G.; Bimler, M.D.; Mayfield, M.M.; Miller, T.E. The evolution of niche overlap and competitive differences. Nat. Ecol. Evol. 2021, 5, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.Q.; Hu, Z.Q.; Liu, X.; Fu, Y.K.; Zhang, W.J. Spatio-Temporal Variation of Total Nitrogen and Ammonia Nitrogen in the Water Source of the Middle Route of the South-To-North Water Diversion Project. Water 2020, 12, 2615. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Li, L.Q.; Liu, Z.H.; Chen, X.N.; Huang, H.Y. Real-Time Control of the Middle Route of South-to-North Water Diversion Project. Water 2021, 13, 97. [Google Scholar] [CrossRef]
- Clifford, C.C.; Heffernan, J.B. Artificial Aquatic Ecosystems. Water 2018, 10, 1096. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, J.K.; Lin, J.Q.; Lei, X.H.; Ding, G.B.; Yuan, R.F. A study on the phytoplankton community structure in the Diaohe River section of the Middle Route of the South-to-North Water Diversion Project in winter. Water Supply 2021, 21, 959–970. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Mi, W.J.; Tu, X.J.; Song, G.F.; Bi, Y.H. Environmental Factors Drive Periphytic Algal Community Assembly in the Largest Long-Distance Water Diversion Channel. Water 2022, 14, 914. [Google Scholar] [CrossRef]
- Das, S.K.; Singh, D. Epiphytic Algae on the Bryophytes of Larsemann Hills, East Antarctica. Natl. Acad. Sci. Lett. 2021, 44, 161–165. [Google Scholar] [CrossRef]
- Azim, M.E. Photosynthetic Periphyton and Surfaces; Academic Press: Oxford, UK, 2009; pp. 184–191. [Google Scholar] [CrossRef]
- Azim, M.E.; Verdegem, M.C.J.; van Dam, A.A.; Beveridge, M. Periphyton. Ecology, Exploitation and Management; CABI Publishing: Oxfordshire, UK, 2005. [Google Scholar]
- Sedano, F.; de Figueroa, J.M.T.; Navarro-Barranco, C.; Ortega, E.; Guerra-Garcia, J.M.; Espinosa, F. Do artificial structures cause shifts in epifaunal communities and trophic guilds across different spatial scales? Mar. Environ. Res. 2020, 158, 104998. [Google Scholar] [CrossRef]
- Becker, L.R.; Ehrenberg, A.; Feldrappe, V.; Kroncke, I.; Bischof, K. The role of artificial material for benthic communities—Establishing different concrete materials as hard bottom environments. Mar. Environ. Res. 2020, 161, 105081. [Google Scholar] [CrossRef] [PubMed]
- Granot, I.; Belmaker, J. Niche breadth and species richness: Correlation strength, scale and mechanisms. Glob. Ecol. Biogeogr. 2020, 29, 159–170. [Google Scholar] [CrossRef]
- Pfeiffer, T.Z.; Mihaljevic, M.; Stevic, F.; Spoljaric, D. Periphytic algae colonization driven by variable environmental components in a temperate floodplain lake. Ann. Limnol.-Int. J. Lim. 2013, 49, 179–190. [Google Scholar] [CrossRef]
- Carscadden, K.A.; Emery, N.C.; Arnillas, C.A.; Cadotte, M.W.; Afkhami, M.E.; Gravel, D.; Livingstone, S.W.; Wiens, J.J. Niche breadth: Causes and consequences for ecology, evolution, and conservation. Q. Rev. Biol. 2020, 95, 179–214. [Google Scholar] [CrossRef]
- Biggs, B.J.F. Eutrophication of streams and rivers: Dissolved nutrient-chlorophyll relationships for benthic algae. J. N. Am. Benthol. Soc. 2000, 19, 17–31. [Google Scholar] [CrossRef]
- Danger, M.; Lacroix, G.; Oumarou, C.; Benest, D.; Meriguet, J. Effects of food-web structure on periphyton stoichiometry in eutrophic lakes: A mesocosm study. Freshw. Biol. 2008, 53, 2089–2100. [Google Scholar] [CrossRef]
