Evaluation of the Antigenotoxic Potential of Two Types of Chayote (Sechium edule) Juices
Abstract
:1. Introduction
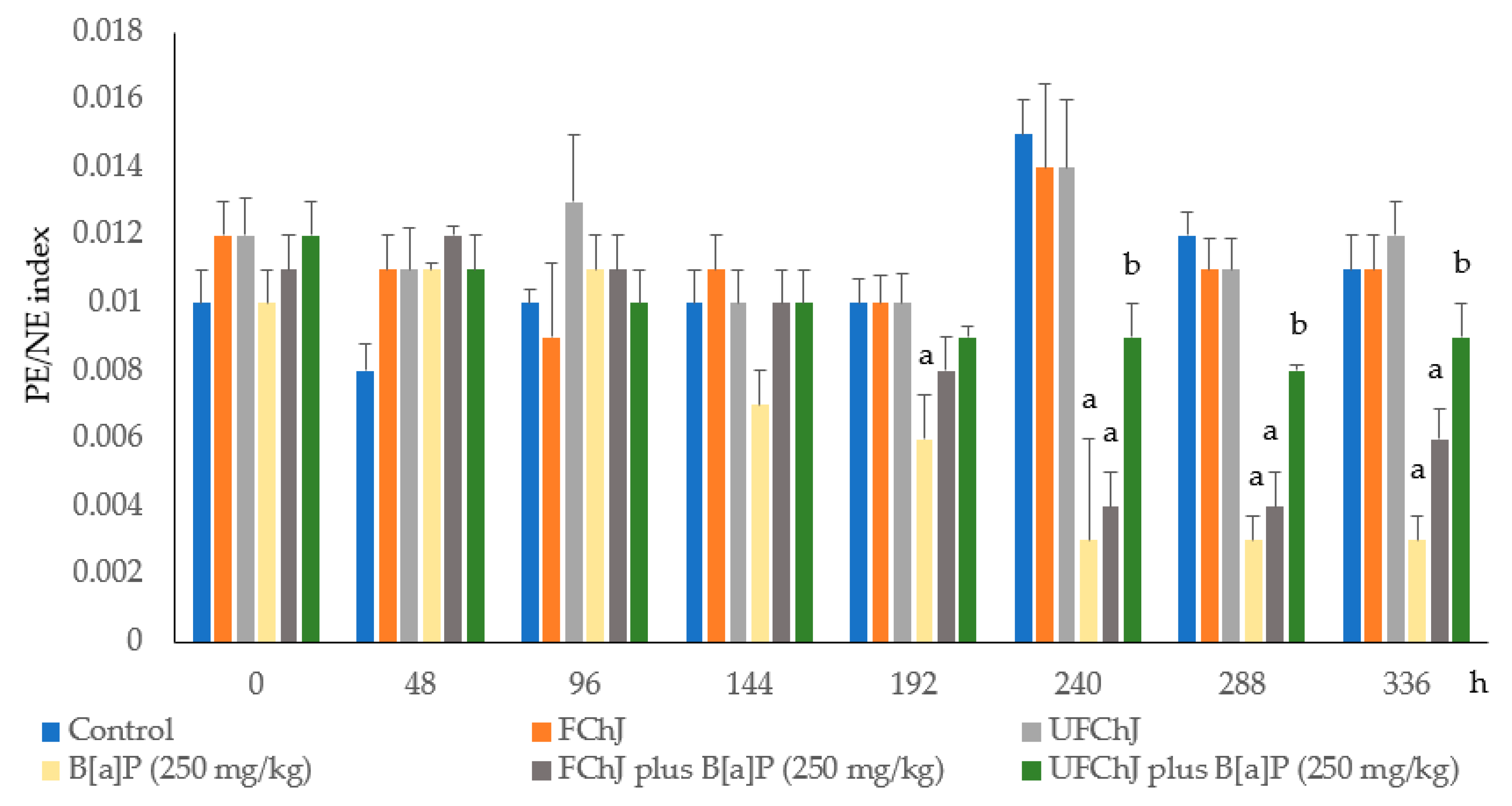
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Plant Material and Obtaining and Preparation of Fresh Chayote Juices
4.4. Antigenotoxicity/Genotoxicity Protocol
4.5. Data Analysis
5. Perspectives and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vieira, E.F.; Pinho, O.; Ferreira, I.M.P.L.V.O.; Delerue-Matos, C. Chayote (Sechium edule): A review of nutritional composition, bioactivities and potential applications. Food Chem. 2019, 275, 557–568. [Google Scholar] [CrossRef]
- Pu, Y.-T.; Luo, Q.; Wen, L.-H.; Li, Y.-R.; Meng, P.-H.; Wang, X.-J.; Tan, G.-F. Origin, Evolution, Breeding, and Omics of Chayote, an Important Cucurbitaceae Vegetable Crop. Front. Plant Sci. 2021, 12, 739091. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.A. Chayote: Pre-columbian origins and dispersal. Hortic. Rev. 2015, 43, 89–143. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Arévalo-Galarza, L.; Avendaño-Arrazate, C.H.; Soto-Hernández, M.; Ruiz-Posadas, L.D.M.; Santiago-Osorio, E.; Acosta-Ramos, M.; Cisneros-Solano, V.M.; Aguirre-Medina, J.F.; Ochoa-Martínez, D. Production, genetics, postharvest management and pharmacological characteristics of Sechium edule (Jacq.) Sw. Fresh Prod. 2007, 1, 41–53. [Google Scholar]
- Hernandez-Uribe, J.P.; Agama-Acevedo, E.; Gonzalez-Soto, R.A.; Bello-Pérez, L.A.; Vargas-Torres, A. Isolation and characterization of Mexican chayote tuber (Sechium edule Sw.). Starch 2011, 63, 32–41. [Google Scholar] [CrossRef]
- Siciliano, T.; De Tommasi, N.; Morelli, I.; Braca, A. Study of flavonoids of Sechium edule (Jacq) Swartz (Cucurbitaceae) different edible organs by liquid chromatography photodiode array mass spectrometry. J. Agric. Food Chem. 2004, 52, 6510–6515. [Google Scholar] [CrossRef] [PubMed]
- Noumedem, J.A.; Mihasan, M.; Lacmata, S.T.; Stefan, M.; Kuiate, J.R.; Kuete, V. Antibacterial activities of the methanol extracts of ten Cameroonian vegetables against Gram-negative multidrug-resistant bacteria. BMC Complement. Altern. Med. 2013, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, A.A.L.; Gomez, J.D.; Cudmani, N.M.; Vattuone, M.A.; Isla, M.I. Antimicrobial activity of nine extracts of Sechium edule (Jacq.) Swartz. Microb. Ecol. Health Dis. 2003, 15, 33–39. [Google Scholar] [CrossRef]
- Yang, M.Y.; Chan, K.C.; Lee, Y.J.; Chang, X.Z.; Wu, C.H.; Wang, C.J. Sechium edule shoot extracts and active components improve obesity and a fatty liver that involved reducing hepatic lipogenesis and adipogenesis in high-fat-diet-fed rats. J. Agric. Food Chem. 2015, 63, 4587–4596. [Google Scholar] [CrossRef]
- Neeraja, K.; Debnath, R.; Firdous, S. Cardioprotective activity of fruits of Sechium edule. Bangladesh J. Pharmacol. 2015, 10, 125–130. [Google Scholar] [CrossRef]
- Wu, C.H.; Ou, T.T.; Chang, C.H.; Chang, X.Z.; Yang, M.Y.; Wang, C.J. The polyphenol extract from Sechium edule shoots inhibits lipogenesis and stimulates lipolysis via activation of AMPK signals in HepG2 cells. J. Agric. Food Chem. 2014, 62, 750–759. [Google Scholar] [CrossRef]
