Effects of Grazing and Leaf Spot Disease on the Structure and Diversity of Phyllosphere Microbiome Communities in Leymus chinensis
Abstract
1. Introduction
2. Results
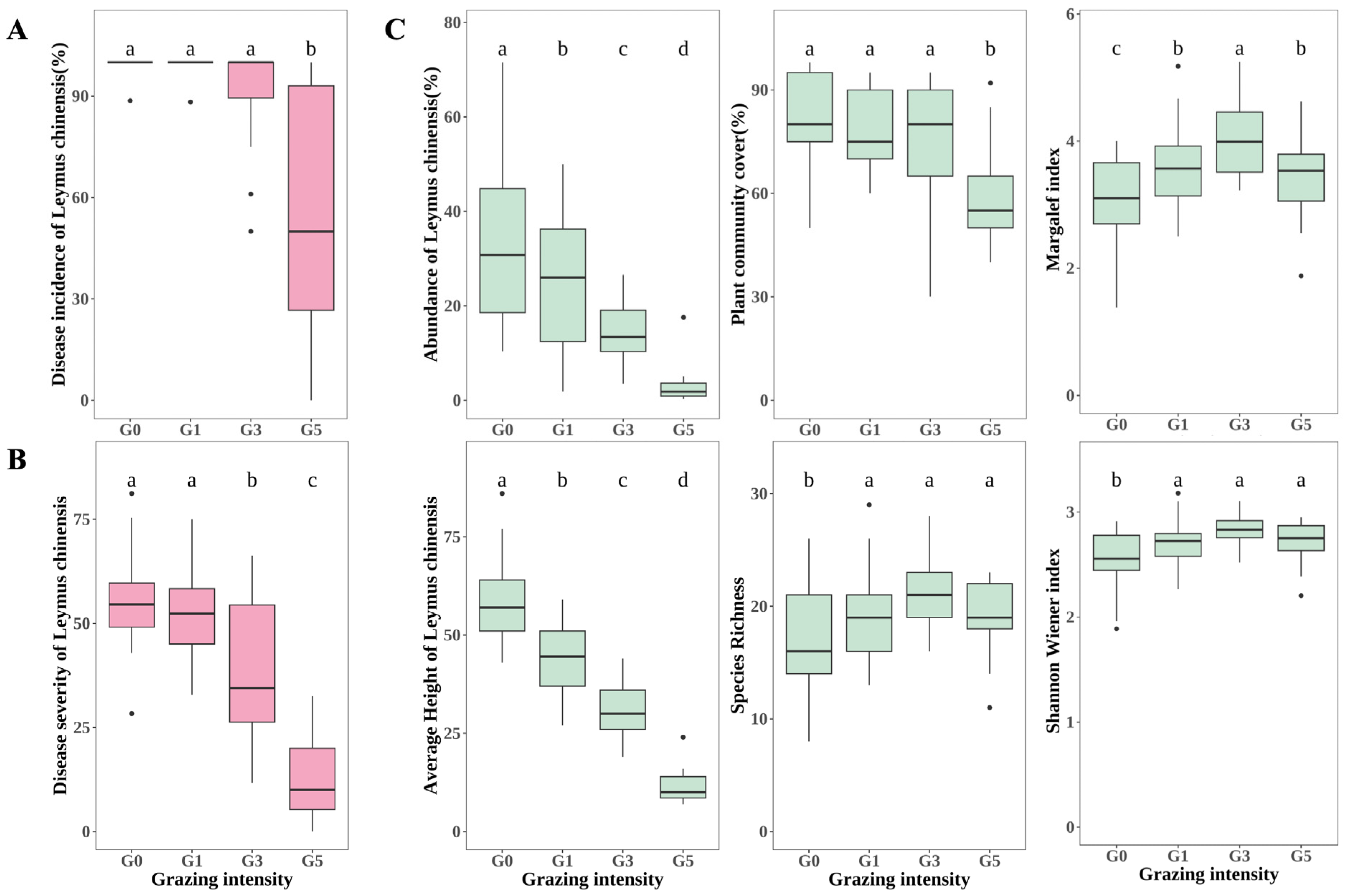
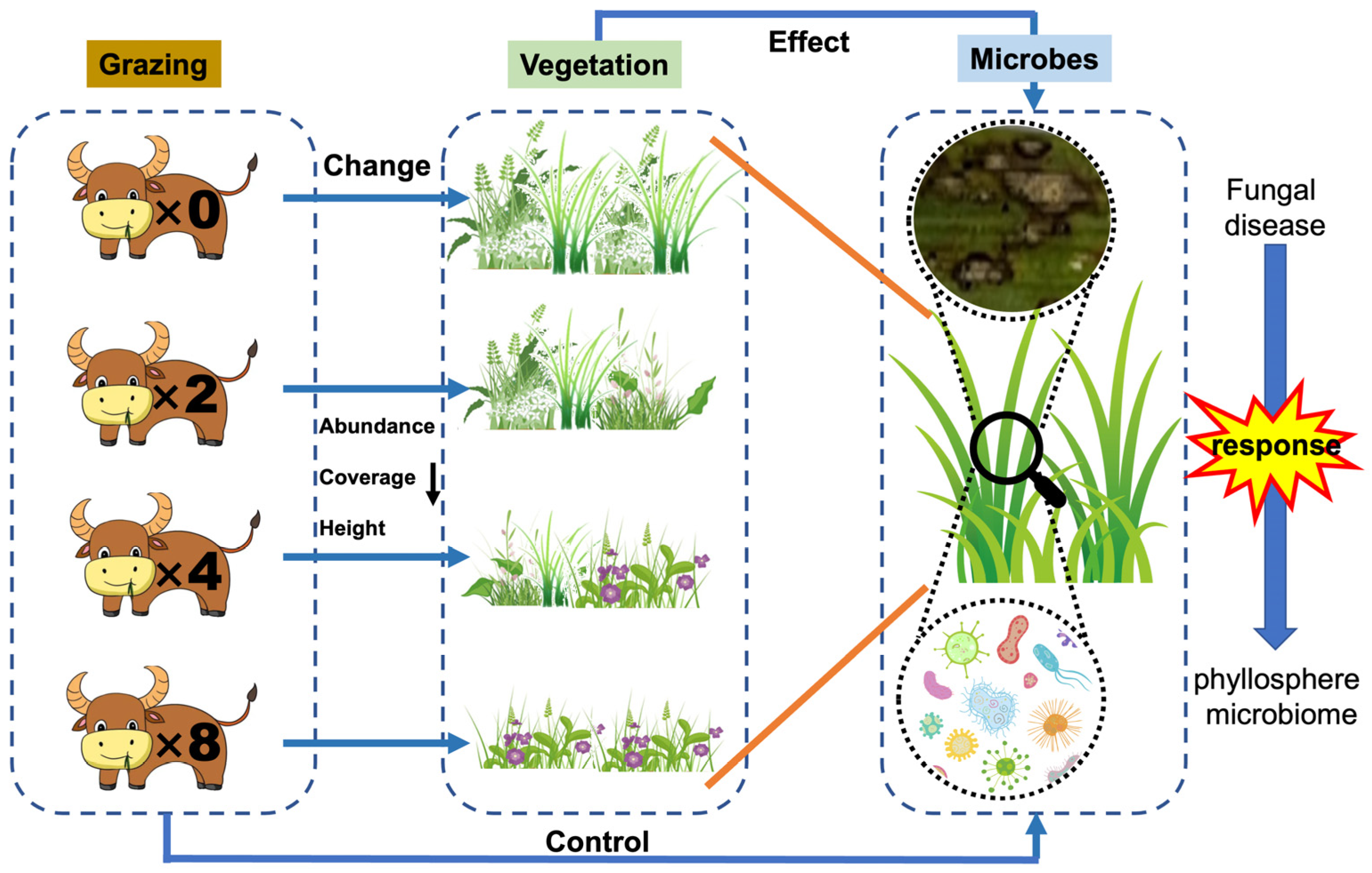
2.1. Effects of Grazing on Leaf Spot Disease and Vegetation of Leymus chinensis
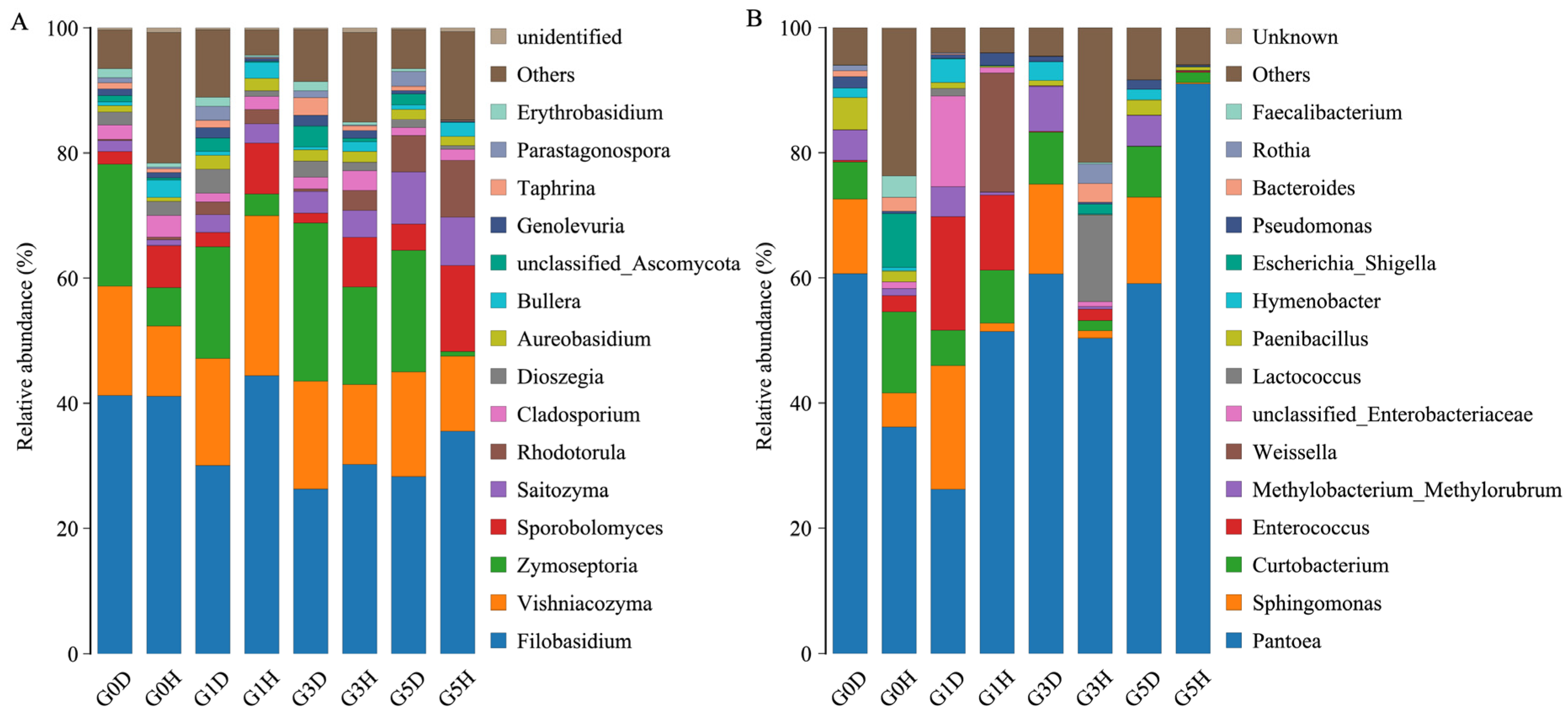
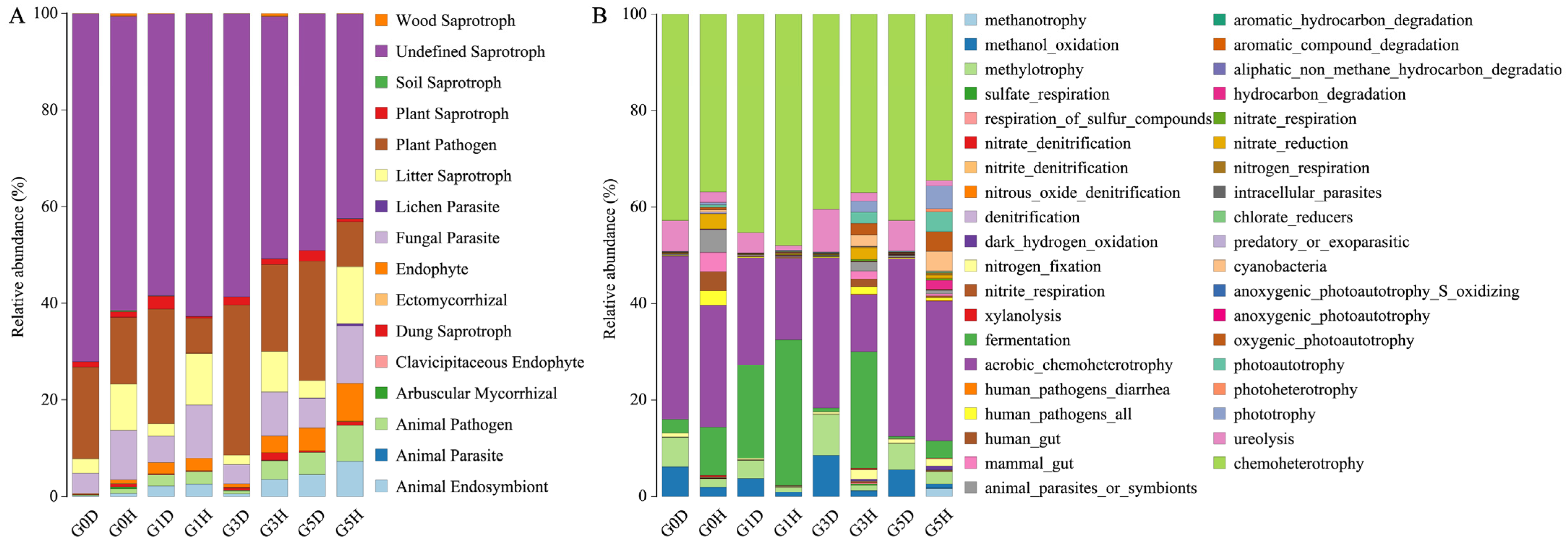
2.2. Microbial Composition
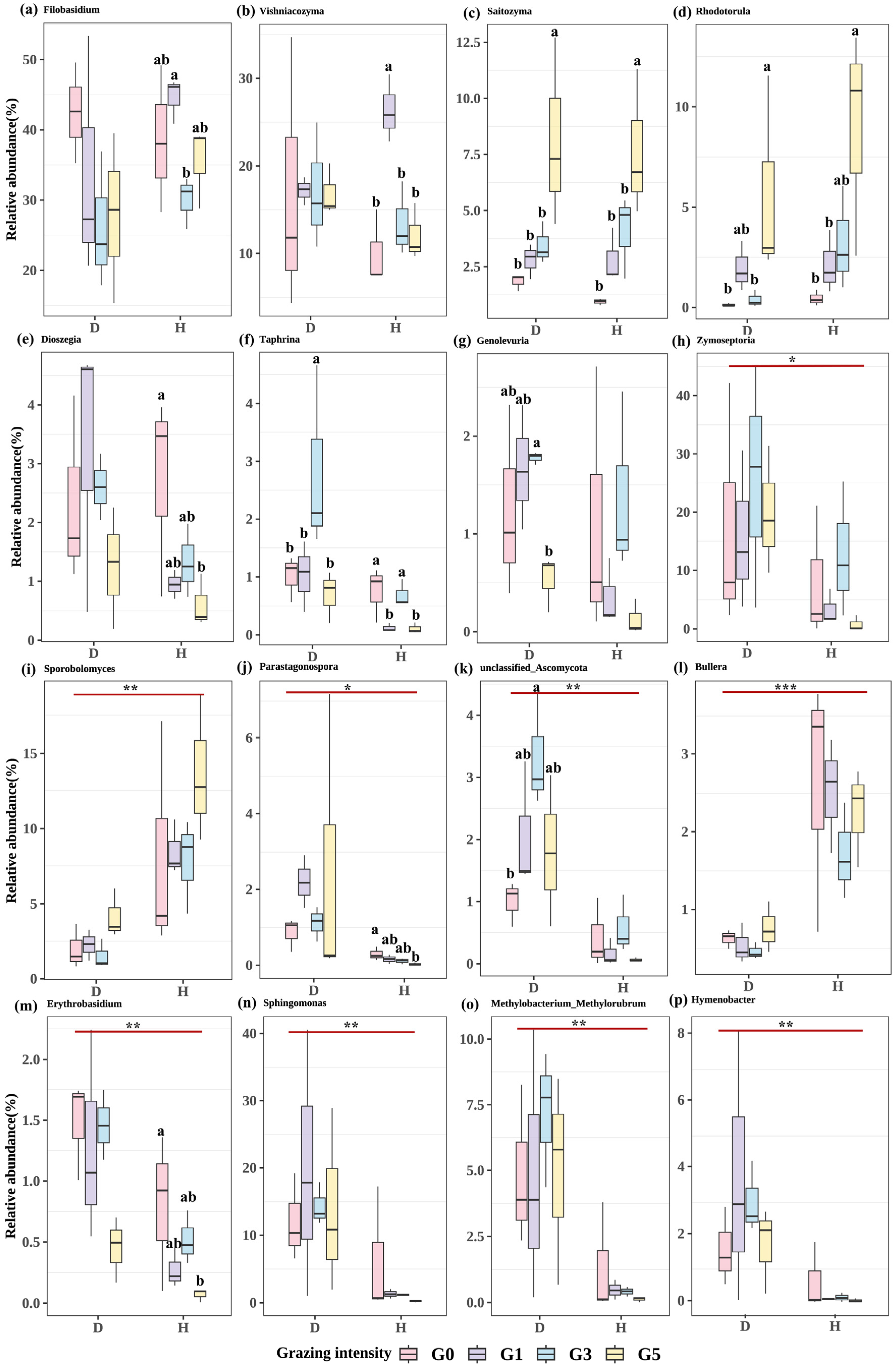
2.2.1. Differences in Microbial Genus Levels across Treatments
2.2.2. Effects of Grazing on the Species Composition of Phyllospheric Micro-Organism
2.2.3. Impact of Leaf Spot Disease on Phyllosphere Microbiome Composition
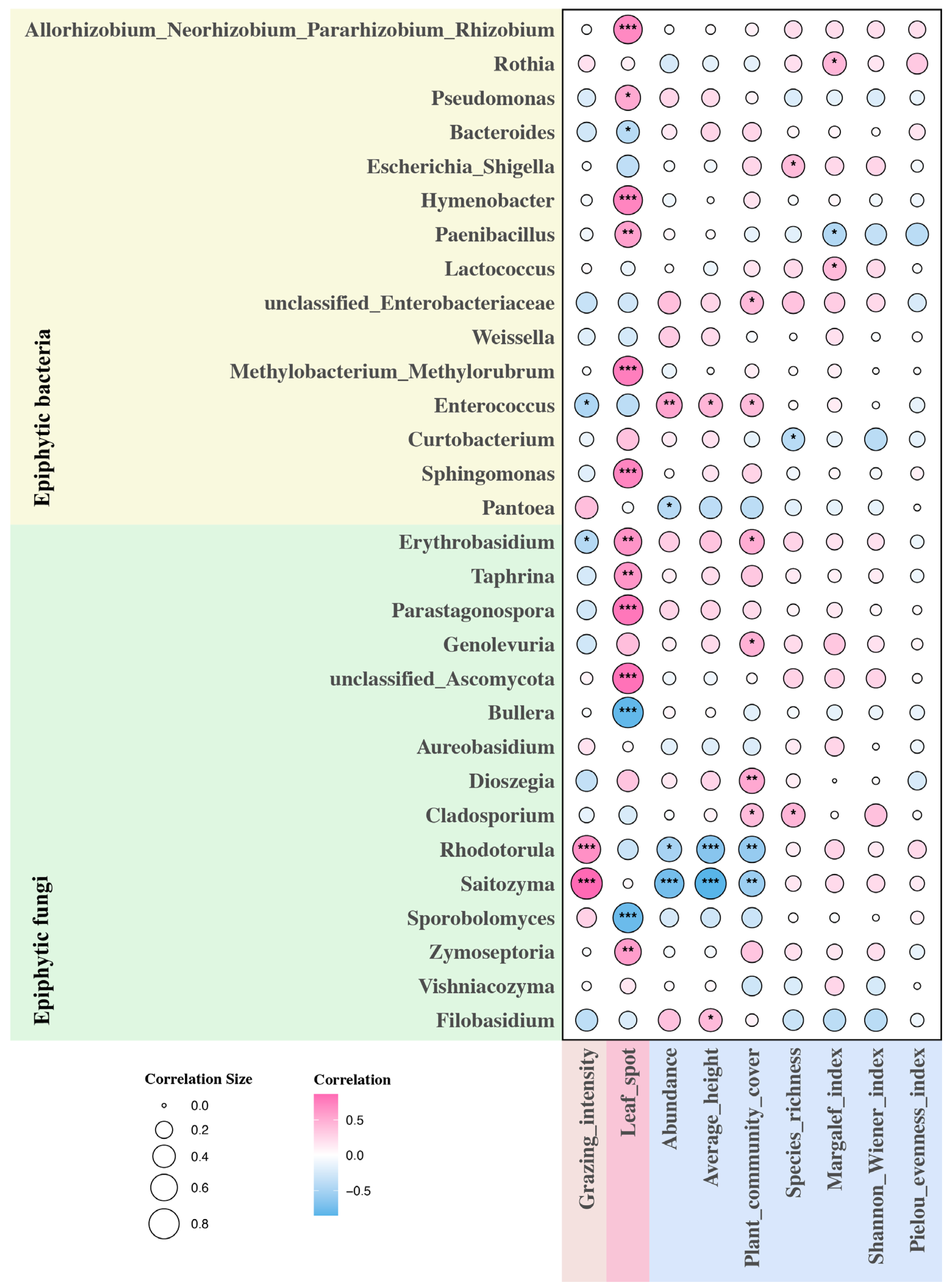
2.3. Environmental Factors and Phyllosphere Microbiome
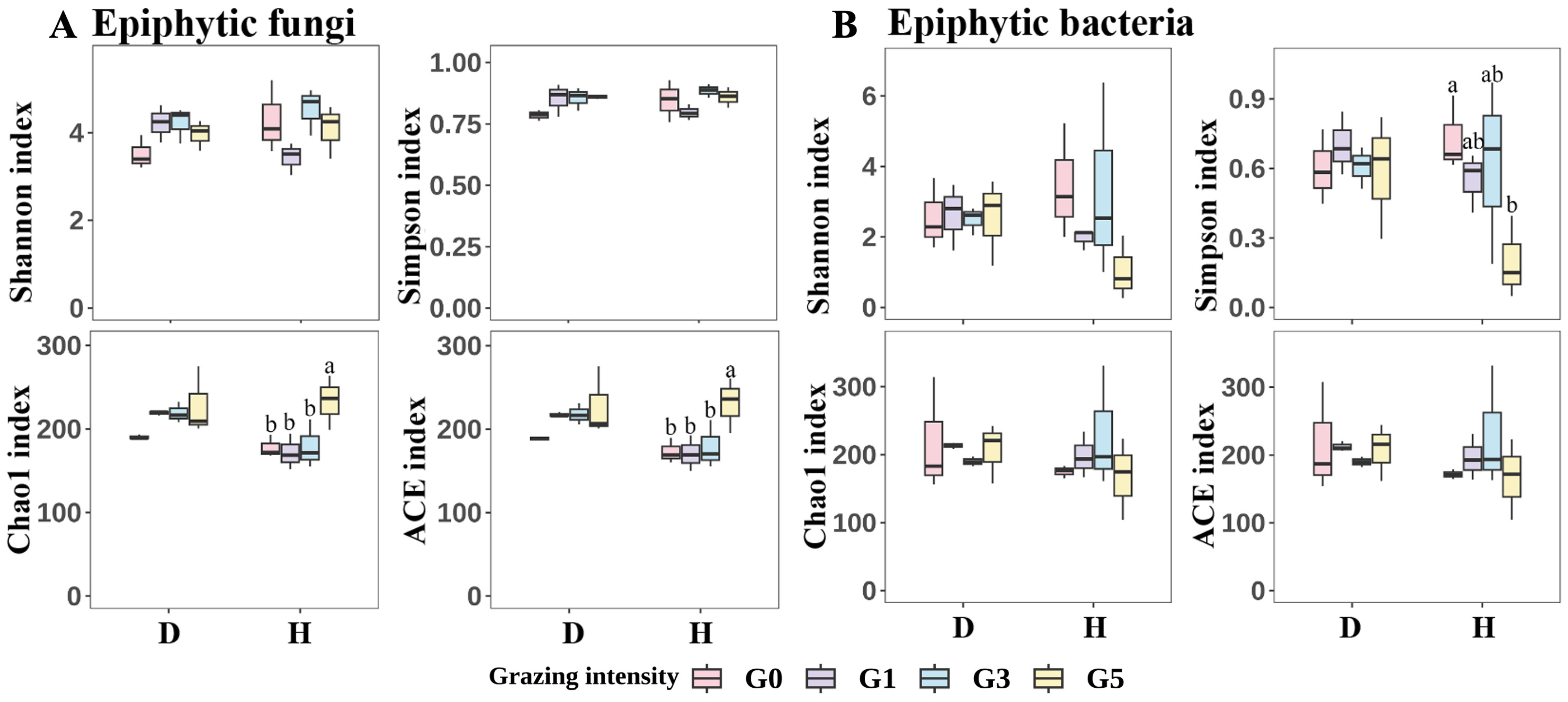
2.4. Microbial Diversity
2.5. Functional Gene Prediction
3. Discussion
3.1. Effects of Grazing on Plant Communities and Leaf Spot Diseases
3.2. Effects of Leaf Spot Disease on Phyllosphere Microbiome
3.3. Effects of Grazing on Phyllosphere Microbiome
4. Materials and Methods
4.1. Experimental Site
4.2. Experimental Plot Design
4.3. Plant and Disease Incidence Investigation
4.4. Phyllospheric Micro-Organism Sampling and Processing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Chen, T.; Nan, Z.; Christensen, M.J. Cattle Grazing Alters the Interaction of Seed-Borne Fungi and Two Foliar Pathogens of Leymus chinensis in a Meadow Steppe. Eur. J. Plant Pathol. 2019, 155, 207–218. [Google Scholar] [CrossRef]
- Wang, H.; Xiang, Y.; Li, L.H.; Bhanbhro, N.; Yang, C.W.; Zhang, Z. Photosynthetic Response and Transcriptomic Profiling Provide Insights into the Alkali Tolerance of Clone Halophyte Leymus Chinensis. Photosynthetica 2020, 58, 780–789. [Google Scholar] [CrossRef]
- Gao, J.; Duan, M.; Hasi, G.; Yang, J.; Yan, C.; Kang, Y.; Qi, Z. Comparison of Two Contrasting Leymus chinensis Accessions Reveals the Roles of the Cell Wall and Auxin in Rhizome Development. J. Plant Physiol. 2023, 287, 154003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nan, Z.; Christensen, M.J.; Xin, X.; Zhang, N. Effects of Rust on Plant Growth and Stoichiometry of Leymuschinensis under Different Grazing Intensities in Hulunber Grassland. Agriculture 2022, 12, 961. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Nan, Z.B. First Report of Leaf Blotch Caused by Parastagonospora Nodorum on Leymus chinensis (Chinese Rye Grass) in China. Plant Dis. 2018, 102, 2661. [Google Scholar] [CrossRef]
