Identification of DREB Family Genes in Banana and Their Function under Drought and Cold Stress
Abstract
:1. Introduction
2. Results
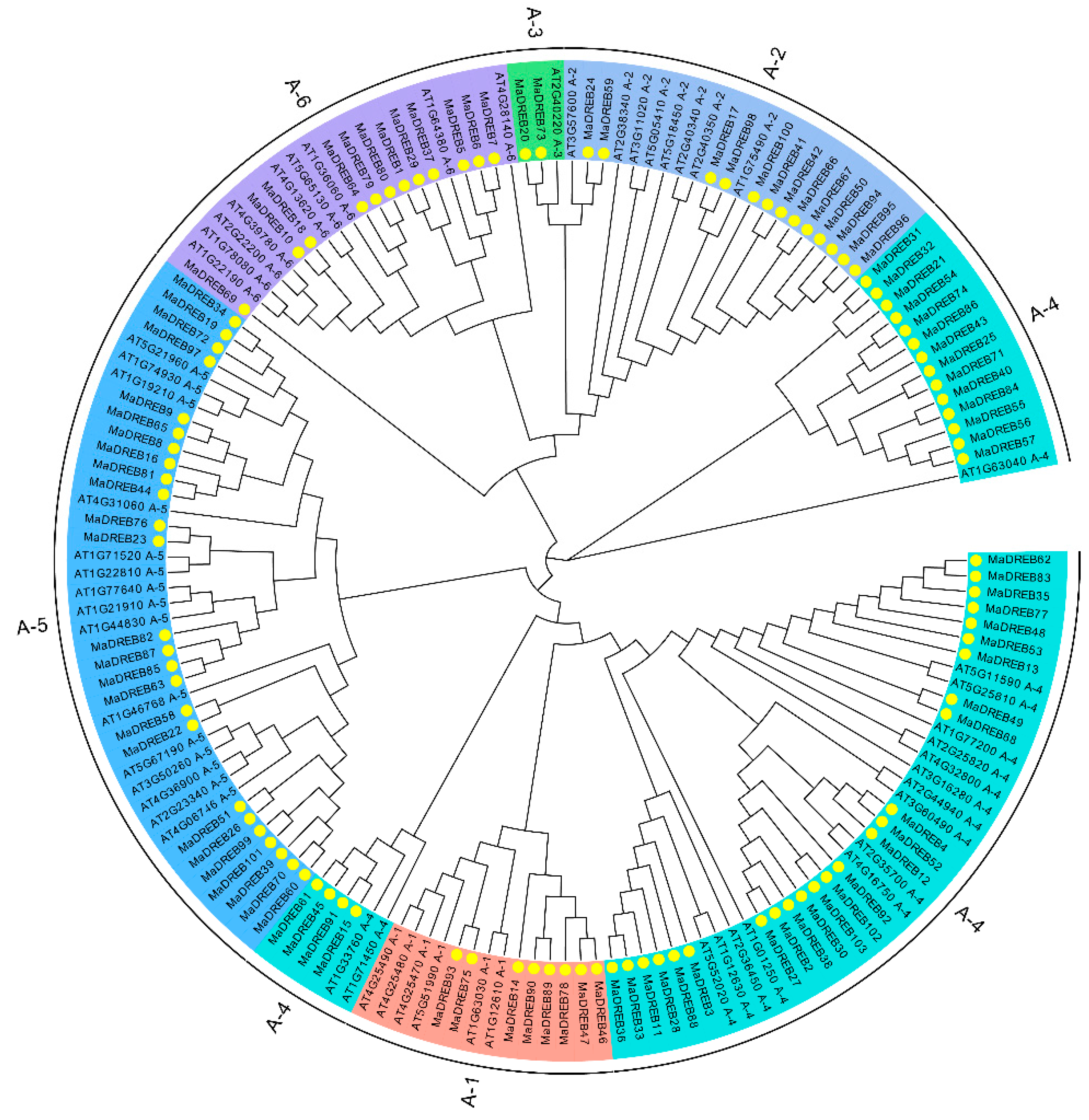
2.1. Phylogenetic Analysis
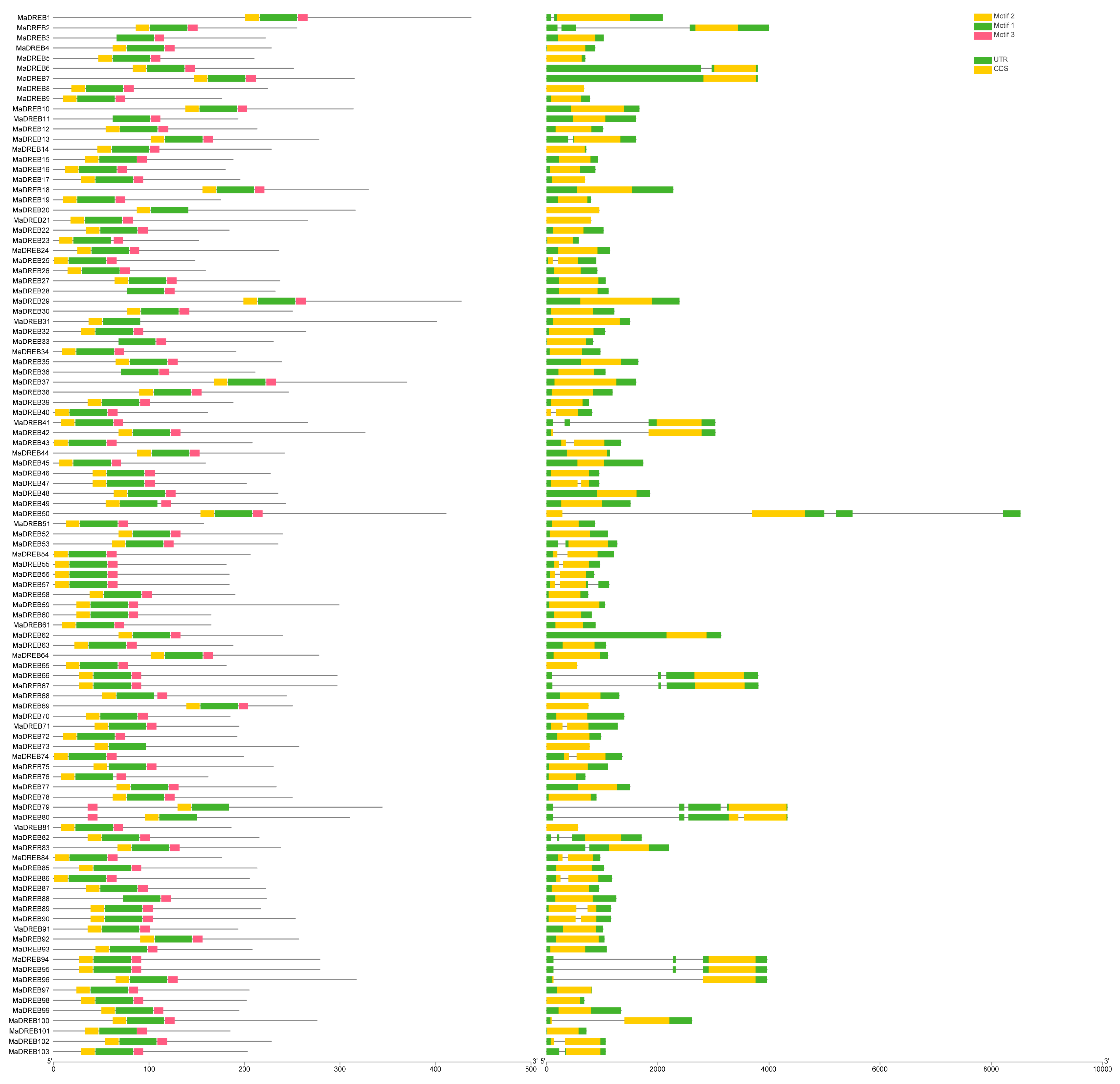
2.2. Analysis of the Structural Domain
2.3. Analysis of MaDREB Promoters Structures
2.4. Collinearity Analysis of MaDREBs
2.5. Protein Interaction Analysis
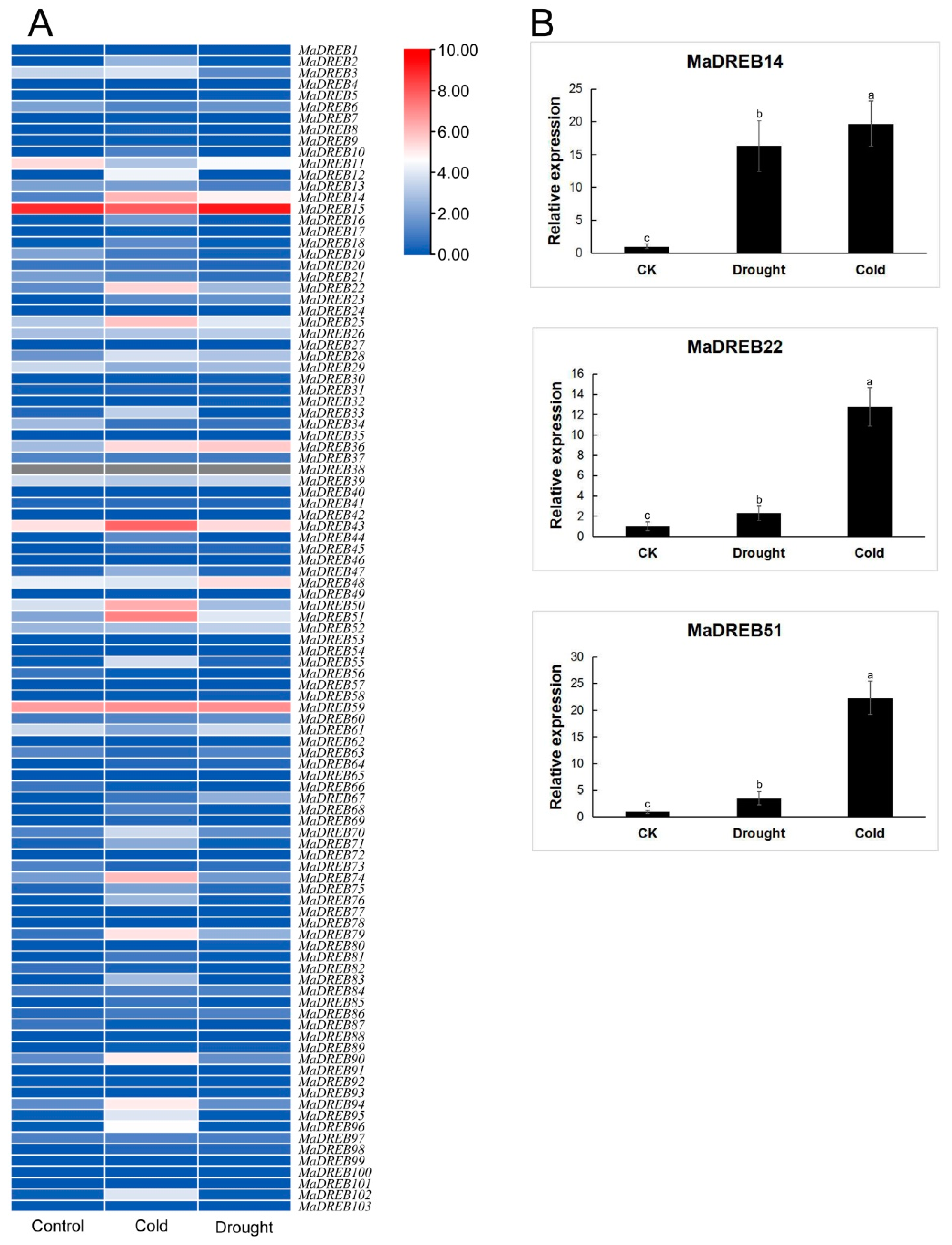
2.6. Three MaDREBs Were Selected to Respond to Drought and Cold Stress
2.7. Overexpression of MaDREB Enhances Transgenic Arabidopsis Tolerance to Drought and Cold Stress
2.8. Chlorophyll Fluorescence Measurements under Drought and Cold Stress
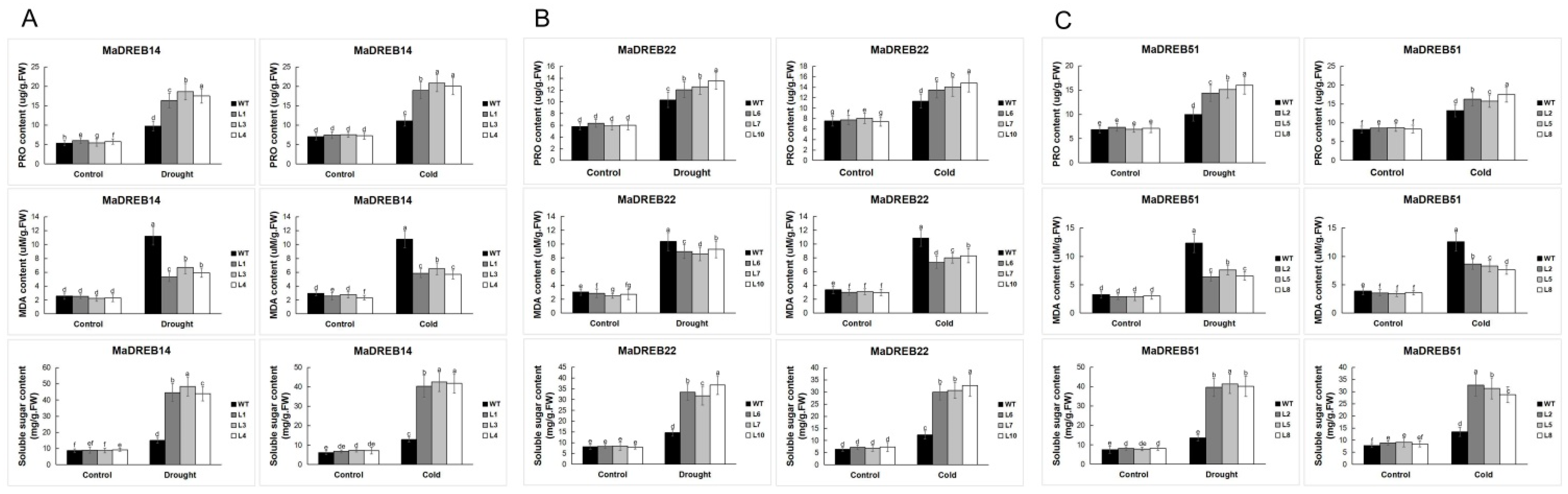
2.9. Measurement of Physiological Indicators in Transgenic Arabidopsis
3. Materials and Methods
3.1. Identification and Gene Family Analysis of DREBs in Banana
3.2. Plant Materials, Transcriptome, and qRT-PCR Analysis
3.3. Cloning and Vector Construction of MaDREB14/22/51
3.4. Plant Transformation and Drought and Cold Treatment
3.5. Analyses of Physiological Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patel, K.; Thankappan, R.; Mishra, G.P.; Mandalia, V.; Kumar, A.; Dobaria, J.R. Transgenic peanut (Arachis hypogaea L.) overexpressing mtlD gene showed improved photosynthetic, physio-biochemical and yield-parameters under soil-moisture deficit stress in lysimeter system. Front. Plant Sci. 2017, 3, 1881. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.S.; Chen, M.; Li, L.C.; Ma, Y.Z. Functions and Application of the AP2/ERF Transcription Factor Family in Crop ImprovementF. J. Integr. Plant Biol. 2011, 53, 570–585. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Peng, T.; Dai, W. Critical cis-Acting Elements and Interacting Transcription Factors: Key Players Associated with Abiotic Stress Responses in Plants. Plant Mol. Biol. Rep. 2014, 32, 303–317. [Google Scholar] [CrossRef]