- Lombardo-Earl, G.; Roman-Ramos, R.; Zamilpa, A.; Herrera-Ruiz, M.; Rosas-Salgado, G.; Tortoriello, J.; Jiménez-Ferrer, E. Extracts and fractions from edible roots of Sechium edule (Jacq.) Sw. with antihypertensive activity. Evid. Based Complement. Altern. Med. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Alvarado, C.; Rojas, A.; Mendoza, S.; Bah, M.; Gutiérrez, D.M.; Hernández-Sandoval, L.; Martínez, M. Vasoactive and antioxidant activities of plants used in Mexican traditional medicine for the treatment of cardiovascular diseases. Pharm. Biol. 2010, 48, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.A.; Guppy, L.J.; Nelson, M. The antihypertensive effects of the Jamaican Cho-Cho (Sechium edule). West Indian Med. J. 2000, 49, 27–31. [Google Scholar] [PubMed]
- Mumtaz, S.M.F.; Ahmed, S.; Dey, S. Antiepileptic and central nervous system depressant activity of Sechium edule fruit extract. Bangladesh J. Pharmacol. 2012, 7, 199–202. [Google Scholar]
- Castro-Alves, V.C.; do Nascimento, J.R.O. Polysaccharides from raw and cooked chayote modulate macrophage function. Food Res. Int. 2016, 81, 171–179. [Google Scholar] [CrossRef]
- Arista-Ugalde, T.L.; Santiago-Osorio, E.; Monroy-García, A.; Rosado-Pérez, J.; Aguiñiga-Sánchez, I.; Cadena-Iñiguez, J.; Gavia-García, G.; Mendoza-Núñez, V.M. Antioxidant and Anti-Inflammatory Effect of the Consumption of Powdered Concentrate of Sechium edule var. nigrum spinosum in Mexican Older Adults with Metabolic Syndrome. Antioxidants 2022, 11, 1076. [Google Scholar] [CrossRef] [PubMed]
- Sateesh, G.; Hussaini, S.F.; Kumar, G.S.; Rao, B.S.S. Anti-ulcer activity of Sechium edule ethanolic fruit extract. Pharm. Innov. 2012, 1, 77–81. [Google Scholar]
- Firdous, S.; Sravanthi, K.; Debnath, R.; Neeraja, K. Protective effect of ethanolic extract and its ethylacetate and n-butanol fractions of Sechium edule fruits against carbon tetrachloride induced hepatic injury in rats. Int. J. Pharm. Sci. 2012, 4, 354–359. [Google Scholar]
- Yen, G.C.; Chen, H.Y.; Peng, H.H. Evaluation of the cytotoxicity, mutagenicity and antimutagenicity of emerging edible plants. Food Chem. Toxicol. 2001, 39, 1045–1053. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Soto-Hernández, M.; Torres-Salas, A.; Aguiñiga-Sánchez, I.; Ruiz-Posadas, L.; Rivera-Martínez, A.R.; Avendaño-Arrazate, C.; Santiago-Osorio, E. The antiproliferative effect of chayote varieties (Sechium edule (Jacq.) Sw.) on tumour cell lines. J. Med. Plant Res. 2013, 7, 455–460. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Bonesi, M.; Menichini, F.; Tenuta, M.C.; Leporini, M.; Tundis, R. Antioxidant and carbohydrate-hydrolysing enzymes potential of Sechium edule (Jacq.) Swartz (Cucurbitaceae) peel, leaves and pulp fresh and processed. Plant Foods Hum. Nutr. 2016, 71, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.Y.; Lin, S.Y.; Lin, K.H.; Liu, Y.F.; Hsu, J.I.; Yang, C.M.; Lai, J.Y. Antioxidant activity in extracts of 27 indigenous Taiwanese vegetables. Nutrients 2014, 6, 2115–2130. [Google Scholar] [CrossRef]