- Bever, J.D.; Mangan, S.A.; Alexander, H.M. Maintenance of Plant Species Diversity by Pathogens. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 305–325. [Google Scholar] [CrossRef]
- Parker, I.M.; Saunders, M.; Bontrager, M.; Weitz, A.P.; Hendricks, R.; Magarey, R.; Suiter, K.; Gilbert, G.S. Phylogenetic Structure and Host Abundance Drive Disease Pressure in Communities. Nature 2015, 520, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Ricklefs, R.E. Intrinsic Dynamics of the Regional Community. Ecol. Lett. 2015, 18, 497–503. [Google Scholar] [CrossRef]
- Packer, C.; Holt, R.D.; Hudson, P.J.; Lafferty, K.D.; Dobson, A.P. Keeping the Herds Healthy and Alert: Implications of Predator Control for Infectious Disease. Ecol. Lett. 2003, 6, 797–802. [Google Scholar] [CrossRef]
- Daleo, P.; Silliman, B.; Alberti, J.; Escapa, M.; Canepuccia, A.; Peña, N.; Iribarne, O. Grazer Facilitation of Fungal Infection and the Control of Plant Growth in South-Western Atlantic Salt Marshes. J. Ecol. 2009, 97, 781–787. [Google Scholar] [CrossRef]
- Liu, M.; Mipam, T.D.; Wang, X.; Zhang, P.; Lin, Z.; Liu, X. Contrasting Effects of Mammal Grazing on Foliar Fungal Diseases: Patterns and Potential Mechanisms. New Phytol. 2021, 232, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lyu, S.; Zhou, S.; Bradshaw, C.J.A. Warming and Fertilization Alter the Dilution Effect of Host Diversity on Disease Severity. Ecology 2016, 97, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Stout, M.J.; Thaler, J.S.; Thomma, B.P.H.J. Plant-Mediated Interactions between Pathogenic Microorganisms and Herbivorous ArthropodS. Annu. Rev. Entomol. 2006, 51, 663–689. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Agrawal, A.A.; Halitschke, R. Salicylate-Mediated Interactions between Pathogens and Herbivores. Ecology 2010, 91, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Wardle, D.A. Herbivore-Mediated Linkages between Aboveground and Belowground Communities. Ecology 2003, 84, 2258–2268. [Google Scholar] [CrossRef]
