Ziziphus mauritiana Lam. Bark and Leaves: Extraction, Phytochemical Composition, In Vitro Bioassays and In Silico Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolics and Flavonoids Content
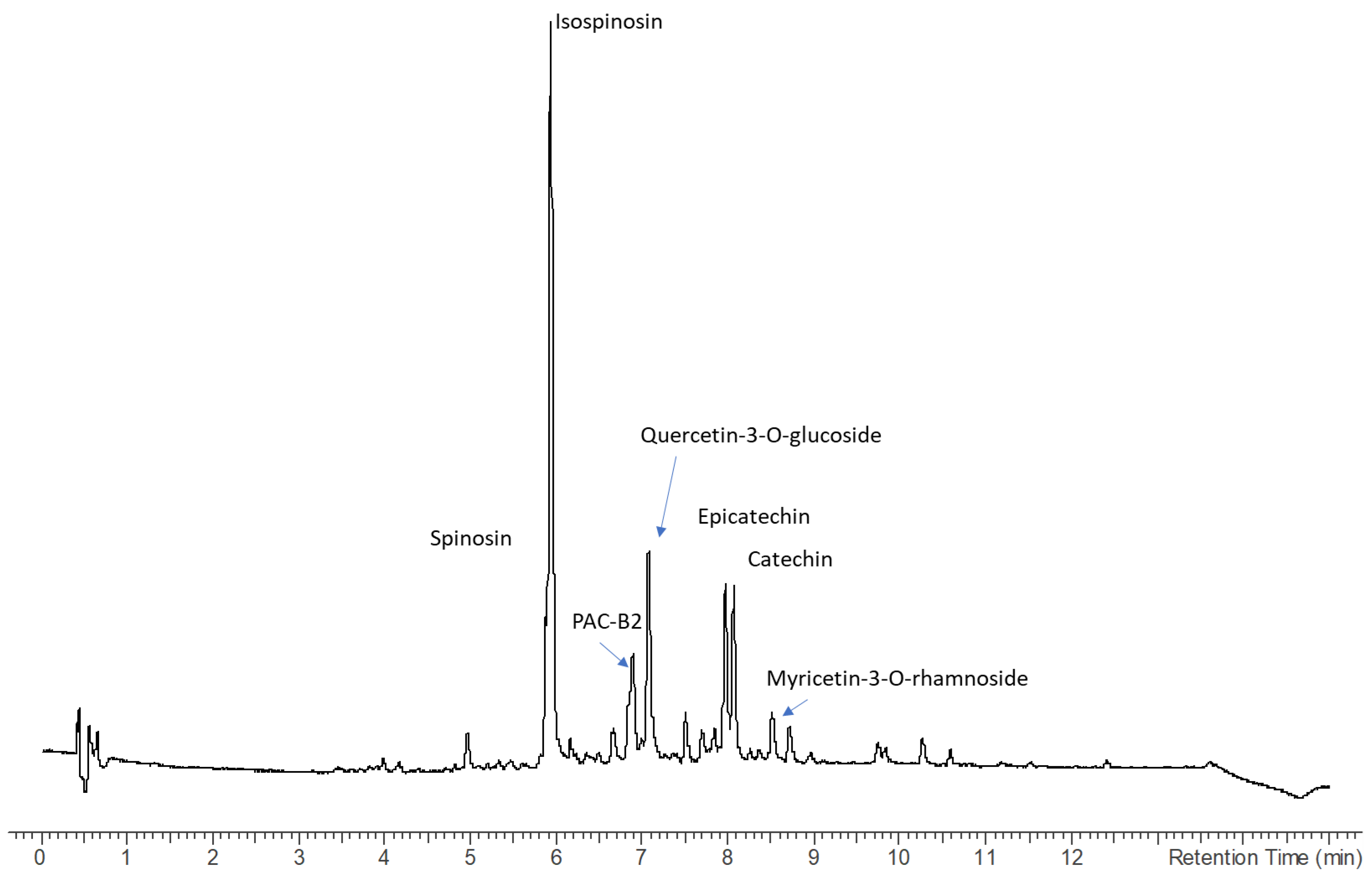
2.2. Chemical Composition by the Comprehensive Analysis of High-Resolution QTOF and Multiple-Stage Mass Spectrometry in Ion Trap
2.3. Antioxidant Capacity
2.4. Enzyme Inhibitory Activity
2.4.1. Neuroprotective Effects
2.4.2. Dermatoprotective Effects
2.4.3. Antidiabetic Effect
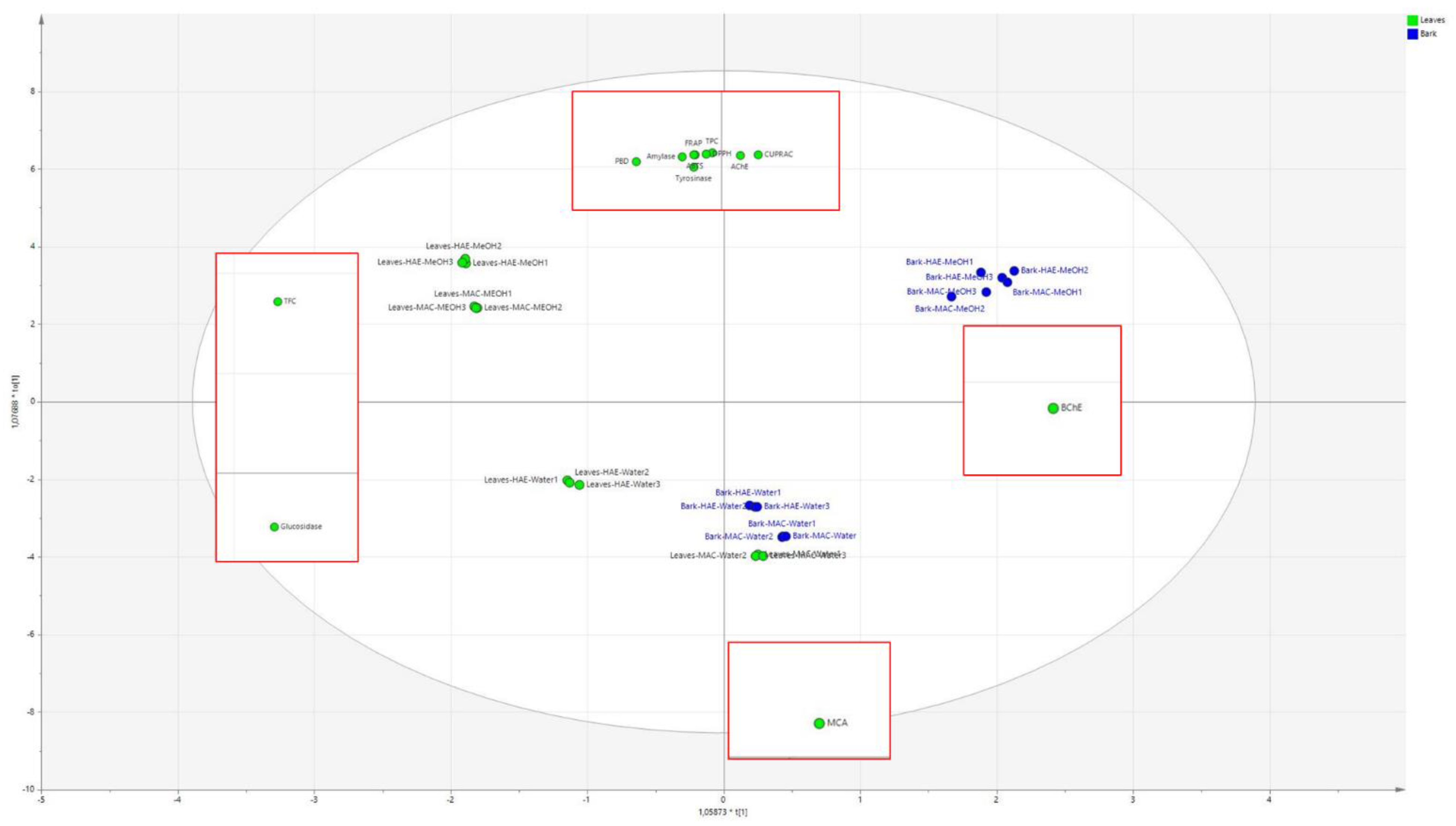
2.5. Multivariate Data Analysis
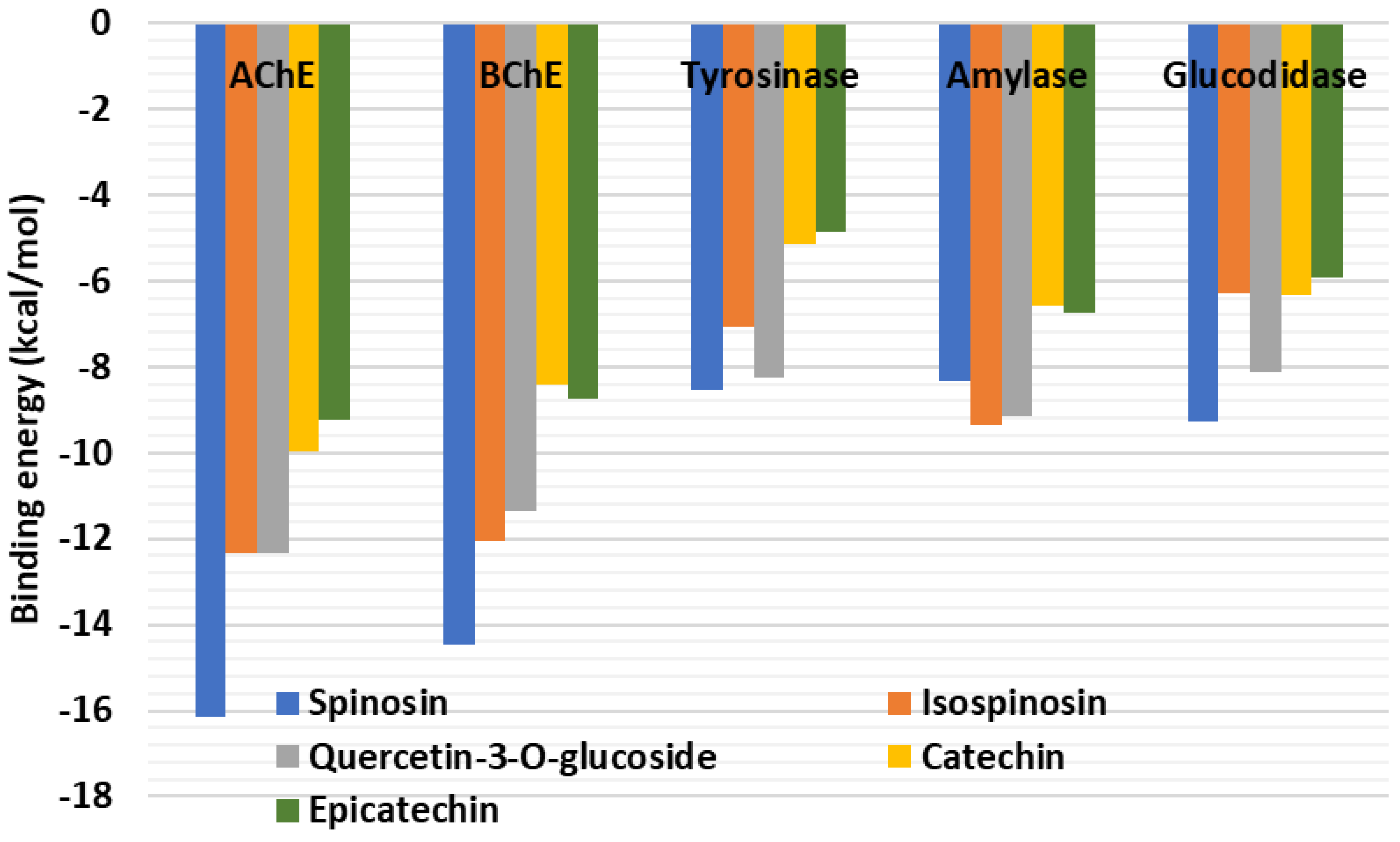
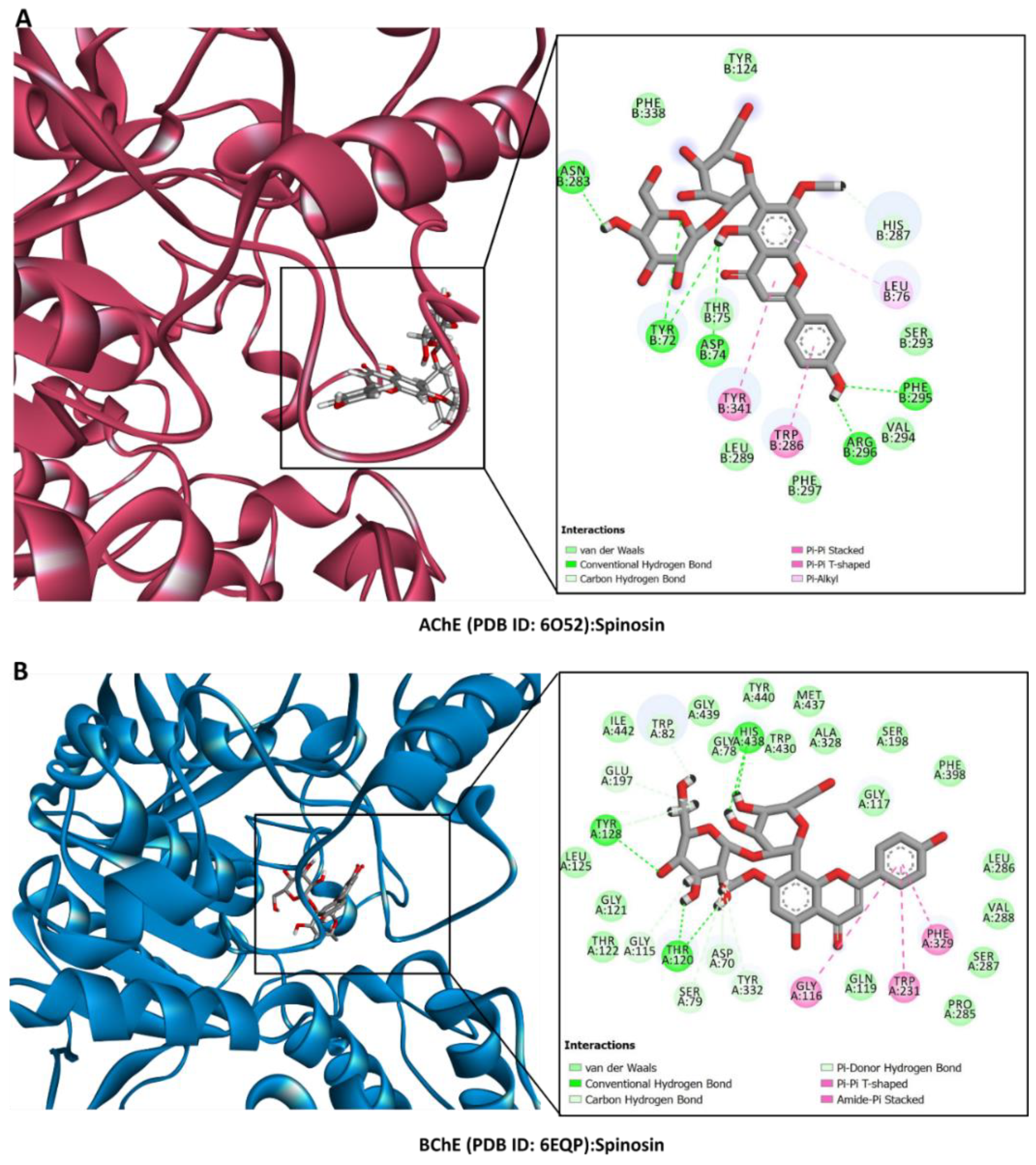
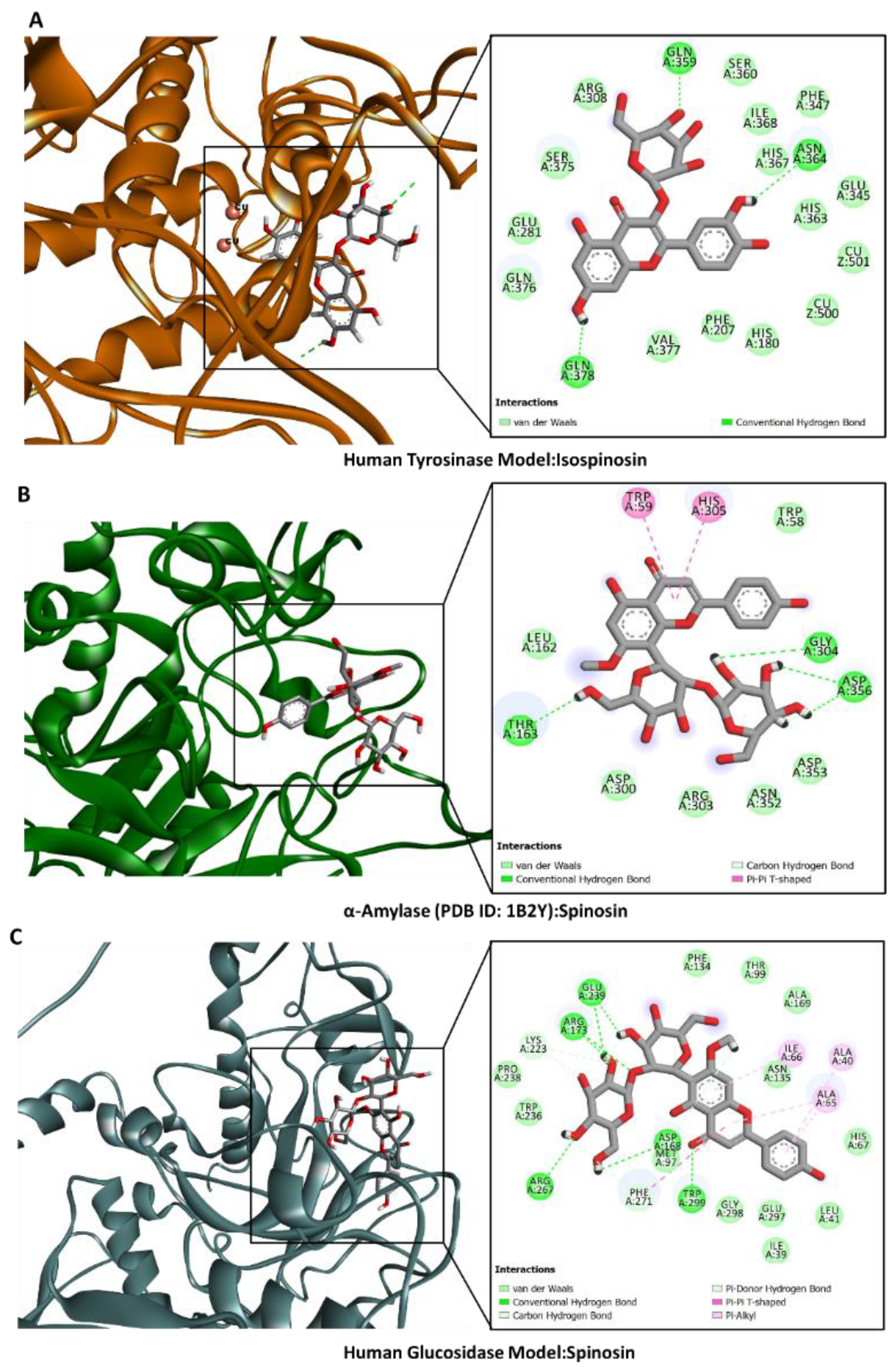
2.6. Molecular Docking
3. Materials and Methods
3.1. Plant Material
3.2. Sample Preparation
3.3. Assay for Total Phenolic and Flavonoid Contents
3.4. High-Resolution LC-QTOF-MS and Multiple-Stage Mass Spectrometry by Ion Trap Analysis