- Dire, G.F.; Almeida, M.C.; Coura, M.F.; Vasconcelos, S.D.; Siqueira, P.R.; Duarte, R.M.; Rodrigues, J.S.; Oliveira, J.C.S.; Fernandes, M.L.; Bernardo-Filho, M. Effects of a chayote (Sechium edule) extract (macerated) on the biochemistry of blood of Wistar rats and on the action against the stannous chloride effect. Pak. J. Biol. Sci. 2007, 10, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, A.A.L.; Gomez, J.D.; Vattuone, M.A.; Lsla, M.I. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Giri, L.; Suyal, R.; Jugran, A.K.; Zucca, P.; Rescigno, A.; Peddio, S.; Bobiş, O.; et al. Antioxidant potential of family Cucurbitaceae with special emphasis on Cucurbita genus: A key to alleviate oxidative stress-mediated disorders. Phytother. Res. 2021, 35, 3533–3557. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Vázquez, M.E.; Soto-Hernandez, M.; Cadena-Iniguez, J.; Santiago-Osorio, E.; Ruiz-Posadas, L.D.M.; Rosas-Acevedo, H. Bio-guided study of an alcoholic extract of Sechium edule (Jacq.) Swartz fruits. Agrociencia 2009, 43, 777–790. [Google Scholar]
- Aguiñiga-Sánchez, I.; Soto-Hernández, M.; Cadena-Iñiguez, J.; Ruíz-Posadas, L.D.M.; Cadena-Zamudio, J.D.; González-Ugarte, A.K.; Weiss-Steider, B.; Santiago-Osorio, E. Fruit extract from a Sechium edule hybrid induce apoptosis in leukaemic cell lines but not in normal cells. Nutr. Cancer. 2015, 67, 250–257. [Google Scholar] [CrossRef]
- Salazar-Aguilar, S.; Ruiz-Posadas, L.M.; Cadena-Iñiguez, J.; Soto-Hernández, M.; Santiago-Osorio, E.; Aguiñiga-Sánchez, I.; Rivera-Martínez, A.R.; Aguirre-Medina, J.F. Sechium edule (Jacq.) Swartz, a New Cultivar with Antiproliferative Potential in a Human Cervical Cancer HeLa Cell Line. Nutrients 2017, 9, 798. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; Fragoso-Antonio, S.; Valadez-Vega, C.; Solano-Solano, G.; Pérez, C.Z.; Sánchez-Gutiérrez, M.; Izquierdo-Vega, J.A.; Gutiérrez-Salinas, J.; Esquivel-Soto, J.; Esquivel-Chirino, C.; et al. Investigation on the protective effects of cranberry against the DNA damage induced by benzo[a]pyrene. Molecules 2012, 17, 4435–4451. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Seeram, N.P.; Nair, M.G. Anticancer and antiinflammatory activities of cucurbitacin from Cucurbita andreana. Cancer Lett. 2003, 189, 11–16. [Google Scholar] [CrossRef]
- Setzer, W.N.; Setzer, M.C. Plant-derived triterpenoids as potential antineoplastic agents. Mini Rev. Med. Chem. 2003, 3, 540–556. [Google Scholar] [CrossRef]
- Metral, E.; Rachidi, W.; Damour, O.; Demarne, F.; Bechetoille, N. Long-term Genoprotection Effect of Sechium edule Fruit Extract against UVA Irradiation in Keratinocytes. Photochem. Photobiol. 2018, 94, 343–350. [Google Scholar] [CrossRef]
- Koklesova, L.; Liskova, A.; Samec, M.; Qaradakhi, T.; Zulli, A.; Smejkal, K.; Kajo, K.; Jakubikova, J.; Behzadi, P.; Pec, M.; et al. Genoprotective activities of plant natural substances in cancer and chemopreventive strategies in the context of 3P medicine. EPMA J. 2020, 11, 261–287. [Google Scholar] [CrossRef]