- Liu, J.L.; Deng, Z.W.; Liang, C.L.; Sun, H.W.; Li, D.L.; Song, J.K.; Zhang, S.L.; Wang, R. Genome-Wide Analysis of RAV Transcription Factors and Functional Characterization of Anthocyanin-Biosynthesis-Related RAV Genes in Pear. Int. J. Mol. Sci. 2021, 22, 5567. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.X.; Chen, S.K.; Wang, P.D.; Peng, D.; Zhang, X.; Li, H.F.; Feng, C.Z. Genome-Wide Analysis of the RAV Gene Family in Wheat and Functional Identification of TaRAV1 in Salt Stress. Int. J. Mol. Sci. 2022, 23, 8834. [Google Scholar] [CrossRef]
- Seo, P.J.; Park, M.J.; Park, C.M. Alternative splicing of transcription factors in plant responses to low temperature stress: Mechanisms and functions. Planta 2013, 237, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Ma, C.; Chen, Y.; Gao, H.; Wang, J. Genome-Wide Screening of AP2 Transcription Factors Involving in Fruit Color and Aroma Regulation of Cultivated Strawberry. Genes 2021, 12, 530. [Google Scholar] [CrossRef]
- Sakuma, Y.; Liu, Q.; Dubouzet, J.G.; Abe, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Biophys. Res. Commun. 2002, 290, 998–1009. [Google Scholar] [CrossRef]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 2006, 40, 411–432. [Google Scholar] [CrossRef]
- Ali, N.; Hadi, F. CBF/DREB transcription factor genes play role in cadmium tolerance and phytoaccumulation in Ricinus communis under molybde-num treatments. Chemosphere 2018, 208, 425–432. [Google Scholar] [CrossRef]
- Eckardt, N.A. DREB Duo Defines Distinct Drought and Cold Response Pathways. Plant Cell. 2019, 31, 1196–1197. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Luo, T.L.; Zhao, H.Y.; Su, Y.L.; Ji, W.Q.; Li, H.F. Identification of wheat DREB genes and functional characterization of TaDREB3 in response to abiotic stress. Gene 2020, 740, 14. [Google Scholar] [CrossRef]
- Kimotho, R.N.; Baillo, E.H.; Zhang, Z. Transcription factors involved in abiotic stress responses in Maize (Zea mays L.) and their roles in enhanced productivity in the post genomics era. PeerJ 2019, 7, e7211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Shen, X.; Yuan, H.; Liu, Y.; Liao, X.; Wang, Q.; Liu, L.; Li, F.; Li, T. Isolation and characterization of dehydration-responsive element-binding factor 2C (MsDREB2C) from Malus sieversii Roem. Plant Cell Physiol. 2013, 54, 1415–1430. [Google Scholar] [CrossRef]