- Zhang, J.C.; Su, J.; Ma, C.; Hu, X.Y.; Teng, H.H. Periphytic Microbial Response to Environmental Phosphate (P) Bioavailability and Its Relevance to P Management in Paddy Fields. Appl. Environ. Microbiol. 2021, 87, 15. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Quiroz-Arita, C.; Monroe, E.A.; Siccardi, A.; Mitchell, J.; Huysman, N.; Davis, R.W. Application of attached algae flow-ways for coupling biomass production with the utilization of dilute non-point source nutrients in the Upper Laguna Madre, TX. Water Res. 2021, 191, 12. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.F.; Chapra, S.C. Evaluating hydraulic habitat suitability of filamentous algae using an unmanned aerial vehicle and acoustic doppler current profiler. J. Environ. Eng. 2020, 146, 04019126. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, H.; Zhang, J.; Yu, J.; Xie, P.; Chen, J. Physiological differences between free-floating and periphytic filamentous algae, and specific submerged macrophytes induce proliferation of filamentous algae: A novel implication for lake restoration. Chemosphere 2020, 239, 124702. [Google Scholar] [CrossRef] [PubMed]
- Dunck, B.; Rodrigues, L.; Lima-Fernandes, E.; Cassio, F.; Pascoal, C.; Cottenie, K. Priority effects of stream eutrophication and assembly history on beta diversity across aquatic consumers, decomposers and producers. Sci. Total Environ. 2021, 797, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Wang, Y.C.; Sun, H.Y.; Tang, J.F. Length-Weight Relationships of Eight Fish Species in the World’s Largest Water Diversion Project. J. Appl. Ichthyol. 2023, 2023, 5512824. [Google Scholar] [CrossRef]
- Lu, H.Y.; Wan, J.J.; Li, J.Y.; Shao, H.B.; Wu, Y.H. Periphytic biofilm: A buffer for phosphorus precipitation and release between sediments and water. Chemosphere 2016, 144, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Khatoon, H.; Shariff, M.; Yusoff, F.M. Immobilized periphytic cyanobacteria for removal of nitrogenouscompounds and phosphorus from shrimp farm wastewater. Turk. J. Biol. 2015, 39, 388–395. [Google Scholar] [CrossRef]
- Bondar-Kunze, E.; Kasper, V.; Hein, T. Responses of periphyton communities to abrupt changes in water temperature and velocity, and the relevance of morphology: A mesocosm approach. Sci. Total Environ. 2021, 768, 11. [Google Scholar] [CrossRef]
- Lizarazo, M.C.G.; Pinilla-Agudelo, G.; Galindo, I.J.E. Functional ecology of periphytic algae in the Colombian Choco: Resource limitations, competition, and environmental variables. Rev. Biol. Trop. 2021, 69, 331–351. [Google Scholar] [CrossRef]
- Sun, M.; Yang, Z.B.; Wawrik, B. Metabolomic Fingerprints of Individual Algal Cells Using the Single-Probe Mass Spectrometry Technique. Front. Plant Sci. 2018, 9, 571. [Google Scholar] [CrossRef] [PubMed]
- Swanson, H.K.; Lysy, M.; Power, M.; Stasko, A.D.; Johnson, J.D.; Reist, J.D. A new probabilistic method for quantifying n-dimensional ecological niches and niche overlap. Ecology 2015, 96, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ellner, S.P.; Snyder, R.E.; Adler, P.B.; Hooker, G. An expanded modern coexistence theory for empirical applications. Ecol. Lett. 2019, 22, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.P.; Turcotte, M.M.; Levine, J.M. Effects of rapid evolution on species coexistence. Proc. Natl. Acad. Sci. USA 2019, 116, 2112–2117. [Google Scholar] [CrossRef]