- Tarantini, A.; Maitre, A.; Lefebvre, E.; Marques, M.; Marie, C.; Ravanat, J.L.; Douki, T. Relative contribution of DNA strand breaks and DNA adducts to the genotoxicity of benzo[a]pyrene as pure compound and in complex mixtures. Mutat. Res. 2009, 671, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, M.; Sawada, M.; Shimada, A.; Suzuki, J.S.; Satoh, M.; Nagase, H. Protective role of metallothionein in benzo[a]pyrene-induced DNA damage. J. Toxicol. Sci. 2009, 34, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Seung-Cheol, J.; Min, K.; Kyeong-Seok, K.; Hyung-Sik, K.; Jung-Suk, S. Protective Effects of Myricetin on Benzo[a]pyrene-Induced 8-Hydroxy-2′-Deoxyguanosine and BPDE-DNA Adduct. Antioxidants 2020, 9, 446. [Google Scholar] [CrossRef]
- Deng, J.; Chen, X.; Wang, D.; Song, Y.; Chen, Y.; Ouyang, D.; Liang, Y.; Sun, Y.; Li, M. Protective effect of hawthorn extract against genotoxicity induced by benzo(<alpha>)pyrene in C57BL/6 mice. Ecotoxicol. Environ. Saf. 2020, 200, 110761. [Google Scholar] [CrossRef]
- Davi Brito, L.; de Souza Araujo, C.; Gomes Silva Moraes Cavalcante, D.; Silva Gomes, A.; Zocoler, M.A.; Yoshihara, E.; Eloizo Job, A.; Ernst Kerche, L. In vivo assessment of antioxidant, antigenotoxic, and antimutagenic effects of bark ethanolic extract from Spondias purpurea L. J. Toxicol. Environ. Health A 2022, 85, 336–352. [Google Scholar] [CrossRef]
- Peramaiyan, P.; Ekambaram, G.; Sakthisekaran, D. Protective Role of Mangiferin against Benzo(a)pyrene Induced Lung Carcinogenesis in Experimental Animals. Biol. Pharm. Bull. 2008, 31, 1053–1058. [Google Scholar]
- Sumedha, S.A.; Marjorie, E.A.; Abhaya, S.B. Effect of cigarette smoke on body weight, food intake and reproductive organs in adults albino rats. Indian J. Exp. Biol. 2006, 44, 562–565. [Google Scholar]
- NRTXDN. Intox Press, Inc., POB 34075, Little Rock, AR 72203. V.1-1979. Neurotoxicology 2007, 28, 630. [Google Scholar]
- Froom, P.; Melamed, S.; Benbassat, J. Smoking cessation and weight gain. J. Fam. Pract. 1998, 46, 460–464. [Google Scholar]
- Kamaraj, S.; Vinodhkumar, R.; Anandakumar, P.; Jagan, S.; Ramakrishnan, G.; Devaki, T. The effects of quercetin on antioxidant status and tumor markers in the lung and serum of mice treated with benzo[a]pyrene. Biol. Pharm. Bull. 2007, 30, 2268–2273. [Google Scholar] [CrossRef]
- Knuckles, M.E.; Inyang, F.; Ramesh, A. Acute and subchronic oral toxicities of benzo[a]pyrene in F-344 rats. Toxicol. Sci. 2001, 61, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Irigaray, P.; Ogier, V.; Jacquenet, S.; Notet, V.; Sibille, P.; Mejean, L.; Bihain, B.E.; Yen, F.T. Benzo(a)pyrene impairs beta-adrenergic stimulation of adipose tissue lipolysis and causes weight gain in mice: A novel molecular mechanism of toxicity for a common food pollutant. FEBS J. 2006, 273, 1362–1372. [Google Scholar] [CrossRef]
- Sengenes, C.; Berlan, M.; De Glisezinski, I.; Lafontan, M.; Galitzky, J. Natriuretic peptides: A new lipolytic pathway in human adipocytes. FASEB J. 2000, 14, 1345–1351. [Google Scholar] [CrossRef]
- Lafontan, M.; Moro, C.; Sengenes, C.; Galitzky, J.; Crampes, F.; Berlan, M. An unsuspected metabolic role for atrial natriuretic peptides: The control of lipolysis, lipid mobilization, and systemic nonesterified fatty acids levels in humans. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2032–2042. [Google Scholar] [CrossRef]
- Jimenez, M.; Leger, B.; Canola, K.; Lehr, L.; Airboit, P.; Seydoux, J.; Russell, A.P.; Giacobino, J.P.; Muzzin, P.; Preitner, F. Beta (1)/beta (2)/beta (3)-adrenoreceptor knockout mice are obese and cold-sensitive but have normal lipolytic responses to fasting. FEBS Lett. 2002, 530, 37–40. [Google Scholar] [CrossRef]
