Germination Promotes Flavonoid Accumulation of Finger Millet (Eleusine coracana L.): Response Surface Optimization and Investigation of Accumulation Mechanism
Abstract
:1. Introduction
2. Results
2.1. Optimum of Germination Conditions by RSM
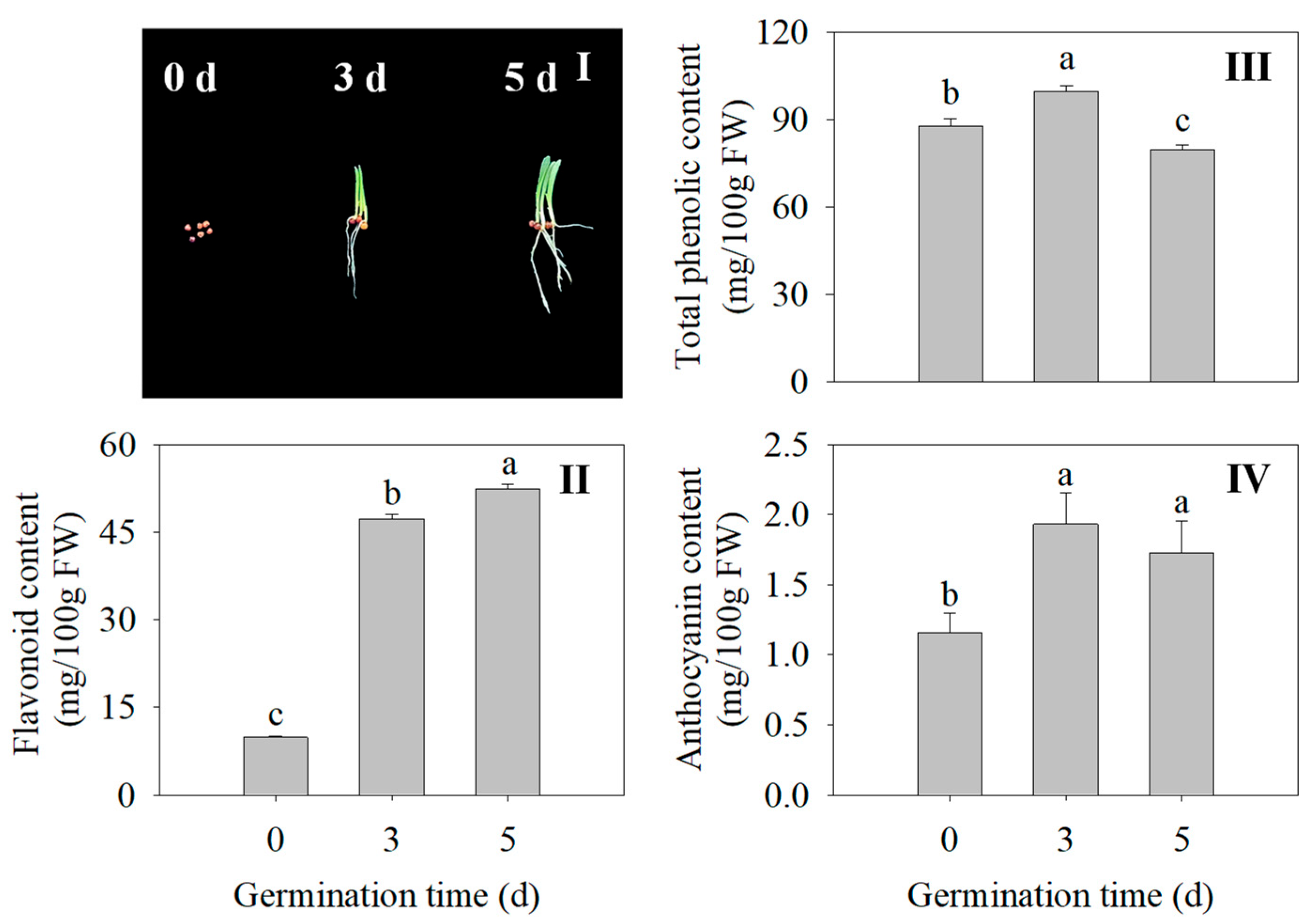
2.2. Effects of Germination Time on Morphology, Flavonoid Content, Total Phenolic Content, and Anthocyanin Content in Finger Millet Sprouts
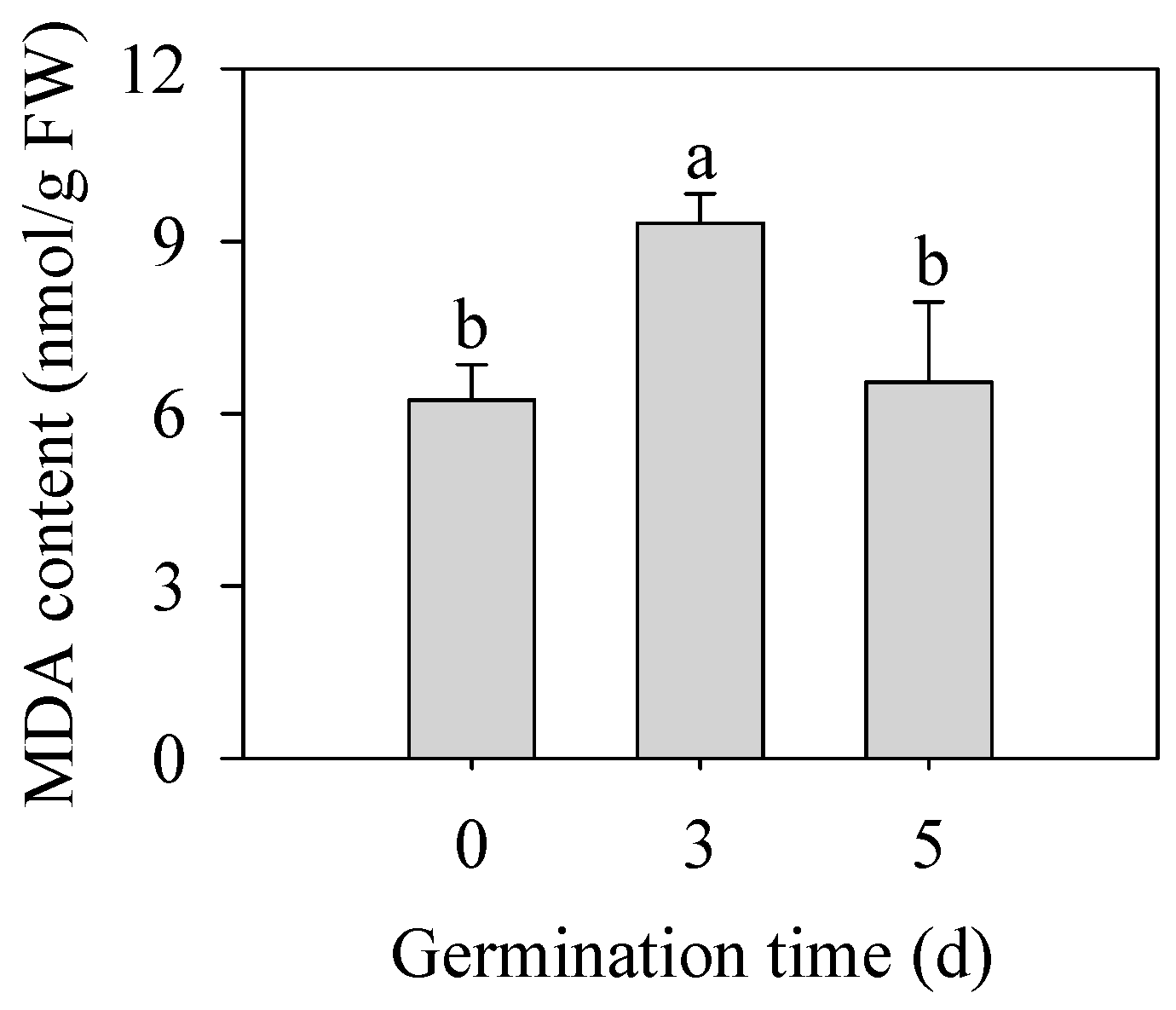
2.3. Effect of Germination Time on the Malondialdehyde (MDA) Content in Finger Mibbllet Sprouts
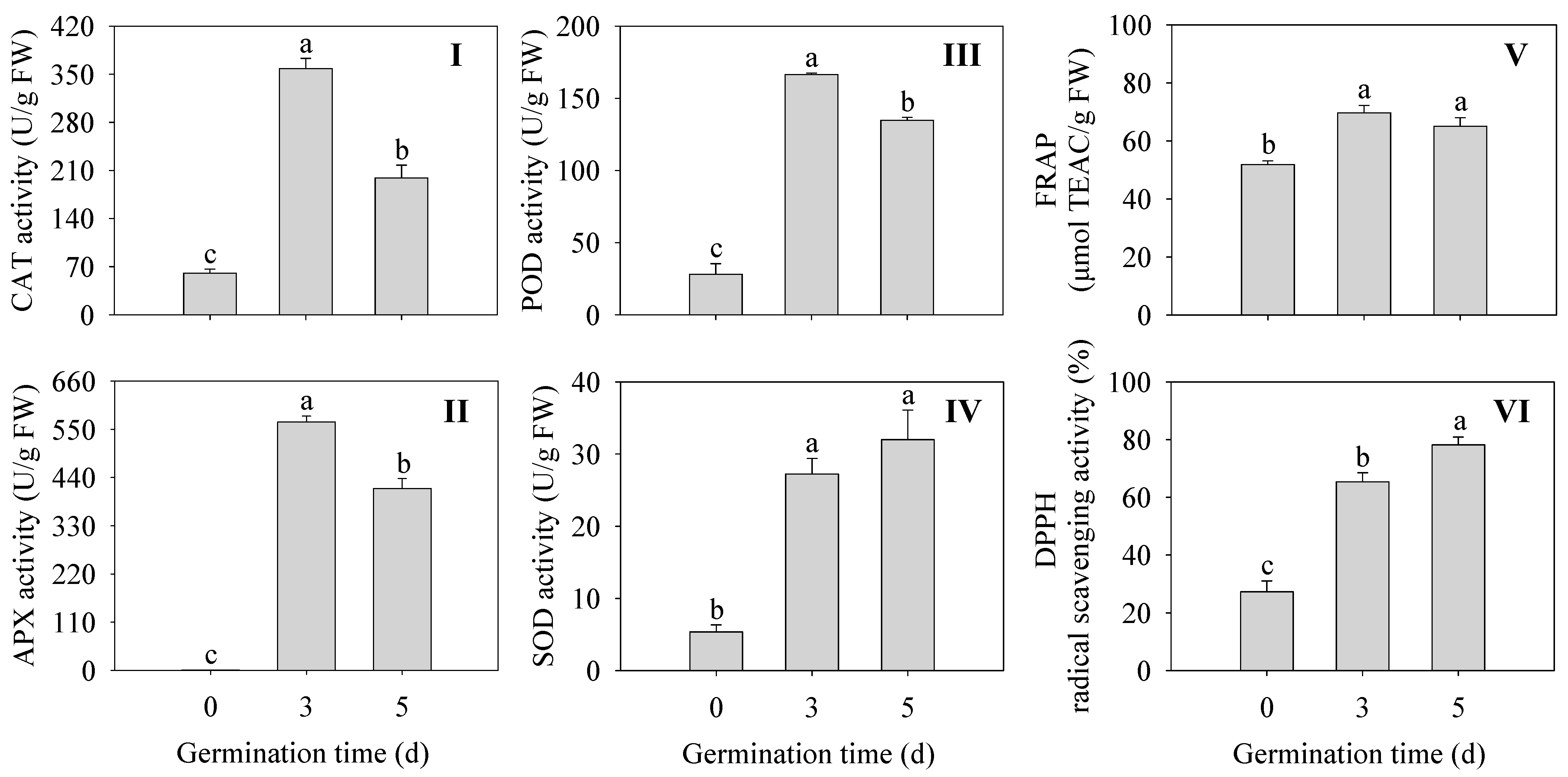
2.4. Effects of Germination Time on the Antioxidant Enzyme Activity and Antioxidant Capacity in Finger Millet Sprouts
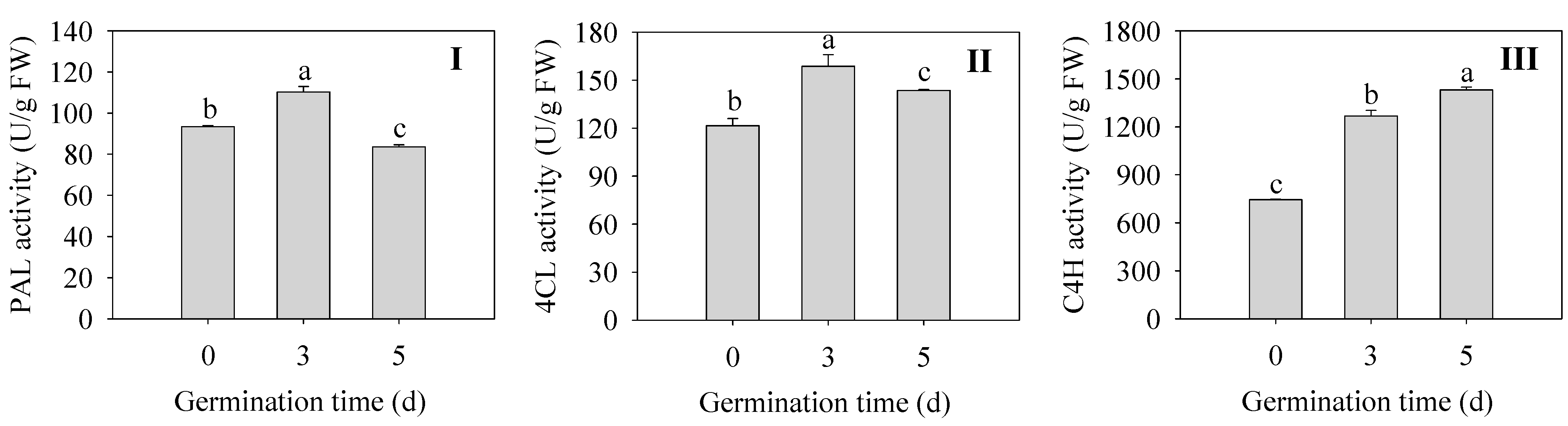
2.5. Effects of Germination Time on Flavonoid Synthetase Activities in Finger Millet Sprouts
2.6. Changes of Relative Expression Levels of Genes of Key Enzymes for Antioxidant Oxidase and Flavonoid Synthesis in Finger Millet Sprouts
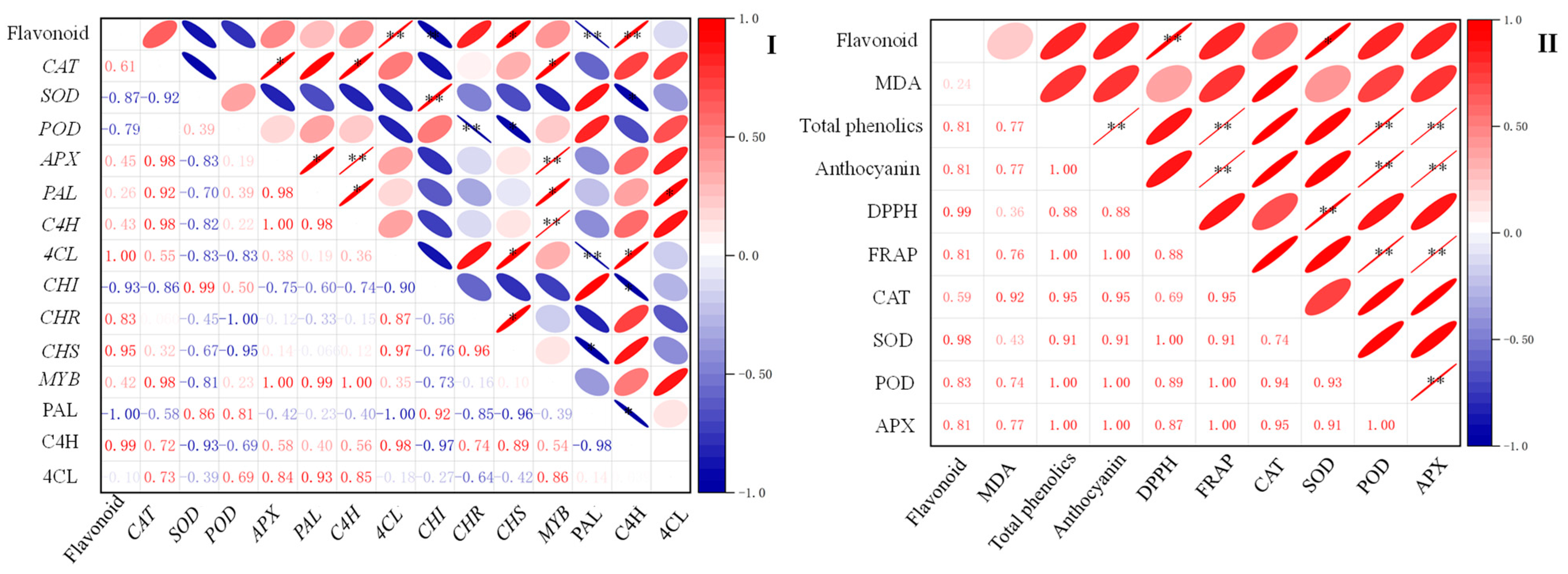
2.7. Correlation between Flavonoid Content and Other Indexes of Finger Millet during Germination
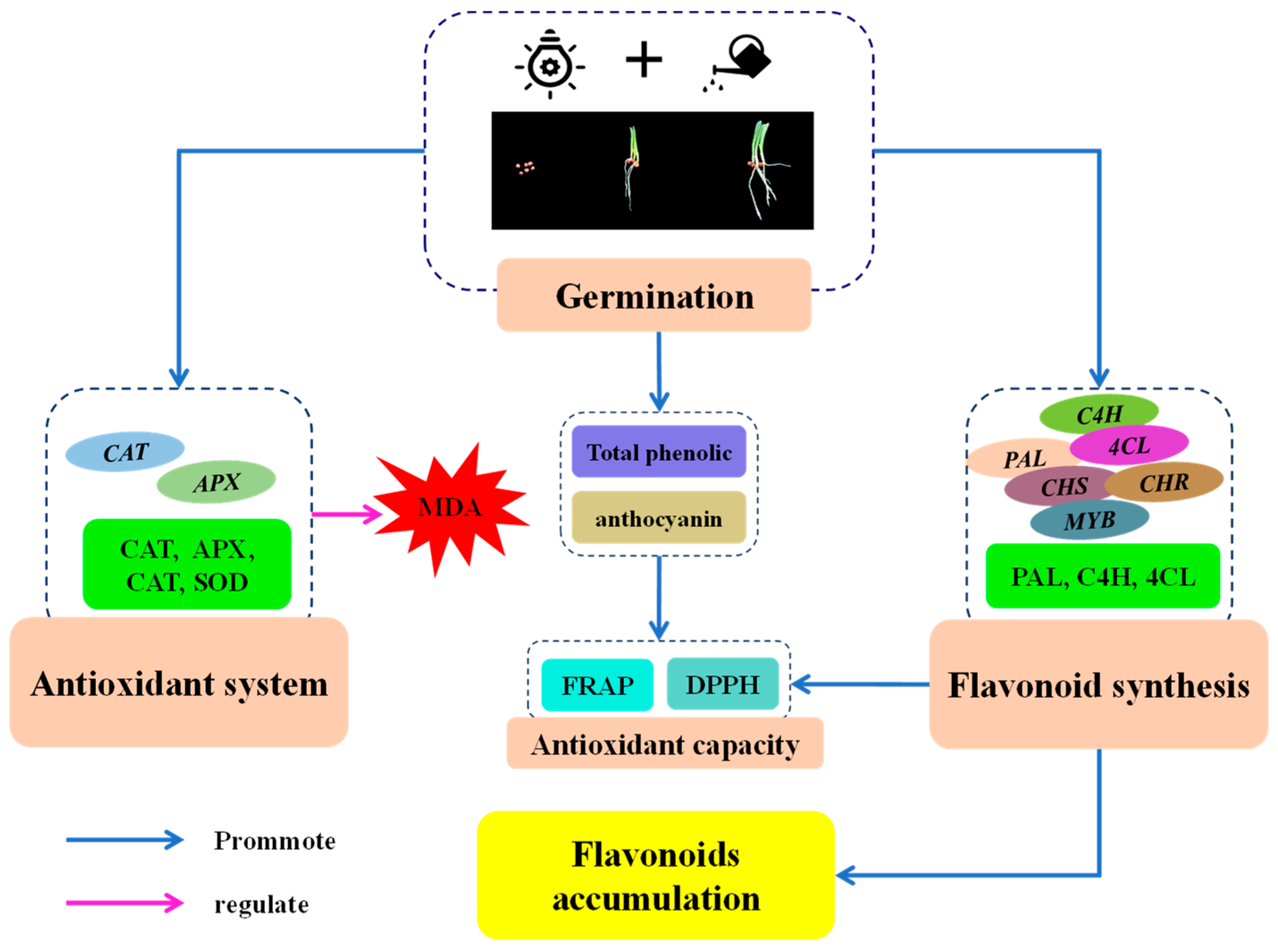
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Cultivation Conditions
4.2. Optimization of Germination Conditions Using RSM
4.3. Extraction and Determination of Flavonoids
4.4. Determination of the Content of Total Phenolic and Anthocyanins
4.5. Determination of the Content of Malondialdehyde
4.6. Determination of Antioxidant Activity
4.7. Determination of Antioxidant Enzyme Activity and Flavonoid Synthetase Activity
4.8. Determination of Relative Gene Expression Levels
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chandra, D.; Satish Chandra, P.; Sharma, A.K. Review of Finger millet [Eleusine coracana (L.) Gaertn)]: A power house of health benefiting nutrients. Food Sci. Hum. Wellness 2016, 5, 149–155. [Google Scholar] [CrossRef]