- Huang, M.; Liu, X.; Cadotte, M.W.; Zhou, S. Functional and Phylogenetic Diversity Explain Different Components of Diversity Effects on Biomass Production. Oikos 2020, 129, 1185–1195. [Google Scholar] [CrossRef]
- Gao, P.; Duan, T.-Y.; Nan, Z.-B.; Christensen, M.J.; Liu, Q.-T.; Meng, F.-J.; Huang, J.-F. The Influence of Irrigation Frequency on the Occurrence of Rust Disease (Melampsora apocyni) and Determination of the Optimum Irrigation Regime in Organic Apocynum Venetum Production. Agric. Water Manag. 2018, 205, 81–89. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, D.; Jiang, F.; Tian, Z.; Feng, X.; Wu, N.; Hou, F.; Kardol, P.; Nan, Z.; Chen, T. Long-Term Heavy Grazing Increases Community-Level Foliar Fungal Diseases by Shifting Plant Composition. J. Appl. Ecol. 2022, 59, 791–800. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the Rhizosphere Microbiome for Disease-Suppressive Bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Yu, K.; Pieterse, C.M.J.; Bakker, P.A.H.M.; Berendsen, R.L. Beneficial Microbes Going Underground of Root Immunity. Plant Cell Environ. 2019, 42, 2860–2870. [Google Scholar] [CrossRef]
- Toju, H.; Peay, K.G.; Yamamichi, M.; Narisawa, K.; Hiruma, K.; Naito, K.; Fukuda, S.; Ushio, M.; Nakaoka, S.; Onoda, Y.; et al. Core Microbiomes for Sustainable Agroecosystems. Nat. Plants 2018, 4, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Naylor, D.; Sadler, N.; Bhattacharjee, A.; Graham, E.B.; Anderton, C.R.; McClure, R.; Lipton, M.; Hofmockel, K.S.; Jansson, J.K. Soil Microbiomes Under Climate Change and Implications for Carbon Cycling. Annu. Rev. Environ. Resour. 2020, 45, 29–59. [Google Scholar] [CrossRef]
- Jiao, N.; Song, X.; Song, R.; Yin, D.; Deng, X. Diversity and Structure of the Microbial Community in Rhizosphere Soil of Fritillaria Ussuriensis at Different Health Levels. PeerJ 2022, 10, e12778. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, A.J.; Cardoni, M.; Gómez-Lama Cabanás, C.; Valverde-Corredor, A.; Villadas, P.J.; Fernández-López, M.; Mercado-Blanco, J. Linking Belowground Microbial Network Changes to Different Tolerance Level towards Verticillium Wilt of Olive. Microbiome 2020, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Kishore, G.K.; Pande, S.; Podile, A.R. Biological Control of Late Leaf Spot of Peanut (Arachis hypogaea) with Chitinolytic Bacteria. Phytopathology 2005, 95, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, A.; Nicosia, M.G.L.D.; Cacciola, S.O.; Droby, S.; Schena, L. Metabarcoding Analysis of Fungal Diversity in the Phyllosphere and Carposphere of Olive (Olea europaea). PLoS ONE 2015, 10, e0131069. [Google Scholar] [CrossRef] [PubMed]
- Bitas, V.; Kim, H.-S.; Bennett, J.W.; Kang, S. Sniffing on Microbes: Diverse Roles of Microbial Volatile Organic Compounds in Plant Health. Mol. Plant-Microbe Interact. 2013, 26, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Steinebrunner, F.; Schiestl, F.P.; Leuchtmann, A. Ecological Role of Volatiles Produced by Epichloë: Differences in Antifungal Toxicity. FEMS Microbiol. Ecol. 2008, 64, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.-Y.; Cao, Y.; Zhang, K.-Q. Metagenomic Insights into Communities, Functions of Endophytes and Their Associates with Infection by Root-Knot Nematode, Meloidogyne Incognita, in Tomato Roots. Sci Rep. 2015, 5, 17087. [Google Scholar] [CrossRef]
- Ma, Y.; Qu, Z.-L.; Liu, B.; Tan, J.-J.; Asiegbu, F.O.; Sun, H. Bacterial Community Structure of Pinus Thunbergii Naturally Infected by the Nematode Bursaphelenchus Xylophilus. Microorganisms 2020, 8, 307. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, L.; Tan, X.; Kong, X.; Yang, J.; Wang, D.; Zhang, D.; Jin, D.; Liu, Y. Pumpkin Powdery Mildew Disease Severity Influences the Fungal Diversity of the Phyllosphere. PeerJ 2018, 6, e4559. [Google Scholar] [CrossRef] [PubMed]
- Manching, H.C.; Balint-Kurti, P.J.; Stapleton, A.E. Southern Leaf Blight Disease Severity Is Correlated with Decreased Maize Leaf Epiphytic Bacterial Species Richness and the Phyllosphere Bacterial Diversity Decline Is Enhanced by Nitrogen Fertilization. Front. Plant Sci. 2014, 5, 403. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Nomura, K.; Wang, X.; Sohrabi, R.; Xu, J.; Yao, L.; Paasch, B.C.; Ma, L.; Kremer, J.; Cheng, Y.; et al. A Plant Genetic Network for Preventing Dysbiosis in the Phyllosphere. Nature 2020, 580, 653–657. [Google Scholar] [CrossRef]
