Abstract
Few researches have explored the production of pharmaceuticals from aquatic plants. Therefore, this study explored, for the first time, the phytochemical composition and bioactivities of ten aquatic plants. Aquatic plant shoots from various Nile River canals were collected, dried, and ground for aqueous extract preparation. Phytochemical composition and antioxidant capacity were assessed using DPPH assays. Extracts were tested for antiparasitic, antibacterial, anti-biofilm, and anticancer activities through standard in vitro assays, measuring IC50 values, and evaluating mechanisms of action, including cell viability and high-content screening assays. The results showed that the aquatic plants were rich in pharmaceutical compounds. The antioxidant capacity of these extracts exceeded that of vitamin C. The extracts showed promising antiparasitic activity against pathogens like Opisthorchis viverrini and Plasmodium falciparum, with IC50 values between 0.7 and 2.5 µg/mL. They also demonstrated low MICs against various pathogenic bacteria, causing DNA damage, increased plasma membrane permeability, and 90% biofilm inhibition. In terms of anticancer activity, extracts were effective against a panel of cancer cell lines, with Ludwigia stolonifera exhibiting the highest efficacy. Its IC50 ranged from 0.5 µg/mL for pancreatic, esophageal, and colon cancer cells to 1.5 µg/mL for gastric cancer cells. Overall, IC50 values for all extracts were below 6 µg/mL, showing significant apoptotic activity, increased nuclear intensity, plasma membrane permeability, mitochondrial membrane permeability, and cytochrome c release, and outperforming doxorubicin. This study highlights the potential of aquatic plants as sources for new, safe, and effective drugs with strong antiparasitic, antibacterial, and anticancer properties.
1. Introduction
Pathogens continually evolve resistance to existing pharmaceuticals, rendering them ineffective. Discovering new compounds can provide alternative treatment options to combat drug-resistant strains of bacteria, viruses, and parasites [1,2,3]. In addition, there are unmet medical needs for various diseases, including cancer, neurodegenerative disorders, and neglected tropical diseases. Exploring new natural compounds offers the potential to develop treatments for these conditions, improving global healthcare. Therefore, exploring new natural, safe pharmaceutical compounds is crucial [4,5,6,7,8,9].
Natural compounds often have a long history of safe use in traditional medicine. Studying these compounds can lead to the development of pharmaceuticals with fewer adverse effects compared to synthetic drugs, which can have detrimental adverse effects on human health. By harnessing natural compounds, sourced sustainably from plants, fungi, and marine organisms, we can develop pharmaceuticals with lower environmental impact [10,11,12]. Earth’s biodiversity is a vast resource for potential pharmaceutical compounds. Exploring diverse ecosystems allows us to discover novel molecules with therapeutic potential, expanding the pharmacological toolkit available to medical professionals [13,14,15,16]. Natural compounds often possess unique chemical structures and biological activities, providing opportunities for drug discovery and innovation. These compounds may target disease pathways not addressed by existing pharmaceuticals, leading to breakthrough treatments [17,18]. The antioxidant activity of plants is the main biological activity that gives plants medicinal importance [19]. The antioxidant activity of plants is related to their chemical constituents [20]. Phenols are the most common plant constituents with antioxidant activity [21].
Aquatic plants have long been recognized as potential sources of pharmaceutical compounds, due to their rich biodiversity and the unique chemical constituents they produce. Researchers have been exploring aquatic plants for bioactive compounds with medicinal properties [22,23,24]. Water hyacinth has been reported to have pharmaceutical components with promising antioxidant, antimicrobial, and anticancer activity [25,26,27,28]. Lotus (Nelumbo nucifera) is another common aquatic plant that has high biologically active compounds such as phenols, flavonoids, and alkaloids, which have shown promise for medicinal application [29]. Water celery (Oenanthe javanica) produces compounds with anti-inflammatory properties, which can be useful in the treatment of inflammatory conditions such as arthritis and asthma [30,31]. Water mint (Mentha aquatica) showed promising phytochemical components with high antioxidant activity and that were efficiently used against skin cancer [32,33]. Researchers have identified anticancer compounds from watercress (Nasturtium officinale) that showed a promising effect in inhibiting the growth of cancer cells or inducing apoptosis [34,35]. Overall, the diverse array of bioactive compounds found in aquatic plants offers great potential for the development of novel pharmaceuticals and therapeutic agents. However, further research is necessary to fully explore and harness the medicinal properties of these plants [23,36]. From this point of view, we tried in this work to investigate the phytochemical components and diverse biological activities (antioxidant, antiparasitic, antibacterial, and anticancer activities) of some aquatic plants. We selected aquatic plants that have not been thoroughly studied for their phytochemical components and biological activity (Supplementary data Table S1, Figure S1). Scirpus maritimus is a perennial, grass-like plant with erect stems and a clump-forming habit, belonging to the Cyperaceae family. Lemna gibba (Gibbous Duckweed) belongs to the Araceae family and is a small, free-floating aquatic plant with a single leaf (frond) and a root hanging below. It often forms dense mats on the surface of still or slow moving freshwater. Ottelia alismoides (Duck Lettuce) belongs to the Hydrocharitaceae family and is an aquatic plant with submerged or floating leaves. The leaves are broad and often have a distinctive pattern of veins, and it produces small, white or yellowish flowers. Ruppia maritima (Widgeon Grass) is a submerged, thread-like aquatic plant with slender stems and narrow leaves, belonging to the Ruppiaceae family. It is highly tolerant of salinity and often found in brackish or saline waters. Zannichellia palustris (Horned Pondweed) belongs to the Zannichelliaceae family and is a submerged aquatic plant with thin, branched stems and narrow, linear leaves. It has small, inconspicuous flowers. Ludwigia stolonifera, belonging to the Onagraceae family, is a creeping or trailing perennial plant with small, yellow flowers and green, oval leaves. It can grow both submerged and emergent. Alisma plantago-aquatica (Water Plantain) belongs to the Alismataceae family and is a perennial herb with broad, lance-shaped leaves and small white or pink flowers arranged in whorls. It grows in shallow water or wet soil. Damasonium alisma (Starfruit) also belongs to the Alismataceae family and is an annual or perennial aquatic plant with rosette-forming leaves and star-shaped white flowers. It produces distinctive star-shaped fruits. Carex divisa (Divided Sedge) belongs to the Cyperaceae family and is a perennial sedge with clump-forming, tufted growth and narrow, grass-like leaves. It produces clusters of small, greenish-brown flower spikes. Leptochloa fusca (Sprangletop) belongs to the Poaceae family and is a perennial grass with a spreading growth habit and long, narrow leaves. It produces open, airy flower panicles [37].
2. Results and Discussion
2.1. Phytochemical Components and Antioxidant Capacity of the Extracts
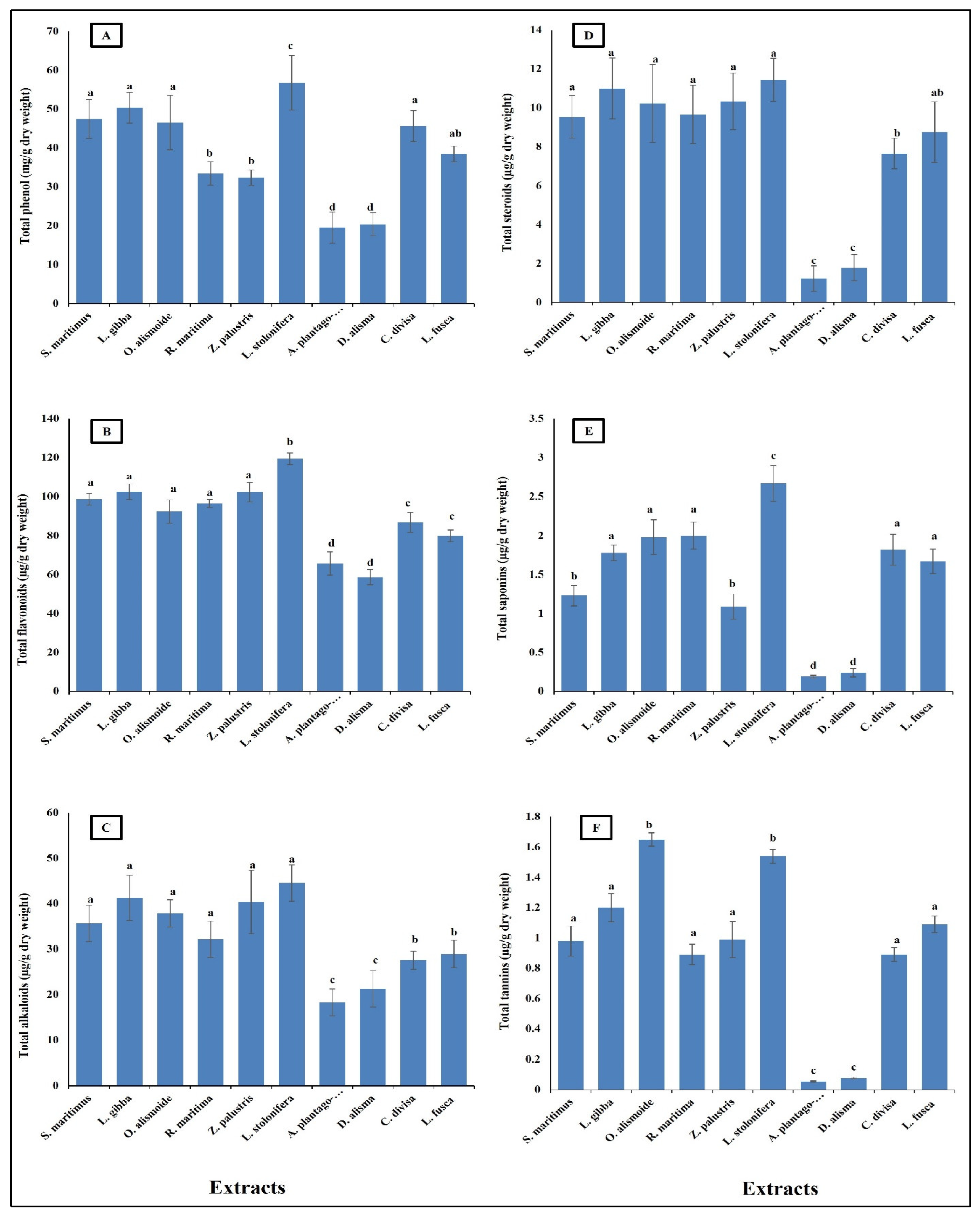
Aquatic plants have long been recognized as potential sources of pharmaceutical compounds, due to their rich biodiversity and the unique chemical constituents they produce [38,39,40]. The present results showed that the aquatic plants were rich in pharmaceutical compounds (Figure 1). Phenols showed to be the most abundant phytochemicals. Ludwigia stolonifera had the highest content of phytochemical components, followed by Scirpus maritimus, Lemna gibba, Otellia alismoide, Ruppia maritima, Zannichellia palustris, Carex divisa, and Leptochloa fusca. The lowest content of phytochemicals was recorded in Alisma plantago-aquatica and Damasonium alisma. Aquatic plants contain a diverse array of secondary metabolites. Each compound plays a specific role in the plant’s ecology and physiology, contributing to its adaptation to the aquatic environment [41,42,43]. Phenolic compounds are common secondary metabolites in aquatic plants. They have antioxidant properties and can help protect the plant from oxidative stress. Tannins are polyphenol compounds known for their astringent properties. They are commonly found in various parts of aquatic plants, including leaves, stems, and roots [44,45]. Tannins can serve as chemical defenses against herbivores by making plant tissues unpalatable or toxic [46,47]. Some aquatic plants produce alkaloids, nitrogen-containing compounds with various biological activities. Alkaloids can act as toxins to deter herbivores or inhibit the growth of competing plants. Saponins are glycosides with foaming properties. They can act as natural surfactants and have been found in some aquatic plants [48,49]. Saponins play a role in defense against different stresses [50]. Steroids are a class of lipids with a characteristic structure consisting of four fused rings. While steroids are more commonly associated with animals, some aquatic plants also produce steroid compounds. Phytosterols, which are plant-derived steroids, are important components of cell membranes in aquatic plants. These compounds help maintain membrane integrity and fluidity, which is crucial for various cellular processes. Phytosterols also have antioxidant properties, protecting cells from oxidative damage caused by reactive oxygen species [51,52,53].
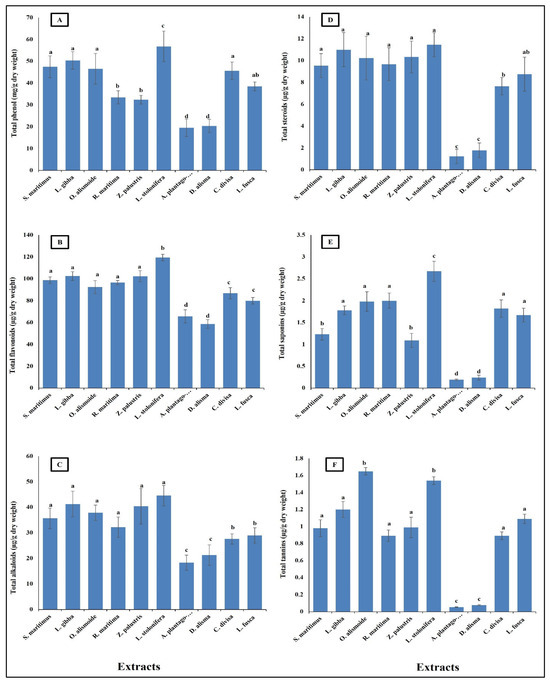
Figure 1.
Phytochemical component content [(A) total phenols (mg/g dry wt), (B) flavonoid, (C) alkaloid, (D) steroid, (E) saponin, (F) tannin (µg/g dry wt)] of different aquatic plants extracts. Column value is the mean of five replicates. The error bars represent standard deviation. Columns followed with different letters are significantly different according to ANOVA test.
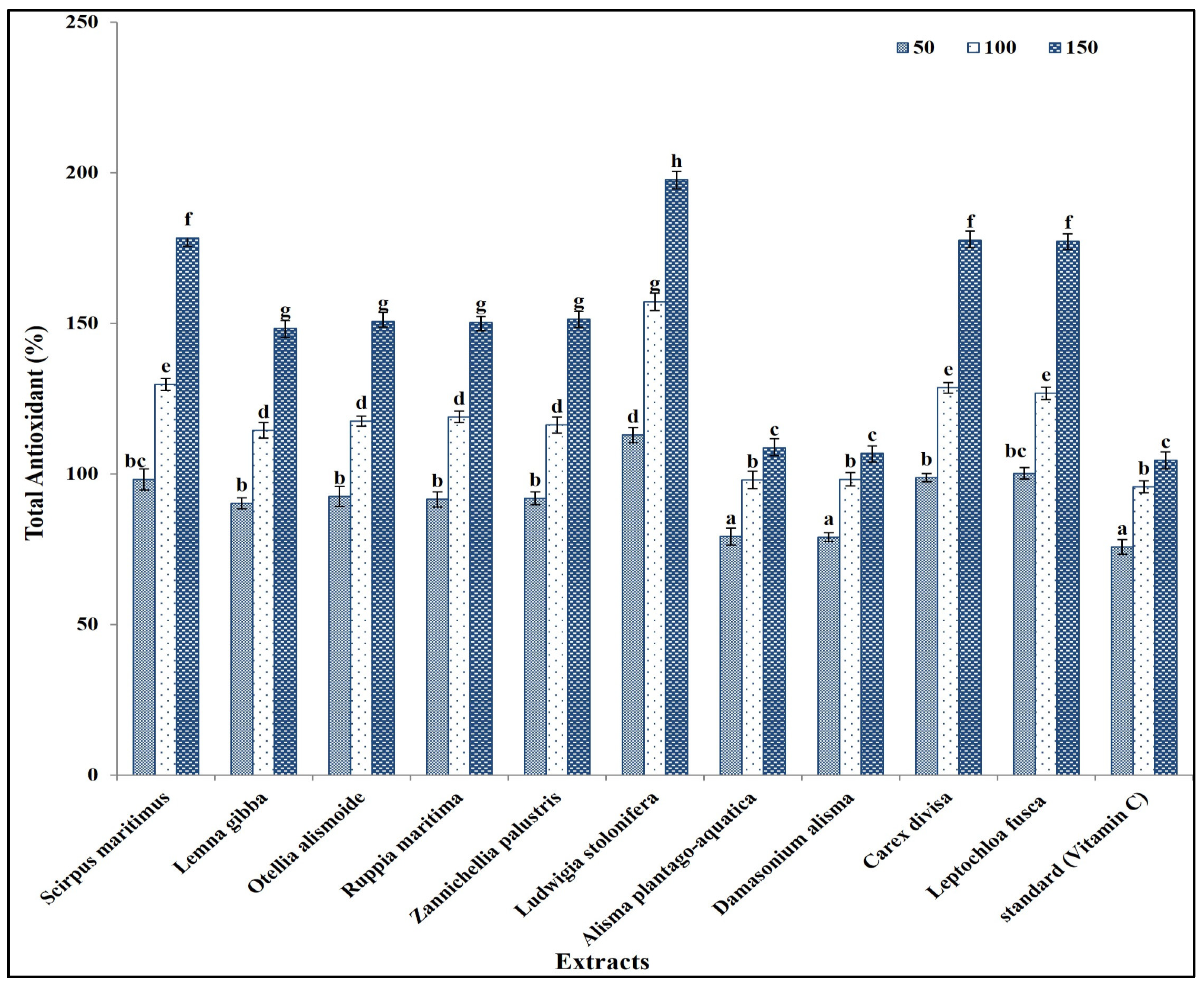
These secondary metabolites are known to contribute to the antioxidant activity of plant extracts, due to their ability to scavenge free radicals and inhibit oxidative processes [54]. The total antioxidant capacity of the prepared extracts was in the same line as the results of the phytochemical components in extracts, as L. stolonifera had the highest antioxidant capacity, followed by C. divisa, L. fusca, S. maritimus, L. gibba, O. alismoide, R. maritima, and Z. palustris. The lowest antioxidant capacity was recorded in A. plantago-aquatica and D. alisma (Figure 2).
