Diclofenac Interacts with Photosynthetic Apparatus: Isolated Spinach Chloroplasts and Thylakoids as a Model System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Buffers
2.2. Chloroplast Isolation
2.3. Thylakoid Preparation
2.4. Exposure Experiments
2.5. Chlorophyll a Fluorescence Measurements (the OJIP Test)
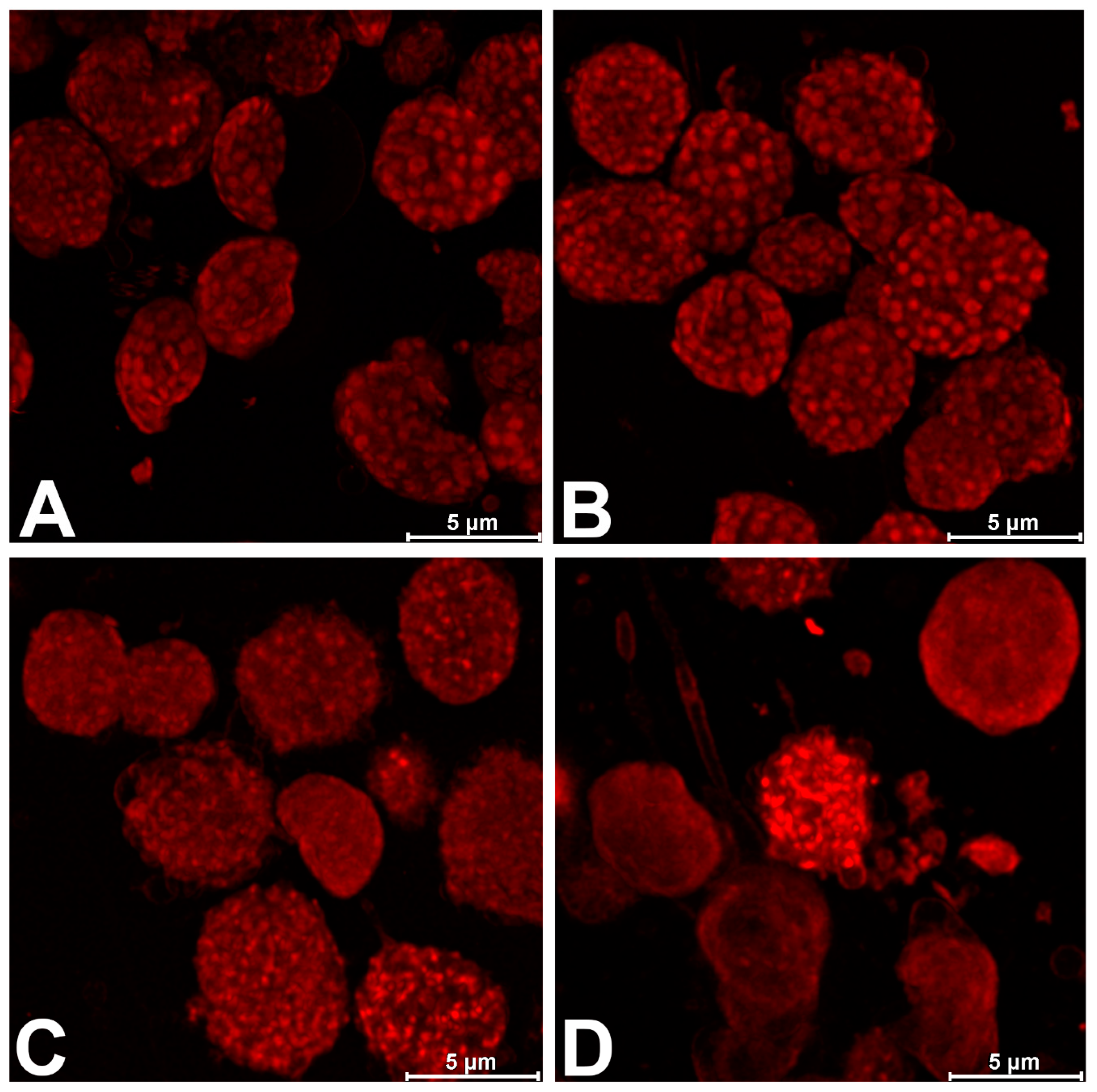
2.6. CLSM and Image Processing
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hejna, M.; Kapuścińska, D.; Aksmann, A. Pharmaceuticals in the aquatic environment: A review on eco-toxicology and the remediation potential of algae. Int. J. Environ. Res. Public Health 2022, 19, 7717. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, P.; Meena, R.A.A.; Palanisami, T.; Ashokkumar, V.; Palvannan, T.; Gu, F.L. Occurrence, interactive effects and ecological risk of diclofenac in environmental compartments and biota—A review. Sci. Total Environ. 2020, 698, 134057. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Dodgen, L.K.; Conkle, J.L.; Gan, J. Plant uptake of pharmaceutical and personal care products from recycled water and biosolids: A review. Sci. Total Environ. 2015, 536, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.K.; Collins, T.J. Human pharmaceuticals in the aquatic environment: A challenge to green chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Paoli, L.; Vichi, M.; Bačkor, M.; Bačkorová, M.; Loppi, S. Toxicity of Diclofenac in the Fern Azolla filiculoides and the Lichen Xanthoria parietina. Bull. Environ. Contam. Toxicol. 2018, 100, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity—A review. Int. J. Environ. 2016, 96, 127–138. [Google Scholar] [CrossRef]
- Gan, T.J. Diclofenac: An update on its mechanism of action and safety profile. Curr. Med. Res. Opin. 2010, 26, 1715–1731. [Google Scholar] [CrossRef]
- Hammad, H.M.; Zia, F.; Bakhat, H.F.; Fahad, S.; Ashraf, M.R.; Wilkerson, C.J.; Shah, G.M.; Nasim, W.; Khosa, I.; Shahid, M. Uptake and toxicological effects of pharmaceutical active compounds on maize. Agric. Ecosyst. Environ. 2018, 258, 143–148. [Google Scholar] [CrossRef]
- Hájková, M.; Kummerová, M.; Zezulka, Š.; Babula, P.; Váczi, P. Diclofenac as an environmental threat: Impact on the photosynthetic processes of Lemna minor chloroplasts. Chemosphere 2019, 224, 892–899. [Google Scholar] [CrossRef]
