1. Introduction
Plants are widely recognized as a rich source of pharmaceutical agents with therapeutic and prophylactic properties, including antioxidant, anti-inflammatory, antimicrobial, and antiproliferative activities [
1]. The multifaceted action of natural extracts makes them valuable in therapeutic applications [
2]. This underscores the importance of exploring plant extracts for new medical uses [
3,
4,
5,
6,
7], such as the investigation of
Passiflora edulis rinds in this study, which looks at their potential to inhibit skin aging and exhibit cytotoxic effects against cancer cells.
P. edulis [
8,
9], commonly known as passion fruit, purple granadilla, or egg fruit, belongs to the
Passifloraceae family, with approximately 60 edible species valued for their economic and medicinal properties. Passion fruit is native to tropical and subtropical regions, consumed fresh or as juice. Traditionally, it has been used in folk medicine for treating insomnia, cough, arthralgia, and constipation [
8,
9]. The fruit is renowned for its rich nutritional content, including vitamins, dietary fiber, minerals, and a variety of bioactive compounds such as polyphenols, triterpenes, flavonoids, and polysaccharides. Given these attributes, expanding the therapeutic applications of
P. edulis is compelling.
Skin aging [
10] is a complex biological process [
11] influenced by intrinsic and extrinsic factors, leading to visible signs such as wrinkles and loss of elasticity [
12,
13,
14,
15]. Some natural products could mitigate skin aging by targeting key enzymes involved in the degradation of the skin matrix [
16], including tyrosinase, elastase, and hyaluronidase. Prior to this study, very little was known about the anti-skin aging properties of
P. edulis, a gap this research aims to fill. Tyrosinase, elastase, and hyaluronidase are enzymes critically involved in skin aging, particularly in the degradation of dermal extracellular matrix components. Their overactivity contributes to visible signs of aging, which can potentially be mitigated by incorporating specific inhibitors into cosmetic formulations. This study evaluates the anti-skin aging potential of
P. edulis (Tainung No. 1) rind extract, focusing particularly on its anti-tyrosinase activity, which plays a crucial role in controlling melanin production.
P. edulis, known for its extensive health-promoting properties, has shown potential in various therapeutic contexts, offering antioxidant, anti-inflammatory, antitumor, anti-obesity, hepatoprotective, and neuroprotective benefits [
8,
9]. This emphasizes the necessity to further explore the functional properties of
P. edulis, including its various subspecies. Consequently, this study broadens the scope of biological activities examined to encompass anti-skin aging potential, antibacterial activity, inhibition of single-stranded DNA-binding proteins (SSB), and cytotoxic effects against oral carcinoma cells, as investigated using
P. edulis var. Tainung No. 1. This particular cultivar [
17], prevalent in Taiwan, is a hybrid of
P. edulis (purple passion fruit) and
P. edulis f.
flavicarpa (yellow passion fruit), with Nantou County being the central cultivation area, contributing about 75% of Taiwan’s total production. The pulp of this fruit is widely consumed, leaving a significant amount of rind as juice-processing by-product. Typically landfilled or used as compost in local areas, these by-products represent a substantial waste management challenge and potential environmental pollutant. Accordingly, this study also aims to explore extended uses for the rind of
P. edulis var. Tainung No. 1, potentially transforming a disposal problem into valuable therapeutic applications.
Antimicrobial drug resistance poses an escalating threat to global public health, with cases of antibiotic-resistant bacterial infections rising alarmingly [
18,
19,
20]. Multidrug-resistant pathogenic bacteria are spreading rapidly across the globe, leading to potentially untreatable conditions. For instance,
Staphylococcus aureus has developed significant antibiotic resistance, contributing to approximately 19,000 deaths annually in the United States alone [
21]. Natural plant extracts, known for their antimicrobial properties, are being explored as alternative therapeutic options [
22]. Accordingly, this study investigated the antibacterial activity of the acetone-derived rind extract of
P. edulis var. Tainung No. 1, which showed activity against
S. aureus and
Pseudomonas aeruginosa.
P. aeruginosa, a prevalent opportunistic pathogen, is notorious for causing nosocomial infections and poses significant risks to immunocompromised patients [
23]. To date, more than 800 β-lactamases have been identified, with at least 120 detected in
P. aeruginosa [
24], underscoring the urgent need for ongoing development of effective small-molecule antibiotics to combat these antibiotic-resistant pathogens.
