Comparative Physiological and Gene Expression Analyses Reveal Mechanisms Involved in Maintaining Photosynthesis Capacity, Alleviating Ion Toxicity and Oxidative Stress of Kentucky Bluegrass under NaCl Treatment
Abstract
:1. Introduction
2. Results
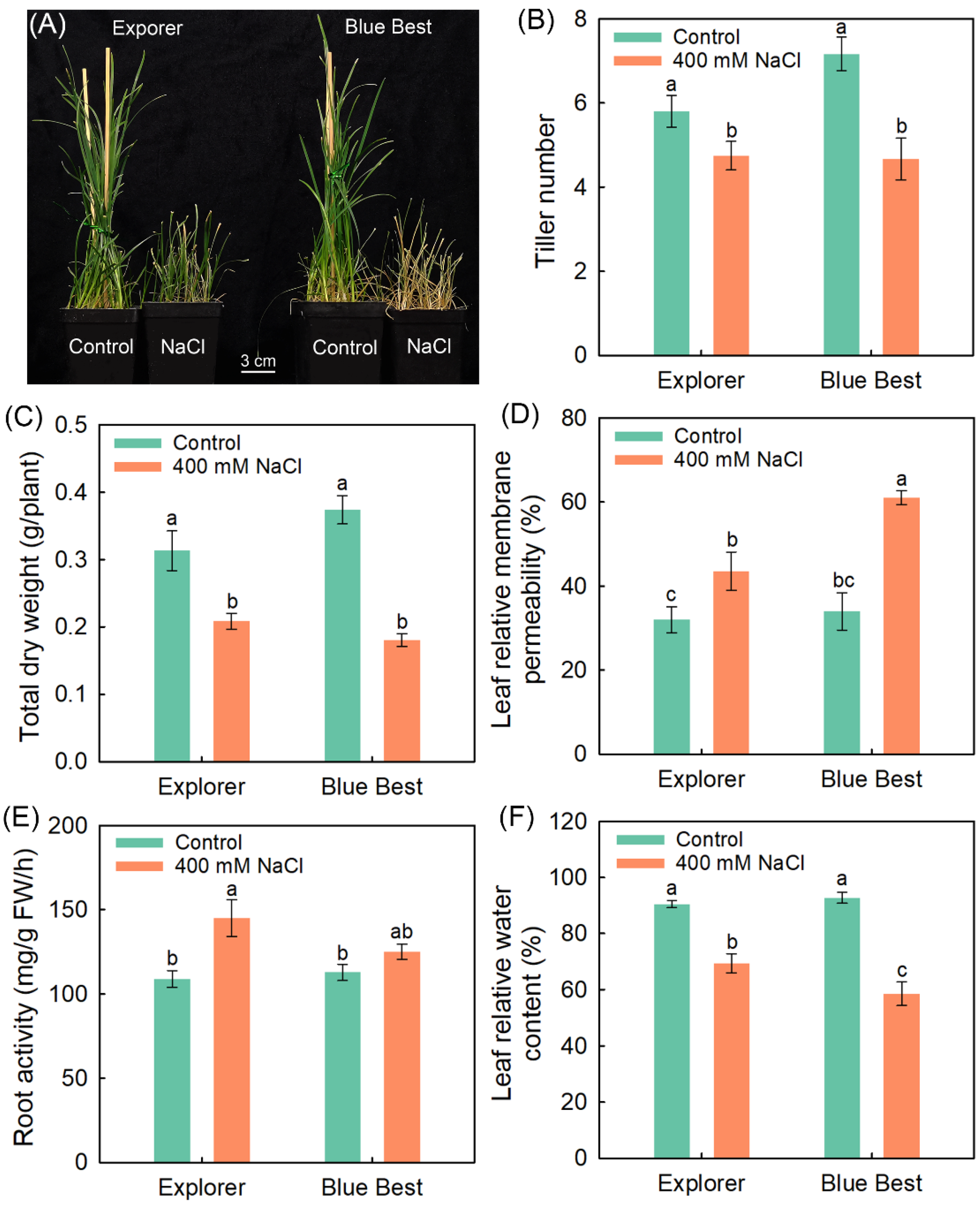
2.1. Effects of NaCl Treatment on the Growth and Photosynthesis of Two Kentucky Bluegrass Cultivars
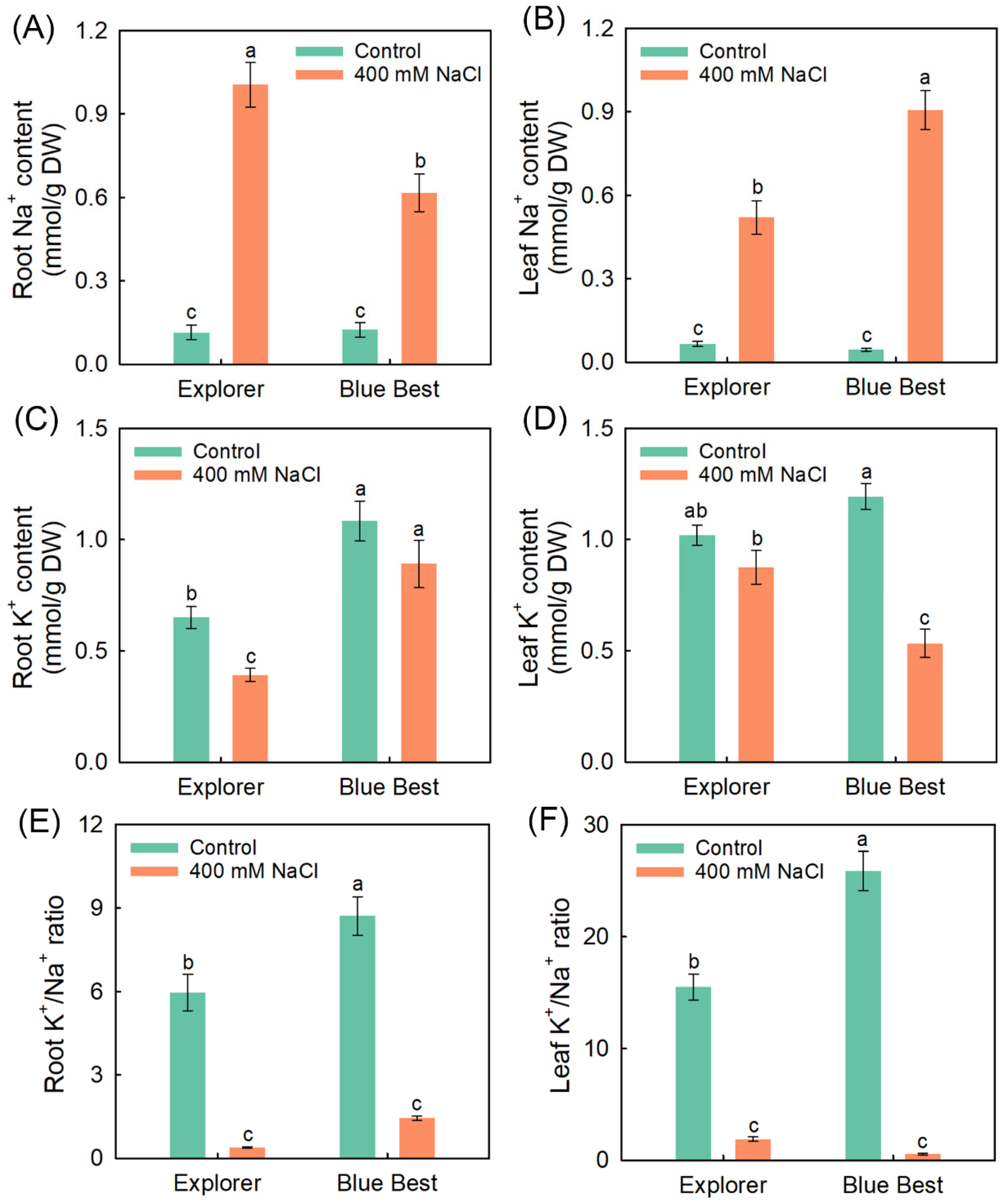
2.2. Effects of NaCl Treatment on Tissue Na+ and K+ Accumulation in Two Kentucky Bluegrass Cultivars
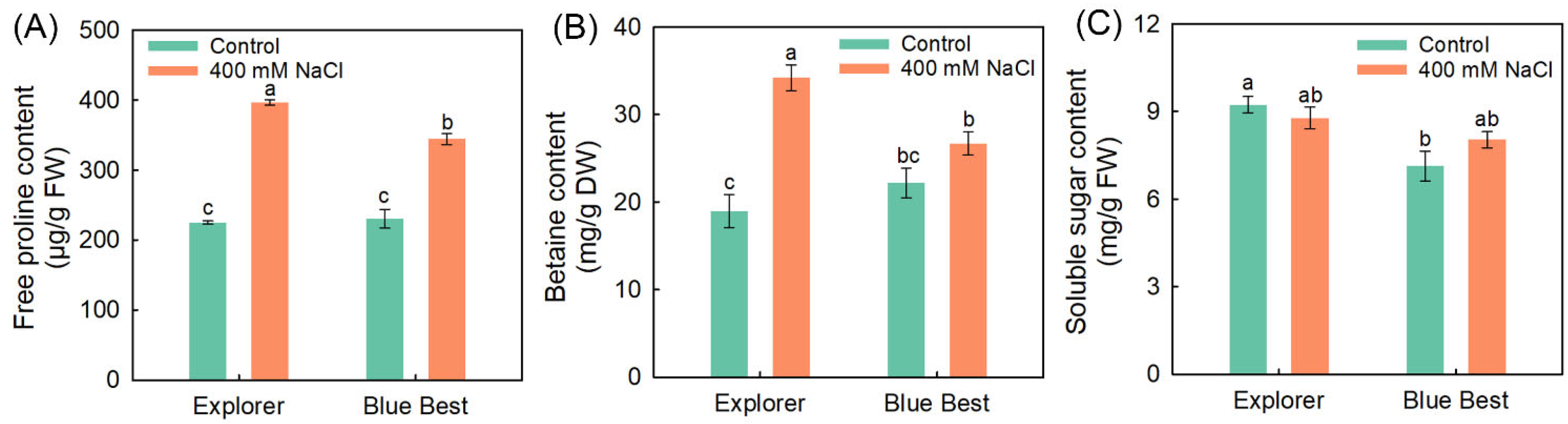
2.3. Effects of NaCl Treatment on Contents of Organic Osmolytes in the Leaves of Two Kentucky Bluegrass Cultivars
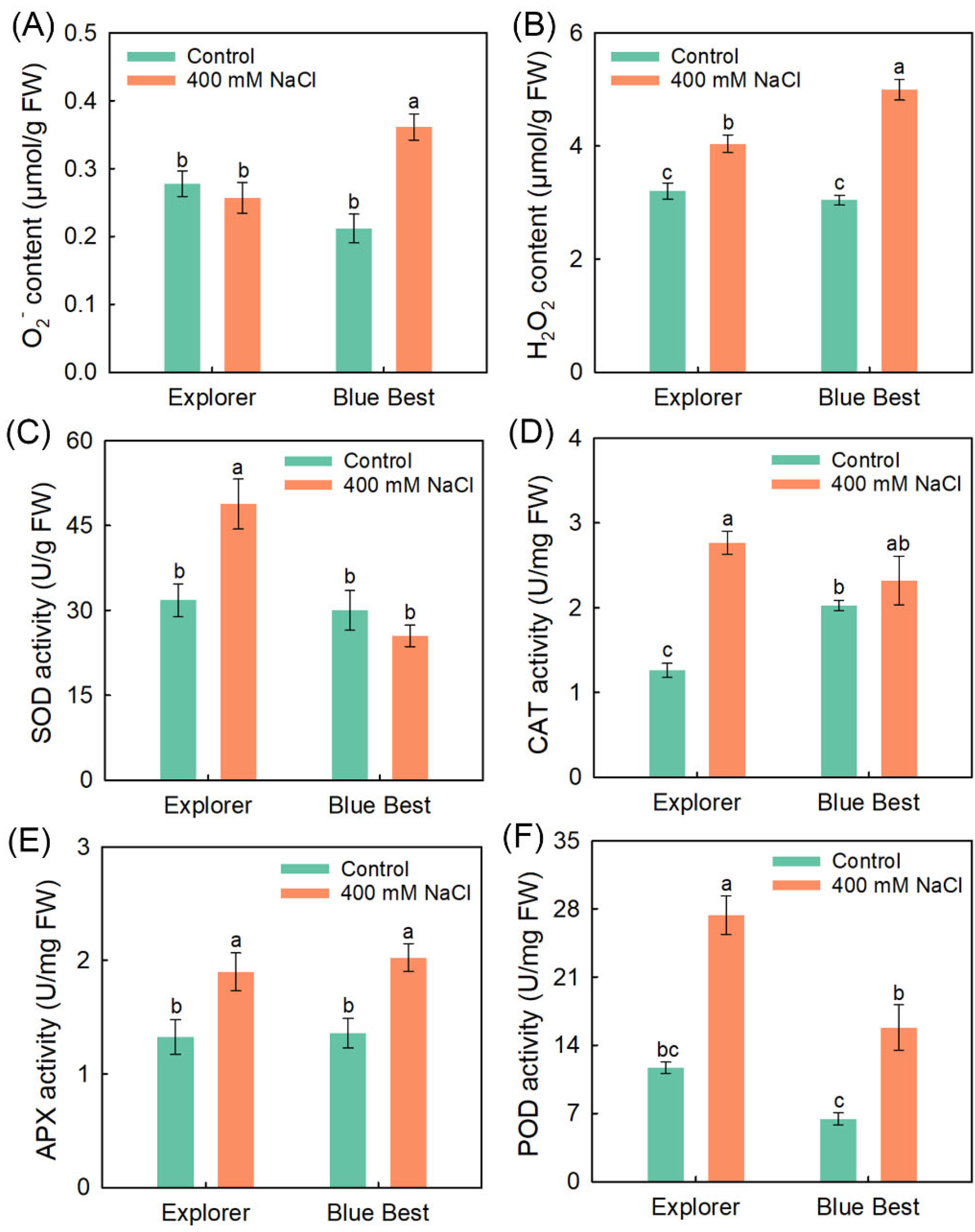
2.4. Effects of NaCl Treatment on ROS Contents and Activities of Antioxidases in the Leaves of Two Kentucky Bluegrass Cultivars
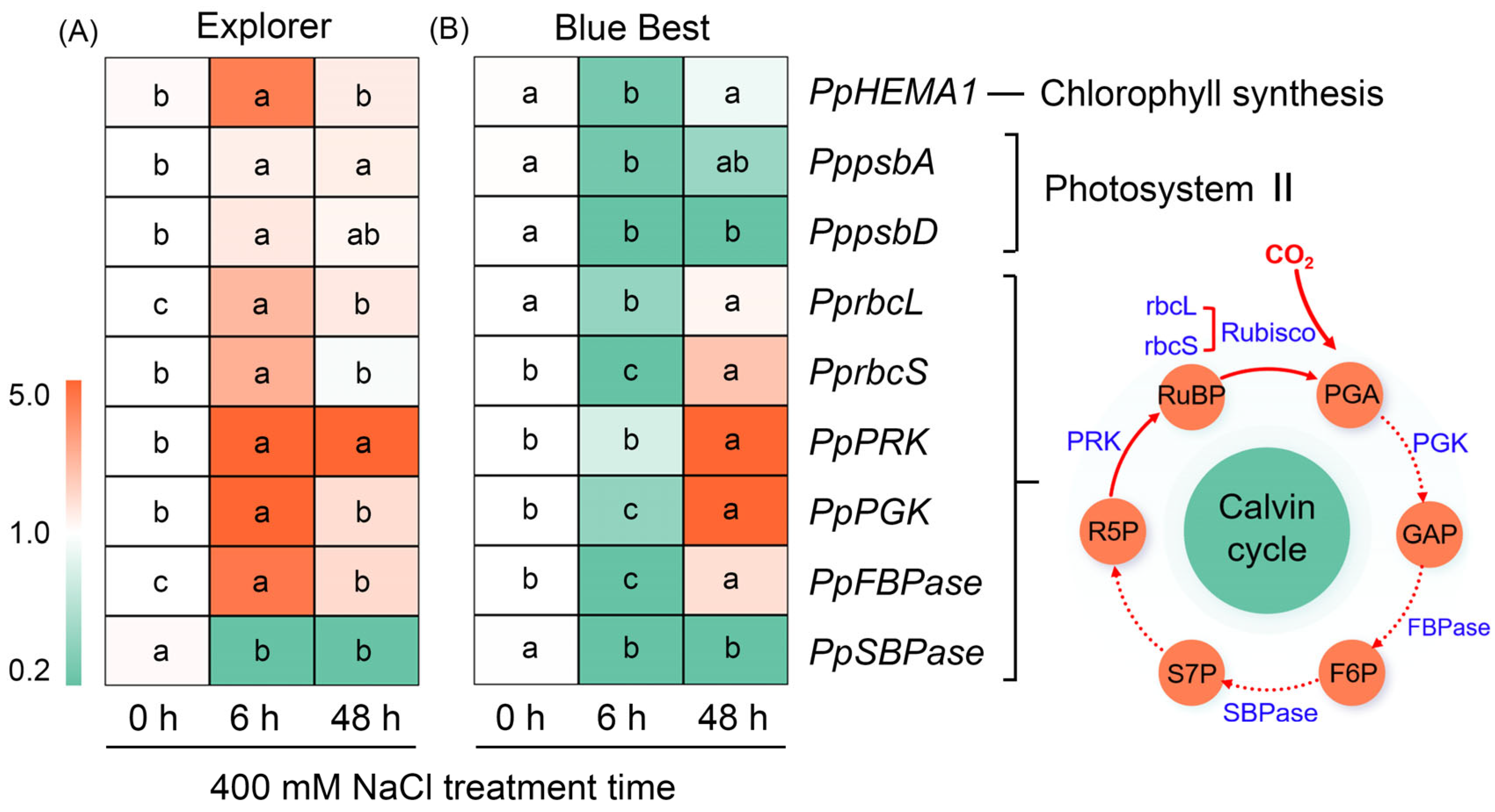
2.5. Effects of NaCl Treatment on the Expression of Key Genes Associated with Photosynthesis in the Leaves of Two Kentucky Bluegrass Cultivars
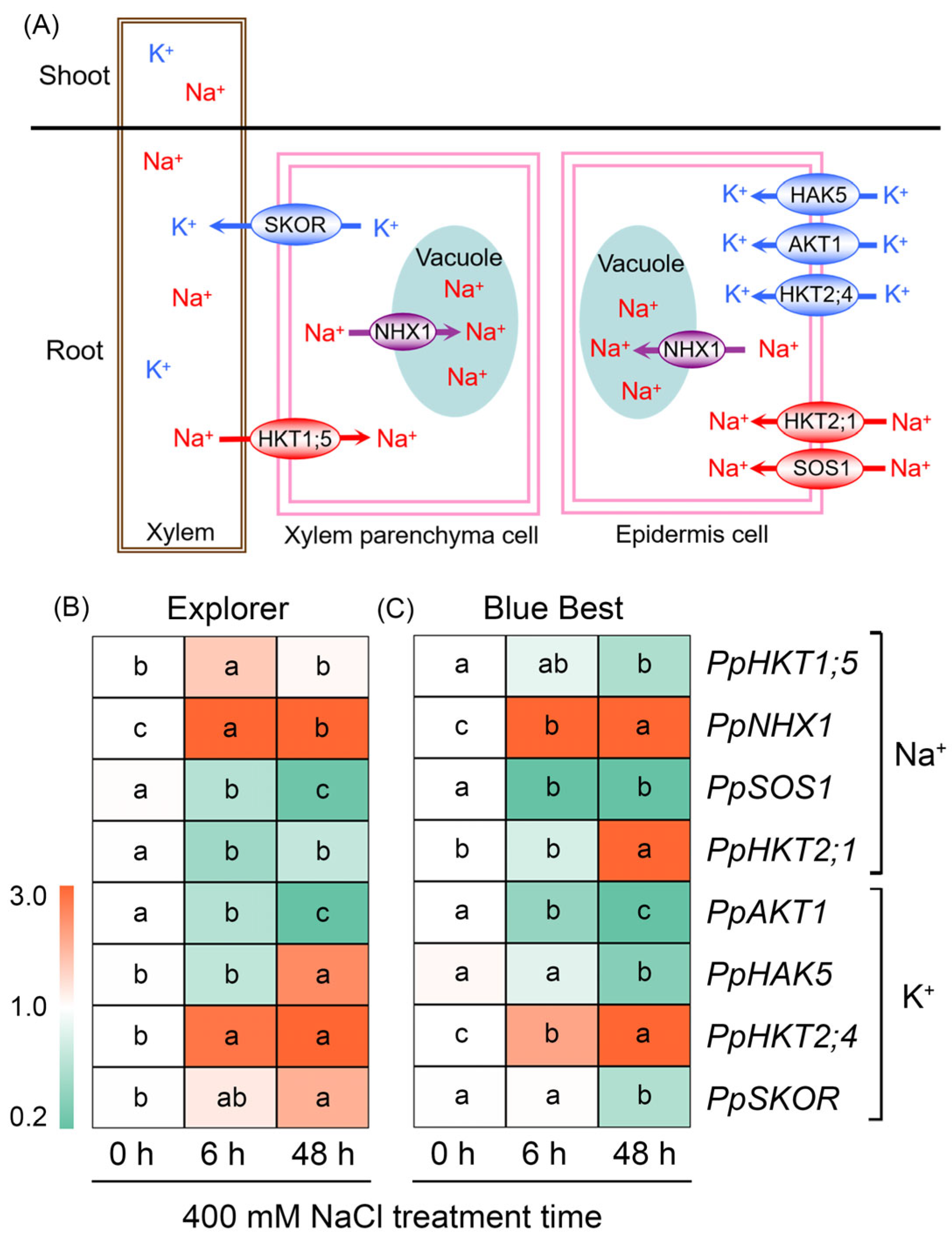
2.6. Effects of NaCl Treatment on the Expression of Key Genes Related to Na+ and K+ Transport in the Roots of Two Kentucky Bluegrass Cultivars
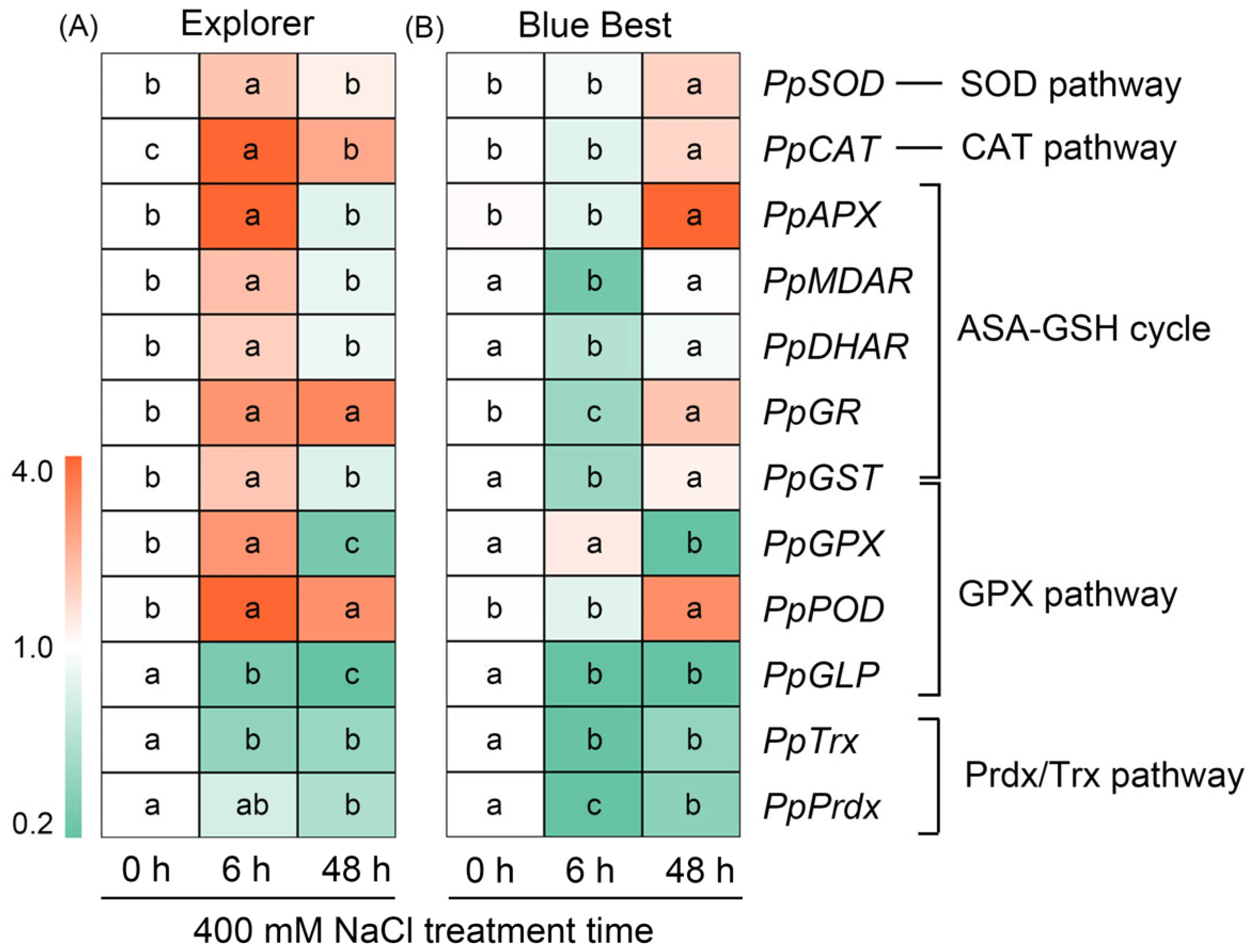
2.7. Effects of NaCl Treatment on the Expression of Key Genes Involved in ROS Degradation in the Leaves of Two Kentucky Bluegrass Cultivars
3. Discussion
3.1. Maintaining a High Photosynthetic Performance by Upregulating the Expression of Photosynthesis-Related Genes Is Important for Salt Tolerance in Kentucky Bluegrass
3.2. Restricting Na+ Overaccumulation and Maintaining K+ Homeostasis in Leaves Play Essential Roles in Kentucky Bluegrass’s Adaptation to Saline Environments
3.3. Enhancing the Ability to Degrade ROS Is Crucial for Kentucky Bluegrass to Cope with Salt Stress