- Kummerová, M.; Zezulka, Š.; Babula, P.; Tříska, J. Possible ecological risk of two pharmaceuticals diclofenac and paracetamol demonstrated on a model plant Lemna minor. J. Hazard. Mater. 2016, 302, 351–361. [Google Scholar] [CrossRef]
- Harshkova, D.; Majewska, M.; Pokora, W.; Baścik-Remisiewicz, A.; Tułodziecki, S.; Aksmann, A. Diclofenac and atrazine restrict the growth of a synchronous Chlamydomonas reinhardtii population via various mechanisms. Aquat. Toxicol. 2021, 230, 105698. [Google Scholar] [CrossRef] [PubMed]
- Harshkova, D.; Zielińska, E.; Aksmann, A. Optimization of a microplate reader method for the analysis of changes in mitochondrial membrane potential in Chlamydomonas reinhardtii cells using the fluorochrome JC-1. J. Appl. Phycol. 2019, 31, 3691–3697. [Google Scholar] [CrossRef]
- Majewska, M.; Harshkova, D.; Pokora, W.; Baścik-Remisiewicz, A.; Tułodziecki, S.; Aksmann, A. Does diclofenac act like a photosynthetic herbicide on green algae? Chlamydomonas reinhardtii synchronous culture-based study with atrazine as reference. Ecotoxicol. Environ. Saf. 2021, 208, 111630. [Google Scholar] [CrossRef]
- Opriș, O.; Lung, I.; Soran, M.L.; Ciorîță, A.; Copolovici, L. Investigating the effects of non-steroidal anti-inflammatory drugs (NSAIDs) on the composition and ultrastructure of green leafy vegetables with important nutritional values. Plant Physiol. Biochem. 2020, 151, 342–351. [Google Scholar] [CrossRef]
- Cummings, B.M.; Needoba, J.A.; Peterson, T.D. Effect of metformin exposure on growth and photosynthetic performance in the unicellular freshwater chlorophyte, Chlorella vulgaris. PLoS ONE 2018, 13, e0207041. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Y.; Nie, X.P.; Liu, W.Q.; Snoeijs, P.; Guan, C.; Tsui, M.T. Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole on photosynthetic apparatus in Selenastrum capricornutum. Ecotoxicol. Environ. Saf. 2011, 74, 1027–1035. [Google Scholar] [CrossRef]
- Wang, H.; Jin, M.; Mao, W.; Chen, C.; Fu, L.; Li, Z.; Du, S.; Liu, H. Photosynthetic toxicity of non-steroidal anti-inflammatory drugs (NSAIDs) on green algae Scenedesmus obliquus. Sci. Total Environ. 2020, 707, 136176. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.; Harshkova, D.; Guściora, M.; Aksmann, A. Phytotoxic activity of diclofenac: Evaluation using a model green alga Chlamydomonas reinhardtii with atrazine as a reference substance. Chemosphere 2018, 209, 989–997. [Google Scholar] [CrossRef]