- Sood, S.K.; Arunava, L.P. Finger Millet [Eleusine coracana (L.) Gaertn.]: A Minor Crop for Sustainable Food and Nutritional Security. Asian J. Chem. 2017, 29, 707–710. [Google Scholar] [CrossRef]
- Shobana, S.; Krishnaswamy, K.; Sudha, V.; Malleshi, N.G.; Anjana, R.M.; Palaniappan, L.; Mohan, V. Finger Millet (Ragi, Eleusine coracana L.): A Review of Its Nutritional Properties, Processing, and Plausible Health Benefits. Adv. Food Nutr. Res. 2013, 69, 1–39. [Google Scholar] [PubMed]
- Ramashia, S.E.; Tonna, A.A.; Eastonce, T.G.; Stephen, M.; Afam, I.O. Processing, nutritional composition and health benefits of finger millet in sub-saharan Africa. J. Food Sci. Technol. 2019, 39, 253–266. [Google Scholar] [CrossRef]
- Mbithi-Mwikya, S.; Camp, J.V.; Yiru, Y.; Huyghebaert, A. Nutrient and antinutrient changes in finger millet (Eleusine coracan) during sprouting. Food Sci. Technol. 2000, 33, 9–14. [Google Scholar] [CrossRef]
- Yenasew, A.; Kelebessa, U. Effect of germination period on physiochemical properties of elite finger millet varieties. Cogent Food Agric. 2022, 8, 2093045. [Google Scholar] [CrossRef]
- Owheruo, J.O.; Ifesan, B.O.; Kolawole, A.O. Physicochemical properties of malted finger millet (Eleusine coracana) and pearl millet (Pennisetum glaucum). J. Food Sci. Nutr. 2019, 7, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Pushparaj, F.S.; Urooj, A. Influence of Processing on Dietary Fiber, Tannin and Protein Digestibility of Pearl Millet. Food Nutr. Sci. 2011, 2, 895–900. [Google Scholar]
- Yin, Y.Q.; Hu, M.X.; Yang, Z.F.; Zhu, J.Y.; Fang, W.M. Salicylic acid promotes phenolic acid biosynthesis for the production of phenol acid-rich barley sprouts. J. Sci. Food Agric. 2024, 104, 5350–5359. [Google Scholar] [CrossRef]