SSB is integral to DNA metabolic processes, including replication, repair, recombination, and replication restart in bacteria [
25,
26,
27]. It binds tightly and cooperatively to single-stranded DNA (ssDNA) irrespective of the DNA sequence [
28]. SSB also forms interactions with numerous proteins involved in DNA metabolism, collectively known as the SSB interactome [
29,
30,
31,
32,
33]. Due to its pivotal roles, SSB is considered a potential target for the development of antibacterial drugs [
34,
35,
36]. The Infectious Disease Society of America (IDSA) identifies a group of antibiotic-resistant bacteria known as the ESKAPE pathogens (
Enterococcus faecium,
Staphylococcus aureus,
Klebsiella pneumoniae,
Acinetobacter baumannii,
Pseudomonas aeruginosa, and
Enterobacter spp.), which can effectively “escape” the effects of antibiotics [
20,
37]. These pathogens are responsible for severe, often lethal diseases and present substantial challenges in treating bacterial infections due to antimicrobial resistance (AMR), contributing to increased morbidity, mortality, and healthcare costs [
18,
19]. Among these,
K. pneumoniae is recognized for causing severe hospital- and community-acquired infections, including pneumonia, liver abscesses, and sepsis [
38]. This study evaluates the activity of
P. edulis rind extracts against
K. pneumoniae SSB (KpSSB), aiming to inhibit DNA replication and reduce the virulence of
K. pneumoniae, thereby mitigating the threat posed by this dangerous pathogen. The bacterial SSB is structurally and functionally conserved, making KpSSB a valuable model for studying the potential effects on SSBs in other bacterial species.
The widespread occurrence of cancer, particularly oral carcinoma, emphasizes the critical need for innovative therapeutic approaches [
39,
40,
41]. Oral cancer is one of the top ten most common cancers globally, primarily occurring in the oral cavity [
42]. Oral squamous cell carcinoma (OSCC), the most common type of head and neck cancer, reports a troubling five-year survival rate of only 50% [
43]. Conventional therapies for oral cancers, such as surgery, chemotherapy, and radiation therapy [
44], often result in significant side effects and can lead to the development of drug resistance [
44]. As a result, there is an increasing interest in natural compounds as supplementary anticancer agents alongside traditional treatments [
1]. This study focuses on the cytotoxic effects of
P. edulis (Tainung No. 1) rind extract, extracted with acetone, on Ca9-22 gingival carcinoma cells. Demonstrating high total phenolic and flavonoid contents, the rind extract from
P. edulis var. Tainung No. 1 has shown potential in preclinical settings for its ability to induce apoptosis and inhibit cell proliferation and migration, crucial factors in effective cancer management.
In this study, we explored the biological properties of P. edulis extract, focusing on its potential for anti-skin aging, antibacterial activity, SSB inhibition, and cytotoxic effects against oral carcinoma cells. Additionally, gas chromatography–mass spectrometry (GC–MS) was employed to tentatively identify the chemical constituents of the extract. The seven most prevalent compounds were further analyzed through molecular docking with both tyrosinase and KpSSB to computationally investigate potential inhibition mechanisms. Overall, our findings underscore the potential novel therapeutic applications of P. edulis rinds, warranting further scientific exploration into their medical uses.
3. Discussion
Passiflora, commonly referred to as “passion fruit”, thrives in tropical and subtropical climates [
9]. This fruit is consumed both fresh and as an ingredient in processed foods. Extracts, juices, and individual compounds derived from passion fruit exhibit diverse health benefits and biological activities, including antioxidant, anti-inflammatory, sedative, and neuroprotective properties [
8,
9]. Accordingly, exploring new therapeutic uses for this fruit is highly warranted. Furthermore, waste from fruits and vegetables, such as the rinds utilized in this study, not only represents a loss of valuable resources but also poses environmental challenges. Efficiently reducing and repurposing food waste to create value-added products can enhance production processes and decrease associated costs. In this study, we discovered that the rind of
P. edulis (Tainung No. 1), extracted with acetone, exhibited high TPC (
Table 3) and TFC (
Table 4), along with notable anti-skin aging (
Table 1), antibacterial (
Table 2), and anti-oral cancer potentials (
Figure 4). This study is the first to evaluate the anti-SSB (
Table 7 and
Figure 2) and anti-skin aging properties (
Table 1) of
P. edulis. GC–MS analysis identified the top 13 compounds in this extract, shedding light on the active components potentially responsible for these biological activities and their possible synergistic interactions. Although HPLC–MS analysis previously identified many constituents in
P. edulis [
52], the compounds identified through this GC–MS study could enrich the existing knowledge of
P. edulis’s chemical profile. Thus, valorizing waste products such as passion fruit rinds is of considerable interest for their added value.