- Melita-Rodríguez, S.; Acosta, H.; Barroso, C. Diuretic effect of chayote juice (Sechium edule) in rats. Rev. Med. Panama 1984, 9, 68–74. [Google Scholar]
- Riviello-Flores, M.L.; Arévalo-Galarza, M.L.; Cadena-Iñiguez, J.; Soto-Hernández, R.M.; Ruiz-Posadas, L.M.; Gómez-Merino, F.C. Nutraceutic Characteristics of the Extracts and Juice of Chayote (Sechium edule (Jacq.) Sw.) Fruits. Beverages 2018, 4, 37. [Google Scholar] [CrossRef]
- Mandey, J.S.; Wolayan, F.R.; Pontoh, C.J.; Kowel, Y.H.S. Effect of orally administrated of cucumber (Cucumis sativus L.) seed juice on the performance and carcass parameters of broiler chickens. IOP Conf. Ser. Earth Environ. Sci. 2020, 492, 012025. [Google Scholar] [CrossRef]
- Penning, T.M.; Ohnishi, S.T.; Ohnishi, T.; Harvey, R.G. Generation of reactive oxygen species during the enzymatic oxidation of polycyclic aromatic hydrocarbon trans-dihydrodiols catalyzed by dihydrodiol dehydrogenase. Chem. Res. Toxicol. 1996, 9, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Uno, S.; Dalton, T.P.; Derkenne, S.; Curran, C.P.; Miller, M.L.; Shertzer, H.G.; Nebert, D.W. Oral exposure to benzo[a]pyrene in the mouse: Detoxication by inducible cytochrome P450 is more important than metabolic activation. Mol. Pharmacol. 2004, 65, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.S.; Brito, L.D.; Tarifa, M.O.; Farah da Silva, N.J.; Rodrigues, K.S.; Cavalcante, D.G.S.M.; Gomes, A.S.; Zocoler, M.A.; Yoshihara, E.; Camparoto, M.L.; et al. Protective effects of bark ethanolic extract from Spondias dulcis Forst F. against DNA damage induced by benzo[a]pyrene and cyclophosphamide. Genet Mol Biol. 2019, 42, 643–654. [Google Scholar] [CrossRef] [PubMed]
- López-Romero, D.; Izquierdo-Vega, J.A.; Morales-González, J.A.; Madrigal-Bujaidar, E.; Chamorro-Cevallos, G.; Sánchez-Gutiérrez, M.; Betanzos-Cabrera, G.; Alvarez-Gonzalez, I.; Morales-González, Á.; Madrigal-Santillán, E. Evidence of Some Natural Products with Antigenotoxic Effects. Part 2: Plants, Vegetables, and Natural Resin. Nutrients 2018, 10, 1954. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor, I.M.; Lemon, P.; Palileo, A.; Bremner, J.B. Antigenotoxic spinasterol from Cucurbita maxima flowers. Mutat. Res. 1996, 360, 89–93. [Google Scholar] [CrossRef] [PubMed]
- İşeri, O.D.; Körpe, D.A.; Yurtcu, E.; Sahin, F.I.; Haberal, M. Copper-induced oxidative damage, antioxidant response and genotoxicity in Lycopersicum esculentum Mill. and Cucumis sativus L. Plant Cell Rep. 2011, 30, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, S.A.; Elelaimy, I.A.; Hassan, A.M.; Ibrahim, H.M.; Elsayad, R.I. Protective effect of pumpkin seed oil against genotoxicity induced by azathioprine. J. Basic Appl. Zool. 2012, 65, 289–298. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Chabra, A.; Naghshvar, F.; Ahmadi, A. The mitigating effect of Citrullus colocynthis (L.) fruit extract against genotoxicity induced by cyclophosphamide in mice bone marrow cells. Sci. World J. 2013, 2013, 980480. [Google Scholar] [CrossRef]