- Huang, C.M.; Quan, X.L.; Yin, Y.Q.; Ding, X.L.; Yang, Z.F.; Zhu, J.Y.; Fang, W.M. Enrichment of Flavonoids in Short-Germinated Black Soybeans (Glycine max L.) Induced by Slight Acid Treatment. Foods 2024, 13, 868. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Xiang, J.; Zhang, J.; Johnson, J.B.; Xu, Y.; Trust, B. Accumulation of γ-aminobutyric acid and modifications of phenolic profiles and antioxidant capacity of foxtail millets during germination. J. Cereal Sci. 2023, 114, 103815. [Google Scholar] [CrossRef]
- Yenasew, A.; Urga, K. Effect of the germination period on functional properties of finger millet flour and sensorial quality of porridge. J. Food Sci. Nutr. 2023, 11, 2336–2343. [Google Scholar] [CrossRef] [PubMed]
- Hithamani, G.; Srinivasan, K. Effect of domestic processing on the polyphenol content and bioaccessibility in finger millet (Eleusine coracana) and pearl millet (Pennisetum glaucum). Food Chem. 2014, 164, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, V.; Jaspreet, K.; Prasad, R.; Sawinder, K.; Jyoti, S.; Ankit, K.; Ashwani, K.; Nitya, S.; Chandra, M.; Mehta, A.; et al. Nutritional significance of finger millet and its potential for using in functional products. Foods Raw Mater. 2024, 12, 110–123. [Google Scholar] [CrossRef]
- Peng, W.; Dong, Y.; Wang, J.; Wang, S.; Wang, N. Effects of exogenous solution treatment on germination, antioxidation and flavonoid biosynthesis of Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn.). Food Biosci. 2023, 56, 103367. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Q.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Ortiz, F.A.; Munoz-Llande, C.B.; Martinez-Villaluenga, C. Time maters: Exploring the dynamics of bioactive compounds content, bioaccessibility and antioxidant activity during (Lupinus angustifolius) germination. Food Res. Int. 2024, 187, 114426. [Google Scholar] [CrossRef] [PubMed]