In vitro studies consistently underscore the health-promoting effects of phytochemicals [
63,
64]. The skin, as the body’s largest and most intricate organ, plays a vital barrier role against external influences [
11]. Its condition greatly affects social perceptions, with youthful and healthy skin typically regarded more favorably [
15]. Aging alters skin structure and function, leading to thinness, dryness, decreased elasticity, rough texture, wrinkles, and dark spots. These changes can significantly affect overall health and quality of life. Hence, the continuous discovery of anti-aging agents is crucial [
65]. Plant-derived compounds [
14], especially secondary metabolites and whole plant extracts, have been thoroughly investigated for their anti-aging benefits [
10]. Polyphenols, known for their potent antioxidant properties, help mitigate aging and photodamage [
66]. Specific compounds such as quercetin and myricetin are recognized for their ability to inhibit enzymes like tyrosinase and hyaluronidase, which are vital in the skin aging process [
67]. This study demonstrates the anti-skin aging potential of
P. edulis (Tainung No. 1), particularly the acetone-extracted rind extract, which exhibits significant inhibition of tyrosinase. These insights highlight the necessity of further research into specific bioactive compounds within the extract that target aging-associated enzymes, particularly tyrosinase, to develop effective anti-aging therapies.
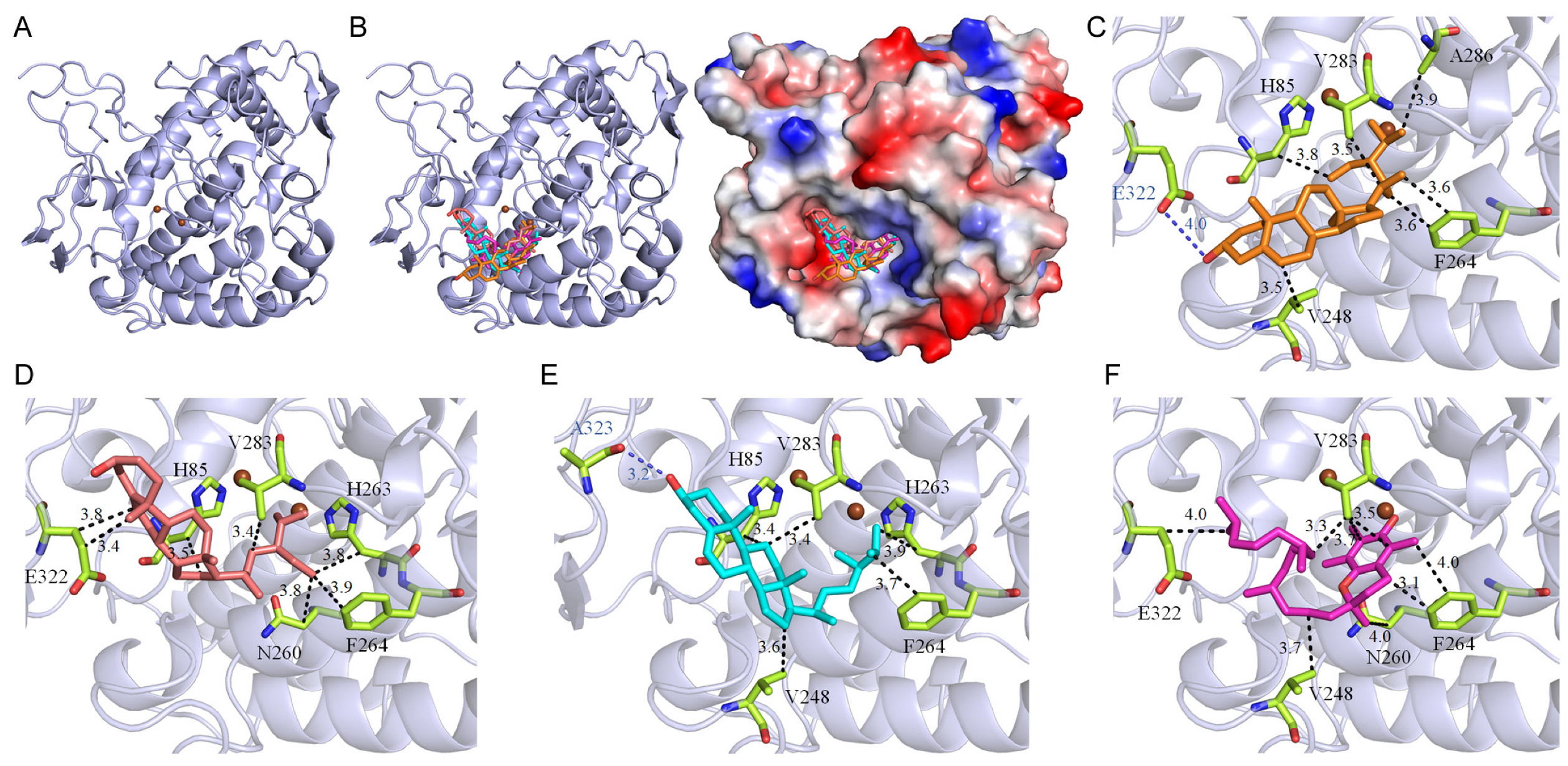
Our findings indicate that the acetone-extracted rind extract of
P. edulis (Tainung No. 1) exhibits strong inhibitory effects on tyrosinase, suggesting potential expanded therapeutic uses. Tyrosinase, a crucial copper-containing enzyme in melanogenesis, facilitates the conversion of L-tyrosine to L-DOPA and its subsequent oxidation to dopaquinone, thereby regulating melanin production [
68]. Recent studies have linked tyrosinase activity not only to age-related skin changes such as spots, photodamage, and pigmentation but also to the development of Alzheimer’s disease [
69,
70]. This neurodegenerative condition is marked by cognitive deterioration, memory loss, and behavioral changes, with its pathology associated with amyloid-beta deposition, neurofibrillary tangles, oxidative stress, a deficit in cholinergic function, and neuroinflammation [