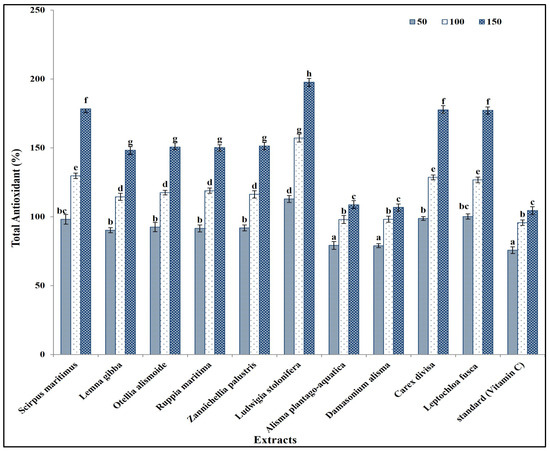
Figure 2.
Antioxidant capacity of different concentrations (50, 100, and 150 mg/mL) of prepared extracts in comparison with standard (Vitamin C). Column value is the mean of five replicates. The error bars represent standard deviation. Columns followed with different letters are significantly different according to ANOVA test.
This is in the same line as Al-Rowaily et al. [55], who reported that antioxidants increased with increasing phytochemical components of certain geophyte sedges and grasses. In addition, Alzandi et al. [56] reported a positive correlation between the antioxidant capacity of a plant extract and its phytochemical component content. Similarly, Kochar et al. [57] reported that antioxidant capacity was related to the secondary metabolites content in a plant extract. The increase in secondary metabolite content in a plant extract correlated with the increase in antioxidant capacity, due to the higher concentration, greater diversity, synergistic interactions, efficient free radical scavenging, and enhanced stability of antioxidants present in the extract [54].
Interestingly, the antioxidant capacity of all prepared extracts was significantly higher than that of vitamin C (the standard) (Figure 2). This is because the antioxidant capacity of plant extracts is often influenced by the combined action of multiple secondary metabolites. Different classes of secondary metabolites may interact synergistically, enhancing the overall antioxidant activity beyond what would be expected based on the individual contributions of each compound [58,59].
2.2. Antiparasitic Activity of the Extracts
Parasitic diseases contribute significantly to the global burden of disease, affecting millions of people worldwide. The rising problem of parasite resistance to the existing antiparasitic drugs makes finding new safe antiparasitic agents an urgent need [60]. Plant extracts and other natural products are promising as effective antiparasitic agents [61,62]. The results in Figure 3 show that aquatics had high antiparasitic activity against Opisthorchis viverrini, Opisthorchis. felineus, Clonorchis sinensis, Plasmodium falciparum, and Leishmania donovani with low IC50 (range between 0.7 and 2.5 µg/mL). The lowest antiparasitic activity was recorded in A. plantago-aquatica and D. alisma with the highest IC50, the other extracts’ antiparasitic activities were not significantly different from each other, but they were significantly higher than that of A. plantago-aquatica and D. alisma with lower IC50 (range between 0.3 and 1.7 µg/mL).
This low IC50 showed the effectiveness of the aquatic extracts as antiparasitic agents. The differences between the studied aquatic plants in their antiparasitic activity is related to the differences in their phytochemical components, as the extracts lowest in phytochemical content were the lowest in antiparasitic activity. This is in the same line as reported in Ali and Mishra [63], where differences in the phytochemical components in each extract led to different biological activities. The low IC50 of the studied aquatic extracts gives them promising antiparasitic activity, as it was reported that crude extracts with IC50 < 20 µg/mL can be considered as antiparasitic agents [64]. The high antiparasitic activity of the aquatic plants was related to their high content of secondary metabolites such as phenols, flavonoids, alkaloids, tannins, saponines, and steroids [62]. Similarly, Ponomarev et al. [65] reported that the anti-Opisthorchis felineus effects of plant-origin materials were more effective than synthetic drugs. Gupta et al. [60] suggested that the future of antiparasitic agents is in natural products.
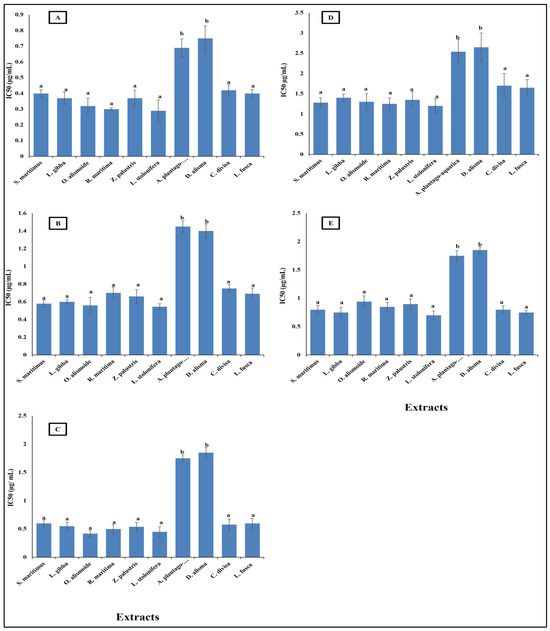
Figure 3.
Antiparasitic activity of different aquatic plant extracts against different parasites [(A) O. viverrini, (B) O. felineus, (C) C. sinensis, (D) P. falciparum, (E) L. donovani]. The IC50 value, indicating the concentration needed to inhibit 50% of parasite growth. Crude extracts with IC50 < 20 µg/mL can be considered as antiparasitic agents [64]. Column value is the mean of five replicates. The error bars represent standard deviation. Columns followed with different letters are significantly different according to ANOVA test.
Figure 3.
Antiparasitic activity of different aquatic plant extracts against different parasites [(A) O. viverrini, (B) O. felineus, (C) C. sinensis, (D) P. falciparum, (E) L. donovani]. The IC50 value, indicating the concentration needed to inhibit 50% of parasite growth. Crude extracts with IC50 < 20 µg/mL can be considered as antiparasitic agents [64]. Column value is the mean of five replicates. The error bars represent standard deviation. Columns followed with different letters are significantly different according to ANOVA test.
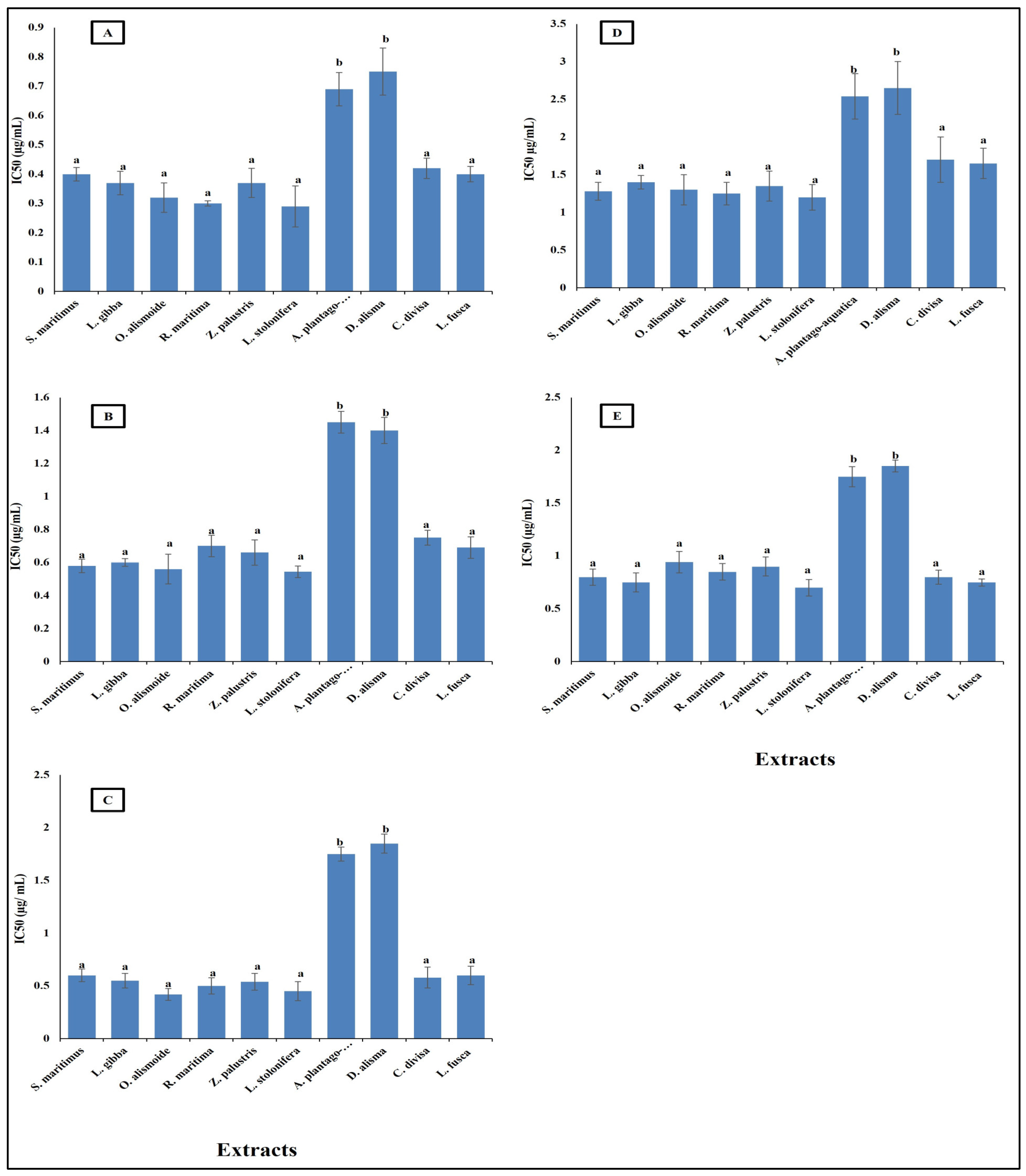
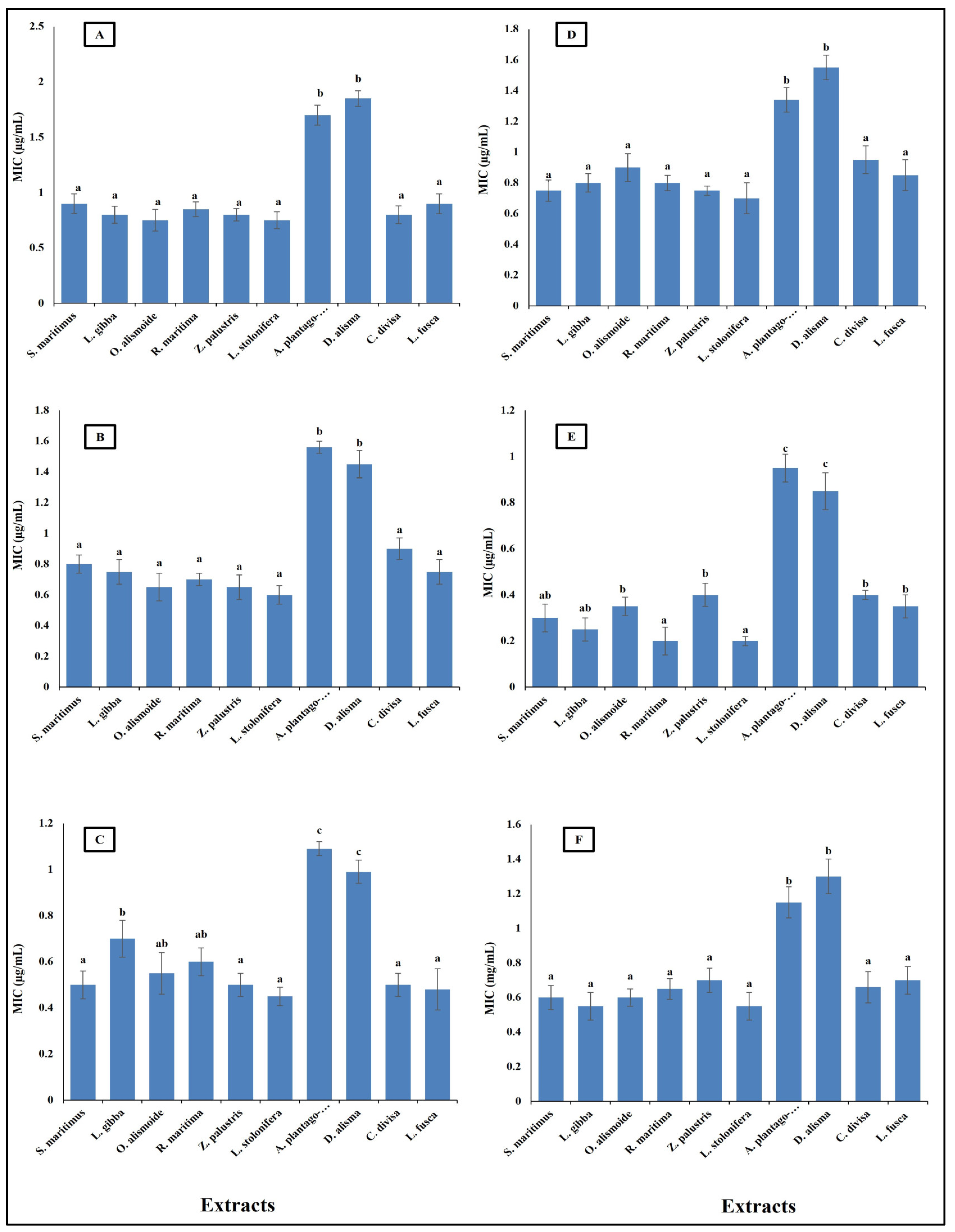
2.3. Antibacterial Activity of the Extracts
Antibiotic resistance poses a critical challenge to modern medicine, necessitating coordinated efforts across healthcare, research, policy, and public education to mitigate its impact and preserve the efficacy of existing antibiotics. New natural and effective antibacterial agents are greatly needed. Aquatic plants have diverse biological active components, such as phenols, alkaloids, flavonoids, saponines, and tannins; thus, these plants are a promising source for safe and effective antibacterial agents [36]. The results in Figure 4 show the antibacterial activity of different aquatic plant extracts against different pathogenic bacteria, in terms of their minimum inhibitory concentration (MIC) (µg/mL). The studied aquatic plants extracts showed a low MIC. Alisma plantago-aquatica and Damasonium alisma extracts showed the highest MIC. This was from 0.9 µg/mL against Salmonella enterica to 1.8 µg/mL against Bacteroides fragilis. The other studied aquatic plants extracts had an MIC lower than unity against the different studied pathogenic bacteria. This low MIC of the extracts makes the studied aquatic plants highly promising antibacterial agents, as it was reported that plant crude extracts with an MIC lower than 8 µg/mL are outstanding antibacterial agents [66]. The different MICs of the extracts are related to the differences in their phytochemicals components. Alzandi et al. [67] reported that plant extracts with different phytochemical components have different antibacterial activity.