- Chadee, A.; Alber, N.A.; Dahal, K.; Vanlerberghe, G.C. The complementary roles of chloroplast cyclic electron transport and mitochondrial alternative oxidase to ensure photosynthetic performance. Front. Plant Sci. 2021, 12, 748204. [Google Scholar] [CrossRef]
- Kromer, S. Respiration during photosynthesis. Annu. Rev. Plant Biol. 2003, 46, 45–70. [Google Scholar] [CrossRef]
- Raghavendra, A.S.; Padmasree, K. Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci. 2003, 8, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Berthold, D.A.; Babcock, G.T.; Yocum, C.F. A highly resolved, oxygen-evolving photosystem II preparation from spinach thylakoid membranes. FEBS Lett. 1981, 134, 231–234. [Google Scholar] [CrossRef]
- Weis, E. Influence of light on the heat sensitivity of the photosynthetic apparatus in isolated spinach chloroplasts. Plant Physiol. 1982, 70, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Walker, D. The Use of the Oxygen Electrode and Fluorescence Probes in Simple Measurements of Photosynthesis; Research Institute for Photosynthesis, University of Sheffield: Sheffield, UK, 1987. [Google Scholar]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant 2016, 38, 102. [Google Scholar] [CrossRef]
- Strassert, R.J.; Srivastava, A. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Spinach chloroplasts scavenge hydrogen peroxide on illumination. Plant Cell Physiol. 1980, 21, 1295–1307. [Google Scholar] [CrossRef]
- Joly, D.; Carpentier, R. Rapid isolation of intact chloroplasts from spinach leaves. Methods Mol. Biol. 2011, 684, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Khorobrykh, S.A.; Ivanov, B.N. Oxygen reduction in a plastoquinone pool of isolated pea thylakoids. Photosynth. Res. 2002, 71, 209–219. [Google Scholar] [CrossRef]
- Aksmann, A.; Pokora, W.; Baścik-Remisiewicz, A.; Dettlaff-Pokora, A.; Tukaj, Z. High hydrogen peroxide production and antioxidative enzymes expression in the Chlamydomonas reinhardtii cia3 mutant with an increased tolerance to cadmium and anthracene. Physiol. Res. 2016, 64, 300–311. [Google Scholar] [CrossRef]