3.5. In Vitro Bioactivity Assays
3.6. Molecular Modeling
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ziada, A.S.; Smith, M.-S.R.; Côté, H.C. Updating the free radical theory of aging. Front. Cell Dev. Biol. 2020, 8, 575645. [Google Scholar] [CrossRef]
- Wawi, M.J.; Mahler, C.; Inguimbert, N.; Marder, T.B.; Ribou, A.-C. A new mitochondrial probe combining pyrene and a triphenylphosphonium salt for cellular oxygen and free radical detection via fluorescence lifetime measurements. Free Radic. Res. 2022, 56, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Maddu, N. Diseases Related to Types of Free Radicals; IntechOpen: London, UK, 2019. [Google Scholar]
- Hawkins, C.L.; Davies, M.J. Role of myeloperoxidase and oxidant formation in the extracellular environment in inflammation-induced tissue damage. Free Radic. Biol. Med. 2021, 172, 633–651. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Baquero, M.; Vento, M.; Cháfer-Pericás, C. Free radicals in Alzheimer’s disease: Lipid peroxidation biomarkers. Clin. Chim. Acta 2019, 491, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, A.; Nilofar; Bouyahya, A.; Zengin, G.; Di Simone, S.C.; Recinella, L.; Leone, S.; Brunetti, L.; Uba, A.I.; Cakilcioğlu, U. Chemical Characterization of Different Extracts from Artemisia annua and Their Antioxidant, Enzyme Inhibitory and Anti-Inflammatory Properties. Chem. Biodivers. 2023, 20, e202300547. [Google Scholar] [CrossRef]
- Barrientos, R.E.; Ahmed, S.; Cortés, C.; Fernández-Galleguillos, C.; Romero-Parra, J.; Simirgiotis, M.J.; Echeverría, J. Chemical fingerprinting and biological evaluation of the endemic Chilean fruit Greigia sphacelata (Ruiz and Pav.) Regel (Bromeliaceae) by UHPLC-PDA-orbitrap-mass spectrometry. Molecules 2020, 25, 3750. [Google Scholar] [CrossRef]