- Perales-Sánchez, J.X.; Reyes-Moreno, C.; Gómez-Favela, M.A.; Milán-Carrillo, J.; Cuevas-Rodríguez, E.; Valdez-Ortiz, A.; Gutiérrez-Dorado, R. Increasing the Antioxidant Activity, Total Phenolic and Flavonoid Contents by Optimizing the Germination Conditions of Amaranth Seeds. Plant Food Hum. Nutr. 2014, 69, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Kruma, Z.; Tatjana, K.; Ruta, G.; Lolita, T.; Evita, S.; Martins, S.; Ievina, S.; Arta, K. Influence of Germination Temperature and Time on Phenolic Content and Antioxidant Properties of Cereals. In Proceedings of the 13th Baltic Conference on Food Science and Technology “FOOD. NUTRITION. WELL-BEING”, Jelgava, Latvia, 2–3 May 2019. [Google Scholar]
- Dey, S.; Saxena, A.; Kumar, Y.; Maity, T.; Tarafdar, A. Optimizing the Effect of Ultrasonication and Germination on Antinutrients and Antioxidants of Kodo (Paspalum Scrobiculatum) and Little (Panicum Sumatrense) Millets. J. Food Sci. Technol. 2023, 60, 2990–3001. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, N.; Wang, S.; Wang, J.; Peng, W. A review: The nutrition components, active substances and flavonoid accumulation of Tartary buckwheat sprouts and innovative physical technology for seeds germinating. Front. Nutr. 2023, 10, 1168361. [Google Scholar] [CrossRef]
- Islam, M.Z.; Park, B.; Jeong, S.; Kang, S.W.; Shin, B.K.; Lee, Y.T. Assessment of biochemical compounds and antioxidant enzyme activity in barley and wheatgrass under water-deficit condition. J. Sci. Food Agric. 