3.4. The Increased Accumulation of Organic Osmolytes Plays Essential Roles in the Osmotic Adjustment of Kentucky Bluegrass
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Determination of Growth-Related Parameters
4.3. Determination of Photosynthesis-Related Parameters
4.4. Determination of Tissue Na+ and K+ Contents
4.5. Determination of the Contents of Free Proline, Betaine, and Soluble Sugars in Leaves
4.6. Determination of the Contents of O2− and H2O2 in Leaves and the Activities of SOD, POD, CAT, and APX
4.7. Real-Time Quantitative PCR Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Xu, R.; Fujiyama, H. Comparison of ionic concentration, organic solute accumulation and osmotic adaptation in Kentucky bluegrass and tall fescue under NaCl stress. Soil Sci. Plant Nutr. 2013, 59, 168–179. [Google Scholar] [CrossRef]
- Dunn, J.H.; Ervin, E.H.; Fresenburg, B.S. Turf performance of mixtures and blends of tall fescue, Kentucky bluegrass, and perennial ryegrass. HortScience 2002, 37, 214–217. [Google Scholar] [CrossRef]
- Zuo, Z.F.; Li, Y.; Mi, X.F.; Li, Y.L.; Zhai, C.Y.; Yang, G.F.; Wang, Z.Y.; Zhang, K. Physiological and lipidomic response of exogenous choline chloride alleviating salt stress injury in Kentucky bluegrass (Poa pratensis). Front. Plant Sci. 2023, 14, 1269286. [Google Scholar] [CrossRef]
- Cui, Y.N.; Li, X.T.; Yuan, J.Z.; Wang, F.Z.; Guo, H.; Xia, Z.R.; Wang, S.M.; Ma, Q. Chloride is beneficial for growth of the xerophyte Pugionium cornutum by enhancing osmotic adjustment capacity under salt and drought stresses. J. Exp. Bot. 2020, 71, 4215–4231. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.G. Osmotic responses of Dunaliella to the changes of salinity. J. Cell. Physiol. 2009, 219, 251–258. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- James, R.A.; Davenport, R.J.; Munns, R. Physiological characterization of two genes for Na+ exclusion in durum wheat, Nax1 and Nax2. Plant Physiol. 