- Aksmann, A.; Tukaj, Z. Intact anthracene inhibits photosynthesis in algal cells: A fluorescence induction study on Chlamydomonas reinhardtii cw92 strain. Chemosphere 2008, 74, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hamada, C. Statistical analysis for toxicity studies. J. Toxicol. Pathol. 2018, 31, 15–22. [Google Scholar] [CrossRef]
- Gwanyanya, A.; MacIanskiene, R.; Mubagwa, K. Insights into the effects of diclofenac and other non-steroidal anti-inflammatory agents on ion channels. J. Pharm. Pharmacol. 2012, 64, 1359–1375. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Mohan, K.; Meena, R.A.A.; Balasubramanian, M.; Chitra, L.; Ganesan, A.R.; Palvannan, T.; Brar, S.K.; Gu, F.L. Hazardous impact of diclofenac on mammalian system: Mitigation strategy through green remediation approach. J. Hazard. Mater. 2021, 419, 126135. [Google Scholar] [CrossRef]
- Alkimin, G.D.; Daniel, D.; Dionísio, R.; Soares, A.M.; Barata, C.; Nunes, B. Effects of diclofenac and salicylic acid exposure on Lemna minor: Is time a factor? Environ. Res. 2019, 177, 108609. [Google Scholar] [CrossRef]
- Siemieniuk, A.; Ludynia, M.; Rudnicka, M. Response of two crop plants, Zea mays L. and Solanum lycopersicum L., to diclofenac and naproxen. Int. J. Mol. Sci. 2021, 22, 8856. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.H.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Haz. Mat. 2010, 175, 45–95. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Boxall, A.; Selby, K. Do pharmaceuticals pose a threat to primary producers? Crit. Rev. Environ. Sci. Technol. 2015, 45, 2565–2610. [Google Scholar] [CrossRef]
- Kaye, Y.; Huang, W.; Clowez, S.; Saroussi, S.; Idoine, A.; Sanz-Luque, E.; Grossmann, A.R. The mitochondrial alternative oxidase from Chlamydomonas reinhardtii enables survival in high light. J. Biol. Chem. 2019, 294, 1380–1395. [Google Scholar] [CrossRef]
- Cleuvers, M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol. Lett. 2003, 142, 185–194. [Google Scholar] [CrossRef]
- Cleuvers, M. Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol. Environ. Saf. 2004, 59, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.; Lúcio, M.; Lima, J.L.; Matos, C.; Reis, S. Effects of diclofenac on EPC liposome membrane properties. Anal. Bioanal. Chem. 2005, 382, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Giraud, M.N.; Motta, C.; Romero, J.J.; Bommelaer, G.; Lichtenberger, L.M. Interaction of indomethacin and naproxen with gastric surface-active phospholipids: A possible mechanism for the gastric toxicity of nonsteroidal anti-inflammatory drugs (NSAIDs). Biochem. Pharmacol. 