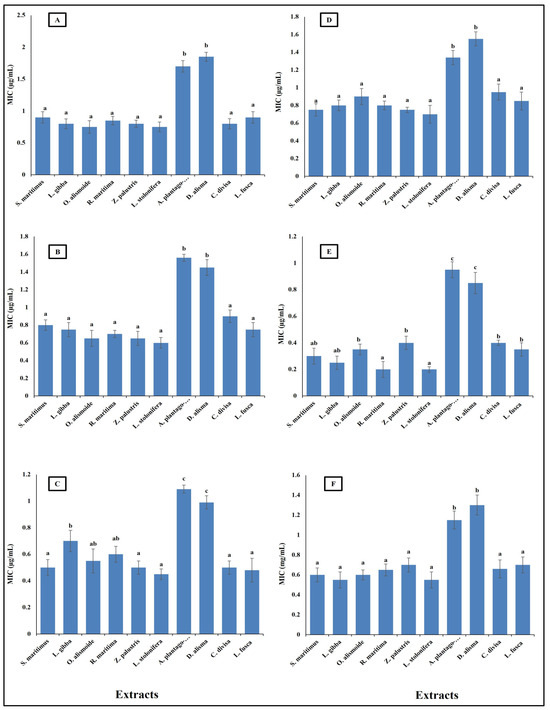
Figure 4.
Antibacterial activity of different aquatic plant extracts against different pathogenic bacteria expressed as MIC, which is the lowest concentration of the assayed agent that inhibits microbial growth. [(A) Bacteroides fragilis, (B) Helicobacter pylori, (C) Fusobacterium nucleatum, (D) Porphyromonas gingivalis, (E) Salmonella enterica, (F) Neisseria gonorrhoeae]. Plant crude extracts with MIC lower than 8 µg/mL are outstanding antibacterial agents [62]. Column value is the mean of five replicates. The error bars represent standard deviation. Columns followed by different letters are significantly different according to ANOVA test.
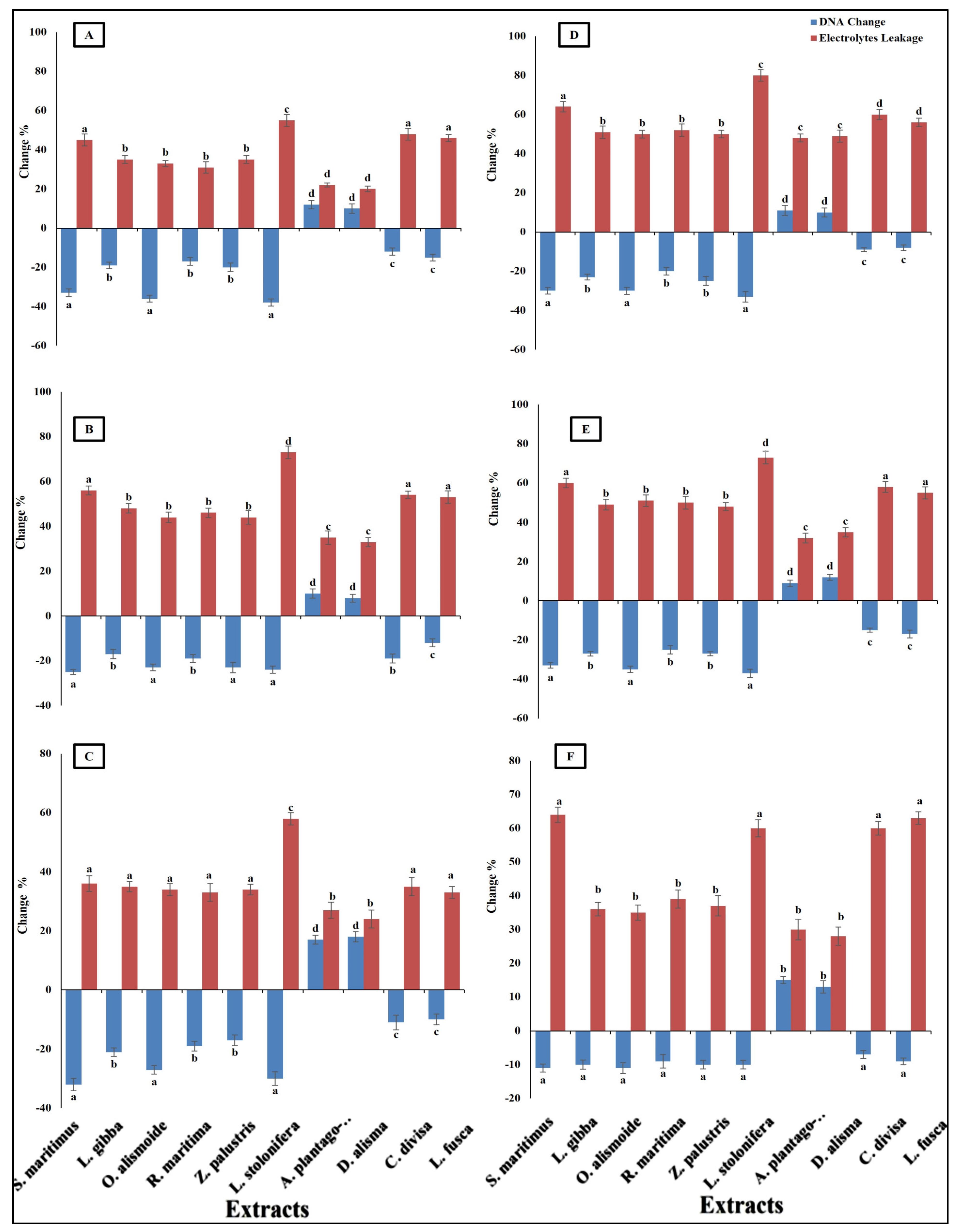
Differences in antibacterial activity against different pathogenic bacteria are related to the different mechanisms through which the antibacterial agent can kill bacterial cells. Antibacterial agents can disturb bacterial cell wall synthesis and plasma membrane permeability. In addition, antibacterial agents can destroy the protein and nucleic acid of bacterial cells [68]. The prepared extracts of aquatic plants showed antibacterial activity through the destruction of DNA (the negative value of the changes in DNA clarified that the DNA in the treated bacterial isolates was lower than that in the non-treated isolates, and this indicated that there had been destruction of the DNA) and an increase in plasma membrane permeability (Figure 5). The results showed that the Ludwigia stolonifera extract was the most effective antibacterial agent and the most effective in the destruction of DNA and in increasing the bacterial membrane permeability. The effect of L. stolonifera extract on DNA change and electrolyte leakage was followed by S. maritimus, O. alismoide, L. gibba, R.maritimac, and Z. palustris. Similarly, Shawky et al. [69] reported the promising antibacterial activity of the genus Ludwigia. The lowest effect was recorded in A. plantago-aquatica and D. alisma. The change in DNA had a positive value that indicated that there was replication in the DNA and no effective destruction in the DNA, as the treated isolates had a DNA content higher than that of the non-treated isolates. In addition, these two extracts showed the lowest electrolyte leakage, which means the lowest adverse effect on the bacterial plasma membrane permeability. The differences in the effects between the studied extracts was due to their different phytochemical components [67].
Figure 5.
Change in DNA and electrolyte leakage (%) in bacterial species [(A) Bacteroides fragilis, (B) Helicobacter pylori, (C) Fusobacterium nucleatum, (D) Porphyromonas gingivalis, (E) Salmonella enterica, (F) Neisseria gonorrhoeae] after treatment, with MIC of each extract. The negative value of the changes in DNA clarified that the DNA in the treated bacterial isolates was lower than that in the non-treated isolates, indicating that there was destruction of DNA. Column value is the mean of five replicates. The error bars represent standard deviation. Columns followed by different letters are significantly different according to ANOVA test.
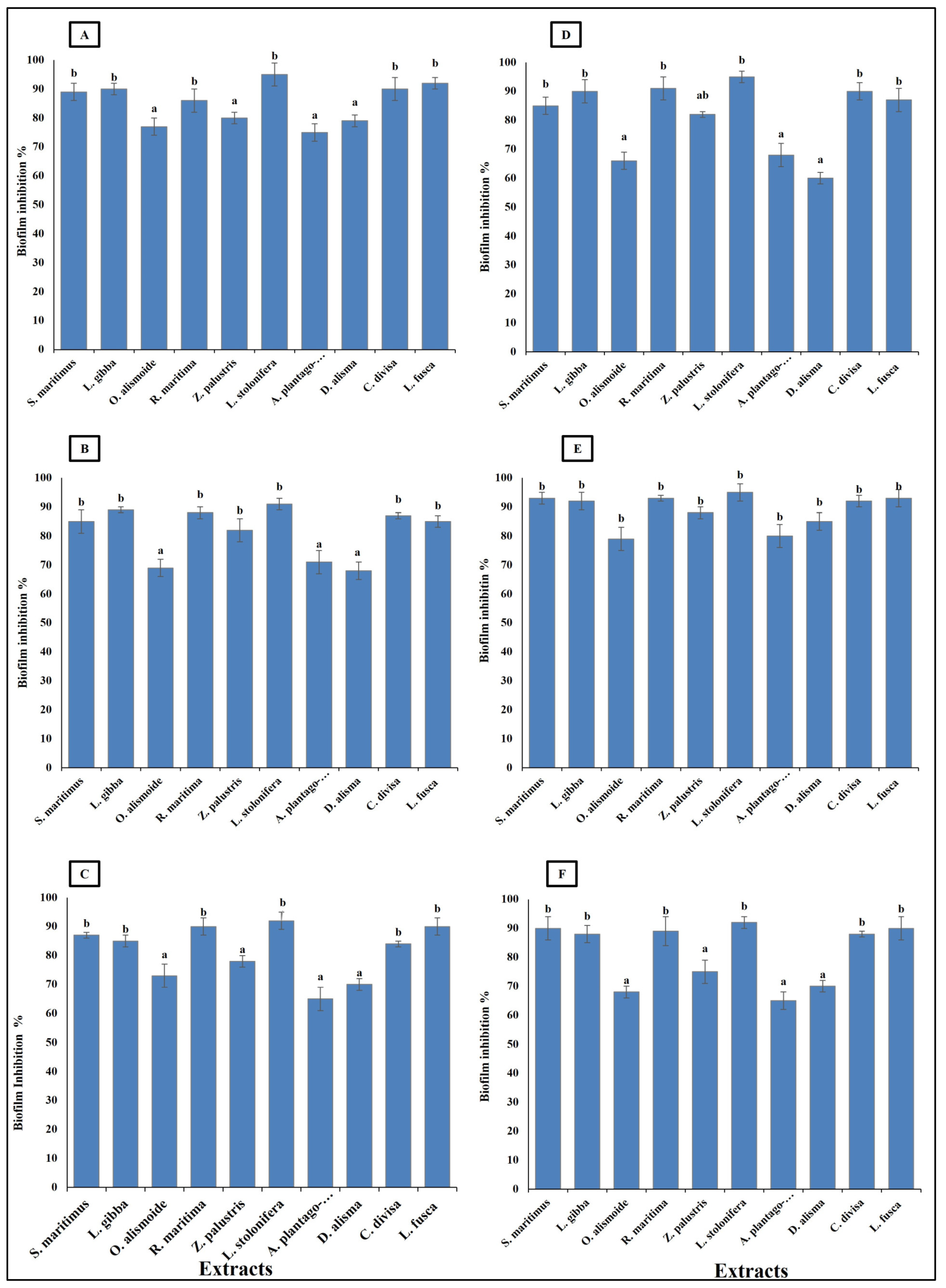
Bacteroides fragilis, Fusobacterium nucleatum, Helicobacter pylori, Porphyromonas gingivalis, Neisseria gonorrhoeae, and Salmonella enterica are considered carcinogenic bacteria, as persistence infection without control is highly related to gastrointestinal cancer [70]. These bacterial species are known for their ability to form biofilms, which enables them to persist in the infection site, causing serious health problems. Thus, it is important that antibacterial agents show biofilm inhibition activity [71]. The studied aquatic plants extract showed the ability to inhibit the formation of bacterial biofilm. The ability of the extracts to inhibit the bacterial biofilm formation was related to their phytochemical components, as the highest phytochemical components (Ludwigia stolonifera extract) showed the highest biofilm inhibition ability, with an inhibition percentage more than 90%. On the other hand, the lowest phytochemical components (Alisma plantago-aquatica, and Damasonium alisma extracts) showed the lowest biofilm inhibition ability, with an inhibition percentage from 60 to 80% (Figure 6). Similarly, Pallavi et al. [72] reported that the anti-biofilm and anti- adherence potential of plant extracts depends on their phytochemical components and active components, which inhibit the secretion of the bacterial extracellular components used in bacterial biofilm formation. Phenols have the ability to inhibit biofilm formation through disruption of the expression of the genes involved in virulence and adherence [73]. Sehgal et al. [74] reported that extracts with hydrolyzed tannins (low tannin content) could not inhibit bacterial biofilm formation, but extracts with high tannin content had the ability to inhibit 90% of bacterial biofilm formation.
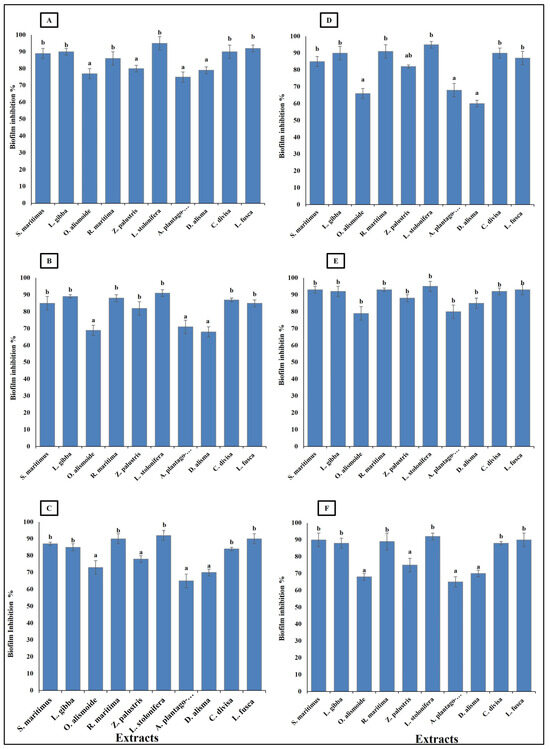
Figure 6.
Biofilm inhibition (%) in bacterial species [(A) Bacteroides fragilis, (B) Helicobacter pylori, (C) Fusobacterium nucleatum, (D) Porphyromonas gingivalis, (E) Salmonella enterica, (F) Neisseria gonorrhoeae], after treatment, with MIC of each extract. Column value is the mean of five replicates. The error bars represent standard deviation. Columns followed by different letters are significantly different according to ANOVA test.