2016, 142, 1537–1547. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Xu, B.; Athman, A.; Conn, S.J.; Jordans, C.; Byrt, C.S.; Hare, R.A.; Tyerman, S.D.; Tester, M.; et al. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat. Biotechnol. 2012, 30, 360–364. [Google Scholar] [CrossRef]
- Akyol, T.Y.; Yilmaz, O.; Uzilday, B.; Uzilday, R.O.; Turkan, I. Plant response to salinity: An analysis of ROS formation, signaling, and antioxidant defense. Turk. J. Bot. 2020, 44, 1–13. [Google Scholar]
- Garg, N.; Manchanda, G. ROS generation in plants: Boon or bane? Plant Biosyst. 2009, 143, 81–96. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Dennison, K.L.; Robertson, W.R.; Lewis, B.D.; Hirsch, R.E.; Sussman, M.R.; Spalding, E.P. Functions of AKT1 and AKT2 potassium channels determined by studies of single and double mutants of Arabidopsis. Plant Physiol. 2001, 127, 1012–1019. [Google Scholar] [CrossRef]
- Ivashikina, N.; Becker, D.; Ache, P.; Meyerhoff, O.; Felle, H.H.; Hedrich, R. K+ channel profile and electrical properties of Arabidopsis root hairs. FEBS Lett. 2001, 508, 463–469. [Google Scholar] [CrossRef]
- Bao, A.K.; Du, B.Q.; Touil, L.; Kang, P.; Wang, Q.L.; Wang, S.M. Co-expression of tonoplast Cation/H+ antiporter and H+-pyrophosphatase from xerophyte Zygophyllum xanthoxylum improves alfalfa plant growth under salinity, drought and field conditions. Plant Biotechnol. J. 2016, 14, 964–975. [Google Scholar] [CrossRef]
- Wang, W.Y.; Liu, Y.Q.; Duan, H.R.; Yin, X.X.; Cui, Y.N.; Chai, W.W.; Song, X.; Flowers, T.J.; Wang, S.M. SsHKT1;1 is coordinated with SsSOS1 and SsNHX1 to regulate Na+ homeostasis in Suaeda salsa under saline conditions. Plant Soil. 2020, 449, 117–131. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. The role of monovalent cation transporters in plant responses to salinity. J. Exp. Bot. 2006, 57, 1137–1147. [Google Scholar] [CrossRef]