- Liang, Y.; Kang, K.; Gan, L.; Ning, S.; Xiong, J.; Song, S.; Xi, L.; Lai, S.; Yin, Y.; Gu, J.; et al. Drought-responsive genes, late embryogenesis abundant group3 (LEA3) and vicinal oxygen chelate, function in lipid accumulation in Brassica napus and Arabidopsis mainly via enhancing photosynthetic efficiency and reducing ROS. Plant Biotechnol. J. 2019, 17, 2123–2142. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, X.; Zhang, L. Structural and functional dynamics of dehydrins: A plant protector protein under abiotic stress. Int. J. Mol. Sci. 2018, 19, 3420. [Google Scholar] [CrossRef]
- Chen, H.L.; Liu, L.P.; Wang, L.X.; Wang, S.H.; Cheng, X.Z. VrDREB2A, a DREB-binding transcription factor from Vigna radiata, increased drought and high-salt tolerance in transgenic Arabidopsis thaliana. J. Plant Res. 2016, 129, 263–273. [Google Scholar] [CrossRef]
- Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Seki, M.; Miura, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Organization and expression of two Arabidopsis DREB2 genes encoding DRE-binding proteins ivolved in dehydration- and high-salinity-responsive gene expression. Plant Mol. Biol. 2000, 42, 657–665. [Google Scholar] [CrossRef]
- Bandeppa, S.; Paul, S.; Thakur, J.K.; Chandrashekar, N.; Umesh, D.K.; Aggarwal, C.; Asha, A.D. Antioxidant, physiological and biochemical responses of drought susceptible and drought tolerant mustard (Brassica juncea L.) genotypes to rhizobacterial inoculation under water defcit stress. Plant Physiol. Biochem. 2019, 143, 19–28. [Google Scholar] [CrossRef]
- Labbo, A.M.; Mehmood, M.; Akhtar, M.N.; Khan, M.J.; Tariq, A.; Sadiq, I. Genome-wide identification of AP2/ERF transcription factors in mung bean (Vigna radiata) and expression profiling of the VrDREB subfamily under drought stress. Crop Pasture Sci. 2018, 69, 1009–1019. [Google Scholar] [CrossRef]
- Mao, D.; Chen, C. Colinearity and Similar Expression Pattern of Rice DREB1s Reveal Their Functional Conservation in the Cold-Responsive Pathway. PLoS ONE 2012, 7, e47275. [Google Scholar] [CrossRef]