- Nilofar; Duran, T.; Uba, A.I.; Cvetanović Kljakić, A.; Božunović, J.; Gašić, U.; Bouyahya, A.; Yıldiztugay, E.; Ferrante, C.; Zengin, G. Extractions of aerial parts of Hippomarathrum scabrum with conventional and green methodologies: Chemical profiling, antioxidant, enzyme inhibition, and anti-cancer effects. J. Sep. Sci. 2023, 47, 2300678. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Usmani, S.; Singh, R.; Singh, N.; Gupta, A.; Ved, A. A panoramic view on phytochemical, nutritional, and therapeutic attributes of Ziziphus mauritiana Lam.: A comprehensive review. Phytother. Res. 2021, 35, 63–77. [Google Scholar] [CrossRef]
- Grice, A. Seed production, dispersal and germination in Cryptostegia grandiflora and Ziziphus mauritiana, two invasive shrubs in tropical woodlands of northern Australia. Aust. J. Ecol. 1996, 21, 324–331. [Google Scholar] [CrossRef]
- El Maaiden, E.; El Kharrassi, Y.; Qarah, N.A.; Essamadi, A.K.; Moustaid, K.; Nasser, B. Genus Ziziphus: A comprehensive review on ethnopharmacological, phytochemical and pharmacological properties. J. Ethnopharmacol. 2020, 259, 112950. [Google Scholar] [CrossRef]
- Alsayari, A.; Wahab, S. Genus Ziziphus for the treatment of chronic inflammatory diseases. Saudi J. Biol. Sci. 2021, 28, 6897–6914. [Google Scholar] [CrossRef] [PubMed]
- Tahergorabi, Z.; Abedini, M.R.; Mitra, M.; Fard, M.H.; Beydokhti, H. “Ziziphus jujuba”: A red fruit with promising anticancer activities. Pharmacogn. Rev. 2015, 9, 99. [Google Scholar] [PubMed]
- Ebrahimi, S.; Mollaei, H.; Hoshyar, R. Ziziphus Jujube: A review study of its anticancer effects in various tumor models invitro and invivo. Cell. Mol. Biol. 2017, 63, 122–127. [Google Scholar] [CrossRef]
- Da Costa Mousinho, N.M.; van Tonder, J.J.; Steenkamp, V. In vitro anti-diabetic activity of Sclerocarya birrea and Ziziphus mucronata. Nat. Prod. Commun. 2013, 8, 1934578X1300800924. [Google Scholar] [CrossRef]
- Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:719349-1 (accessed on 12 May 2024).