- Ma, Q.; Hu, J.; Zhou, X.R.; Yuan, H.J.; Kumar, T.; Luan, S.; Wang, S.M. ZxAKT1 is essential for K+ uptake and K+/Na+ homeostasis in the succulent xerophyte Zygophyllum xanthoxylum. Plant J. 2017, 90, 48–60. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, X.; Wang, L.; Cao, Y.; Song, W.; Shi, J.; Lai, J.; Jiang, C. A HAK family Na+ transporter confers natural variation of salt tolerance in maize. Nat. Plants 2019, 5, 1297–1308. [Google Scholar] [CrossRef]
- Nouri, M.Z.; Moumeni, A.; Komatsu, S. Abiotic stresses: Insight into gene regulation and protein expression in photosynthetic pathways of plants. Int. J. Mol. Sci. 2015, 16, 20392–20416. [Google Scholar] [CrossRef]
- Sui, N.; Yang, Z.; Liu, M.; Wang, B. Identification and transcriptomic profiling of genes involved in increasing sugar content during salt stress in sweet sorghum leaves. BMC Genom. 2015, 16, 534. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Bao, A.K.; Chai, W.W.; Wang, W.Y.; Zhang, J.L.; Li, Y.X.; Wang, S.M. Transcriptomic analysis of the succulent xerophyte Zygophyllum xanthoxylum in response to salt treatment and osmotic stress. Plant Soil 2016, 402, 343–361. [Google Scholar] [CrossRef]
- McCormac, A.C.; Fischer, A.; Kumar, A.M.; Söll, D.; Terry, M.J. Regulation of HEMA1 expression by phytochrome and a plastid signal during de-etiolation in Arabidopsis thaliana. Plant J. 2001, 25, 549–561. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front. Plant Sci. 2017, 8, 1739. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Tang, M.; Jin, X.Q.; Li, H.; Chen, L.S.; Wang, Q.L.; Sun, A.Z.; Yi, Y.; Guo, F.Q. Regulation of Calvin-Benson cycle enzymes under high temperature stress. aBIOTECH 2022, 3, 65–77. [Google Scholar] [CrossRef]
- Shi, H.Z.; Quintero, F.J.; Pardo, J.M.; Zhu, J.K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 2002, 14, 465–477. [Google Scholar] [CrossRef]