2.4. Anticancer Activity of the Extracts
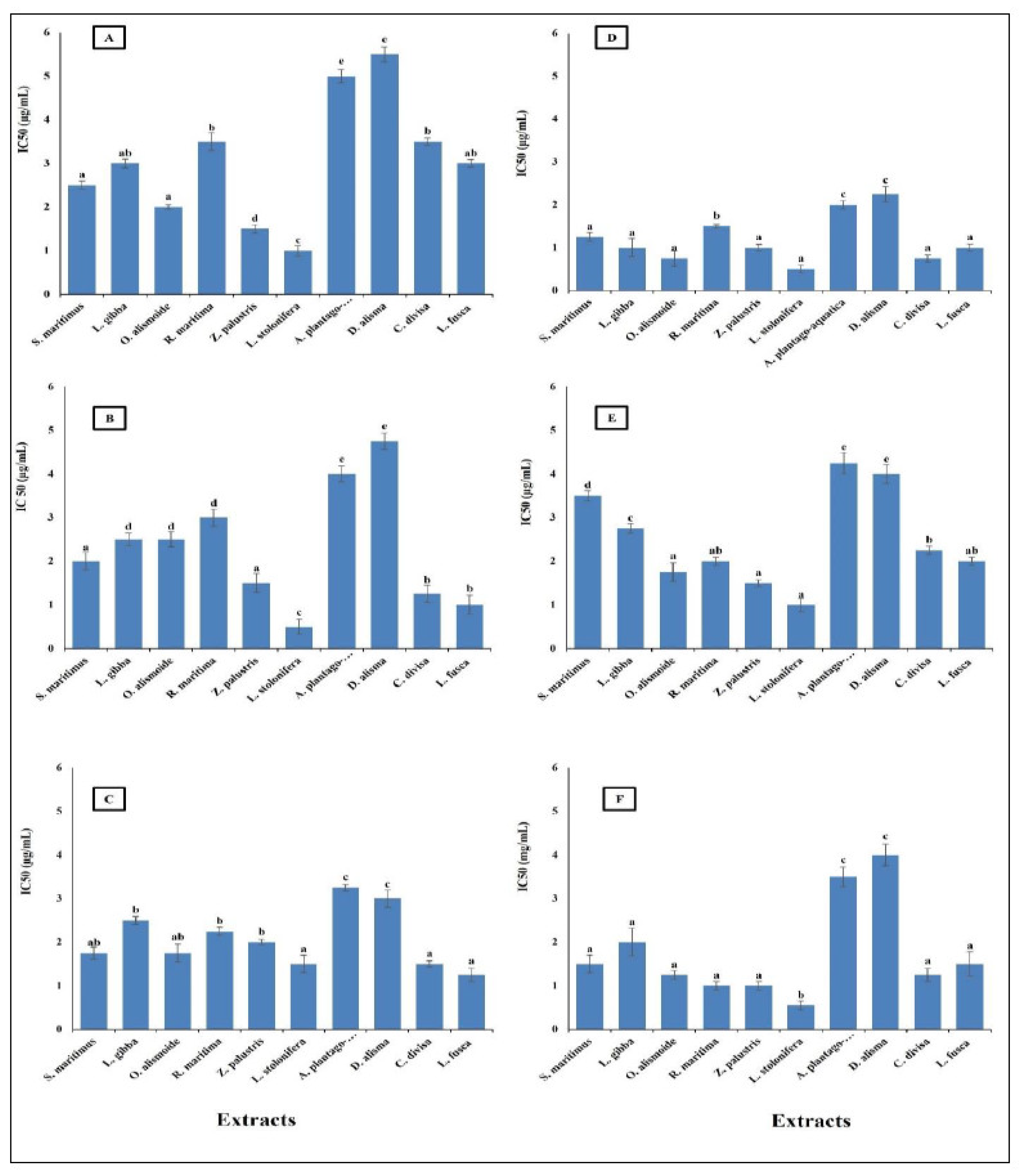
Cancer is a significant global health problem that poses severe challenges to healthcare systems worldwide. It is characterized by the uncontrolled growth and spread of abnormal cells, which can invade and damage surrounding tissues and organs [75]. Cancer is one of the leading causes of death globally. According to the World Health Organization (WHO), there were approximately 19.3 million new cases and 10 million cancer deaths in 2020 [76]. The most common cancers include breast, lung, and the different gastrointestinal cancers [77]. Finding new safe effective anticancer agents has become an urgent need [76]. The results in Figure 7 show that the studied aquatic plants extract showed promising anticancer activity against the different cancer cell lines tested. The highest anticancer activity was recorded in the L. stolonifera extract, with the lowest IC50 range from 0.5 µg/mL against pancreatic cancer cell line (AsPC-1), esophagus cancer cell line (KYSE-410), and colon cancer cell line (HCT116) to 1.5 µg/mL against gasteric cancer cell line (ClS-145). The lowest anticancer activity was in D. alisma with the highest IC50, which ranged from 2.25 µg/mL against esophagus cancer cell line (KYSE-410) to 5.5 µg/mL against gasteric cancer cell line (ClS-145). In general, the IC50 of the prepared extracts was lower than 6 µg/mL against the different cancer cell lines. This low IC50 is considered very promising for anticancer agents, as the American National Cancer Institute (NCI) guidelines reported that a crude extract can be considered an anticancer agent when its IC50 is less than 30 μg/mL [78]. Similarly, Jamaludin et al. [79] reported the effective anticancer activity of certain marine sponges with an IC50 lower than 30 µg/mL. The difference in the anticancer activity between the extracts was related to the difference in their phytochemical components. Huang et al. [80] reported a relationship between the anticancer activity of plant extracts and their phytochemical compositions.
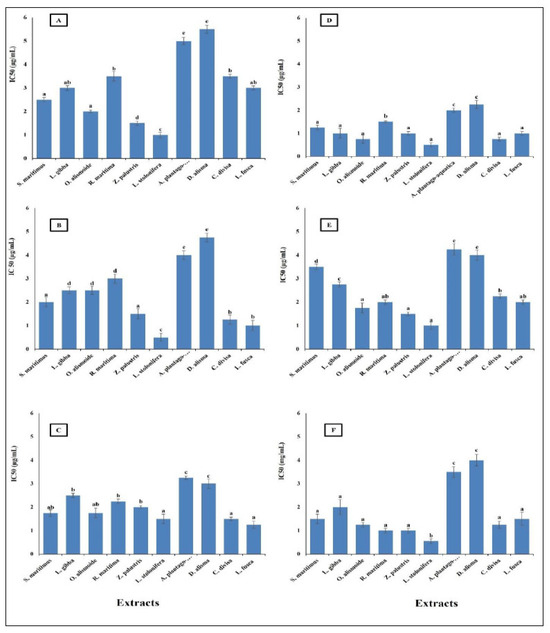
Figure 7.
Anticancer activity of different aquatic plant extracts on different human cancer cell lines. (A) Gasteric cancer (CLS-145), (B) Pancreatic cancer (AsPC-1), (C) Liver cancer (HepG2), (D) Esophagus cancer (KYSE-410), (E) Breast cancer (MCF-7), (F) Colon cancer (HCT116). The American National Cancer Institute (NCI) guidelines reported that a crude extract can be considered an anticancer agent when its IC50 is less than 30 μg/mL [74]. Column value is the mean of five replicates. The error bars represent standard deviation. Columns followed by different letters are significantly different according to ANOVA test.
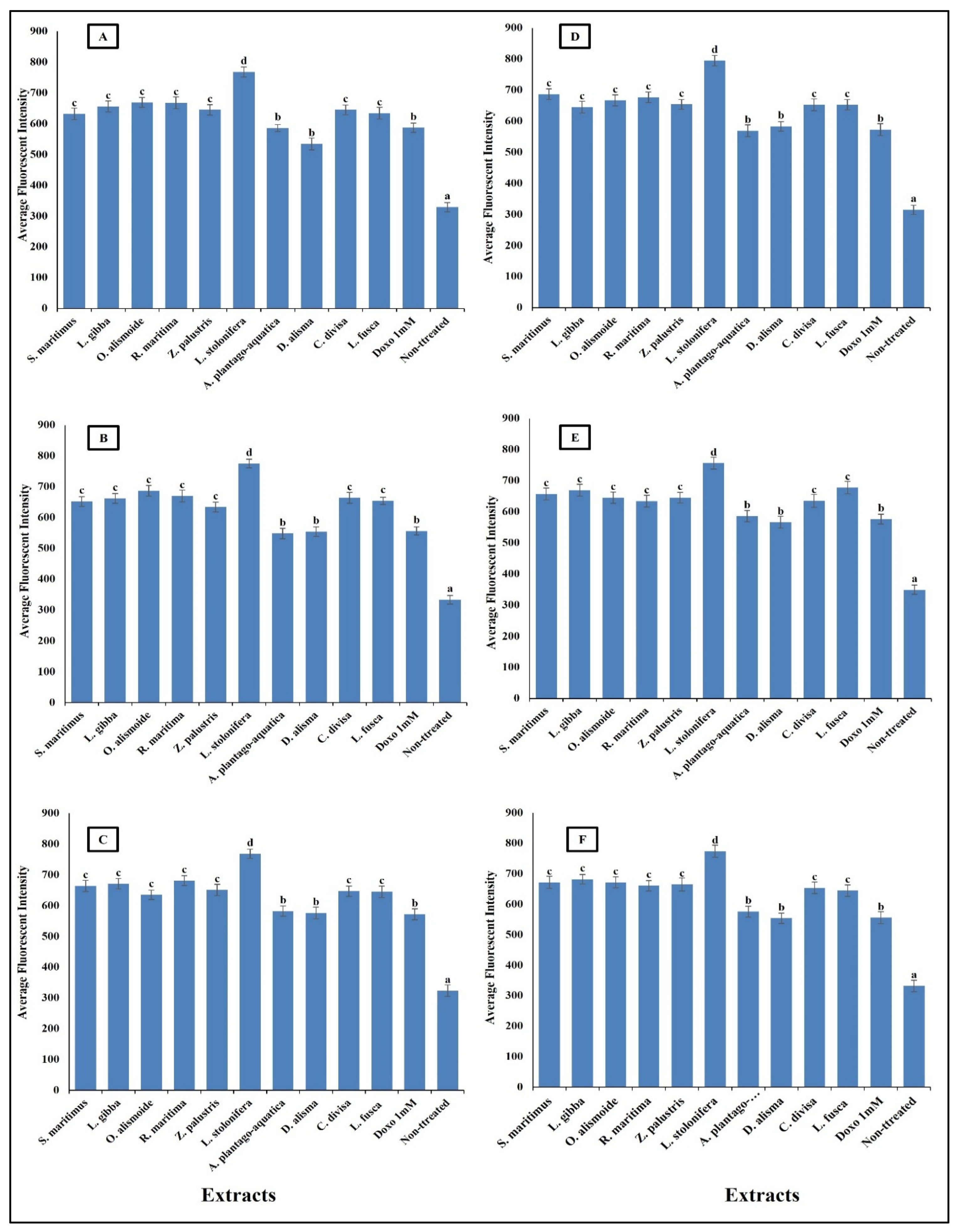
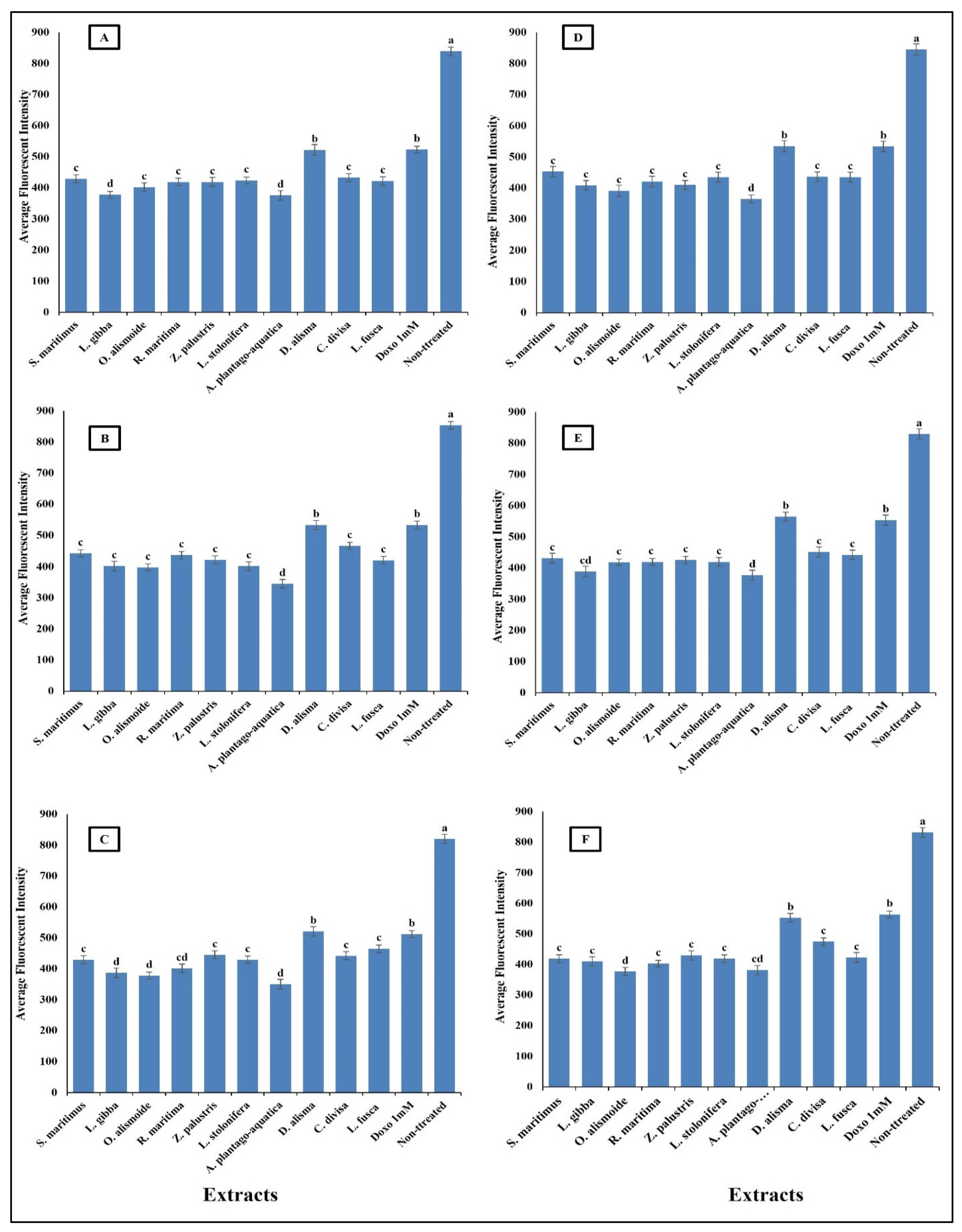
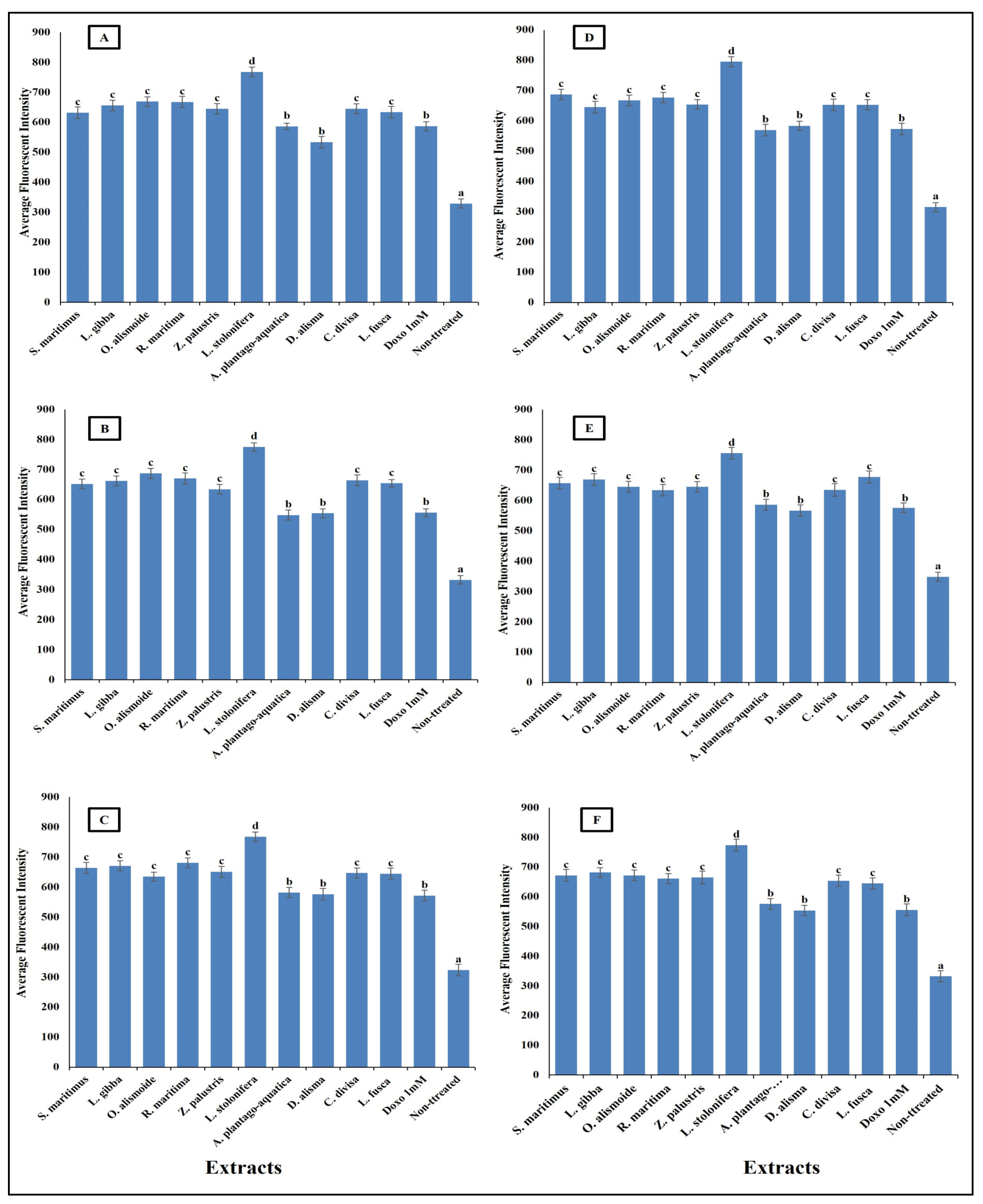
Anticancer agents affect cancer cells through multiple mechanisms, often targeting specific cellular structures and functions. Thus, to evaluate the mechanism by which the prepared extracts destroyed the cancer cells, we evaluated the change in nuclear intensity, plasma membrane permeability, mitochondrial membrane permeability, and cytochrome C release as a response to the different IC50 levels of the prepared extract and 1 mM doxorubicin (positive control) for comparison of their effectiveness. The results in Figure 8 show that nuclear intensity increased under treatment with the aquatic plant extracts or doxorubicin. However, the treatment with aquatic plant extracts caused a higher increase in cancer cell nuclear intensity than doxorubicin. Changes in nuclear intensity revealed a change in the nucleic acids and chromatin, which disturbed the cell cycle [81]. Anticancer agents can alter nuclear intensity by inducing DNA damage, disrupting the cell cycle, and promoting apoptosis. Many anticancer agents cause DNA breaks or cross-links, or interfere with DNA replication and transcription. This leads to increased nuclear intensity, due to DNA condensation during apoptosis [82,83]. Changes in plasma membrane permeability are a hallmark of apoptosis and can be triggered by various anticancer agents [84,85]. The studied aquatic plant extracts increased the plasma membrane permeability of the cancer cells more than doxorubicin (Figure 9). Anticancer agents can activate apoptotic pathways, leading to externalization of phosphatidylserine and increased membrane permeability [86,87]. At higher concentrations, some anticancer drugs may induce necrosis, causing loss of membrane integrity and uncontrolled release of cellular contents [88,89].