- Delfanian, M.; Esmaeilzadeh Kenari, R.; Sahari, M.A. Utilization of Jujube fruit (Ziziphus mauritiana Lam.) extracts as natural antioxidants in stability of frying oil. Int. J. Food Prop. 2016, 19, 789–801. [Google Scholar] [CrossRef]
- Zozio, S.; Servent, A.; Cazal, G.; Mbéguié-A-Mbéguié, D.; Ravion, S.; Pallet, D.; Abel, H. Changes in antioxidant activity during the ripening of jujube (Ziziphus mauritiana Lamk). Food Chem. 2014, 150, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-W.; Fan, L.-P.; Ding, S.-D.; Ding, X.-L. Nutritional composition of five cultivars of Chinese jujube. Food Chem. 2007, 103, 454–460. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, L.; Ye, S.; Ye, Y.; Ren, F. Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujuba Mill.) from China. Food Chem. Toxicol. 2010, 48, 1461–1465. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Márquez, K.; Márquez, N.; Ávila, F.; Cruz, N.; Burgos-Edwards, A.; Pardo, X.; Carrasco, B. Oleuropein-Enriched Extract From Olive Mill Leaves by Homogenizer-Assisted Extraction and Its Antioxidant and Antiglycating Activities. Front. Nutr. 2022, 9, 895070. [Google Scholar] [CrossRef]
- Yang, C.-H.; Li, R.-X.; Chuang, L.-Y. Antioxidant activity of various parts of Cinnamomum cassia extracted with different extraction methods. Molecules 2012, 17, 7294–7304. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, A.; Kouadio Ibrahime, S.; Nilofar; Claudio, F.; Ozan Emre, E.; Ouattara Katinan, E.; Gokhan, Z. Online-HPLC Strategies, Antioxidant and Enzyme Inhibitory Activities of Different Extracts of Mondia whitei Leaves. J. Biol. Regul. Homeost. Agents 2023, 37, 6029–6039. [Google Scholar]
- Al-Reza, S.M.; Bajpai, V.K.; Kang, S.C. Antioxidant and antilisterial effect of seed essential oil and organic extracts from Zizyphus jujuba. Food Chem. Toxicol. 2009, 47, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Uddin, N.; Ali, N.; Uddin, Z.; Nazir, N.; Zahoor, M.; Rashid, U.; Ullah, R.; Alqahtani, A.S.; Alqahtani, A.M.; Nasr, F.A. Evaluation of cholinesterase inhibitory potential of different genotypes of Ziziphus nummularia, their HPLC-UV, and molecular docking analysis. Molecules 2020, 25, 5011. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.U.; Raza, M.A.; Saeed, A.; Ahmed, M.; Hussain, T. Variations in morphological characters and antioxidant potential of different plant parts of four Ziziphus Mill. species from the Cholistan. Plants 2021, 10, 2734. [Google Scholar] [CrossRef]
- Kim, J.H.; Hart, H.T.; Stevens, J.F. Alkaloids of some Asian Sedum species. Phytochemistry 1996, 41, 1319–1324. [Google Scholar] [CrossRef]
- Kumar, B.; Khan, S.A.; Akhtar, M.J. Phytochemicals and therapeutic potential of Punica granatum L. Herbs Spices Their Roles Nutraceuticals Funct. Foods 2023, 1, 171–209. [Google Scholar]
- Du, J.; Zhong, B.; Subbiah, V.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Lc-esi-qtof-ms/ms profiling and antioxidant activity of phenolics from custard apple fruit and by-products. Separations 2021, 8, 62. [Google Scholar] [CrossRef]
- Xue, X.; Zhao, A.; Wang, Y.; Ren, H.; Du, J.; Li, D.; Li, Y. Composition and content of phenolic acids and flavonoids among the different varieties, development stages, and tissues of Chinese Jujube (Ziziphus jujuba Mill.). PLoS ONE 2021, 16, e0254058. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.H.; Yan, F.; E, S.; Xiong, P.; Tang, S.N.; Yu, K.Q.; Zhang, M.; Cheng, Y.C.; Cai, W. Comprehensive characterization of multiple components of Ziziphus jujuba Mill using UHPLC-Q-Exactive Orbitrap Mass Spectrometers. Food Sci. Nutr. 2022, 10, 4270–4295. [Google Scholar] [CrossRef] [PubMed]
- Tuenter, E.; Foubert, K.; Staerk, D.; Apers, S.; Pieters, L. Isolation and structure elucidation of cyclopeptide alkaloids from Ziziphus nummularia and Ziziphus spina-christi by HPLC-DAD-MS and HPLC-PDA-(HRMS)-SPE-NMR. Phytochemistry 2017, 138, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Won, T.H.; Kang, S.S.; Shin, J. Simultaneous analysis of bioactive metabolites from Ziziphus jujuba by HPLC–DAD–ELSD–MS/MS. J. Pharm. Investig. 2012, 42, 21–31. [Google Scholar] [CrossRef]