2022, 102, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.A.; Semenova, N.A.; Dorokhov, A.S.; Proshkin, Y.A.; Godyaeva, M.M.; Vodeneev, V.; Sukhov, V.; Panchenko, V.; Chilingaryan, N.O. Influence of pulsed, scanning and constant (16- and 24-h) modes of LED irradiation on the physiological, biochemical and morphometric parameters of lettuce plants (Lactuca sativa L.) while cultivated in vertical farms. Agriculture 2022, 12, 1998. [Google Scholar] [CrossRef]
- Rawat, M.; Aditi, V.; Muskan, R.; Aniket, C.; Arvandana, L.P.; Akanksha, J.; Anjali, B. A comprehensive review on nutraceutical potential of underutilized cereals and cereal-based products. J. Agric. Food Res. 2023, 12, 100619. [Google Scholar] [CrossRef]
- Pasternak, T.; Palme, K.; Pérez-Pérez, J.M. Role of reactive oxygen species in the modulation of auxin flux and root development in Arabidopsis thaliana. Plant J. 2023, 114, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T.; Whiteman, M. Harnessing Evolutionary Toxins for Signaling: Reactive Oxygen Species, Nitric Oxide and Hydrogen Sulfide in Plant Cell Regulation. Front. Plant Sci. 2017, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, T.; Perez-Perez, J.M. Optimization of ROS Measurement and Localization in Plant Tissues: Challenges and Solutions; Protocols.io: Berkeley, CA, USA, 2021. [Google Scholar] [CrossRef]
- Xie, C.; Wang, P.; Gu, Z.X.; Yang, R.Q. Spermidine alleviates oxidative damage and enhances phenolic compounds accumulation in barley seedlings under UV-B stress. J. Sci. Food Agric. 2023, 103, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Udeh, H.O.; Duodu, K.G.; Jideani, A.I. Malting Period Effect on the Phenolic Composition and Antioxidant Activity of Finger Millet (Eleusine coracana L. Gaertn) Flour. Molecules 2018, 23, 2091. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Lee, H. Changes in enzymatic activity and in vitro protein digestibility of four millet varieties upon germination and quality evaluation of cookies prepared from germinated millet composite flours. J. Food Process. Preserv. 2022, 46, e16854. [Google Scholar] [CrossRef]