- Yasir, M.; Sultana, B.; Singh-Nigam, P.; Owusu-Apenten, R. Antioxidant and genoprotective activity of selected cucurbitaceae seed extracts and LC-ESIMS/MS identification of phenolic components. Food Chem. 2016, 199, 307–313. [Google Scholar] [CrossRef]
- Shayesteh, R.; Kamalinejad, M.; Adiban, H.; Kardan, A.; Keyhanfar, F.; Eskandari, M.R. Cytoprotective Effects of Pumpkin (Cucurbita Moschata) Fruit Extract against Oxidative Stress and Carbonyl Stress. Drug Res. 2017, 67, 576–582. [Google Scholar] [CrossRef]
- Aguiñiga-Sánchez, I.; Cadena-Íñiguez, J.; Santiago-Osorio, E.; Gómez-García, G.; Mendoza-Núñez, V.M.; Rosado-Pérez, J.; Ruíz-Ramos, M.; Cisneros-Solano, V.M.; Ledesma-Martínez, E.; Delgado-Bordonave, A.J.; et al. Chemical analyses and in vitro and in vivo toxicity of fruit methanol extract of Sechium edule var. nigrum spinosum. Pharm. Biol. 2017, 55, 1638–1645. [Google Scholar] [CrossRef]
- Putri, D.P.; Putri-Wahyuningtyas, A.; Al-Baarri, A.N.; Maharani, N. The effect of chayote leaves (Sechium edule)’s flavonoid fraction on the reduction of the serum uric acid levels through the inhibition of xanthine oxidase activity. Potravin. Slovak J. Food Sci. 2021, 15, 1049–1055. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Aguiñiga-Sánchez, I.; Uriostegui-Arias, M.T.; Santiago-Osorio, E.; Ruiz-Posadas, L.M.; Soto-Hernández, M. Antiproliferative Effect of Sechium edule (Jacq.) Sw., cv. Madre Negra Extracts on Breast Cancer In Vitro. Separations 2022, 9, 230. [Google Scholar] [CrossRef]
- Costa da Rocha Galucio, N.; de Araujo Moyses, D.; Rodrigo Souza Pina, J.; Santana Barbosa Marinho, P.; Cardoso Gomes Junior, P.; Neves Cruz, J.; Vieira Vale, V.; Salim Khayat, A.; do Rosario Marinho, A.M. Antiproliferative, genotoxic activities and quantification of extracts and cucurbitacin B obtained from Luffa operculata (L.) Cogn. Arab. J. Chem. 2022, 15, 103589. [Google Scholar] [CrossRef]
- da Cruz, G.K.; Morgan Martins, M.I.; Techera Antunes, F.T.; Hubner de Souza, A.; Wiilland, E.F.; Nascimento Picada, J.; da Silva Brum, L.F. Evaluation of the efficacy and toxicity of oral and topical pumpkin oil on the hair growth of mice. Acta Histochem. 2022, 124, 151894. [Google Scholar] [CrossRef] [PubMed]
- Kleijn, A.F.; Mutter, M.; Akingbasote, J.A.; Meetro, J.; Simon, R.R.; Muntendam, P.; Frommhagen, M.; Schols, H.A. Toxicological evaluation of a pumpkin-derived pectin preparation: In vitro genotoxicity studies and a 13-week oral toxicity study in Sprague-Dawley rats. Toxicol. Res. 2024, 13, tfae004. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kaur, V.; Kumar, S.; Kaur, S. Phytoconstituents as apoptosis inducing agents: Strategy to combat cancer. Cytotechnology 2016, 68, 531–563. [Google Scholar] [CrossRef]
- De Flora, S.; Ferguson, L.R. Overview of mechanisms of cancer chemopreventive agents. Mutat. Res. 2005, 591, 8–15. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Bronzetti, G.; De Flora, S. Mechanistic approaches to chemoprevention of mutation and cancer. Mutat. Res. 2005, 591, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, S3479–S3485. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Philpott, M.; Karunasinghe, N. Dietary cancer and prevention using antimutagens. Toxicology 2004, 198, 147–159. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, I.K.; Povey, A.C.; Bingham, S.; Cardis, E. Systematic modulation by human diet levels of dietary fibre and beef on metabolism and disposition of benzo[a]pyrene in the gastrointestinal tract of Fischer F344 rats. Carcinogenesis 1990, 11, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Roberton, A.M.; Watson, M.E.; Triggs, C.M.; Harris, P.J. The effects of a soluble-fibre polysaccharide on the adsorption of carcinogens to insoluble dietary fibres. Chem. Biol. Interact. 1995, 95, 245–255. [Google Scholar] [CrossRef]
- Schwab, C.E.; Huber, W.W.; Parzefall, W.; Hietsch, G.; Kassie, F.; Schulte-Hermann, R.; Knasmuller, S. Search for compounds that inhibit the genotoxic and carcinogenic effects of heterocyclic aromatic amines. Crit. Rev. Toxicol. 2000, 30, 1–69. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Smith, T.J.; Hong, J.Y. Cytochrome P-450 enzymes as targets for chemoprevention against chemical carcinogenesis and toxicity: Opportunities and limitations. Cancer Res. 1994, 54, 1982s–1986s. [Google Scholar] [PubMed]
- Tuenter, E.; Creylman, J.; Verheyen, G.; Pieters, L.; Van Miert, S. Development of a classification model for the antigenotoxic activity of flavonoids. Bioorg. Chem. 2020, 98, 103705. [Google Scholar] [CrossRef]
- Aguiñiga-Sánchez, I.; Soto-Hernández, M.; Cadena-Iñiguez, J.; Suwalsky, M.; Colina, J.R.; Castillo, I.; Rosado-Pérez, J.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Phytochemical Analysis and Antioxidant and Anti-Inflammatory Capacity of the Extracts of Fruits of the Sechium Hybrid. Molecules 2020, 25, 4637. [Google Scholar] [CrossRef]
- Del Ángel Coronel, O.A.; León-García, E.; Vela-Gutiérrez, G.; De la Cruz Medina, J.; García Varela, R.; García, H. Chayote (Sechium edule (Jacq.) Swartz). In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Yahia Elhadi, M., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 979–992. [Google Scholar]


| Day | Control | FChJ | UFChJ | B[a]P (250 mg/kg) | FChJ Plus B[a]P (250 mg/kg) | UFChJ Plus B[a]P (250 mg/kg) |
|---|---|---|---|---|---|---|
| 0 | 22.39 ± 1.70 | 23.05 ± 1.66 | 21.75 ± 1.59 | 23.05 ± 0.66 | 22.75 ± 0.83 | 22.05 ± 1.71 |
| 1 | 23.15 ± 2.31 | 23.55 ± 2.31 | 22.39 ± 1.71 | 24.15 ± 2.32 | 23.55 ± 2.32 | 23.05 ± 2.32 |
| 2 | 24.05 ± 2.45 a | 24.75 ± 0.84 | 23.75 ± 0.83 a | 24.75 ± 2.59 | 24.75 ± 2.15 a | 24.15 ± 2.46 a |
| 3 | 24.75 ± 2.51 a | 25.85 ± 2.32 a | 24.55 ± 2.32 a | 26.55 ± 1.91 a,b | 25.25 ± 0.99 a | 25.15 ± 2.59 a |
| 4 | 25.15 ± 2.31 a | 26.10 ± 1.71 a | 25.05 ± 2.59 a | 27.92 ± 0.96 a,b | 26.05 ± 0.96 a,c | 25.75 ± 2.32 a,c |
| 5 | 26.05 ± 2.43 a | 26.75 ± 2.46 a | 26.15 ± 2.46 a | 28.20 ± 2.15 a,b | 27.05 ± 0.92 a | 26.75 ± 2.46 a |
| 6 | 27.12 ± 2.52 a | 27.32 ± 2.59 a | 27.25 ± 2.59 a | 29.15 ± 2.03 a,b | 28.10 ± 1.03 a | 28.12 ± 2.59 a |
| 7 | 28.02 ± 2.31 a | 28.75 ± 2.32 a | 28.22 ± 2.32 a | 31.73 ± 1.40 a,b | 29.05 ± 1.40 a,c | 28.75 ± 2.32 a,c |
| 8 | 29.21 ± 2.41 a | 30.05 ± 2.46 a | 29.15 ± 2.46 a | 33.33 ± 2.12 a,b | 31.91 ± 0.62 a,b | 29.75 ± 2.46 a,c |
| 9 | 31.05 ± 2.52 a | 31.25 ± 2.59 a | 31.55 ± 2.59 a | 34.10 ± 1.94 a,b | 32.75 ± 1.37 a,b | 30.75 ± 2.59 a,c |