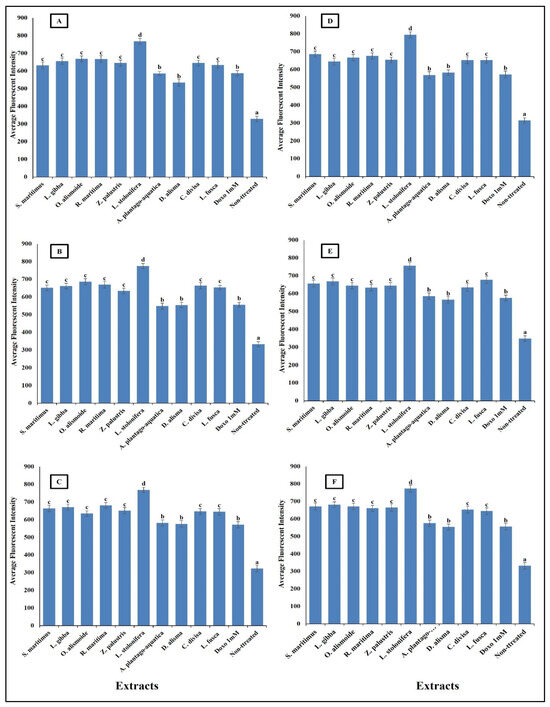
Figure 8.
Effect of IC50 of different aquatic plant extracts and Doxo 1 mM on nuclear intensity of different human cancer cells. (A) Gasteric cancer (CLS-145), (B) Pancreatic cancer (AsPC-1), (C) Liver cancer (HepG2), (D) Esophagus cancer (KYSE-410), (E) Breast cancer (MCF-7), (F) Colon cancer (HCT116). Nuclear intensity refers to the mean fluorescence intensity measured within the nuclear region of cells stained with Hoechst 33258 (λ ex = 352 nm, λ_em = 461 nm). Visualization was conducted using a Cellomics ArrayScan HCS reader (Thermo Scientific, Waltham, MA, USA), and quantification of fluorescence intensity was performed using a Cell Health Profiling bioapplication module. The relationship between nuclear intensity and average fluorescence intensity is directly proportional, providing insights into the distribution and localization of the fluorescent marker within the cells. Column value is the mean of five replicates. The error bars represent standard deviation. Columns followed by different letters are significantly different according to ANOVA test.
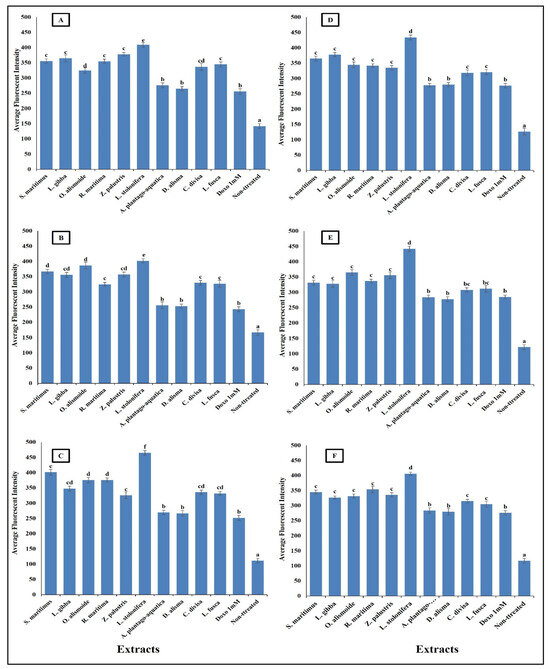
Figure 9.
Effect of IC50 of different aquatic plant extracts and Doxo 1 mM on plasma membrane permeability of different human cancer cells. (A) Gasteric cancer (CLS-145), (B) Pancreatic cancer (AsPC-1), (C) Liver cancer (HepG2), (D) Esophagus cancer (KYSE-410), (E) Breast cancer (MCF-7), (F) Colon cancer (HCT116). Plasma membrane permeability refers to the ability of the plasma membrane to allow certain molecules or ions to pass through it by diffusion or active transport mechanisms. The average fluorescence intensity is used as an indicator of plasma membrane permeability. When the plasma membrane becomes more permeable, there is an increased influx or efflux of fluorescent dyes (cell permeability dye (Excitation 491/Emission 509), leading to changes in the average fluorescence intensity measured within the cells. Column value is the mean of five replicates. The error bars represent standard deviation. Columns followed with different letters are significantly different according to ANOVA test.
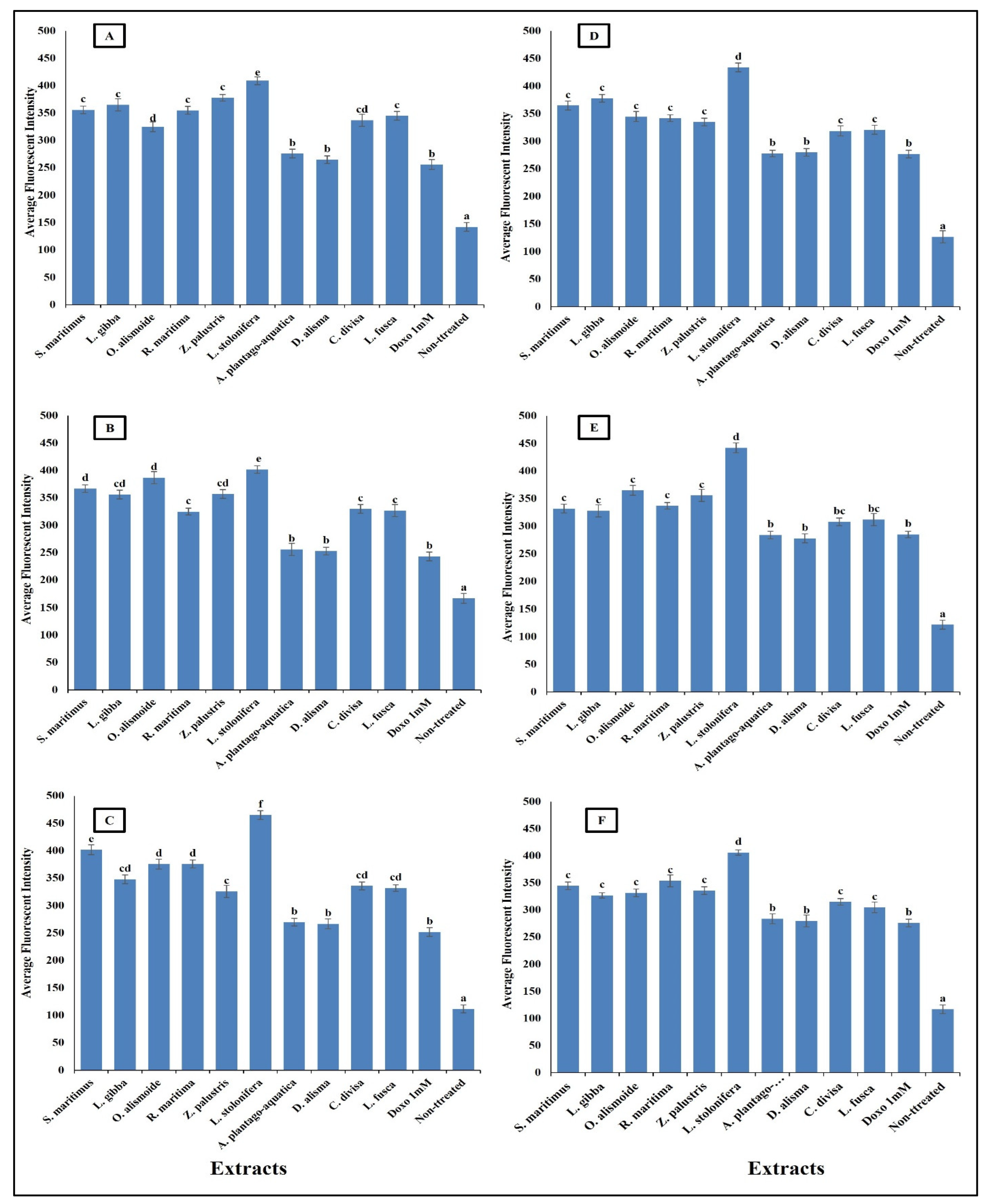
Mitochondrial membrane permeability (MMP) is an indicator for apoptosis [90]. An MMP dye was used to assess the functionality of active mitochondria, as it accumulates in organelles that maintain inner membrane potential. The measurement of MMP was based on the mean intensity of the dye within the mitochondria; a lower fluorescence intensity indicated a greater negative impact on mitochondrial function and an increase in the MMP [91]. The studied aquatic plant extracts significantly increased the MMP of the different cancer cells (Figure 10). Many anticancer agents increase mitochondrial membrane permeability, leading to mitochondrial dysfunction and apoptosis. Anticancer agents promote the release of pro-apoptotic proteins that permeabilize the outer mitochondrial membrane [92,93]. Increasing MMP leads to cytochrome c release. The release of cytochrome c from mitochondria into the cytosol is a critical step in the intrinsic apoptotic pathway, often induced by anticancer agents [94]. The results in Figure 11 clarify the significant increase in cytochrome c release after treatment with the prepared aquatic plant extracts and doxorubicin, but the cytochrome c release was the highest in the case of aquatic plant extract treatment. Once in the cytosol, cytochrome c binds to Apaf-1, leading to the formation of apoptosome and activation of caspase-9, which subsequently activates caspase-3 and other executioner caspases, triggering a cascade of proteolytic events that lead to the orderly dismantling of the cell. This cascade ensures that apoptosis proceeds efficiently, allowing for the removal of damaged or unwanted cells without eliciting an inflammatory response [95,96,97,98].
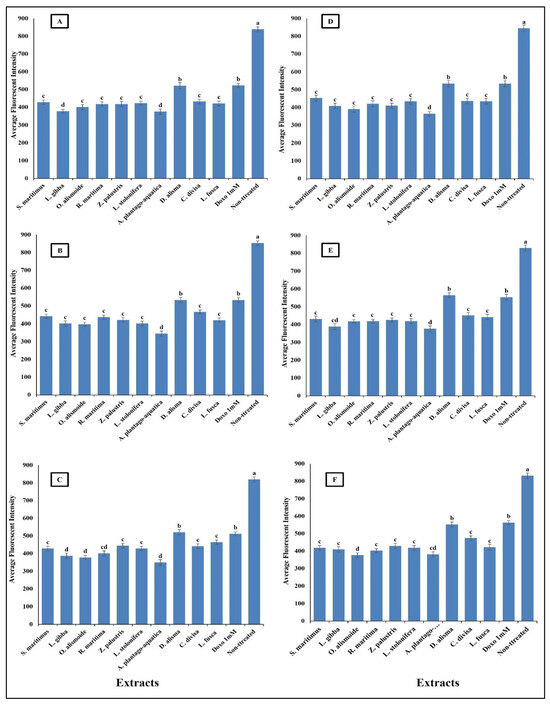
Figure 10.
Effect of IC50 of different aquatic plant extracts and Doxo 1 mM on mitochondrial membrane permeability (MMP) of different human cancer cells. (A) Gasteric cancer (CLS-145), (B) Pancreatic cancer (AsPC-1), (C) Liver cancer (HepG2), (D) Esophagus cancer (KYSE-410), (E) Breast cancer (MCF-7), (F) Colon cancer (HCT116). MPP measurement was based on the mean intensity of MMP dye (Excitation 552/Emission 576) penetrating the mitochondria; the lower the fluorescent intensity, the higher the effect against the mitochondria. Column value is the mean of five replicates. The error bars represent standard deviation. Columns followed by different letters are significantly different according to ANOVA test.
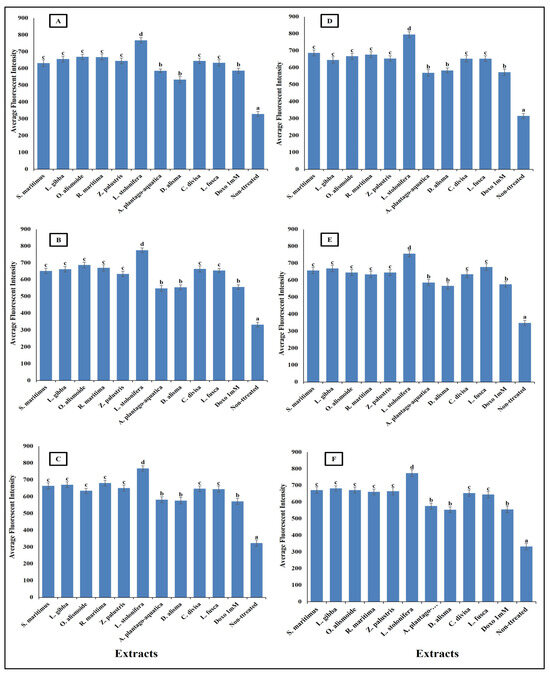
Figure 11.