- Liu, N.; Zhong, N.Q.; Wang, G.L.; Li, L.J.; Liu, X.L.; He, Y.K.; Xia, G.X. Cloning and functional characterization of PpDBF1 gene encoding a DRE-binding transcription factor from Physcomitrella patens. Planta 2007, 226, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Jha, B.; Agarwal, P.K. A dehydration-responsive element binding (DREB) transcription factor from the succulent halophyte Salicornia brachiata enhances abiotic stress tolerance in transgenic tobacco. Mar. Biotechnol. 2014, 16, 657–673. [Google Scholar] [CrossRef]
- Shen, Y.G.; Zhang, W.K.; He, S.J.; Zhang, J.S.; Liu, Q.; Chen, S.Y. An EREBP/AP2-type protein in Triticum aestivum was a DRE-binding transcription factor induced by cold, dehydration and ABA stress. Theor. Appl. Genet. 2003, 106, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Jaglo-Ottosen, K.R.; Gilmour, S.J.; Zarka, D.G.; Schabenberger, O.; Thomashow, M.F. Arabidopsis CBF1 overexpression induces cor genes and enhances freezing tolerance. Science 1998, 280, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liao, J.Y.; Ling, Q.Q.; Xi, Y.; Qian, Y.X. Genome-wide identification and expression profiling analysis of maize AP2/ERF superfamily genes reveal essential roles in abiotic stress Tolerance. BMC Genom. 2022, 23, 125. [Google Scholar] [CrossRef] [PubMed]
- Dossa, K.; Wei, X.; Li, D.; Fonceka, D.; Zhang, Y.X.; Wang, L.H.; Yu, J.Y.; Boshou, L.; Diouf, D.; Cisse, N. Insight into the AP2/ERF transcription factor superfamily in sesame and expression profiling of DREB subfamily under drought stress. BMC Plant Boil. 2016, 16, 171. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, D.; Ma, F.; Xing, W.; Huang, D.; Wu, B.; Chen, J.; Chen, D.; Xu, B.; Xu, Y. Genome-wide identification and expression analyses of the aquaporin gene family in passion fruit (Passiflora edulis), revealing PeTIP3-2 to be involved in drought stress. Int. J. Mol. Sci. 2022, 23, 5720. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, P.; Ma, F.; Huang, D.; Xing, W.; Wu, B.; Sun, P.; Xu, B.; Song, S. Characterization of the NAC Transcription Factor in Passion Fruit (Passiflora edulis) and Functional Identification of PeNAC-19 in Cold Stress. Plants 2023, 12, 1393. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, W.; Ma, F.; Huang, D.; Xing, W.; Wu, B.; Sun, P.; Chen, D.; Xu, B.; Song, S. Characterization of the Passion Fruit (Passiflora edulis Sim) bHLH Family in Fruit Development and Abiotic Stress and Functional Analysis of PebHLH56 in Cold Stress. Horticulturae 2023, 9, 272. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Xu, Y.; Xing, W.T.; Wu, B.; Huang, D.M.; Ma, F.N. Identification of the passion fruit (Passiflora edulis Sims) MYB family in fruit development and abiotic stress, and functional analysis of PeMYB87 in abiotic stress. Front. Plant Sci. 2023, 14, 1124351. [Google Scholar] [CrossRef]
- Hu, W.; Ding, Z.H.; Tie, W.W.; Yan, Y.; Liu, Y.; Wu, C.L.; Liu, J.H.; Wang, J.S.; Peng, M.; Xu, B.Y.; et al. Comparative physiological and transcriptomic analyses provide integrated insight into osmotic, cold, and salt stress tolerance mechanisms in banana. Sci. Rep. 2017, 22, 43007. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Ma, F.N.; Zhou, H.W.; Yang, H.T.; Huang, D.M.; Xing, W.T.; Wu, B.; Li, H.L.; Hu, W.B.; Song, S.; Xu, Y. WRKY Transcription Factors in Passion Fruit Analysis Reveals Key PeWRKYs Involved in Cold Stress and Flavonoid Biosynthesis. Int. J. Biol. Macromol. 2024, 256, 128063. [Google Scholar] [CrossRef]
- Li, X.P.; Tian, A.G.; Luo, G.Z.; Gong, Z.Z.; Zhang, J.S.; Chen, S.Y. Soybean DRE-binding transcription factors that are responsive to abiotic stress. Theor. Appl. Genet. 2005, 110, 1355–1362. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998, 10, 1391–1406. [Google Scholar] [CrossRef]
- Kume, S.; Kobayashi, F.; Ishibashi, M.; Ohno, R.; Nakamura, C.; Takumi, S. Differential and coordinated expression of Cbf and Cor/Lea genes during long-term cold acclimation in two wheat cultivars showing distinct levels of freezing tolerance. Genes Genet. Syst. 2005, 80, 185–197. [Google Scholar] [CrossRef]