1999, 57, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Tomisato, W.; Tanaka, K.I.; Katsu, T.; Kakuta, H.; Sasaki, K.; Tsutsumi, S.; Hoshino, T.; Aburaya, M.; Li, D.; Tsuchiya, T.; et al. Membrane permeabilization by non-steroidal anti-inflammatory drugs. Biochem. Biophys. Res. Commun. 2004, 323, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J.; Williams, W.P. Environmentally induced changes in chloroplast membranes and their effects on photosynthetic function. In Photosynthetic Mechanisms and the Environment; Barber, J., Baker, N.R., Eds.; Elsevier Science Publishers Company: Amsterdam, The Netherlands; New York, NY, USA; Oxford, UK, 1985; Volume 6, pp. 1–47. [Google Scholar]
- Sato, N.; Hagio, M.; Wada, H.; Tsuzuki, M. Requirement of phosphatidylglycerol for photosynthetic function in thylakoid membranes. Proc. Natl. Acad. Sci. USA 2000, 97, 10655–10660. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wang, H.; Ki, J.S. Chloroacetanilides inhibit photosynthesis and disrupt the thylakoid membranes of the dinoflagellate Prorocentrum minimum as revealed with metazachlor treatment. Ecotoxicol. Environ. Saf. 2021, 211, 111928. [Google Scholar] [CrossRef]
- Chauhan, J.; Prathibha, M.D.; Singh, P.; Choyal, P.; Mishra, U.N.; Saha, D.; Kumar, R.; Anuragi, H.; Pandey, S.; Bose, B.; et al. Plant photosynthesis under abiotic stresses: Damages, adaptive, and signaling mechanisms. Plant Stress 2023, 10, 100296. [Google Scholar] [CrossRef]
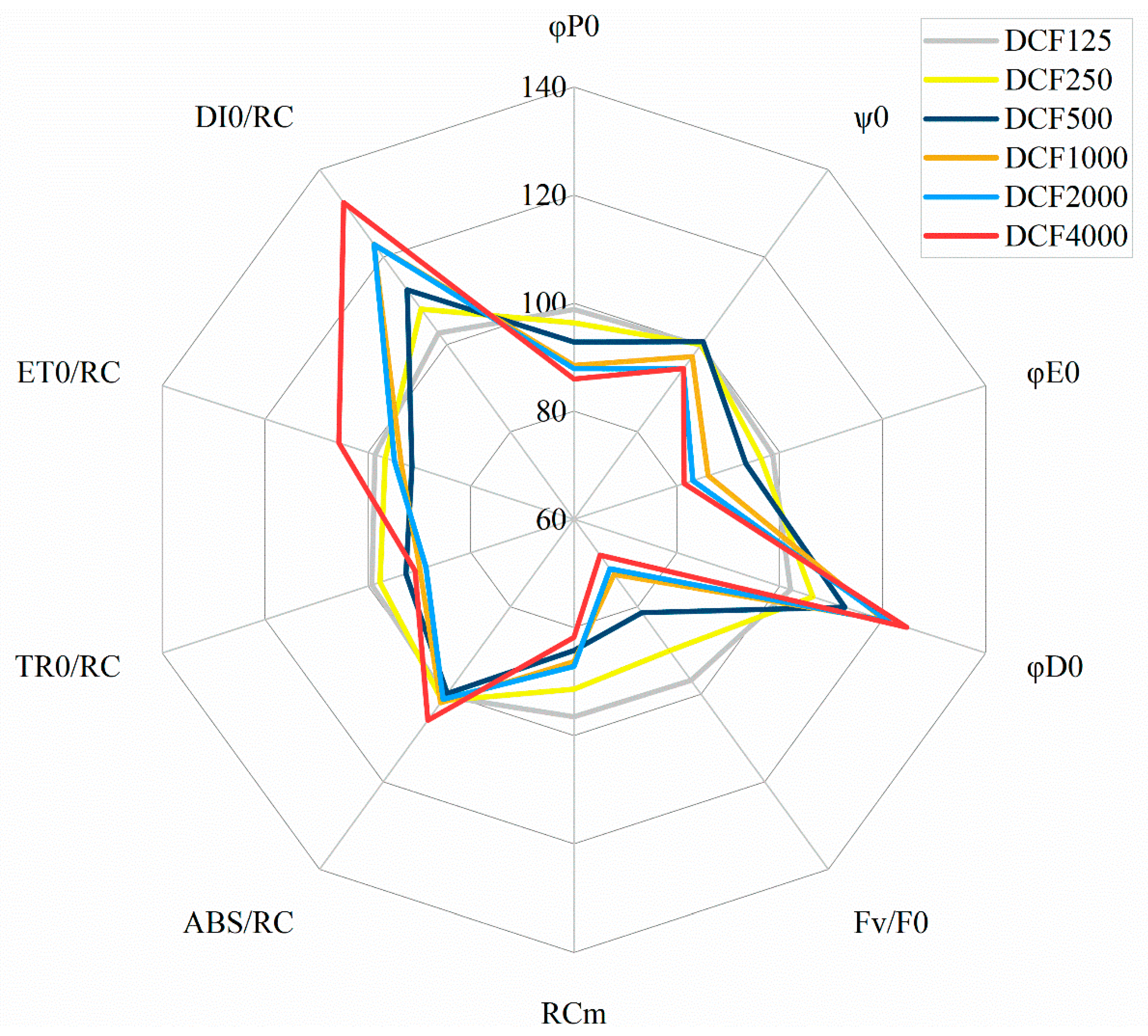

| φP0 | ѱ0 | φE0 | φD0 | ABS/RC | TR0/RC | ET0/RC | DI0/RC | FV/F0 | RCM | |
|---|---|---|---|---|---|---|---|---|---|---|
| C | 0.64 ± 0.006 | 0.71 ± 0.004 | 0.45 ± 0.004 | 0.36 ± 0.006 | 2.39 ± 0.032 | 1.52 ± 0.021 | 0.60 ± 0.012 | 0.87 ± 0.019 | 1.91 ± 0.047 | 637 ± 15.21 |
| DCF125 | 0.63 ± 0.005 | 0.71 ± 0.003 | 0.44 ± 0.004 | 0.37 ± 0.005 | 2.40 ± 0.013 | 1.51 ± 0.007 | 0.59 ± 0.008 | 0.89 ± 0.017 | 1.85 ± 0.044 | 615 ± 13.08 |
| DCF250 | 0.61 * ± 0.008 | 0.71 ± 0.004 | 0.43 ± 0.007 | 0.39 * ± 0.008 | 2.43 ± 0.027 | 1.49 ± 0.012 | 0.58 ± 0.010 | 0.94 ± 0.029 | 1.72 * ± 0.060 | 582 * ± 17.62 |