- Nemes-Kókai, Z.; Borics, G.; Csépes, E.; Lukács, A.; Török, P.; T-Krasznai, E.; Bácsi, I.; B-Béres, V. Role of microhabitats in shaping diversity of periphytic diatom assemblages. Hydrobiologia 2024, 851, 959–972. [Google Scholar] [CrossRef]
- da Silva, J.C.; Gubiani, E.A.; Neves, M.P.; Delariva, R.L. Coexisting small fish species in lotic neotropical environments: Evidence of trophic niche differentiation. Aquat. Ecol. 2017, 51, 275–288. [Google Scholar] [CrossRef]
- Martin, C.H.; Genner, M.J. High niche overlap between two successfully coexisting pairs of Lake Malawi cichlid fishes. Can. J. Fish Aquat. Sci. 2009, 66, 579–588. [Google Scholar] [CrossRef]
- Mruzek, J.L.; Budnick, W.R.; Larson, C.A.; Luc, D.K.; Passy, S. Stronger niche than dispersal effects on α- and β-diversity of stream algae, insects, and fish across latitudes in the United States. Glob. Ecol. Biogeogr. 2022, 31, 2453–2462. [Google Scholar] [CrossRef]
- Oliveira, P.H.F.; Machado, K.B.; Teresa, F.B.; de Carvalho, R.A.; Ferreira, M.E.; Tejerina-Garro, F.L.; Carvalho, P.; Ferragut, C.; Melo, A.S.; Nabout, J.C. Spatial distance explains the periphyton metacommunity structure of a neotropical stream network. Hydrobiologia 2023, 850, 1869–1884. [Google Scholar] [CrossRef]
- Taormina, B.; Percheron, A.; Marzloff, M.P.; Caisey, X.; Quillien, N.; Lejart, M.; Desroy, N.; Dugornay, O.; Tancray, A.; Carlier, A. Succession in epibenthic communities on artificial reefs associated with marine renewable energy facilities within a tide-swept environment. ICES J. Mar. Sci. 2020, 77, 2656–2668. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Sexton, J.P.; McIntyre, P.J.; Angert, A.L.; Rice, K.J. Evolution and Ecology of Species Range Limits. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 415–436. [Google Scholar] [CrossRef]
- Lima, M.S.; Schneck, F.; They, N.H.; Crossetti, L.O.; Bohnenberger, J.E.; McMahon, K.D.; Marques, D.D. Turnover is replaced by nestedness with increasing geographical distance in bacterial communities of coastal shallow lakes. Mar. Freshw. Res. 2020, 71, 1086–1098. [Google Scholar] [CrossRef]
- Bevilacqua, S.; Terlizzi, A. Nestedness and turnover unveil inverse spatial patterns of compositional and functional beta-diversity at varying depth in marine benthos. Divers. Distrib. 2020, 26, 743–757. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Global Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Segre, H.; Ron, R.; De Malach, N.; Henkin, Z.; Mandel, M.; Kadmon, R. Competitive exclusion, beta diversity, and deterministic vs. stochastic drivers of community assembly. Ecol. Lett. 2014, 17, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- MEEPRC. Technical Guidelines for Water Ecological Monitoring—Aquatic Organism Monitoring and Evaluation of Rivers (on Trial); MEEPRC: Beijing, China, 2024. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/shjbh/xgbzh/202305/t20230511_1029711.shtml (accessed on 15 June 2024).
- Hu, H.; Wei, Y. The Freshwater Algae of China—Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006. [Google Scholar]
- Guiry, M.D.G. AlgaeBase. Available online: https://www.algaebase.org (accessed on 3 April 2024).