Effect of IC50 of different aquatic plant extracts and Doxo 1 mM cytocrome c release in different human cancer cells. (A) Gasteric cancer (CLS-145), (B) Pancreatic cancer (AsPC-1), (C) Liver cancer (HepG2), (D) Esophagus cancer (KYSE-410), (E) Breast cancer (MCF-7), (F) Colon cancer (HCT116). Cytochrome c release from the mitochondria into the cytosol is a key event in the apoptotic pathway. In our study, we quantified cytochrome c release by measuring the average fluorescence intensity of a cytochrome c-specific fluorescent probe. Visualization was conducted using a Cellomics ArrayScan HCS reader (Thermo Scientific), and quantification of fluorescence intensity was performed using a Cell Health Profiling bioapplication module. When cytochrome c is released from the mitochondria, it binds to the fluorescent probe, resulting in an increase in fluorescence intensity. Column value is the mean of five replicates. The error bars represent standard deviation. Columns followed with different letters are significantly different according to ANOVA test.
Interestingly, the plant crude extracts possessed a considerably higher activity than the pure standard bioactive compound (Doxorubicin). Crude extracts can sometimes be more effective than pure standard anticancer agents. This can be due to the presence of a mixture of compounds in the crude extracts that can work together synergistically to enhance the overall anticancer effect [99]. These compounds may act on different targets or pathways, providing a broader and more effective response. While pure compounds typically target a specific molecule or pathway, crude extracts may affect multiple targets simultaneously. This can be beneficial in cancer treatment, where multiple pathways are often dysregulated [100]. Cancer cells can develop resistance to single-agent therapies over time. The complex mixture of compounds in crude extracts may reduce the likelihood of the development of resistance [101,102].
3. Materials and Methods
3.1. Aquatic Plants Extract Preparation
Shoot systems of Scirpus maritimus, Lemna gibba, Otellia alismoide, Ruppia maritima, Zannichellia palustris, Ludwigia stolonifera, Alisma plantago-aquatica, Damasonium alisma, Carex divisa, and Leptochloa fusca were collected from various branched canals of the Nile River in Egypt, Sharqia Governorate (30.7° N 31.63° E), in the period from January 2023 to June 2023. Plants were identified according to the local people and Zahran [37]. After drying, the shoot systems were finely ground, and aqueous extracts were prepared following the method of Naguib and Tantawy [103]. The ground powder (100 g) was extracted in 50 mL dist. water for 24 h on an orbital shaker (100 rpm) at 30 °C. After incubation, the mixture was filtrated through Whitman filter paper No. 1, the filtrate was dried, and designated as the plant extracts.
3.2. Phytochemical Components and Antioxidant Capacity of the Extracts
The antioxidant capacity and phytochemical composition of the extracts were evaluated through various assays.
The total antioxidant capacity of different concentrations (50, 100, and 150 mg/mL) of prepared extracts was determined in comparison with a standard (Vitamin C) using a DPPH radical scavenging assay, following Blois [104]. The reaction mixture consisted of 1.6 mL of prepared extract (with different concentrations) and 2.4 mL of 0.1 mM DPPH, shaken well and incubated in the dark at room temperature for 30 min. Absorbance was measured at 517 nm after incubation. The percentage of DPPH radical scavenging activity was calculated using the following equation:
where Ac is the absorbance of the control and As is the absorbance of the sample.
%DPPH Radical Scavenging Activity = (Ac − As)/Ac × 100
Phytochemical components including tannins, saponins, steroids, alkaloids, flavonoids, and phenols were quantified following methods described by Harbourne [105] with adjustments by Trease and Evans [106] for tannin, saponin, steroid, and alkaloid contents. Total flavonoid content was measured as per Pallab et al. [107], using an aluminum chloride colorimetric assay, while total phenol content was determined according to Julkunen-Tiitto [108].
Tannin content was measured by adding 0.3 mL of 0.1 N FeCl3 in 0.1 N HCl (3 mL) to 2 mL of plant extracts, along with 0.3 mL of 0.0008 M potassium ferricyanide. The absorbance was recorded at 720 nm, and tannin concentration was calculated using a standard curve.
Steroid content was measured by adding cholesterol color reagent to plant extracts and incubating the mixture for 35 min at room temperature. Absorbance was recorded at 550 nm, and steroid concentration was determined using a cholesterol standard curve.
Saponin content was measured by evaporating 0.5 mL of plant extract to dryness, then adding a fresh solution of vanillin acetic acid and perchloric acid. After incubation, the absorbance was recorded at 550 nm, and saponin concentration was determined using a sapogenin standard curve.
Alkaloid content was measured by adding 60% H2SO4 to the plant extract and incubating the mixture at room temperature for 3 h. Absorbance was recorded at 565 nm, and alkaloid concentration was calculated using an atropine standard curve.
Total flavonoid content was measured by incubating the plant extract with a sodium nitrite solution, followed by the addition of aluminum chloride and NaOH. Absorbance was recorded at 510 nm, and total flavonoid content was calculated using a quercetin standard curve.
Total phenol content was measured by mixing the plant extract with Folin–Ciocalteu reagent and Na2CO3. After incubation, absorbance was recorded at 725 nm, and phenol concentration was determined using a pyrogallol standard curve.
3.3. Antiparasitic Activity
Adult worms of Opisthorchis viverrini, Opisthorchis felineus, and Clonorchis sinensis were cultured at 37 °C for 24 h in RPMI 1640 medium containing 1% glucose, 0.1 mg/mL streptomycin, and penicillin, within a CO2 incubator. To calculate the IC50, various concentrations of extracts were introduced into the medium, and both treated and untreated cultures were then cultured for an additional 24 h. The viability of the worms was examined under an inverted microscope, and IC50 values were determined using a dose–response curve according to the method described by Pakharukova et al. [109].
Different concentrations of extracts were assessed for their antimalarial activity in vitro against the chloroquine-resistant FCB1 strain of Plasmodium falciparum, with the IC50 being determined as a measure of activity [110]. In a standard in vitro assay for antimalarial activity, Plasmodium falciparum cultures were synchronized and incubated with various concentrations of plant extracts or compounds in a 96-well plate at 37 °C for 48 h. Parasite growth was assessed using microscopy to determine parasitemia. The percentage inhibition of parasite growth was calculated by comparing treated samples to controls, and the IC50 value, indicating the concentration needed to inhibit 50% of parasite growth, was determined to evaluate the efficacy of the test samples.
Regarding leishmanicidal activity in vitro, Leishmania donovani was employed, and the antileishmanial screening followed the protocol outlined by Mbongo et al. [111]. In an in vitro assay for leishmanicidal activity, Leishmania donovani were cultured with various concentrations of plant extracts and incubated in a 96-well plate at 26 °C for 72 h. Parasite viability was assessed using direct counting under a microscope after staining. The percentage inhibition of parasite growth was calculated by comparing treated samples to controls; and the IC50 value, indicating the concentration needed to inhibit 50% of parasite growth, was determined to evaluate the efficacy of the test samples.
3.4. Antibacterial Activity
3.4.1. Pathogenic Bacterial Strains
Pathogenic Gram negative bacteria Bacteroides fragilis (ATCC 29762), Fusobacterium nucleatum (ATCC 25586), Helicobacter pylori (ATCC 51407), Porphyromonas gingivalis (ATCC 33277), Neisseria gonorrhoeae (ATCC 43070), and Salmonella enterica (ATCC 14028) were obtained from the American Type Culture Collection (Manassas, VA, USA).
3.4.2. Determination the Minimal Inhibitory Concentration (MIC) of Each Extract against Different Pathogenic Bacteria
The MIC of dried extracts was evaluated through a broth dilution assay. Extracts were dissolved, serially diluted in brain heart infusion broth, inoculated with bacteria, and incubated. Following incubation, turbidity was assessed at OD 600 nm. MIC was the lowest concentration of the studied extract that inhibited the microbial growth [112].
3.4.3. Antibacterial Mechanism
Determination of Bacterial Cell Membrane Permeability
Permeability changes were expressed as the percentage of relative electric conductivity [113]. After culturing for 12 h at 37 °C, bacteria were centrifuged at 1500× g for 10 min. Cells were washed with 5% glucose until their electric conductivities approximated that of 5% glucose, defining them as isotonic bacteria. The electric conductivities of the prepared aquatic extracts were evaluated after adding 5% glucose and the MIC of each extract (designated as A). Isotonic bacteria were then incubated with the MIC of each extract at 37 °C for 12 h, after which, conductivities were measured and recorded (designated as B). For reference, the conductivity of bacteria in 5% glucose was treated for 5 s in boiling water and noted as (C). The relative electric conductivity was calculated using the following equation:
Determination of Changes in Bacterial DNA Content
Bacterial suspension with OD600 nm = 2.0 was treated with the MIC of extracts at 37 °C for 12 h, and bacterial precipitation was obtained by refrigerated centrifugation (10,000× g, 1 min). Bacterial genomic DNA extraction kits (Tian Gen Biotech Co., Ltd., Beijing, China) were utilized to determine intracellular DNA content. The change in bacterial DNA content was calculated using the following equation:
3.4.4. Anti-Biofilm Activity
The effectiveness of aquatic extracts in inhibiting biofilm formation was evaluated using the crystal violet staining technique. Biofilms cultivated in 96-well flat-bottom polystyrene plates were exposed to the MIC of the extracts and incubated at 37 °C for 24 h. Following incubation, each plate was rinsed with distilled water to remove any planktonic cells and air-dried. Subsequently, 125 µL of 0.1% crystal violet dye was added to stain the adherent biofilm. The biofilm was dissolved by adding 130 µL of 30% acetic acid, and the absorbance of each plate was measured at 550 nm using a Microplate absorbance reader to assess biofilm inhibition [114].
3.5. Anticancer Activity
3.5.1. Cell Viability Assay
Various cancer cell lines (gastric cancer cell line (CLS-145), pancreatic cancer cell line (AsPC-1), liver cancer cell line (HepG2), colon cancer cell line (HCT116), esophagus cancer cell line (KYSE-410), and breast cancer cell line (MCF-7) from Cell Line Service (Eppelheim, Germany) were obtained and cultured. The 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) method was employed for cell viability assays. In this assay, cells were incubated with the yellow tetrazolium salt (MTT). Viable cells with active metabolism reduced MTT to purple formazan crystals. After incubation, the formazan crystals were solubilized using DMSO. The absorbance of the solution was then measured at 570 nm using a spectrophotometer. The intensity of the color correlated with the number of viable cells, allowing for the determination of the cell viability and cytotoxicity of test compounds.
3.5.2. High-Content Screening Assay
A high-content screening (HCS) assay was conducted to assess toxicity on the different studied cancer cells, examining various parameters such as nuclear intensity, membrane permeability, mitochondria membrane permeability, and cytochrome c. Cancer cells (105) were seeded in twelve-well plates and incubated at 37 °C with 5% CO2 for 24 h. Subsequently, cells were treated with IC50 concentrations of different prepared extracts, alongside untreated cells (negative control) and cells treated with 1 mM doxorubicin (positive control). After another 24 h incubation period, MMP dye (Excitation 552/Emission 576) and cell permeability dye (Excitation 491/Emission 509) were applied to living cells, followed by a 1 h incubation. Fixation (4% formaldehyde, 15 min) and permeabilization (0.1% Triton X-100 in PBS) were then carried out, followed by blocking with 3% bovine serum albumin and incubation with cytochrome c primary mouse antibody for 1 h. Samples were washed three times with wash buffer I (1:6 PBS), followed by addition of goat anti-mouse secondary antibodies conjugated with DyLightTM 649. Cells were rinsed with wash buffer II (1:6 PBS with 1% Tween-20) and stained with Hoechst 33258 (λ ex = 352 nm, λ_em = 461 nm) to visualize nuclei. Visualization was conducted using a Cellomics ArrayScan HCS reader (Thermo Scientific). Quantification of the fluorescence intensity of each dye, to examine parameters such as nuclear intensity, membrane permeability, mitochondria membrane permeability, and cytochrome c, was performed using a Cell health profiling bioapplication module [115].
Nuclear intensity was determined as the mean fluorescence intensity measured within the nuclear region of cells stained with Hoechst 33258. The relationship between nuclear intensity and average fluorescence intensity is directly proportional, i.e., an increase in nuclear intensity leads to an increase in fluorescence intensity. This provided insights into the distribution and localization of the fluorescent marker within the cells.
Plasma membrane permeability refers to the ability of the plasma membrane to allow certain molecules or ions to pass through it by diffusion or active transport mechanisms. The average fluorescence intensity is used as an indicator of plasma membrane permeability. When the plasma membrane becomes more permeable, there is an increased influx or efflux of fluorescent dyes (cell permeability dye (Excitation 491/Emission 509), leading to changes in the average fluorescence intensity measured within the cells.
MPP measurement was based on the mean intensity of MMP dye penetrating the mitochondria; the lower the fluorescent intensity, the higher the effect against the mitochondria.
Cytochrome c release from the mitochondria into the cytosol is a key event in the apoptotic pathway. In our study, we quantified cytochrome c release by measuring the average fluorescence intensity of a cytochrome c-specific fluorescent probe. When cytochrome c is released from the mitochondria, it binds to the fluorescent probe, resulting in an increase in fluorescence intensity.
3.6. Statistical Analysis
Data analysis was conducted using SPSS software (version 14), with results expressed as mean ± SD. An ANOVA test was used for comparing mean values.
4. Conclusions
Aquatic plants are a rich source of pharmaceutical compounds such as phenols, tannins, saponins, alkaloids, and flavonoids. Among the studied plants, Ludwigia stolonifera exhibited the highest levels of phytochemicals, followed by Scirpus maritimus, Lemna gibba, Ottelia alismoides, Ruppia maritima, Zannichellia palustris, Carex divisa, and Leptochloa fusca. The lowest levels were found in Alisma plantago-aquatica and Damasonium alisma. Extracts from these plants’ shoots showed significant antioxidant, antibacterial, antiparasitic, and anticancer activities, surpassing the antioxidant capacity of vitamin C. The extracts had IC50 values for antiparasitic activity ranging from 0.7 to 2.5 µg/mL and demonstrated low minimum inhibitory concentrations (MIC) against various pathogenic bacteria, causing DNA damage, increased plasma membrane permeability, and 90% biofilm inhibition. They were also effective against a range of cancer cell lines, with IC50 values below 6 µg/mL, indicating significant apoptotic activity, increased nuclear intensity, plasma membrane permeability, mitochondrial membrane permeability, and cytochrome c release, and outperforming doxorubicin. This study underscores the potential of aquatic plants as sources of new, safe, and effective drugs with strong antiparasitic, antibacterial, and anticancer properties. Future research should focus on identifying specific pharmaceutical compounds in each plant and conducting in vivo studies to compare these extracts with standard medications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13152148/s1, Figure S1: Studied aquatic plants; Table S1: English name, scientific name, classification, and plant morphological characters.
Author Contributions
Conceptualization F.A., H.A.A., I.J., A.A.A.O., M.M.A., L.Y.A.A.-H., N.E.A.-H., S.M.E. and D.M.N.; methodology F.A., H.A.A., I.J., A.A.A.O., M.M.A., L.Y.A.A.-H., N.E.A.-H., S.M.E. and D.M.N.; software, D.M.N.; validation, F.A., H.A.A., I.J., A.A.A.O., M.M.A., L.Y.A.A.-H., N.E.A.-H., S.M.E. and D.M.N.; formal analysis, F.A., H.A.A., I.J., A.A.A.O., M.M.A., L.Y.A.A.-H., N.E.A.-H., S.M.E. and D.M.N.; investigation, F.A., H.A.A., I.J., A.A.A.O., M.M.A., L.Y.A.A.-H., N.E.A.-H., S.M.E. and D.M.N.; resources, F.A., H.A.A., I.J., A.A.A.O., M.M.A., L.Y.A.A.-H., N.E.A.-H., S.M.E. and D.M.N.; data curation, F.A., H.A.A., I.J., A.A.A.O., M.M.A., L.Y.A.A.-H., N.E.A.-H., S.M.E. and D.M.N.; writing—original draft preparation, D.M.N.; writing—review and editing, F.A., H.A.A., I.J., A.A.A.O., M.M.A., L.Y.A.A.-H., N.E.A.-H., S.M.E. and D.M.N.; project administration, F.A.; funding acquisition, F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Taif University, Saudi Arabia, Project No. (TU-DSPP-2024-227).
Data Availability Statement
All data generated in this study are found in the manuscript.