| DCF500 | 0.59 * ± 0.008 | 0.71 ± 0.004 | 0.42 * ± 0.006 | 0.41 * ± 0.008 | 2.39 ± 0.020 | 1.41 * ± 0.018 | 0.55 * ± 0.012 | 0.98 * ± 0.023 | 1.55 * ± 0.051 | 537 * ± 16.08 |
| DCF1000 | 0.56 * ± 0.008 | 0.69 ± 0.008 | 0.39 * ± 0.009 | 0.44 * ± 0.008 | 2.44 ± 0.045 | 1.37 * ± 0.015 | 0.56 ± 0.014 | 1.07 * ± 0.038 | 1.38 * ± 0.044 | 550 * ± 11.62 |
| DCF2000 | 0.56 * ± 0.006 | 0.67 * ± 0.014 | 0.37 * ± 0.012 | 0.44 * ± 0.006 | 2.42 ± 0.039 | 1.35 * ± 0.012 | 0.57 ± 0.014 | 1.07 * ± 0.031 | 1.36 * ± 0.033 | 555 * ± 9018 |
| DCF4000 | 0.55 * ± 0.009 | 0.67 * ± 0.013 | 0.37 * ± 0.012 | 0.45 * ± 0.009 | 2.53 * ± 0.044 | 1.38 * ± 0.011 | 0.64 ± 0.012 | 1.15 * ± 0.041 | 1.30 * ± 0.046 | 521 * ± 7.88 |
| φP0 | ѱ0 | φE0 | φD0 | ABS/RC | TR0/RC | ET0/RC | DI0/RC | FV/F0 | RCM | |
|---|---|---|---|---|---|---|---|---|---|---|
| C | 0.69 ± 0.011 | 0.59 ± 0.007 | 0.41 ± 0.009 | 0.31 ± 0.011 | 2.05 ± 0.050 | 1.41 ± 0.015 | 0.58 ± 0.015 | 0.64 ± 0.038 | 2.53 ± 0.139 | 914 ± 52.18 |
| DCF1000 | 0.65 ± 0.016 | 0.56 * ± 0.009 | 0.36 * ± 0.012 | 0.35 ± 0.016 | 2.36 * ± 0.094 | 1.53 * ± 0.024 | 0.68 * ± 0.024 | 0.83 * ± 0.071 | 2.16 ± 0.157 | 729 ± 63.68 |
| DCF2000 | 0.56 * ± 0.023 | 0.52 * ± 0.011 | 0.29 * ± 0.018 | 0.44 * ± 0.023 | 3.10 * ± 0.173 | 1.72 * ± 0.025 | 0.79 * ± 0.020 | 1.38 * ± 0.150 | 1.50 * ± 0.138 | 448 * ± 47.46 |
| DCF4000 | 0.23 * ± 0.020 | 0.41 * ± 0.020 | 0.09 * ± 0.012 | 0.77 * ± 0.020 | 9.13 * ± 0.761 | 1.98 * ± 0.041 | 0.80 * ± 0.043 | 7.15 * ± 0.772 | 0.33 * ± 0.036 | 85 * ± 9.065 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewska, M.; Kapusta, M.; Aksmann, A. Diclofenac Interacts with Photosynthetic Apparatus: Isolated Spinach Chloroplasts and Thylakoids as a Model System. Plants 2024, 13, 2189. https://doi.org/10.3390/plants13162189
Majewska M, Kapusta M, Aksmann A. Diclofenac Interacts with Photosynthetic Apparatus: Isolated Spinach Chloroplasts and Thylakoids as a Model System. Plants. 2024; 13(16):2189. https://doi.org/10.3390/plants13162189
Chicago/Turabian StyleMajewska, Monika, Małgorzata Kapusta, and Anna Aksmann. 2024. "Diclofenac Interacts with Photosynthetic Apparatus: Isolated Spinach Chloroplasts and Thylakoids as a Model System" Plants 13, no. 16: 2189. https://doi.org/10.3390/plants13162189