- Apse, M.P.; Blumwald, E. Na+ transport in plants. FEBS Lett. 2007, 581, 2247–2254. [Google Scholar] [CrossRef]
- Laurie, S.; Feeney, K.A.; Maathuis, F.J.M.; Heard, P.J.; Brown, S.J.; Leigh, R.A. A role for HKT1 in sodium uptake by wheat roots. Plant J. 2002, 32, 139–149. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, Y.; Wang, Z.; Wang, Z.Q.; Shi, J.; Liang, X.; Song, W.; Chen, Q.; Lai, J.; Jiang, C. A retrotransposon in an HKT1 family sodium transporter causes variation of leaf Na+ exclusion and salt tolerance in maize. New Phytol. 2018, 217, 1161–1176. [Google Scholar] [CrossRef]
- Horie, T.; Brodsky, D.E.; Costa, A.; Kaneko, T.; Schiavo, F.L.; Katsuhara, M.; Schroeder, J.I. K+ transport by the OsHKT2;4 transporter from rice with atypical Na+ transport properties and competition in permeation of K+ over Mg2+ and Ca2+ Ions. Plant Physiol. 2009, 156, 1493–1507. [Google Scholar] [CrossRef] [PubMed]
- Rubio, F.; Nieves-Cordones, M.; Aleman, F.; Martinez, V. Relative contribution of AtHAK5 and AtAKT1 to K+ uptake in the high-affinity range of concentrations. Physiol. Plant. 2008, 134, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, L.; Luan, S. Intracellular K+ sensing of SKOR, a shaker-type K+ channel from Arabidopsis. Plant J. 2006, 46, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.C.; Johnston, W.J.; Golob, C.T. Residue management, seed production, crop development, and turf quality in diverse Kentucky bluegrass germplasm. Crop Sci. 2003, 43, 1091–1099. [Google Scholar] [CrossRef]
- Xia, H.; Cao, Z.; Yu, M.; Li, Z.; Nie, C.; Zhang, P.; Guo, H. Tolerance of 30 Kentucky bluegrass varieties to NaCl stress during the seedling stage. Pratacultural Sci. 2023, 12, 3124–3137. [Google Scholar]