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington DC, USA, 2017. [Google Scholar]
- Ranjard, L.; Dequiedt, S.; Prevost-Boure, N.C.; Thioulouse, J.; Saby, N.P.A.; Lelievre, M.; Maron, P.A.; Morin, F.E.R.; Bispo, A.; Jolivet, C.; et al. Turnover of soil bacterial diversity driven by wide-scale environmental heterogeneity. Nat. Commun. 2013, 4, 1434. [Google Scholar] [CrossRef] [PubMed]
- Huber, P.; Metz, S.; Unrein, F.; Mayora, G.; Sarmento, H.; Devercelli, M. Environmental heterogeneity determines the ecological processes that govern bacterial metacommunity assembly in a floodplain river system. ISME 2020, 14, 2951–2966. [Google Scholar] [CrossRef] [PubMed]
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
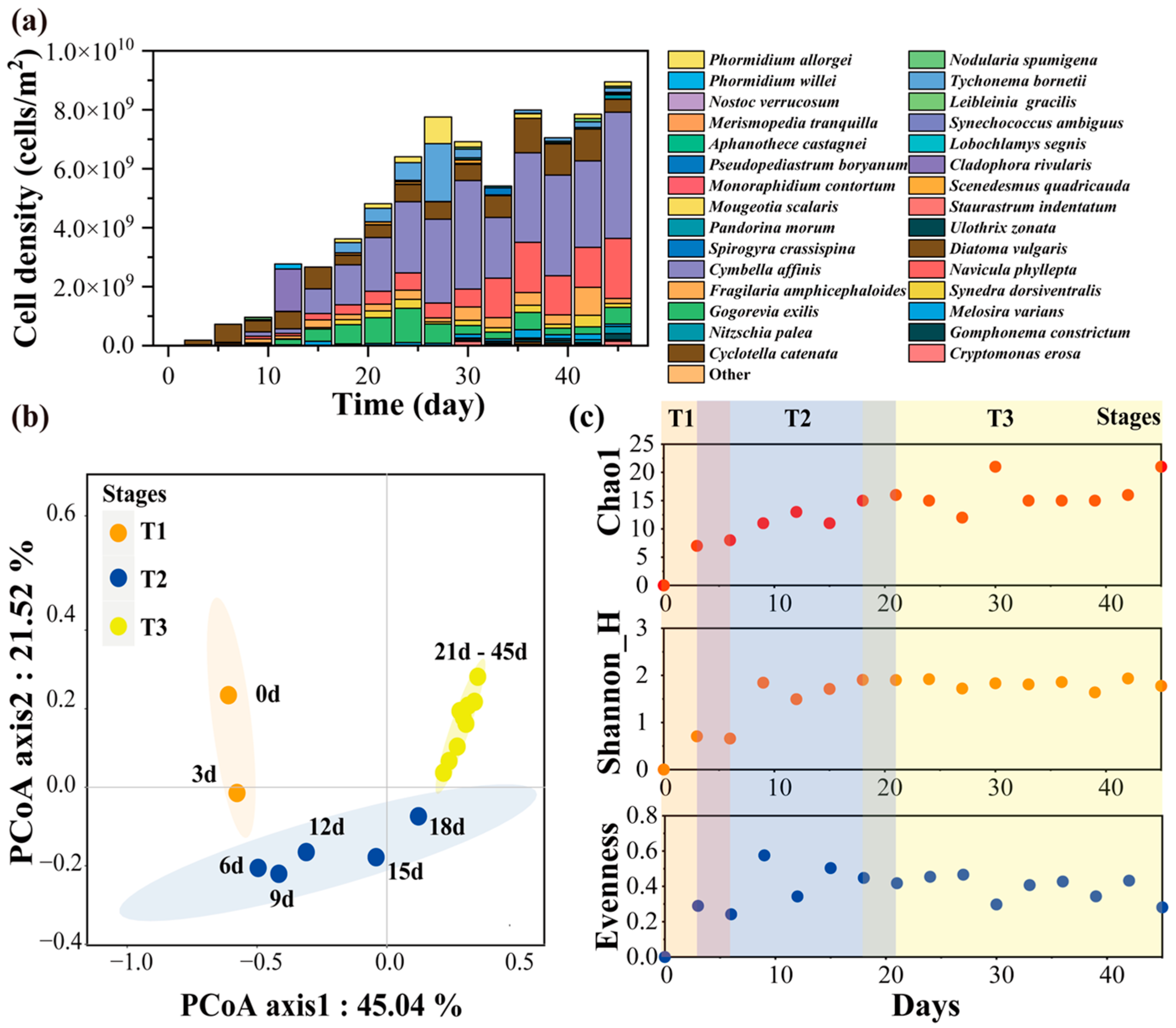

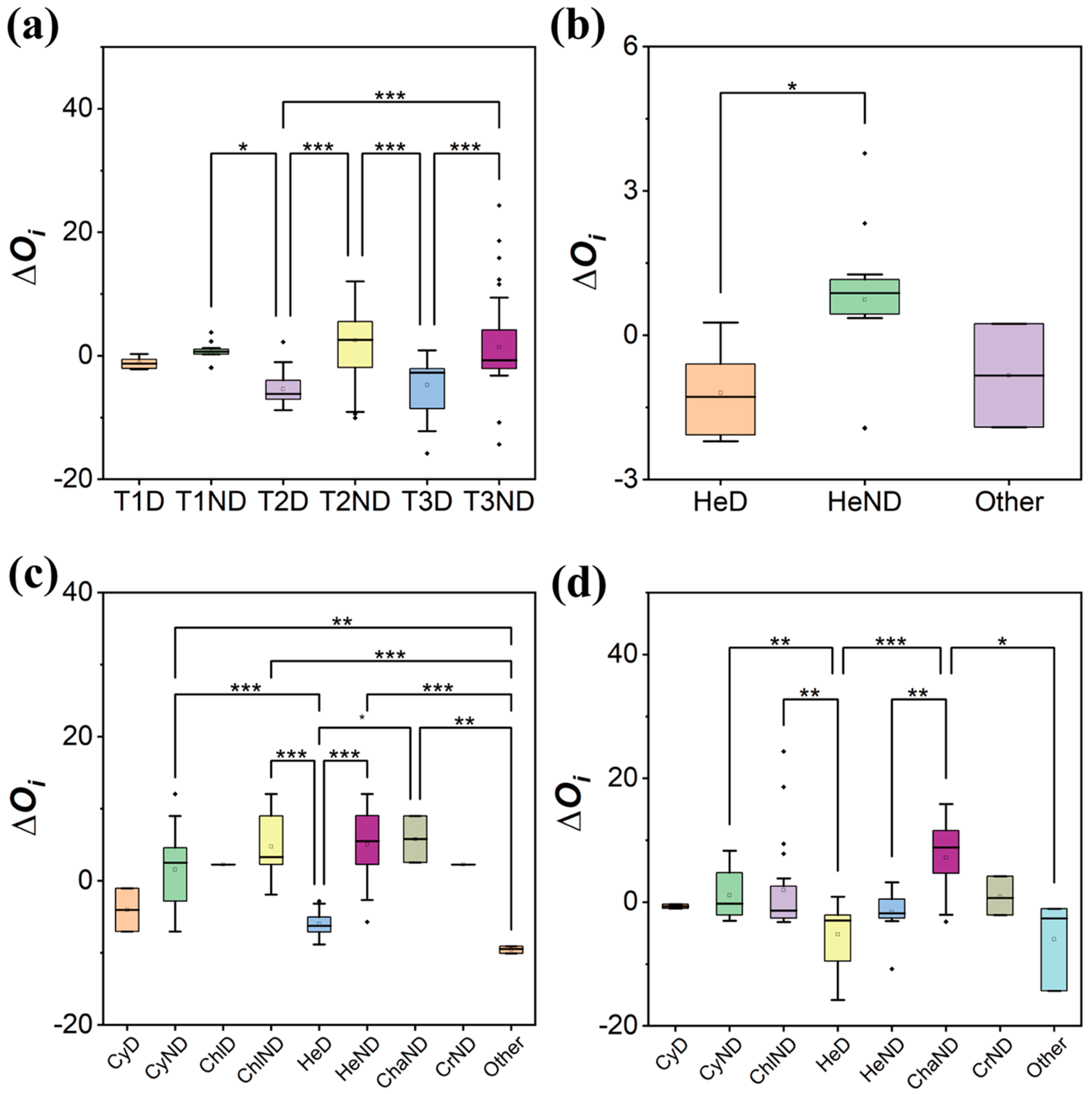
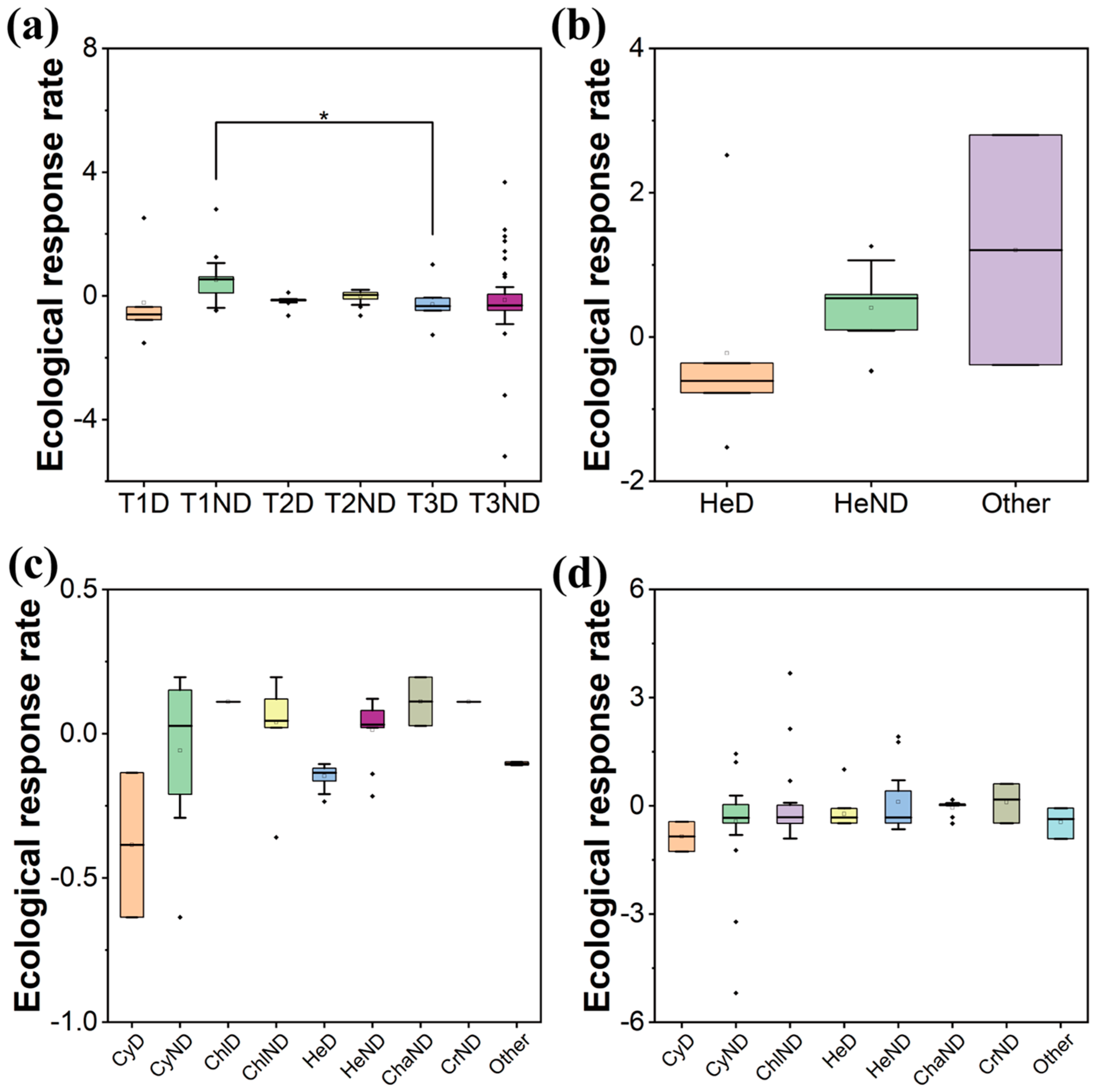
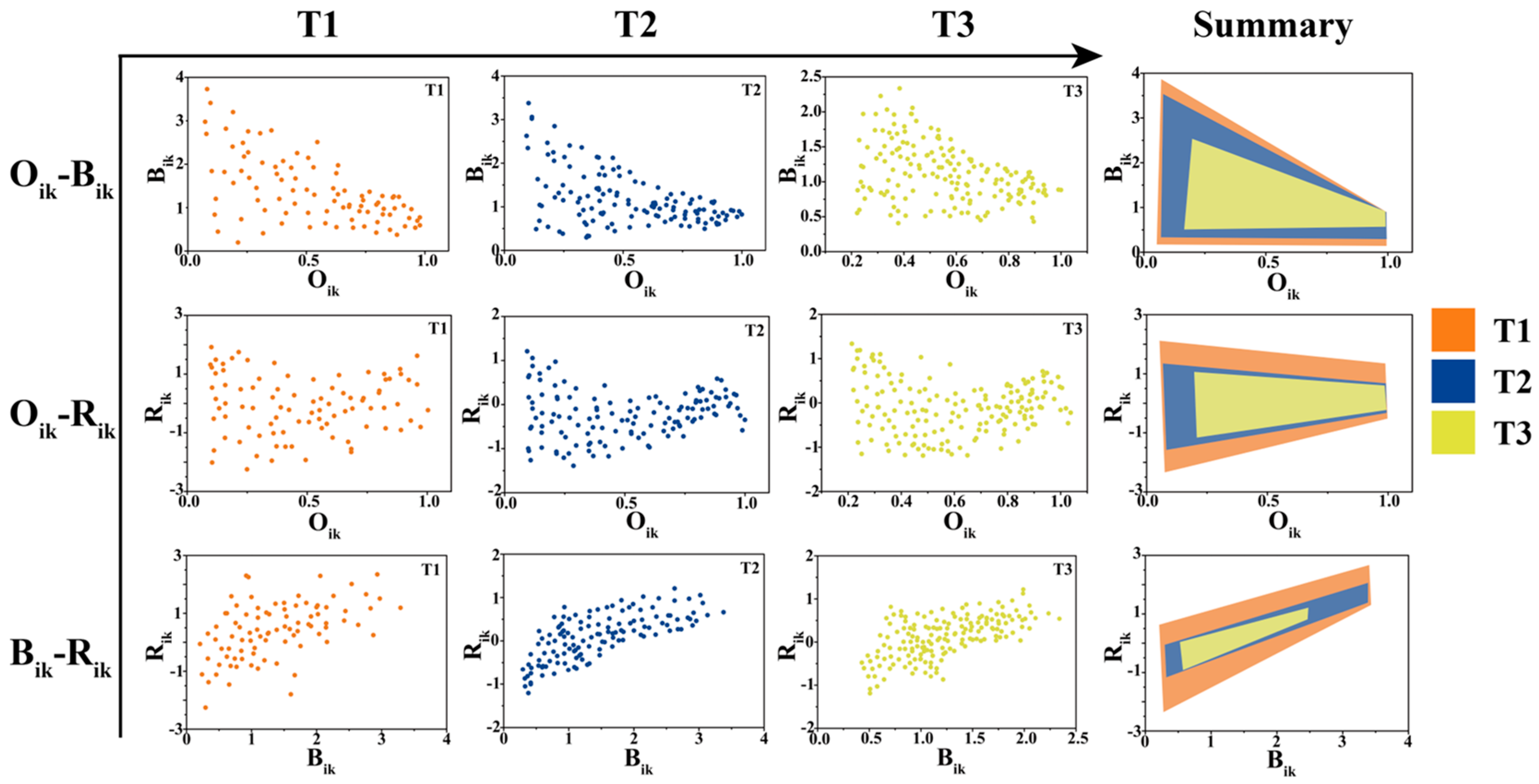
| Stage | Phylum | Species | Dominant Species |
|---|---|---|---|
| T1 (Sobs = 27) | Heterokontophyta | Diatoma vulgaris | D |