- Rakkammal, K.; Pandian, S.; Maharajan, T.; Ceasar, S.A.; Sohn, S.I.; Ramesh, M. Humic acid regulates gene expression and activity of antioxidant enzymes to inhibit the salt-induced oxidative stress in finger millet. Cereal Res. Commun. 2024, 52, 397–411. [Google Scholar] [CrossRef]
- Singh, A.K.; Rehal, J.; Kaur, A.; Jyot, G. Enhancement of attributes of cereals by germination an fermentation: A review. Crit. Rev. Food Sci. 2015, 55, 1575–1589. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa, buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Pasko, P.; Barton, H.K.; Zagrodzki, P.; Gorinstein, S.; Folta, M.; Zachwieja, Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem. 2009, 115, 994–998. [Google Scholar] [CrossRef]
- Xu, M.W.; Rao, J.J.; Chen, B.C. Phenolic compounds in germinated cereal and pulse seeds: Classification, transformation, and metabolic process. Crit. Rev. Food Sci. 2020, 60, 740–759. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, P.M.; Sreerama, Y.N. Impact of Processing on the Phenolic Profiles of Small Millets: Evaluation of Their Antioxidant and Enzyme Inhibitory Properties Associated with Hyperglycemia. Food Chem. 2015, 169, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Luo, L.; Zheng, L.Q. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Rosinski, J.; Atchley, W. Molecular Evolution of the Myb Family of Transcription Factors: Evidence for Polyphyletic Origin. J. Mol Evol. 1998, 46, 74–83. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Lee, H. MYB-related transcription factors function as regulators of the circadian clock and anthocyanin biosynthesis in Arabidopsis. Plant Signal. Behav. 2016, 11, e1139278. [Google Scholar] [CrossRef]