- Guo, H.; Zhang, L.; Cui, Y.N.; Wang, S.M.; Bao, A.K. Identification of candidate genes related to salt tolerance of the secretohalophyte Atriplex canescens by transcriptomic analysis. BMC Plant Biol. 2019, 19, 213. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Munns, R.; Shabala, S.; Gilliham, M.; Pogson, B.; Tyerman, S. Chloroplast function and ion regulation in plants growing on saline soils: Lessons from halophytes. J. Exp. Bot. 2017, 68, 3129–3143. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, R.; Shabala, S. Stomata in a saline world. Curr. Opin. Plant Biol. 2018, 46, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Farquhar, G.D. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 2007, 143, 78–87. [Google Scholar] [CrossRef]
- Turan, S.; Tripathy, B.C. Salt-stress induced modulation of chlorophyll biosynthesis during de-etiolation of rice seedlings. Physiol. Plant. 2015, 153, 477–491. [Google Scholar] [CrossRef]
- Ma, Q.; Yue, L.J.; Zhang, J.L.; Wu, G.Q.; Bao, A.K.; Wang, S.M. Sodium chloride improves photosynthesis and water status in the succulent xerophyte Zygophyllum xanthoxylum. Tree Physiol. 2012, 32, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Z.; Qiao, Z.; Guo, X.; Wen, Y.; Zhou, Y.; Yao, C.; Fan, H.; Wang, B.; Han, G. The RING zinc finger protein LbRZF1 promotes salt gland development and salt tolerance in Limonium bicolor. J. Integr. Plant Biol. 2024, 66, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Nie, C.Y.; Li, Z.; Kang, J.; Wang, X.L.; Cui, Y.N. Physiological and transcriptional analyses provide insight into maintaining ion homeostasis of sweet sorghum under salt stress. Int. J. Mol. Sci. 2023, 24, 11045. [Google Scholar] [CrossRef] [PubMed]