| 10 | 32.05 ± 2.31 a | 32.55 ± 2.32 a | 32.75 ± 2.32 a | 33.69 ± 1.74 a | 32.75 ± 1.51 a | 33.05 ± 2.32 a |
| 11 | 33.05 ± 2.42 a | 33.75 ± 2.46 a | 33.21 ± 2.46 a | 34.02 ± 1.21 a | 34.02 ± 1.21 a | 34.05 ± 2.46 a |
| 12 | 34.29 ± 2.55 a | 34.12 ± 2.59 a | 35.75 ± 2.59 a | 35.12 ± 0.83 a | 34.75 ± 0.26 a | 34.75 ± 2.59 a |
| 13 | 36.75 ± 2.31 a | 36.55 ± 2.32 a | 36.05 ± 2.32 a | 36.35 ± 1.16 a | 36.12 ± 1.16 a | 36.05 ± 2.32 a |
| 14 | 37.45 ± 2.42 a | 37.05 ± 2.46 a | 37.75 ± 2.46 a | 38.05 ± 1.46 a | 37.15 ± 2.46 a | 37.05 ± 2.46 a |
| Day | Control | FChJ | UFChJ | B[a]P (250 mg/kg) | FChJ Plus B[a]P (250 mg/kg) | UFChJ Plus B[a]P (250 mg/kg) |
|---|---|---|---|---|---|---|
| 0 | 2.91 ± 0.08 | 3.00 ± 0.0 | 2.85 ± 0.08 | 2.97 ± 0.12 | 3.05 ± 0.0 | 3.03 ± 0.01 |
| 1 | 2.98 ± 0.12 | 3.00 ± 0.07 | 3.05 ± 0.26 | 3.08 ± 0.36 | 3.10 ± 0.02 | 3.09 ± 0.08 |
| 2 | 3.03 ± 0.09 | 3.03 ± 0.04 | 3.06 ± 0.22 | 3.16 ± 0.09 | 3.17 ± 0.07 | 3.13 ± 0.14 |
| 3 | 3.13 ± 0.12 | 3.10 ± 0.07 | 3.19 ± 0.26 | 3.17 ± 0.11 | 3.19 ± 0.01 | 3.18 ± 0.17 |
| 4 | 3.25 ± 0.12 | 3.16 ± 0.07 | 3.20 ± 0.26 | 3.22 ± 0.11 | 3.19 ± 0.01 | 3.20 ± 0.26 |
| 5 | 3.35 ± 0.12 | 3.33 ± 0.07 | 3.30 ± 0.26 | 3.32 ± 0.11 | 3.37 ± 0.01 | 3.35 ± 0.17 |
| 6 | 3.50 ± 0.09 | 3.48 ± 0.26 | 3.89 ± 0.22 | 3.66 ± 0.09 | 3.44 ± 0.02 | 3.47 ± 0.04 |
| 7 | 3.87 ± 0.23 | 3.60 ± 0.08 | 4.06 ± 0.41 | 3.89 ± 0.05 | 3.72 ± 0.02 | 3.55 ± 0.04 |
| 8 | 4.02 ± 0.30 | 3.95 ± 0.26 | 4.00 ± 0.22 | 4.62 ± 0.10 a,b | 4.78 ± 0.01 a,b | 3.90 ± 0.07 |
| 9 | 4.07 ± 0.30 | 3.98 ± 0.26 | 4.00 ± 0.26 | 4.89 ± 0.30 a,b | 4.60 ± 0.26 a | 4.00 ± 0.17 c |
| 10 | 4.00 ± 0.10 | 4.02 ± 0.04 | 4.06 ± 0.36 | 4.95 ± 0.14 a,b | 4.73 ± 0.22 a,b | 4.05 ± 0.05 c |
| 11 | 4.06 ± 0.23 | 4.04 ± 0.23 | 4.09 ± 0.24 | 4.99 ± 0.17 a,b | 4.04 ± 0.11 c | 4.02 ± 0.07 c |
| 12 | 4.00 ± 0.07 | 4.06 ± 0.01 | 4.05 ± 0.14 | 4.75 ± 0.04 a,b | 4.02 ± 0.07 c | 3.99 ± 0.12 c |
| 13 | 3.99 ± 0.11 | 3.97 ± 0.10 | 3.98 ± 0.07 | 4.83 ± 0.07 a,b | 3.98 ± 0.04 c | 4.03 ± 0.01 c |
| 14 | 4.02 ± 0.09 | 4.08 ± 0.02 | 4.00 ± 0.02 | 4.95 ± 0.05 a,b | 4.00 ± 0.01 c | 4.06 ± 0.02 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madrigal-Santillán, E.; Portillo-Reyes, J.; Morales-González, J.A.; Garcia-Melo, L.F.; Serra-Pérez, E.; Vidović, K.; Sánchez-Gutiérrez, M.; Álvarez-González, I.; Madrigal-Bujaidar, E. Evaluation of the Antigenotoxic Potential of Two Types of Chayote (Sechium edule) Juices. Plants 2024, 13, 2132. https://doi.org/10.3390/plants13152132
Madrigal-Santillán E, Portillo-Reyes J, Morales-González JA, Garcia-Melo LF, Serra-Pérez E, Vidović K, Sánchez-Gutiérrez M, Álvarez-González I, Madrigal-Bujaidar E. Evaluation of the Antigenotoxic Potential of Two Types of Chayote (Sechium edule) Juices. Plants. 2024; 13(15):2132. https://doi.org/10.3390/plants13152132
Chicago/Turabian StyleMadrigal-Santillán, Eduardo, Jacqueline Portillo-Reyes, José A. Morales-González, Luis F. Garcia-Melo, Estrella Serra-Pérez, Kristijan Vidović, Manuel Sánchez-Gutiérrez, Isela Álvarez-González, and Eduardo Madrigal-Bujaidar. 2024. "Evaluation of the Antigenotoxic Potential of Two Types of Chayote (Sechium edule) Juices" Plants 13, no. 15: 2132. https://doi.org/10.3390/plants13152132