- Yin, Y.Q.; Tian, X.; Yang, J.; Yang, Z.F.; Tao, J.; Fang, W.M. Melatonin mediates isoflavone accumulation in germinated soybeans (Glycine max L.) under ultraviolet-B stress. Plant Physiol. Biochem. 2022, 175, 23–32. [Google Scholar] [CrossRef]
- Lazo-Vélez, M.A.; Guardado-Félix, D.; Avilés-González, J.; Romo-López, I.; Serna-Saldívar, S.O. Effect of germination with sodium selenite on the isoflavones and cellular antioxidant activity of soybean (Glycine max). Food Sci. Technol. 2018, 93, 64–70. [Google Scholar] [CrossRef]
- Wang, S.F.; Zhang, X.J.; Fan, Y.H.; Wang, Y.T.; Yang, R.Q.; Wu, J.R.; Xu, J.H.; Tu, K. Effect of magnetic field pretreatment on germination characteristics, phenolic biosynthesis, and antioxidant capacity of quinoa. Plant Physiol. Biochem. 2024, 212, 108734. [Google Scholar] [CrossRef]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Yang, D.D.; Chen, Y.; Guo, F.X.; Huang, B.T.; Okyere, S.A.; Wang, H. Comparative analysis of chemical composition, antioxidant and antimicrobial activities of leaves, leaf tea and root from Codonopsis pilosula. Ind. Crops Prod. 2019, 142, 111844. [Google Scholar] [CrossRef]
- Zhou, H.F.; Chen, Y.; Wang, Z.Y.; Xie, C.; Ye, D.; Guo, A.R.; Xie, A.R.; Xing, J.; Zheng, J. Preparation, characterization and antioxidant activity of cobalt polysaccharides from Qingzhuan Dark Tea. Heliyon 2023, 9, e15503. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Wang, G.; Wu, L.Y.; Zhang, H.; Wu, W.J.; Zhang, M.M.; Li, X.F.; Wu, H. Regulation of the Phenylpropanoid Pathway: A Mechanism of Selenium Tolerance in Peanut (Arachis hypogaea L.) Seedlings. J. Agric. Food Chem. 2016, 64, 3626–3635. [Google Scholar] [CrossRef]