71]. Tyrosinase’s byproduct, L-DOPA, is implicated in neurotoxic effects, inflammatory responses, and increased tau protein phosphorylation [
72]. Additionally, heightened tyrosinase activity, particularly in the substantia nigra, contributes to neuromelanin production [
73]. Given the considerable side effects associated with current Alzheimer’s treatments, targeting tyrosinase inhibition could represent a dual-purpose therapeutic approach, addressing both skin aging and the progression of Alzheimer’s disease. All seven major compounds from the extract successfully docked into the active site of tyrosinase (
Figure 1). These compounds demonstrated binding energies ranging from −5.0 kcal/mol to −7.8 kcal/mol, with stigmasterol exhibiting the highest affinity at −7.8 kcal/mol, surpassing even kojic acid, a well-known tyrosinase inhibitor, which displayed a binding energy of −6.1 kcal/mol (
Table 5). Additionally, stigmast-5-en-3-ol, campesterol, and vitamin E from the extract also exhibited higher affinities for tyrosinase compared to kojic acid, highlighting the potential of the
P. edulis (Tainung No. 1) rind extract for drug development. The cooperative action of these compounds (
Table 6), by individually docking into the enzyme’s active site and obstructing substrate access, suggests a collective mechanism that inhibits tyrosinase activity through varied binding poses. Therefore, the potent inhibitory effects of
P. edulis (Tainung No. 1) rind extract on tyrosinase warrant further investigation into its potential as a source of tyrosinase inhibitors for applications in both dermatological and neurotherapeutic domains.
Although acetone is less safe compared to ethanol as an extraction solvent, it remains effective for extracting natural products. For example, using acetone as the solvent has proven beneficial for enhancing TPC (
Table 3) and TFC (
Table 4). For future applications of the acetone-extracted
P. edulis (Tainung No. 1) rind extract, it is crucial to ensure the complete volatilization of acetone from the extract.
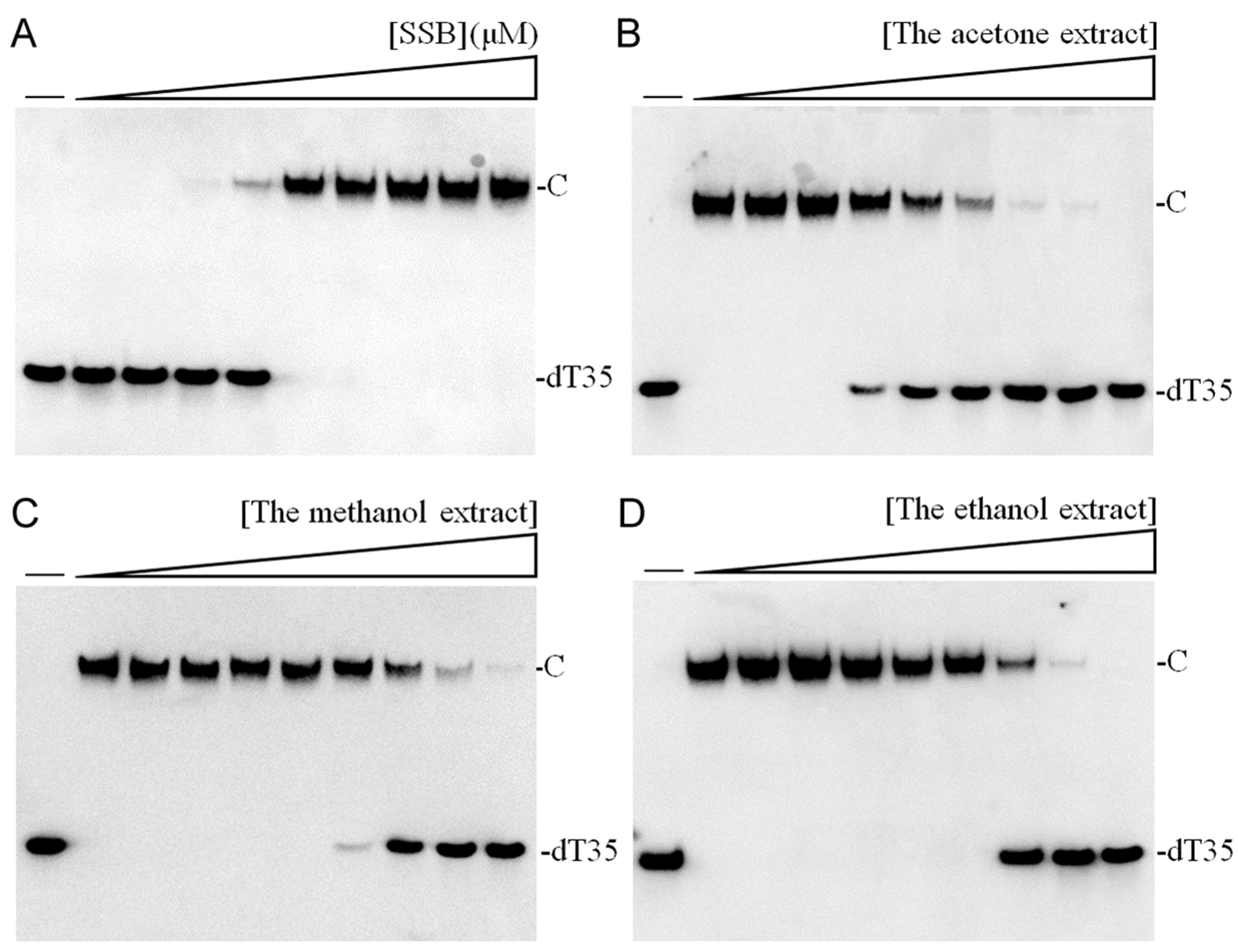
In this study, we explored the acetone-extracted rind of
P. edulis (Tainung No. 1) and discovered its potential to inhibit the activity of SSB in