- Singh, P.; Bajpai, V.; Gupta, A.; Gaikwad, A.N.; Maurya, R.; Kumar, B. Identification and quantification of secondary metabolites of Pterocarpus marsupium by LC–MS techniques and its in-vitro lipid lowering activity. Ind. Crops Prod. 2019, 127, 26–35. [Google Scholar] [CrossRef]
- Xu, T.; Kuang, T.; Du, H.; Li, Q.; Feng, T.; Zhang, Y.; Fan, G. Magnoflorine: A review of its pharmacology, pharmacokinetics and toxicity. Pharmacol. Res. 2020, 152, 104632. [Google Scholar] [CrossRef]
- Singh, B.; Pandey, V.B. An N-formyl cyclopeptide alkaloid from Zizyphus nummularia bark. Phytochemistry 1995, 38, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.P.D.; Pandey, V.B.; Shah, A.H.; Eckhardt, G. Cyclopeptide Alkaloids from Zizyphus nummularia. J. Nat. Prod. 1987, 50, 235–237. [Google Scholar] [CrossRef]
- Tuenter, E.; Ahmad, R.; Foubert, K.; Amin, A.; Orfanoudaki, M.; Cos, P.; Maes, L.; Apers, S.; Pieters, L.; Exarchou, V. Isolation and Structure Elucidation by LC-DAD-MS and LC-DAD-SPE-NMR of Cyclopeptide Alkaloids from the Roots of Ziziphus oxyphylla and Evaluation of Their Antiplasmodial Activity. J. Nat. Prod. 2016, 79, 2865–2872. [Google Scholar] [CrossRef]
- de Souza, L.M.; Cipriani, T.R.; Iacomini, M.; Gorin, P.A.J.; Sassaki, G.L. HPLC/ESI-MS and NMR analysis of flavonoids and tannins in bioactive extract from leaves of Maytenus ilicifolia. J. Pharm. Biomed. Anal. 2008, 47, 59–67. [Google Scholar] [CrossRef]
- Symma, N.; Hensel, A. Advanced analysis of oligomeric proanthocyanidins: Latest approaches in liquid chromatography and mass spectrometry based analysis. Phytochem. Rev. 2022, 21, 809–833. [Google Scholar] [CrossRef]
- Xie, P.-J.; You, F.; Huang, L.-X.; Zhang, C.-H. Comprehensive assessment of phenolic compounds and antioxidant performance in the developmental process of jujube (Ziziphus jujuba Mill.). J. Funct. Foods 2017, 36, 233–242. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Dahiru, D.; Obidoa, O. Evaluation of the antioxidant effects of Ziziphus mauritiana Lam. leaf extracts against chronic ethanol-induced hepatotoxicity in rat liver. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 39–45. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Gong, G.; Ma, R.; Xu, F.; Yan, T.; Wu, B.; Jia, Y. Spinosin inhibits aβ1-42 production and aggregation via activating Nrf2/HO-1 pathway. Biomol. Ther. 2020, 28, 259. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Koley, T.K.; Walia, S.; Nath, P.; Awasthi, O.; Kaur, C. Nutraceutical composition of Zizyphus mauritiana Lamk (Indian ber): Effect of enzyme-assisted processing. Int. J. Food Sci. Nutr. 2011, 62, 276–279. [Google Scholar] [CrossRef]
- El-Shahir, A.A.; El-Wakil, D.A.; Abdel Latef, A.A.H.; Youssef, N.H. Bioactive Compounds and Antifungal Activity of Leaves and Fruits Methanolic Extracts of Ziziphus spina-christi L. Plants 2022, 11, 746. [Google Scholar] [CrossRef]
- Tsegaye, M.; Alemu, T.; Dilnessa, A.; Tolessa, A.; Tantu, T.; Bekalu, Y.; Haile, F. Effect of storage condition on the nutritional and anti-nutritional composition of kurkura (Ziziphus mauritiana Lam.) fruit from North-Eastern Ethiopia. Heliyon 2023, 9, e17380. [Google Scholar] [CrossRef] [PubMed]
- Dare, R.G.; Nakamura, C.V.; Ximenes, V.F.; Lautenschlager, S.O. Tannic acid, a promising anti-photoaging agent: Evidences of its antioxidant and anti-wrinkle potentials, and its ability to prevent photodamage and MMP-1 expression in L929 fibroblasts exposed to UVB. Free Radic. Biol. Med. 2020, 160, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.; Becker, K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010, 120, 945–959. [Google Scholar] [CrossRef]
- Small, D.H.; Mok, S.S.; Bornstein, J.C. Alzheimer’s disease and Aβ toxicity: From top to bottom. Nat. Rev. Neurosci. 2001, 2, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Mehfooz, H.; Saeed, A.; Sharma, A.; Albericio, F.; Larik, F.A.; Jabeen, F.; Channar, P.A.; Flörke, U. Dual Inhibition of AChE and BChE with the C-5 substituted derivative of Meldrum’s acid: Synthesis, structure elucidation, and molecular docking studies. Crystals 2017, 7, 211. [Google Scholar] [CrossRef]