- Xu, H.; You, C.; Tan, B.; Xu, L.; Liu, Y.; Wang, M.; Xu, Z.; Sardans, J.; Peñuelas, J. Effects of Livestock Grazing on the Relationships between Soil Microbial Community and Soil Carbon in Grassland Ecosystems. Sci. Total Environ. 2023, 881, 163416. [Google Scholar] [CrossRef] [PubMed]
- Mushinski, R.M.; Zhou, Y.; Hyodo, A.; Casola, C.; Boutton, T.W. Interactions of Long-Term Grazing and Woody Encroachment Can Shift Soil Biogeochemistry and Microbiomes in Savanna Ecosystems. Geoderma 2024, 441, 116733. [Google Scholar] [CrossRef]
- Jing, L.; Mipam, T.D.; Ai, Y.; Jiang, A.; Gan, T.; Zhang, S.; Liu, J.; Tian, L. Grazing Intensity Alters Soil Microbial Diversity and Network Complexity in Alpine Meadow on the Qinghai-Tibet Plateau. Agric. Ecosyst. Environ. 2023, 353, 108541. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Delgado-Baquerizo, M. Functional Groups of Soil Fungi Decline under Grazing. Plant Soil. 2018, 426, 51–60. [Google Scholar] [CrossRef]
- Wang, L.; Delgado-Baquerizo, M.; Wang, D.; Isbell, F.; Liu, J.; Feng, C.; Liu, J.; Zhong, Z.; Zhu, H.; Yuan, X.; et al. Diversifying Livestock Promotes Multidiversity and Multifunctionality in Managed Grasslands. Proc. Natl. Acad. Sci. USA 2019, 116, 6187–6192. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Le Bagousse-Pinguet, Y.; Delgado-Baquerizo, M.; Eldridge, D.J.; Saiz, H.; Berdugo, M.; Gozalo, B.; Ochoa, V.; Guirado, E.; García-Gómez, M.; et al. Grazing and Ecosystem Service Delivery in Global Drylands. Science 2022, 378, 915–920. [Google Scholar] [CrossRef]
- Eskelinen, A.; Harpole, W.S.; Jessen, M.-T.; Virtanen, R.; Hautier, Y. Light Competition Drives Herbivore and Nutrient Effects on Plant Diversity. Nature 2022, 611, 301–305. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Miao, H.; Zhang, C.; Zou, H.; Yang, Y.; Zhang, Z.; Liu, J. Appropriate Livestock Grazing Alleviates the Loss of Plant Diversity and Maintains Community Resistance in Alpine Meadows. J. Environ. Manag. 2024, 351, 119850. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, J.; Tang, Y.; Li, Z.; Liu, H.; Wang, L.; Wu, Y.; Liang, C. Plant Functional Traits and Biodiversity Can Reveal the Response of Ecosystem Functions to Grazing. Sci. Total Environ. 2023, 899, 165636. [Google Scholar] [CrossRef]