- Olías, R.; Eljakaoui, Z.; Li, J.; Morales, P.A.D.; Marín-Manzano, M.C.; Pardo, J.M.; Belver, A. The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs. Plant Cell Environ. 2009, 32, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Hauser, F.; Schroeder, J.I. HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci. 2009, 14, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Costa, A.; Kim, T.H.; Han, M.J.; Horie, R.; Leung, H.Y.; Miyao, A.; Hirochika, H.; An, G.; Schroeder, J.I. Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. Embo J. 2007, 26, 3003–3014. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Shabala, L. Ion transport and osmotic adjustment in plants and bacteria. Biomol. Concepts 2011, 2, 407–419. [Google Scholar] [CrossRef]
- Hoque, M.A.; Okuma, E.; Banu, M.N.A.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. J. Plant Physiol. 2007, 164, 553–561. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, G.; Bian, X.; Zhao, Q. Effects of root interaction and nitrogen fertilization on the chlorophyll content, root activity, photosynthetic characteristics of intercropped soybean and microbial quantity in the rhizosphere. Plant Soil Environ. 2013, 59, 80–88. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Wang, S.M.; Zhang, J.L.; Flowers, T.J. Low-affinity Na+ uptake in the halophyte Suaeda maritima. Plant Physiol. 2007, 145, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Pan, Y.Q.; Guo, H.; Wang, S.M.; Zhao, B.; Zhang, J.L.; Ma, Q.; Yin, H.J.; Bao, A.K. The photosynthesis, Na+/K+ homeostasis and osmotic adjustment of Atriplex canescens in response to salinity. Front. Plant Sci. 2016, 7, 848. [Google Scholar] [CrossRef] [PubMed]
- Pe’er, S.; Cohen, Y. Sugar accumulation in tobacco plants systemically protected against blue mold (Peronospora tabacina). Phytoparasitica 1987, 15, 307–315. [Google Scholar] [CrossRef]