- Akbudak, M.A.; Filiz, E.; Kontbay, K.J.B. DREB2 (dehydration-responsive element-binding protein 2) type transcription factor in sorghum (Sorghum bicolor): Genome-wide identification, characterization and expression profiles under cadmium and salt stress. 3 Biotech. 2018, 8, 426–441. [Google Scholar] [CrossRef]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, X.; Martin, G.B. The Pto kinase conferring resistance to tomato bacterial speck disease interacts with proteins that bind a cis-element of pathogenesis-related genes. EMBO J. 2014, 16, 3207–3218. [Google Scholar] [CrossRef]
- Ohme-Takagi, M.; Shinshi, H. Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell. 1995, 7, 173–182. [Google Scholar] [CrossRef]
- Guo, B.; Wei, Y.; Xu, R.; Lin, S.; Luan, H.; Lv, C.; Zhang, X.; Song, X.; Xu, R. Genome-wide analysis of APETALA2/Ethylene-responsive factor (AP2/ERF) gene family in barley (Hordeum vulgare L.). PLoS ONE 2016, 11, e0161322. [Google Scholar] [CrossRef]
- Qin, F.; Kakimoto, M.; Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Tran, L.P.; Shino-zaki, K.; Yamaguchi-Shinozaki, K. Regulation and functional analysis of ZmDREB2A in response to drought and heat stress in Zea mays L. Plant Cell Physiol. 2017, 50, 54–69. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, L.T.; Nie, L.B.; Zheng, Y.S.; Zhu, S.D.; Hou, J.F.; Li, R.J.; Chen, G.H.; Tang, X.Y.; Wang, C.G.; et al. Genome-wide analysis of the DREB family genes and functional identification of the involvement of BrDREB2B in abiotic stress in wucai (Brassica campestris L.). BMC Genom. 2022, 23, 598. [Google Scholar] [CrossRef]
- Sheng, S.; Guo, X.Y.; Wu, C.Z.; Xiang, Y.C.; Duan, S.H.; Yang, W.Q.; Le, W.R.; Cao, F.C.; Liu, L.H. Genome-wide identification and expression analysis of DREB genes in alfalfa (Medicago sativa) in response to cold stress. Plant Signal Behav. 2022, 17, 2081420. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Wang, H.; Xin, H.; Yang, X.; Yan, J.; Li, J.; Tran, L.-S.P.; Shinozaki, K.; Yamaguchi-Shinozaki, K.; et al. Genome-wide analysis of ZmDREB genes and their association with natural variation in drought tolerance at seedling stage of Zea mays L. PLoS Genet. 2013, 9, e1003790. [Google Scholar] [CrossRef]
- Su, J.C.; Song, S.L.; Wang, Y.T.; Zeng, Y.P.; Dong, T.Y.; Ge, X.Y.; Duan, H.Y. Genome-wide identification and expression analysis of DREB family genes in cotton. BMC Plant Biol. 2023, 23, 169. [Google Scholar] [CrossRef]