Acknowledgments
The authors extend their appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2024-227).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ahmed, F.; Shamim, N.J.; Sharma, H.K.; Grewal, A.S.; Pandita, D.; Lather, V. Combating Antimicrobial Resistance: A paradigm shift from general to precision medicine. Chem. Biol. Lett. 2024, 11, 662. [Google Scholar] [CrossRef]
- Patil, P.A.; Bobde, K.A.; Masurkar, S.A. Combating Antimicrobial Resistance: The Role of New Biotechnological Tools. Nat. Camp. 2024, 24, 142–150. Available online: https://museonaturalistico.it/index.php/journal/article/view/49 (accessed on 1 May 2024).
- Sahoo, P. Complementary supramolecular drug associates in perfecting the multidrug therapy against multidrug resistant bacteria. Front. Immunol. 2024, 15, 1352483. [Google Scholar] [CrossRef] [PubMed]
- Bentivegna, E.; Galastri, S.; Onan, D.; Martelletti, P. Unmet Needs in the Acute Treatment of Migraine. Adv. Ther. 2024, 41, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Olewinska, E.; Famulska, A. Heart failure with mildly reduced and preserved ejection fraction: A review of disease burden and remaining unmet medical needs within a new treatment landscape. Heart Fail. Rev. 2024, 29, 631–662. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xu, H.; Zhang, Y.; Liu, S.; Xu, S.; Xie, Y.; Xiao, J.; Hu, T.; Xiao, H. Identifying the unmet needs of post-treatment colorectal cancer survivors: A critical literature review. Eur. J. Oncol. Nurs. 2024, 70, 102570. [Google Scholar] [CrossRef] [PubMed]
- Stout, N.L.; Boatman, D.; Rice, M.; Branham, E.; Miller, M.; Salyer, R. Unmet Needs and Care Delivery Gaps Among Rural Cancer Survivors. J. Patient Exp. 2024, 11, 23743735241239865. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.; Luykx, J.J.; Strube, W.; Hasan, A. Challenges, unmet needs and future directions—A critical evaluation of the clinical trial landscape in schizophrenia research. Expert Rev. Clin. Pharmacol. 2024, 17, 11–18. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Mease, P.; Kerschbaumer, A.; Voll, R.E.; Breedveld, F.C.; Smolen, J.S.; Gottenberg, J.-E.; Baraliakos, X.; Kiener, H.P.; Aletaha, D.; et al. Unmet need in rheumatology: Reports from the Advances in Targeted Therapies meeting, 2023. Ann. Rheum. Dis. 2024, 83, 409–416. [Google Scholar] [CrossRef]
- Aggarwal, G.; Sharma, M.; Singh, R.; Sharma, U. Plant-based natural product chemistry: An overview of the multistep journey involved in scientific validation of traditional knowledge. Stud. Nat. Prod. Chem. 2024, 80, 327–377. [Google Scholar] [CrossRef]
- Chunarkar-Patil, P.; Kaleem, M.; Mishra, R.; Ray, S.; Ahmad, A.; Verma, D.; Bhayye, S.; Dubey, R.; Singh, H.N.; Kumar, S. Anticancer Drug Discovery Based on Natural Products: From Computational Approaches to Clinical Studies. Biomedicines 2024, 12, 201. [Google Scholar] [CrossRef]
- Singh, K.; Singh, G.; Bhushan, B.; Kumar, S.; Dhurandhar, Y.; Dixit, P. A comprehensive pharmacological review of Atractylodes Macrocephala: Traditional uses, phytochemistry, pharmacokinetics, and therapeutic potential. Pharmacol. Res.-Mod. Chin. Med. 2024, 10, 100394. [Google Scholar] [CrossRef]
- Ahmed, S.; Jamil, S. Chemical Pharmacognosy in natural drug discovery-bridging folk wisdom and modern medicine. J. Pharmacogn. Phytochem. 2024, 13, 391–398. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidate 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Davis, C.C.; Choisy, P. Medicinal plants meet modern biodiversity science. Curr. Biol. 2024, 34, R158–R173. [Google Scholar] [CrossRef]
- Jain, H.; Aggarwal, N.K. From Pond to Pill: Microalgae’s Role in Vegan Pharmaceuticals’. In Harnessing Microbial Potential for Multifarious Applications. Energy, Environment, and Sustainability; Bala, K., Ghosh, T., Kumar, V., Sangwan, P., Eds.; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Jin, F.; Fan, P.; Wu, Y.; Yang, Q.; Li, J.; Liu, H. Efficacy and Mechanisms of Natural Products as Therapeutic Interventions for Chronic Respiratory Diseases. Am. J. Chin. Med. 2024, 52, 57–88. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, M.; Huang, H.; Jin, W. Drug repurposing for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 92. [Google Scholar] [CrossRef]
- Aras, A.; Dogru, M.; Bursal, E. Determination of antioxidant potential of Nepeta nuda subsp. lydiae. Anal. Chem. Lett. 2016, 6, 758–765. [Google Scholar] [CrossRef]
- Bursal, E.; Aras, A.; Kılıç, Ö.; Buldurun, K. Chemical constituent and radical scavenging antioxidant activity of Anthemis kotschyana Boiss. Nat. Prod. Res. 2020, 35, 4794–4797. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, M.A.; Taslimi, P.; Kılıç, Ö.; Gülçin, İ.; Dey, A.; Bursal, E. Unravelling the phenolic compound reserves, antioxidant and enzyme inhibitory activities of an endemic plant species, Achillea pseudoaleppica. J. Biomol. Struct. Dyn. 2021, 41, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.H.; Azha, N.U.; Farooq, R.; Sheikh, S.A.; Ganie, M.; Parray, M.N.; Mushtaq, H.; Hameed, I.; Lone, M.A. Exploring the potential of marine natural products in drug development: A comprehensive review. Phytochem. Lett. 2024, 59, 124–135. [Google Scholar] [CrossRef]
- Maisha, M.H.; Jui, Z.S.; Begum, N. Ethnomedicinal and ethnobotanical uses of aquatic flora by local inhabitants of Gopalganj District, Bangladesh. J. Med. Plants Stud. 2024, 12, 157–165. [Google Scholar] [CrossRef]
- Pereira, L.; Cotas, J. Seaweed: A sustainable solution for greening drug manufacturing in the pursuit of sustainable healthcare. Explor. Drug Sci. 2024, 2, 50–84. [Google Scholar] [CrossRef]
- Elagib, S.M. Antiparasitic activity of Eichhornia crassipes leaves extract. Biocatal. Agric. Biotechnol. 2020, 24, 101556. [Google Scholar] [CrossRef]
- Eden, W.T.; Wahyuono, S.; Cahyono, E.; Astuti, P. Phytochemical, Antioxidant, and Cytotoxic Activity of Water Hyacinth (Eichhornia crassipes) Ethanol Extract. Trop. J. Nat. Prod. Res. 2023, 7, 3606–3612. [Google Scholar] [CrossRef]
- Powthong, P.; Suntornthiticharoen, P. Comparative analysis of antioxidant, antimicrobial, and tyrosinase inhibitory activities of Centella asiatica (l.) Urb and Eichhornia crassipes (mart.) Solms. J. Med. Pharm. Allied Sci. 2023, 12, 5931–5938. [Google Scholar] [CrossRef]
- Ratnani, R.D.; Arianti, F.D.; Sasongko, N.A. Exploring the potential of water hyacinth weed (Pontederia crassipes) as an environmentally friendly antifungal to realize sustainable development in lakes: A review. Case Stud. Chem. Environ. Engin 2024, 9, 100702. [Google Scholar] [CrossRef]
- Lata, S.; Lata, R.; Ram, R.B. Lotus: A Sacred, Valuable and Sustainable Aquatic Plant. Sustain. Agri Food Environ. Res. 2024, 12, 1. [Google Scholar]
- Ma, Q.; Guan, Y.; Sang, Z.; Wei, R. Anti-Inflammatory Phenylpropanoid Derivatives from the Aerial Parts of Oenanthe javanica. Chem. Nat. Compd. 2021, 57, 752–756. [Google Scholar] [CrossRef]
- Bae, I.A.; Ha, J.W.; Boo, Y.C. Chlorogenic Acid, a Component of Oenanthe javanica (Blume) DC., Attenuates Oxidative Damage and Prostaglandin E2 Production Due to Particulate Matter 10 in HaCaT Keratinocytes. Cosmetics 2023, 10, 60. [Google Scholar] [CrossRef]
- Shahdadi, F.; Salehi Sardoei, A. Antioxidant Activity of Methanolic Extracts of Borago Officinalis, Teucrium Polium, Mentha Aquatica and Allium Taradox. Arch. Med. Lab. Sci. 2023, 9, 1–7 (e4). [Google Scholar] [CrossRef]
- Chang, C.; Chen, Y.; Shyur, L. Phytocompounds from essential oil of Mentha aquatica L. Cv. Lime prevent vemurafenib-promoted skin carcinogenesis via inhibiting HRASQ61L keratinocytes and reprogramming macrophage activities. Phytomedicine 2024, 122, 155161. [Google Scholar] [CrossRef]
- Nilash, A.B.; Jahanbani, J.; Jolehar, M. Effect of nasturtium extract on oral cancer. Adv. Biomed. Res. 2023, 12, 53. [Google Scholar] [CrossRef]
- Zaman, S.; Ahmad, R.; Abdulaziz Binobead, M.; Ragab Abdel Gawwad, M.; Soliman Elshikh, M.; Gafforov, Y.; Mehmood Abbasi, A. Polyphenolic contents and antioxidant potential in Nasturtium officinale. J. King Saud. Univ.-Sci. 2024, 36, 103223. [Google Scholar] [CrossRef]
- Suman, B.; Singh, S.P. Diversity of Aquatic Medicinal Angiosperms of District Hamirpur, Himachal Pradesh, India. Ecol. Environ. Conserv. 2024, 30, S368–S374. [Google Scholar] [CrossRef]
- Zahran, M.A. Hydrophytes of the Nile in Egypt. In The Nile. Monographiae Biologicae; Dumont, H.J., Ed.; Springer: Dordrecht, The Netherlands, 2009; Volume 89, pp. 463–478. [Google Scholar] [CrossRef]
- Pooja, K.; Rani, S.; Rana, V.; Pal, G.K. Aquatic plants as a natural source of antimicrobial and functional ingredients. In Functional and Preservative Properties of Phytochemicals; Prakash, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 93–118. [Google Scholar]
- Ferrara, L. Seaweeds are a Future Resource in Food as a Source of RawMaterials and Bio Functional Compounds. Int. J. Pharm. Res. Appl. 2023, 8, 512–527. [Google Scholar]
- Wang, M.; Hu, W.; Wang, Q.; Yang, B.; Kuang, H. Extraction, purification, structural characteristics, biological activities, and application of the polysaccharides from Nelumbo nucifera Gaertn. (lotus): A review. Int. J. Biol. Macromol. 2023, 226, 562–579. [Google Scholar] [CrossRef]
- Dávid, C.Z.; Hohmann, J.; Vasas, A. Chemistry and Pharmacology of Cyperaceae Stilbenoids: A Review. Molecules 2021, 26, 2794. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. (Eds.) Secondary Metabolites. In Plant Physiology, Development and Metabolism; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Sadeghi, A.; Rajabiyan, A.; Nabizade, N.; Meygoli Nezhad, N.; Zarei-Ahmady, A. Seaweed-derived phenolic compounds as diverse bioactive molecules: A review on identification, application, extraction and purification strategies. Int. J. Biol. Macromol. 2024, 266, 131147. [Google Scholar] [CrossRef]
- Zhou, H.C.; Kang, H.X.; Wei, J.; Gao, C.J.; Hussain, M.; Fu, Y.J.; Li, M.D.; Li, F.L.; Xu, S.J.L.; Lee, F.W.F.; et al. Microcosm study on fate and dynamics of mangrove tannins during leaf litter leaching. Ecol. Process 2023, 12, 37. [Google Scholar] [CrossRef]
- Iqbal, N.; Poór, P. Plant Protection by Tannins Depends on Defence-Related Phytohormones. J. Plant Growth Regul. 2024, 1–18. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, H.; Chen, S. Progress on synthesis of benzylisoquinoline alkaloids in sacred lotus (Nelumbo nucifera). Med. Plant Biol. 2023, 2, 20. [Google Scholar] [CrossRef]
- Yernazarova, G.I.; Ramazanova, A.A.; Turasheva, S.K.; Almalki, F.A.; Hadda, T.B.; Orazova, S.B.; Madenova, A.K.; Admanova, G.B.; Korul’kin, D.Y.; Sabdenalieva, G.M.; et al. Extraction, Purification and Characterisation of four new alkaloids from the water plant Pistia stratiotes: POM Analyses and Identification of Potential Pharmacophore Sites. Res. J. Pharm. Technol. 2023, 16, 3410–3416. [Google Scholar] [CrossRef]
- Abd El-Gwaid, F.S.; El Saied, A.S.; El-Swaify, Z.A.; Salah El Din, R.A. A Comparative Evaluation of Phytochemical and Antimicrobial Properties of Selected Aquatic and Terrestrial Halophyte Plants Growing in Egypt. Int. Theor. Appl. Res. 2023, 2, 169–182. [Google Scholar] [CrossRef]
- Hoang, C.K.; Le, C.H.; Nguyen, D.T.; Tran, H.T.N.; Luu, C.V.; Le, H.M.; Tran, H.T.H. Steroid Components of Marine-Derived Fungal Strain Penicillium levitum N33.2 and Their Biological Activities. Mycobiol 2023, 51, 246–255. [Google Scholar] [CrossRef]
- Obakan Yerlikaya, P.; Arısan, E.D.; Mehdizadehtapeh, L.; Uysal-onganer, P.; Gürkan, A. The Use of Plant Steroids in Viral Disease Treatments: Current Status and Future Perspectives. Eur. J. Biol. 2024, 82, 86–94. [Google Scholar] [CrossRef]
- Strzemski, M.; Adamec, L.; Dresler, S.; Mazurek, B.; Dubaj, K.; Stolarczyk, P.; Feldo, M.; Płachno, B.J. Shoots and Turions of Aquatic Plants as a Source of Fatty Acids. Molecules 2024, 29, 2062. [Google Scholar] [CrossRef]
- Osama, M.; Wei, C.R.; Saleem, R.; Unar, A.A.; Unar, K.; Siyal, F.J.; Shaikh, B.; Baig, S.G.; Siddiq, A. Aquatic Plants with Anti-Inflammatory and Anti-Oxidant Activities. J. Surv. Fish. Sci. 2023, 10, 3802–3806. [Google Scholar] [CrossRef]
- Al-Rowaily, S.L.; Abd-ElGawad, A.M.; Alghanem, S.M.; Al-Taisan, W.A.; El-Amier, Y.A. Nutritional Value, Mineral Composition, Secondary Metabolites, and Antioxidant Activity of Some Wild Geophyte Sedges and Grasses. Plants 2019, 8, 569. [Google Scholar] [CrossRef]
- Alzandi, A.A.; Taher, E.A.; Al-Sagheer, N.A.; Al-Khulaidi, A.W.; Azizi, M.; Naguib, D.M. Phytochemical components, antioxidant and anticancer activity of 18 major medicinal plants in Albaha region, Saudi Arabia. Biocatal. Agric. Biotechnol. 2021, 34, 102020. [Google Scholar] [CrossRef]
- Kochar, N.; Vyas, J.; Vyas, K.; Chandewar, A.; Mundhada, D. Secondary metabolite estimation and antioxidant potential assessment of purple bell Thunbergia erecta (Benth.) T. Anderson. Int. Second. Metab. 2024, 11, 23–36. [Google Scholar] [CrossRef]
- Kalemba, M.R.K.; Makhuvele, R.; Njobeh, P.B. Phytochemical screening, antioxidant activity of selected methanolic plant extracts and their detoxification capabilities against AFB1 toxicity. Heliyon 2024, 10, e24435. [Google Scholar] [CrossRef]
- Łyczko, J.; Jamroz, E.; Kocowicz, A.; Kawałko, D. Antioxidant capacity sources of soils under different land uses. Sci. Rep. 2024, 14, 8394. [Google Scholar] [CrossRef]
- Gupta, N.; Shalaby, S.; Awad, M.A.; Shalaby, S. The future of antiparasitic therapy. In Advances in Antiparasitic Therapies and Drug Delivery; Kesharwani, P., Gupta, N., Eds.; Elsiever: Amsterdam, The Netherlands, 2024; pp. 391–405. [Google Scholar] [CrossRef]
- Rezaeilaal, A.; Nasoori, H.; Shamsnia, H.S.; Samanian, A.; Qavami, N.; Momtaz, S.; Jamialahmadi, T.; Emami, S.A.; Sahebkar, A. Traditional medicine and natural products as antiparasitic agents. In Advances in Antiparasitic Therapies and Drug Delivery; Kesharwani, P., Gupta, N., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 33–90. [Google Scholar] [CrossRef]
- Saqlain, M.; Wasif, Z.; Ali, Q.; Hayat, S. Anti-parasitic Activities of Medicinal Plants. J. Life Soc. Sci. 2024, 3, 21. Available online: https://bbasrjlifess.com/index.php/home/article/view/21 (accessed on 1 May 2024).