- Mitchell, C.E.; Tilman, D.; Groth, J.V. Effects of Grassland Plant Species Diversity, Abundance, and Composition on Foliar Fungal Disease. Ecology 2002, 83, 1713–1726. [Google Scholar] [CrossRef]
- Mitchell, C.E.; Reich, P.B.; Tilman, D.; Groth, J.V. Effects of Elevated CO2, Nitrogen Deposition, and Decreased Species Diversity on Foliar Fungal Plant Disease. Glob. Chang. Biol. 2003, 9, 438–451. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Keesing, F. Effects of Host Diversity on Infectious Disease. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 157–182. [Google Scholar] [CrossRef]
- Li, P.-D.; Zhu, Z.-R.; Zhang, Y.; Xu, J.; Wang, H.; Wang, Z.; Li, H. The Phyllosphere Microbiome Shifts toward Combating Melanose Pathogen. Microbiome 2022, 10, 56. [Google Scholar] [CrossRef]
- Seybold, H.; Demetrowitsch, T.J.; Hassani, M.A.; Szymczak, S.; Reim, E.; Haueisen, J.; Lübbers, L.; Rühlemann, M.; Franke, A.; Schwarz, K.; et al. A Fungal Pathogen Induces Systemic Susceptibility and Systemic Shifts in Wheat Metabolome and Microbiome Composition. Nat Commun. 2020, 11, 1910. [Google Scholar] [CrossRef] [PubMed]
- Croll, D.; Crous, P.W.; Pereira, D.; Mordecai, E.A.; McDonald, B.A.; Brunner, P.C. Genome-Scale Phylogenies Reveal Relationships among Parastagonospora Species Infecting Domesticated and Wild Grasses. Persoonia 2021, 46, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Quaedvlieg, W.; Kema, G.H.J.; Groenewald, J.Z.; Verkley, G.J.M.; Seifbarghi, S.; Razavi, M.; Gohari, A.M.; Mehrabi, R.; Crous, P.W. Zymoseptoria Gen. Nov.: A New Genus to Accommodate Septoria-like Species Occurring on Graminicolous Hosts. Persoonia-Mol. Phylogeny Evol. Fungi 2011, 26, 57–69. [Google Scholar] [CrossRef]
- Meile, L.; Garrido-Arandia, M.; Bernasconi, Z.; Peter, J.; Schneller, A.; Bernasconi, A.; Alassimone, J.; McDonald, B.A.; Sánchez-Vallet, A. Natural Variation in Avr3D1 from Zymoseptoria sp. Contributes to Quantitative Gene-for-Gene Resistance and to Host Specificity. New Phytol. 2023, 238, 1562–1577. [Google Scholar] [CrossRef]