| Heterokontophyta | Fragilaria amphicephaloides | D | |
| Heterokontophyta | Navicula phyllepta | D | |
| Heterokontophyta | Gogorevia exilis | ND | |
| Heterokontophyta | Cymbella affinis | ND | |
| Heterokontophyta | Synedra dorsiventralis | ND | |
| Other (n = 21) | ND | ||
| T2 (Sobs = 87) | Cyanobacteria | Phormidium willei | D |
| Cyanobacteria | Leibleinia gracilis | ND | |
| Cyanobacteria | Nodularia spumigena | ND | |
| Cyanobacteria | Phormidium allorgei | ND | |
| Chlorophyta | Cladophora rivularis | D | |
| Chlorophyta | Monoraphidium contortum | ND | |
| Chlorophyta | Pseudopediastrum boryanum | ND | |
| Heterokontophyta | Gogorevia exilis | D | |
| Heterokontophyta | Cymbella affinis | D | |
| Heterokontophyta | Diatoma vulgaris | D | |
| Heterokontophyta | Fragilaria amphicephaloides | D | |
| Heterokontophyta | Melosira varians | D | |
| Heterokontophyta | Navicula phyllepta | D | |
| Heterokontophyta | Cyclotella catenata | ND | |
| Heterokontophyta | Gomphonema constrictum | ND | |
| Heterokontophyta | Nitzschia palea | ND | |
| Heterokontophyta | Synedra dorsiventralis | ND | |
| Cryptista | Cryptomonas erosa | ND | |
| Other (n = 69) | ND | ||
| T3 (Sobs = 131) | Cyanobacteria | Aphanothece castagnei | ND |
| Cyanobacteria | Leibleinia gracilis | ND | |
| Cyanobacteria | Merismopedia tranquilla | ND | |
| Cyanobacteria | Nodularia spumigena | ND | |
| Cyanobacteria | Nostoc verrucosum | ND | |
| Cyanobacteria | Phormidium allorgei | ND | |
| Cyanobacteria | Synechococcus ambiguus | ND | |
| Cyanobacteria | Tychonema bornetii | ND | |
| Chlorophyta | Ulothrix zonata | D | |
| Chlorophyta | Monoraphidium contortum | D | |
| Chlorophyta | Lobochlamys segnis | ND | |
| Chlorophyta | Pandorina morum | ND | |
| Chlorophyta | Pseudopediastrum boryanum | ND | |
| Chlorophyta | Scenedesmus quadricauda | ND | |
| Heterokontophyta | Gogorevia exilis | ND | |
| Heterokontophyta | Cymbella affinis | ND | |
| Heterokontophyta | Diatoma vulgaris | ND | |