- Yu, P.; Song, X.; Zhang, W.; Yao, Y.; Ren, J.; Wang, L.; Liu, W.; Meng, Z.; Meng, X. Analysis of ginseng rusty root symptoms transcriptome and its pathogenesis directed by reactive oxygen species theory. Plant Direct 2024, 8, e586. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W.; Ren, G.; Cao, Y.; Di, J.; Wang, Y.; Zhang, L. Selenium treatment regulated the accumulation of reactive oxygen species and the expressions of related genes in postharvest broccoli. Agronomy 2024, 14, 1047. [Google Scholar] [CrossRef]
- Cui, Y.N.; Lin, Z.R.; Cai, M.M.; Liu, R.W.; Wang, S.M.; Ma, Q. PcCLCg is involved in the accumulation of Cl− in shoots for osmotic adjustment and salinity resistance in the Cl−-tolerant xerophyte Pugionium cornutum. Plant Soil 2023, 487, 283–298. [Google Scholar] [CrossRef]
- Duan, H.R.; Ma, Q.; Zhang, J.L.; Hu, J.; Bao, A.K.; Wei, L.; Wang, Q.; Luan, S.; Wang, S.M. The inward-rectifying K+ channel SsAKT1 is a candidate involved in K+ uptake in the halophyte Suaeda salsa under saline condition. Plant Soil 2015, 395, 173–187. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Yan, S.-J.; Liu, C.; Guo, H.; Cui, Y.-N. Comparative Physiological and Gene Expression Analyses Reveal Mechanisms Involved in Maintaining Photosynthesis Capacity, Alleviating Ion Toxicity and Oxidative Stress of Kentucky Bluegrass under NaCl Treatment. Plants 2024, 13, 2107. https://doi.org/10.3390/plants13152107
Wang R, Yan S-J, Liu C, Guo H, Cui Y-N. Comparative Physiological and Gene Expression Analyses Reveal Mechanisms Involved in Maintaining Photosynthesis Capacity, Alleviating Ion Toxicity and Oxidative Stress of Kentucky Bluegrass under NaCl Treatment. Plants. 2024; 13(15):2107. https://doi.org/10.3390/plants13152107
Chicago/Turabian StyleWang, Rong, Shi-Jie Yan, Chao Liu, Huan Guo, and Yan-Nong Cui. 2024. "Comparative Physiological and Gene Expression Analyses Reveal Mechanisms Involved in Maintaining Photosynthesis Capacity, Alleviating Ion Toxicity and Oxidative Stress of Kentucky Bluegrass under NaCl Treatment" Plants 13, no. 15: 2107. https://doi.org/10.3390/plants13152107