- Stukenbrock, E.H.; Quaedvlieg, W.; Javan-Nikhah, M.; Zala, M.; Crous, P.W.; McDonald, B.A. Zymoseptoria Ardabiliae and Z. Pseudotritici, Two Progenitor Species of the Septoria Tritici Leaf Blotch Fungus Z. Tritici (Synonym: Mycosphaerella Graminicola). Mycologia 2012, 104, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- De Tenório, D.A.; de Medeiros, E.V.; Lima, C.S.; da Silva, J.M.; de Barros, J.A.; Neves, R.P.; Laranjeira, D. Biological Control of Rhizoctonia Solani in Cowpea Plants Using Yeast. Trop. Plant Pathol. 2019, 44, 113–119. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The Rhizosphere Microbiome: Significance of Plant Beneficial, Plant Pathogenic, and Human Pathogenic Microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Yang, N.; Wen, Z.; Sun, X.; Chai, Y.; Ma, Z. Wheat Microbiome Bacteria Can Reduce Virulence of a Plant Pathogenic Fungus by Altering Histone Acetylation. Nat. Commun. 2018, 9, 3429. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-Mediated Stress Resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Mazzola, M. Diversity and Natural Functions of Antibiotics Produced by Beneficial and Plant Pathogenic Bacteria. Annu. Rev. Phytopathol. 2012, 50, 403–424. [Google Scholar] [CrossRef] [PubMed]
- Ritpitakphong, U.; Falquet, L.; Vimoltust, A.; Berger, A.; Métraux, J.-P.; L’Haridon, F. The Microbiome of the Leaf Surface of Arabidopsis Protects against a Fungal Pathogen. New Phytol. 2016, 210, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Wees, S.C.M.V.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Masenya, K.; Thompson, G.D.; Tekere, M.; Makhalanyane, T.P.; Pierneef, R.E.; Rees, D.J.G. Pathogen Infection Influences a Distinct Microbial Community Composition in Sorghum RILs. Plant Soil 2021, 463, 555–572. [Google Scholar] [CrossRef]
- Lee, S.E.; Ten, L.N.; Park, Y.; Maeng, S.; Zhang, J.; Kim, M.-K.; Cha, I.-T.; Lee, K.-E.; Lee, B.-H.; Jung, H.-Y.; et al. Hymenobacter Busanensis Sp. Nov., Radiation-Resistant Species Isolated from Soil in South Korea. Arch. Microbiol. 2021, 203, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Lindow, S.E.; Leveau, J.H.J. Phyllosphere Microbiology. Curr. Opin. Biotechnol. 2002, 13, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; Civitello, D.J.; Halliday, F.W.; Hudson, P.J.; Lafferty, K.D.; Wood, C.L.; Mordecai, E.A. Towards Common Ground in the Biodiversity-Disease Debate. Nat. Ecol. Evol. 2020, 4, 24–33. [Google Scholar] [CrossRef]