| Heterokontophyta | Fragilaria amphicephaloides | D | |
| Heterokontophyta | Navicula phyllepta | D | |
| Heterokontophyta | Synedra dorsiventralis | D | |
| Heterokontophyta | Cyclotella catenata | D | |
| Heterokontophyta | Gomphonema constrictum | D | |
| Heterokontophyta | Melosira varians | D | |
| Heterokontophyta | Nitzschia palea | ND | |
| Charophyta | Mougeotia scalaris | ND | |
| Charophyta | Spirogyra crassispina | ND | |
| Charophyta | Staurastrum indentatum | ND | |
| Cryptista | Cryptomonas erosa | ND | |
| Other (n = 103) | ND |
| Stage | T1 | T2 | T3 |
|---|---|---|---|
| TN | 1.18 ± 0.04 a | 1.12 ± 0.03 a | 1.10 ± 0.09 a |
| NH3 | 0.06 ± 0.01 a | 0.04 ± 0.01 a | 0.06 ± 0.02 a |
| NO3−-N | 1.09 ± 0.02 a | 1.05 ± 0.02 a | 1.05 ± 0.03 a |
| TP | 0.03 ± 0.01 a | 0.02 ± 0.01 a | 0.01 ± 0.00 a |
| PO43−-P | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| CODMn | 1.63 ± 0.33 a | 1.91 ± 0.25 a | 2.06 ± 0.14 a |
| T | 23.67 ± 2.14 a | 24.96 ± 0.58 a | 25.73 ± 0.11 a |
| DO | 11.52 ± 0.18 a | 11.39 ± 0.55 a | 11.48 ± 0.49 a |
| SPC | 217.95 ± 1.48 a | 220.84 ± 3.61 a | 211.13 ± 10.61 a |
| pH | 8.42 ± 0.01 a | 8.44 ± 0.01 a | 8.42 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Tu, X.; Bi, Y.; Song, G.; Mi, W. Competition in the Periphytic Algal Community during the Colonization Process: Evidence from the World’s Largest Water Diversion Project. Plants 2024, 13, 2067. https://doi.org/10.3390/plants13152067
Zhu Y, Tu X, Bi Y, Song G, Mi W. Competition in the Periphytic Algal Community during the Colonization Process: Evidence from the World’s Largest Water Diversion Project. Plants. 2024; 13(15):2067. https://doi.org/10.3390/plants13152067
Chicago/Turabian StyleZhu, Yuxuan, Xiaojie Tu, Yonghong Bi, Gaofei Song, and Wujuan Mi. 2024. "Competition in the Periphytic Algal Community during the Colonization Process: Evidence from the World’s Largest Water Diversion Project" Plants 13, no. 15: 2067. https://doi.org/10.3390/plants13152067