K. pneumoniae (
Figure 2). SSB is crucial for DNA replication and cellular survival, highlighting its significance as a target for anti-pathogenic therapies [
34,
57,
74,
75].
K. pneumoniae is noted for its resistance to antibiotics and is one of the ESKAPE pathogens [
20,
23,
76], which can effectively “escape” the effects of conventional antibiotics. The inhibition of essential proteins like SSB offers a promising avenue for developing new antimicrobial strategies [
77,
78]. Targeting DNA replication and repair mechanisms has been a foundational approach in antibiotic development, exemplified by the success of quinolones and aminocoumarins, which inhibit bacterial DNA gyrase and topoisomerase IV [
79,
80]. Given the profound antibacterial properties of various plant extracts, continuing the search for effective SSB inhibitors is of significant interest. Our findings underscore the potential of
P. edulis (Tainung No. 1) extract as a therapeutic agent against bacterial infections by targeting this critical DNA replication protein (
Table 8 and
Figure 3). Currently, our laboratory is investigating specific bioactive compounds within the extract that could serve as potent antibacterial agents. Additionally, the correlation between the extract’s toxicity against oral carcinoma cells and its inhibition of ssDNA-binding activity merits further investigation.
The acetone-extracted rind of
P. edulis (Tainung No. 1) exhibited cytotoxic properties against Ca9-22 oral carcinoma cells, manifesting through inhibition of cell migration and proliferation and induction of apoptosis (
Figure 4). The GC–MS analysis tentatively identified key compounds within the extract, including stigmast-5-en-3-ol, vitamin E, palmitic acid, stigmasterol, linoleic acid, campesterol, octadecanoic acid, heptacosane, sitostenone, eicosane, squalene, pentacosane, and docosane. The primary constituents, notably stigmast-5-en-3-ol [
81], vitamin E [
82], palmitic acid [
83], stigmasterol [
84,
85], linoleic acid [
86], campesterol [
84,
87], are recognized for their anticancer activities. Future investigations should focus on the synergistic and polypharmacological effects of these bioactive molecules, aiming to determine the most effective combinations and concentrations for cancer therapy.