- Bergmann, G.E.; Leveau, J.H.J. A Metacommunity Ecology Approach to Understanding Microbial Community Assembly in Developing Plant Seeds. Front. Microbiol. 2022, 13, 877519. [Google Scholar] [CrossRef] [PubMed]
- Custer, G.F.; Bresciani, L.; Dini-Andreote, F. Ecological and Evolutionary Implications of Microbial Dispersal. Front. Microbiol. 2022, 13, 855859. [Google Scholar] [CrossRef]
- Nan, Z.B. Investigation and evaluation of pasture diseases[A]. In Methods of Research in Grass Science; Ren, J.-Z., Ed.; China Agricultural Publishing House: Beijing, China, 1998; pp. 214–232. (In Chinese) [Google Scholar]
- Li, M.; Hong, L.; Ye, W.; Wang, Z.; Shen, H. Phyllosphere Bacterial and Fungal Communities Vary with Host Species Identity, Plant Traits and Seasonality in a Subtropical Forest. Environ. Microbiome 2022, 17, 29. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Y.; Jin, Y.; Han, X.; Malik, K.; Li, C.; Yu, B. Effects of Grazing and Leaf Spot Disease on the Structure and Diversity of Phyllosphere Microbiome Communities in Leymus chinensis. Plants 2024, 13, 2128. https://doi.org/10.3390/plants13152128
Qian Y, Jin Y, Han X, Malik K, Li C, Yu B. Effects of Grazing and Leaf Spot Disease on the Structure and Diversity of Phyllosphere Microbiome Communities in Leymus chinensis. Plants. 2024; 13(15):2128. https://doi.org/10.3390/plants13152128
Chicago/Turabian StyleQian, Yani, Yuanyuan Jin, Xinyao Han, Kamran Malik, Chunjie Li, and Binhua Yu. 2024. "Effects of Grazing and Leaf Spot Disease on the Structure and Diversity of Phyllosphere Microbiome Communities in Leymus chinensis" Plants 13, no. 15: 2128. https://doi.org/10.3390/plants13152128
APA StyleQian, Y., Jin, Y., Han, X., Malik, K., Li, C., & Yu, B. (2024). Effects of Grazing and Leaf Spot Disease on the Structure and Diversity of Phyllosphere Microbiome Communities in Leymus chinensis. Plants, 13(15), 2128. https://doi.org/10.3390/plants13152128