- Ali, H.S.; Mishra, S. Natural Products as Antiparasitic, Antifungal, and Antibacterial Agents. In Drugs from Nature: Targets, Assay Systems and Leads; Haridas, M., Abdulhameed, S., Francis, D., Kumar, S.S., Eds.; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Tajbakhsh, E.; Kwenti, T.E.; Kheyri, P.; Nezaratizade, S.; Lindsay, D.S.; Khamesipour, F. Antiplasmodial, antimalarial activities and toxicity of African medicinal plants: A systematic review of literature. Malar. J. 2021, 20, 349. [Google Scholar] [CrossRef]
- Ponomarev, D.; Lvova, M.; Mordvinov, V.; Chidunchi, I.; Dushkin, A.; Avgustinovich, D. Anti-Opisthorchis felineus effects of artemisinin derivatives: An in vitro study. Acta Trop. 2024, 254, 107196. [Google Scholar] [CrossRef]
- Kuete, V. Potential of African medicinal plants against Enterobacteria: Classification of plants antibacterial agents. Adv. Bot. Res. 2023, 106, 151–335. [Google Scholar] [CrossRef]
- Alzandi, A.A.; Taherc, E.A.; Azizi, M.; Al-Sagheer, N.A.; Al-Khulaidi, A.W.; Naguib, D.M. Antibacterial Activity of Some Medicinal Plants in Al Baha Region, Saudi Arabia, Against Carcinogenic Bacteria Related to Gastrointestinal Cancers. J. Gastrointest. Cancer 2023, 54, 51–55. [Google Scholar] [CrossRef]
- Jena, B.; Singh, S.S.; Chakrabortty, S.; Behera, S.K.; Tripathy, S.K.; Lundborg, C.S.; Kumar, R.; Ali Khan, M.; Jeon, B.H.; Mishra, A. Understanding the antibacterial mechanism of a phytochemical derived from Urginea indica against Methicillin-Resistant Staphylococcus aureus: A phytochemical perspective to impede antibiotics resistance. J. Ind. Eng. Chem. 2024. [Google Scholar] [CrossRef]
- Shawky, E.M.; Elgindi, M.R.; Baky, M.H. Phytochemical and biological diversity of genus Ludwigia: Acomprehensive review. ERU Res. J. 2023, 2, 447–474. [Google Scholar] [CrossRef]
- Wang, B.; Deng, J.; Donati, V.; Merali, N.; Frampton, A.E.; Giovannetti, E.; Deng, D. The Roles and Interactions of Porphyromonas gingivalis and Fusobacterium nucleatum in Oral and Gastrointestinal Carcinogenesis: A Narrative Review. Pathogens 2024, 13, 93. [Google Scholar] [CrossRef]
- Chamlagain, M.; Hu, J.; Sionov, R.V.; Steinberg, D. Anti-bacterial and anti-biofilm activities of arachidonic acid against the cariogenic bacterium Streptococcus mutans. Front. Microbiol. 2024, 15, 1333274. [Google Scholar] [CrossRef]
- Pallavi, P.; Sahoo, P.P.; Sen, S.K.; Raut, S. Comparative evaluation of anti-biofilm and anti-adherence potential of plant extracts against Streptococcus mutans: A therapeutic approach for oral health. Microb. Pathogen. 2024, 188, 106514. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, K.; Zheng, S.; Wang, Y.; Ren, Q.; Li, H.; Ding, L.; Li, W.; Zhangc, L. Antibacterial Effect of Caffeic Acid Phenethyl Ester on Cariogenic Bacteria and Streptococcus mutans Biofilms. Antimicrob. Agents Chemother. 2020, 64, e00251-20. [Google Scholar] [CrossRef]
- Sehgal, R.; Sharma, A.K.; Singh, B.J.; Saini, R.V.; Saini, A.K.; Beniwal, V. Augmenting the antioxidant, anti-bacterial and anti-carcinogenic potential of Terminalia chebula and Terminalia bellirica after tannin acyl hydrolase mediated biotransformation. Biocatal. Agric. Biotechnol. 2024, 56, 103045. [Google Scholar] [CrossRef]
- Mansour, R.; Abdel-Razeq, H.; Al-Hussaini, M.; Shamieh, O.; Al-Ibraheem, A.; Al-Omari, A.; Mansour, A.H. Systemic Barriers to Optimal Cancer Care in Resource-Limited Countries: Jordanian Healthcare as an Example. Cancers 2024, 16, 1117. [Google Scholar] [CrossRef]
- Bray, F.; Laversannec, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Lin, H.Y.; Park, J.Y. Epidemiology of Cancer. In Anesthesia for Oncological Surgery; Huang, J., Huang, J., Liu, H., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Abdul Aziz, M.Y. Antiproliferative and Apoptosis-Inducing Effects of Ethanolic Extract of Morus Alba Leaves on Human Chronic Myeloid Leukaemia K-562 Cell Lines. Asian J. Med. Biomed. 2024, 8, 163–171. [Google Scholar] [CrossRef]
- Jamaludin, N.A.; Bakar, K.; Saidin, J. In Vitro Biological Activity of Three Marine Sponges From Theonella and Haliclona Genera Collected From Bidong Island, Terengganu, Malaysia. Malays. Appl. Biol. 2023, 52, 51–59. [Google Scholar] [CrossRef]
- Huang, X.; Arjsri, P.; Srisawad, K.; Yodkeeree, S.; Dejkriengkraikul, P. Exploring the Anticancer Potential of Traditional Thai Medicinal Plants: A Focus on Dracaena loureiri and Its Effects on Non-Small-Cell Lung Cancer. Plants 2024, 13, 290. [Google Scholar] [CrossRef]
- Pochechueva, T.V.; Schwenzer, N.; Kohl, T.; Brandenburg, S.; Kaltenecker, G.; Wollnik, B.; Lehnart, S.E. 3D Super-Resolution Nuclear Q-FISH Imaging Reveals Cell-Cycle-Related Telomere Changes. Int. J. Mol. Sci. 2024, 25, 3183. [Google Scholar] [CrossRef]
- Prabhu, K.S.; Kuttikrishnan, S.; Ahmad, N.; Habeeba, U.; Mariyam, Z.; Suleman, M.; Bhat, A.A.; Uddin, S. H2AX: A key player in DNA damage response and a promising target for cancer therapy. Biomed. Pharmacotherap. 2024, 175, 116663. [Google Scholar] [CrossRef]
- Wu, T.; Liu, W.; Chen, H.; Hou, L.; Ren, W.; Zhang, L.; Hu, J.; Chen, H.; Chen, C. Toxoflavin analog D43 exerts antiproliferative effects on breast cancer by inducing ROS-mediated apoptosis and DNA damage. Sci. Rep. 2024, 14, 4008. [Google Scholar] [CrossRef] [PubMed]
- Manzano, J.A.H.; Abellanosa, E.A.; Aguilar, J.P.; Brogi, S.; Yen, C.-H.; Macabeo, A.P.G.; Austriaco, N. Globospiramine from Voacanga globosa Exerts Robust Cytotoxic and Antiproliferative Activities on Cancer Cells by Inducing Caspase-Dependent Apoptosis in A549 Cells and Inhibiting MAPK14 (p38α): In Vitro and Computational Investigations. Cells 2024, 13, 772. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Baban, M.M.; Azzam, A.O.; Issa, J.J.; Ali, A.Y.; AlSuwais, A.K.; Allala, S.; AL Kury, L.T. Allicin and Cancer Hallmarks. Molecules 2024, 29, 1320. [Google Scholar] [CrossRef]
- Drozdowska, M.; Piasna-Słupecka, E.; Such, A.; Dziadek, K.; Krzyściak, P.; Kruk, T.; Duraczyńska, D.; Morawska-Tota, M.; Jamróz, E. Design and In Vitro Activity of Furcellaran/Chitosan Multilayer Microcapsules for the Delivery of Glutathione and Empty Model Multilayer Microcapsules Based on Polysaccharides. Materials 2024, 17, 2047. [Google Scholar] [CrossRef] [PubMed]
- Dye, S.; Mondal, A.; Aash, A.; Mukherjee, R.; Kolay, S.; Murmu, N.; Murmu, N.; Giri, B.; Molla, M.R. Poly-β-thioester-Based Cross-Linked Nanocarrier for Cancer Cell Selectivity over Normal Cells and Cellular Apoptosis by Triggered Release of Parthenolide, an Anticancer Drug. ACS Appl. Bio Mater. 2024, 7, 1214–1228. [Google Scholar] [CrossRef]
- Narayanaswamy, V.; Rah, B.; Al-Omari, I.A.; Kamzin, A.S.; Khurshid, H.; Muhammad, J.S.; Obaidat, I.M.; Issa, B. Evaluation of Antiproliferative Properties of CoMnZn-Fe2O4 Ferrite Nanoparticles in Colorectal Cancer Cells. Pharmaceuticals 2024, 17, 327. [Google Scholar] [CrossRef]
- Wendlocha, D.; Kubina, R.; Krzykawski, K.; Mielczarek-Palacz, A. Selected Flavonols Targeting Cell Death Pathways in Cancer Therapy: The Latest Achievements in Research on Apoptosis, Autophagy, Necroptosis, Pyroptosis, Ferroptosis, and Cuproptosis. Nutrients 2024, 16, 1201. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, H.; Qin, H.; Wang, H.; Wang, H. Norcantharidin Sensitizes Colorectal Cancer Cells to Radiotherapy via Reactive Oxygen Species–DRP1-Mediated Mitochondrial Damage. Antioxidants 2024, 13, 347. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wan, J.; Gao, X.; Wei, Y.; Fang, J.; Shen, B. Versatile Fluorescence Lifetime-Based Copper Probe to Quantify Mitochondrial Membrane Potential and Reveal Its Interaction with Protein Aggregation. Anal. Chem. 2024, 96, 6493–6500. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-J.; Moon, D.-O.; Park, J.-Y.; Kim, N.; Lee, S.H.; Ryu, H.W.; Huh, Y.H.; Lee, H.-S.; Kim, M.-O. Rotundifuran Induces Ferroptotic Cell Death and Mitochondria Permeability Transition in Lung Cancer Cells. Biomedicines 2024, 12, 576. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Xu, J.; Lu, C.; Gao, K.; Hu, Y.; Xue, C.; Yan, X. Nano-flow cytometry unveils mitochondrial permeability transition process and multi-pathway cell death induction for cancer therapy. Cell Death Discov. 2024, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, G.; Alagar Yadav, S. Synergistic inhibitory actions of resveratrol, epigallocatechin-3-gallate, and diallyl trisulfide against skin cancer cell line A431 through mitochondrial caspase dependent pathway: A combinational drug approach. Med. Oncol. 2024, 41, 64. [Google Scholar] [CrossRef]
- Morse, P.T.; Arroum, T.; Wan, J.; Pham, L.; Vaishnav, A.; Bell, J.; Pavelich, L.; Malek, M.H.; Sanderson, T.H.; Edwards, B.F.P.; et al. Phosphorylations and Acetylations of Cytochrome c Control Mitochondrial Respiration, Mitochondrial Membrane Potential, Energy, ROS, and Apoptosis. Cells 2024, 13, 493. [Google Scholar] [CrossRef]
- Nwaechefu, O.; Adeoye, B.; Lateef, I.; Olorunsogo, O. Cajanus cajan induces mitochondrial-mediated apoptosis via caspase activation and cytochrome c release. Comp. Clin. Pathol. 2024, 33, 207–222. [Google Scholar] [CrossRef]
- Zhou, Z.; Arroum, T.; Luo, X.; Kang, R.; Lee, Y.J.; Tang, D.; Hüttemann, M.; Song, X. Diverse functions of cytochrome c in cell death and disease. Cell Death Differ. 2024, 31, 387–404. [Google Scholar] [CrossRef]
- Pezzani, R.; Salehi, B.; Vitalini, S.; Iriti, M.; Zuñiga, F.A.; Sharifi-Rad, J.; Martorell, M.; Martins, N. Synergistic Effects of Plant Derivatives and Conventional Chemotherapeutic Agents: An Update on the Cancer Perspective. Medicina 2019, 55, 110. [Google Scholar] [CrossRef] [PubMed]
- Herranz-López, M.; Losada-Echeberría, M.; Barrajón-Catalán, E. The Multitarget Activity of Natural Extracts on Cancer: Synergy and Xenohormesis. Medicines 2019, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Doxorubicin resistance in breast cancer cells is mediated by extracellular matrix proteins. BMC Cancer 2018, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, L.; Bernards, R. Rational combinations of targeted cancer therapies: Background, advances and challenges. Nat. Rev. Drug Discov. 2023, 22, 213–234. [Google Scholar] [CrossRef] [PubMed]
- Naguib, D.M.; Tantawy, A.A. Anticancer effect of some fruits peels aqueous extracts. Orient. Pharm. Exp. Med. 2019, 19, 415–420. [Google Scholar] [CrossRef]
- Blois, M. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Harbourne, J.B. Phytochemical Methods: A Guide to Modern Technology of Plant Analysis, 2nd ed.; Chapman and Hall: New York, NY, USA, 1973; pp. 88–185. ISBN 978-0-412-57260-9. [Google Scholar]
- Trease, G.E.; Evans, W.C. Phenols and phenolic glycosides. In Trease and Evans Pharmacology and Bikere; Tindall: London, UK, 1996; pp. 832–836. [Google Scholar]
- Pallab, K.; Tapan, B.; Tapas, P.; Ramenc, K. Estimation of total flavonoids content (TPC) and antioxidant activities of methanolic whole plant extract of Biophytum sensitivum Linn. J. Drug Deliv. Ther. 2013, 3, 33–37. Available online: https://pdfs.semanticscholar.org/33e0/a651949abde7f11a31509b4e5906fb1512d7.pdf (accessed on 1 June 2023).
- Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Pakharukova, M.Y.; Samsonov, V.A.; Serbina, E.A.; Mordvinov, V.A. A study of tribendimidine effects in vitro and in vivo on the liver fluke Opisthorchis felineus. Parasit. Vectors 2019, 12, 23. [Google Scholar] [CrossRef]
- Akendengue, B.; Ngou-Milama, E.; Roblot, F.; Laurens, A.; Hocquemiller, R.; Grellier, P.; Frappier, F. Antiplasmodial activity of Uvaria klaineana. Planta Med. 2002, 68, 167–169. [Google Scholar] [CrossRef]
- Mbongo, N.; Loiseau, P.; Lawrence, F.; Bories, C.; Craciunescu, D.G.; Robert-Gero, M. In vitro sensitivity of Leishmania donovani to organometallic derivatives of pentamidine. Parasitol. Res. 1997, 83, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, T.; Sharma, A.; Akhterc, J.; Pathania, R. The small molecule IITR08027 restores the antibacterial activity of fluoro-quinolones against multidrug-resistant Acinetobacter baumannii by efflux inhibition. Int. J. Antimicrob. Agents 2017, 50, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Diao, W.-R.; Hu, Q.-P.; Zhang, H.; Xu, J.-G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Sandasi, M.; Leonard, C.M.; Viljoen, A.M. The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes. Lett. Appl. Microbiol. 2010, 50, 30–35. [Google Scholar] [CrossRef]
- Byrne, F.; Prina-Mello, A.; Whelan, A.; Mohamed, B.M.; Davies, A.; Gun’ko, Y.K.; Coey, J.M.D.; Volkov, Y. High content analysis of the biocompatibility of nickel nanowires. J. Magn. Magn. Mater. 2009, 321, 1341–1345. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).