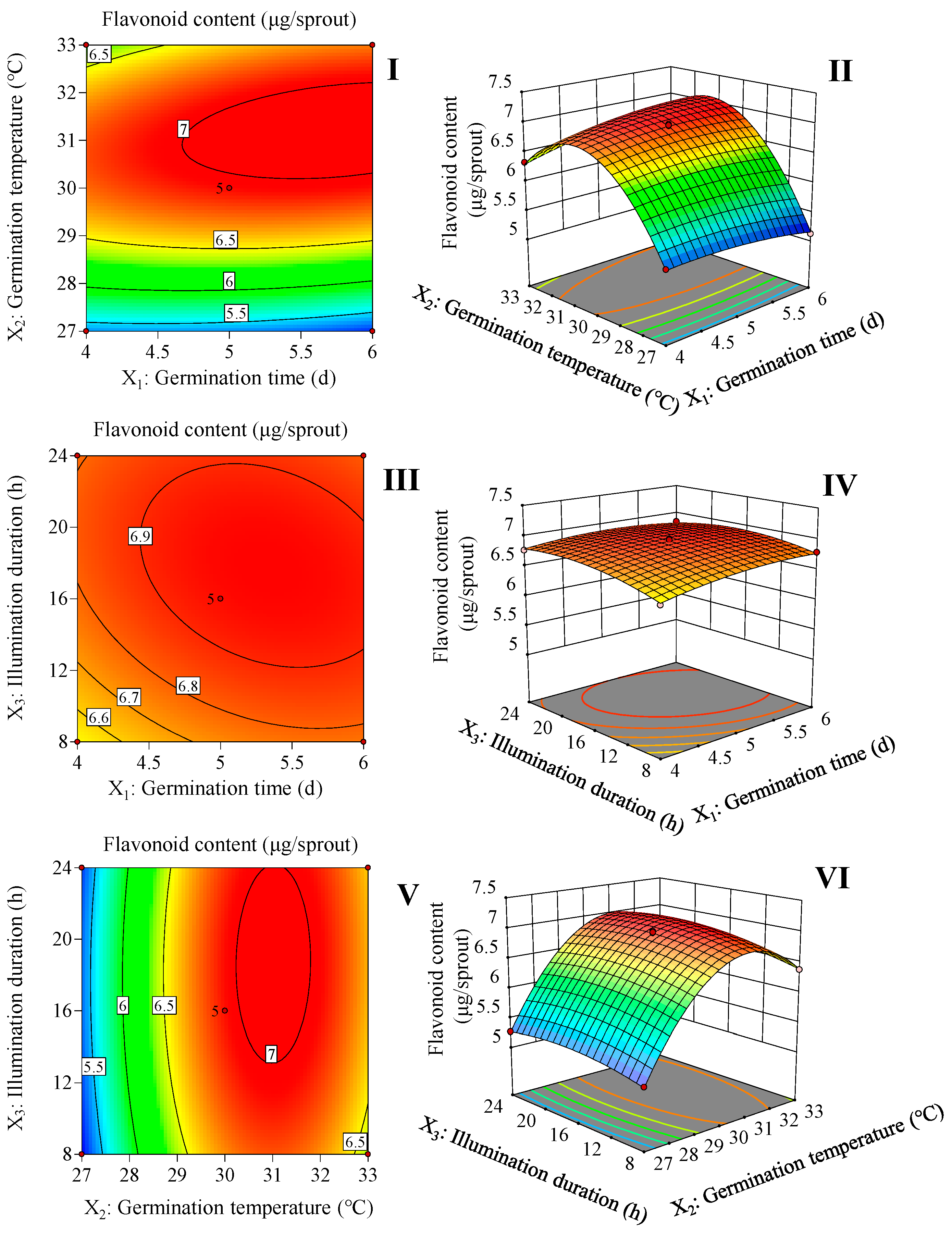

| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 7.62 | 9 | 0.8466 | 702.31 | <0.0001 ** |
| X1: Germination time | 0.0388 | 1 | 0.0388 | 32.19 | 0.0008 ** |
| X2: Germination temperature | 3.28 | 1 | 3.28 | 2723.19 | <0.0001 ** |
| X3: Illumination duration | 0.0525 | 1 | 0.0525 | 43.52 | 0.0003 ** |
| X1 X2 | 0.1130 | 1 | 0.1130 | 93.77 | <0.0001 ** |
| X1 X3 | 0.0122 | 1 | 0.0122 | 10.11 | 0.0155 * |
| X2 X3 | 0.0025 | 1 | 0.0025 | 2.07 | 0.1931 |
| X12 | 0.0321 | 1 | 0.0321 | 26.64 | 0.0013 ** |
| X22 | 3.88 | 1 | 3.88 | 3217.86 | <0.0001 ** |
| X32 | 0.0771 | 1 | 0.0771 | 63.93 | <0.0001 ** |
| Residual | 0.0084 | 7 | 0.0012 | ||
| Lack of Fit | 0.0051 | 3 | 0.0017 | 2.00 | 0.2566 |
| Pure Error | 0.0034 | 4 | 0.0008 | ||
| Cor Total | 7.63 | 16 | |||
| C.V. % = 0.5436 R2 = 0.9989 | Adjusted R2 = 0.9975 | Predicted R2 = 0.9887 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yang, J.; Yin, Y. Germination Promotes Flavonoid Accumulation of Finger Millet (Eleusine coracana L.): Response Surface Optimization and Investigation of Accumulation Mechanism. Plants 2024, 13, 2191. https://doi.org/10.3390/plants13162191
Zhang J, Yang J, Yin Y. Germination Promotes Flavonoid Accumulation of Finger Millet (Eleusine coracana L.): Response Surface Optimization and Investigation of Accumulation Mechanism. Plants. 2024; 13(16):2191. https://doi.org/10.3390/plants13162191
Chicago/Turabian StyleZhang, Jing, Jia Yang, and Yongqi Yin. 2024. "Germination Promotes Flavonoid Accumulation of Finger Millet (Eleusine coracana L.): Response Surface Optimization and Investigation of Accumulation Mechanism" Plants 13, no. 16: 2191. https://doi.org/10.3390/plants13162191