- Kumar, T.S.; Park, J.I.; Jung, H.J.; Nou, I.S. Genome-wide analysis of the distribution of AP2/ERF transcription factors reveals duplication and CBFs genes elucidate their potential function in Brassica oleracea. BMC Genom. 2014, 15, 422–434. [Google Scholar] [CrossRef]
- Koralewski, T.; Krutovsky, K. Evolution of exon–intron structure and alternative splicing. PLoS ONE 2011, 6, e18055. [Google Scholar] [CrossRef]
- Jin, J.H.; Wang, M.; Zhang, H.X.; Khan, A.; Wei, A.M.; Luo, D.X.; Gong, Z.H. Genome-wide identification of the AP2/ERF transcription factor family in pepper (Capsicum annuum L.). Genome 2018, 61, 663–674. [Google Scholar] [CrossRef]
- Li, P.; Chai, Z.; Lin, P.; Huang, C.; Huang, G.; Xu, L.; Deng, Z.; Zhang, M.; Zhang, Y.; Zhao, X. Genome-wide identification and expression analysis of AP2/ERF transcription factors in sugarcane (Saccharum spontaneum L.). BMC Genom. 2020, 21, 1–17. [Google Scholar] [CrossRef]
- Fang, Z.W.; Xu, X.Y.; Gao, J.F.; Wang, P.K.; Liu, Z.X.; Feng, B.L. Characterization of FeDREB1 promoter involved in cold- and drought-inducible expression from common buckwheat (Fagopyrum esculentum). Genet. Mol. Res. 2015, 14, 7990–8000. [Google Scholar] [CrossRef]
- Liu, X.Q.; Zhu, J.J.; Wei, C.J.; Guo, Q.; Bian, C.K.; Xiang, Z.H.; Zhao, A.C. Genome-wide identification and characterization of the DREB transcription factor gene family in mulberry. Biol. Plant. 2015, 59, 253–265. [Google Scholar] [CrossRef]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 2012, 1819, 86–96. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Gupta, A.; Soni, D.; Garg, R.; Pathre, U.V.; Nath, P.; Sane, A.P. Ectopic expression of a tomato DREB gene affects several ABA processes and influences plant growth and root architecture in an age-dependent manner. J. Plant Physiol. 2017, 214, 97–107. [Google Scholar] [CrossRef]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress. In Survival Strategies in Extreme Cold and Desiccation. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Xi, Y.; Chen, X.L.; Cheng, Z.M. Genome-wide identification of AP2/EREBP in Fragaria vesca and expression pattern analysis of the FvDREB subfamily under drought stress. BMC Plant Biol. 2021, 21, 295. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, G.; Liu, X.; Wang, Z.; Zhang, M.; Zhang, J. Genome-wide identification and expression profiling of DREB genes in Saccharum spontaneum. BMC Genom. 2021, 22, 456–470. [Google Scholar] [CrossRef]
- Mallikarjuna, G.; Mallikarjuna, K.; Reddy, M.K.; Kaul, T. Expression of OsDREB2A transcription factor confers enhanced dehydration and salt stress tolerance in rice (Oryza sativa L.). Biotechnol. Lett. 2011, 33, 1689–1697. [Google Scholar] [CrossRef]
- Mizoi, J.; Ohori, T.; Moriwaki, T.; Kidokoro, S.; Todaka, D.; Maruyama, K.; Kusakabe, K.; Osakabe, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. GmDREB2A;2, a Canonical Dehydration-Responsive element-binding protein2-Type Transcription Factor in Soybean, Is Posttranslationally Regulated and Mediates Dehydration-Responsive Element-Dependent Gene Expression. Plant Physiol. 2013, 161, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc. Natl. Acad. Sci. USA 2006, 49, 18822–18827. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, K.; Yang, Y.; Wu, J.; Chen, F.; Yu, D. Molecular cloning, expression profiling and trans-activation property studies of a DREB 2 -like gene from chrysanthemum (Dendranthema vestitum). J. Plant Res. 2008, 121, 215–226. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Zhang, Y.; Ma, F.; Zhao, J.; Yang, H.; Song, S.; Zhang, S. Identification of DREB Family Genes in Banana and Their Function under Drought and Cold Stress. Plants 2024, 13, 2119. https://doi.org/10.3390/plants13152119
Xu Y, Zhang Y, Ma F, Zhao J, Yang H, Song S, Zhang S. Identification of DREB Family Genes in Banana and Their Function under Drought and Cold Stress. Plants. 2024; 13(15):2119. https://doi.org/10.3390/plants13152119
Chicago/Turabian StyleXu, Yi, Yanshu Zhang, Funing Ma, Jingxi Zhao, Huiting Yang, Shun Song, and Shaoling Zhang. 2024. "Identification of DREB Family Genes in Banana and Their Function under Drought and Cold Stress" Plants 13, no. 15: 2119. https://doi.org/10.3390/plants13152119