- Xu, F.; He, B.; Xiao, F.; Yan, T.; Bi, K.; Jia, Y.; Wang, Z. Neuroprotective effects of spinosin on recovery of learning and memory in a mouse model of Alzheimer’s disease. Biomol. Ther. 2019, 27, 71. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, H.-T.; Wang, D.; Yang, C.-R.; Xu, M.; Zhang, Y.-J. New spinosin derivatives from the seeds of Ziziphus mauritiana. Nat. Prod. Bioprospecting 2013, 3, 93–98. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Tolueinia, B.; Hashemi, A.; Ebrahimi, L.; Fesahat, F. Antioxidant and cholinesterase inhibitory activity of a new peptide from Ziziphus jujuba fruits. Am. J. Alzheimer’s Dis. Other Dement. 2013, 28, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Del Marmol, V.; Beermann, F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef]
- Mermer, A.; Demirci, S. Recent advances in triazoles as tyrosinase inhibitors. Eur. J. Med. Chem. 2023, 259, 115655. [Google Scholar] [CrossRef]
- Priestley, G.C. Molecular Aspects of Dermatology; John Wiley & Sons: Hoboken, NJ, USA, 1993. [Google Scholar]
- Kim, Y.-J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. CMLS 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.M.; Hwang, Y.-H.; Yang, J.-H.; Ma, J.Y.; Lee, B. Spinosin is a flavonoid in the seed of Ziziphus jujuba that prevents skin pigmentation in a human skin model. J. Funct. Foods 2019, 54, 449–456. [Google Scholar] [CrossRef]
- Ratner, R.E. Controlling postprandial hyperglycemia. Am. J. Cardiol. 2001, 88, 26–31. [Google Scholar] [CrossRef] [PubMed]
- McCue, P.; KWON, Y.I.; Shetty, K. Anti-amylase, anti-glucosidase and anti-angiotensin i-converting enzyme potential of selected foods. J. Food Biochem. 2005, 29, 278–294. [Google Scholar] [CrossRef]
- Ohmura, C.; Tanaka, Y.; Mitsuhashi, N.; Atsum, Y.; Matsuoka, K.; Onuma, T.; Kawamori, R. Efficacy of low-dose metformin in Japanese patients with type 2 diabetes mellitus. Curr. Ther. Res. 1998, 59, 889–895. [Google Scholar] [CrossRef]
- Nilofar; Eyupoglu, O.E.; Nazzaro, F.; Fratianni, F.; Ahmed, S.; Ferrante, C.; Senkardes, I.; Zengin, G. An analytical framework combining online high-performance liquid chromatography methodologies and biological properties of different extracts of Leonurus cardiaca. J. Sep. Sci. 2023, 47, 2300695. [Google Scholar] [CrossRef]
- Rahman, M.M.; Dhar, P.S.; Anika, F.; Ahmed, L.; Islam, M.R.; Sultana, N.A.; Cavalu, S.; Pop, O.; Rauf, A. Exploring the plant-derived bioactive substances as antidiabetic agent: An extensive review. Biomed. Pharmacother. 2022, 152, 113217. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef] [PubMed]
- Small, D.H.; Michaelson, S.; Sberna, G. Non-classical actions of cholinesterases: Role in cellular differentiation, tumorigenesis and Alzheimer’s disease. Neurochem. Int. 1996, 28, 453–483. [Google Scholar] [CrossRef]
- Suksamrarn, S.; Saenkham, A.; Nakapong, S.; Lomchoey, N. Potent a–Glucosidase inhibitory activity of some Ziziphus plants. KKU Sci. J. 2017, 45, 438–446. [Google Scholar]
- Khaleel, S. Anti-oz-glucosidase, anti-o-amylase and antiinflammatory effects of leaf extracts of Ziziphus spina-christi (sedr) grown in Jordan. Res. J. Biol. Sci. 2018, 13, 1–7. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Iyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Gerlits, O.; Ho, K.-Y.; Cheng, X.; Blumenthal, D.; Taylor, P.; Kovalevsky, A.; Radić, Z. A new crystal form of human acetylcholinesterase for exploratory room-temperature crystallography studies. Chem.-Biol. Interact. 2019, 309, 108698. [Google Scholar] [CrossRef] [PubMed]
- Maurus, R.; Begum, A.; Williams, L.K.; Fredriksen, J.R.; Zhang, R.; Withers, S.G.; Brayer, G.D. Alternative Catalytic Anions Differentially Modulate Human α-Amylase Activity and Specificity. Biochemistry 2008, 47, 3332–3344. [Google Scholar] [CrossRef] [PubMed]
- Rosenberry, T.; Brazzolotto, X.; Macdonald, I.; Wandhammer, M.; Trovaslet-Leroy, M.; Darvesh, S.; Nachon, F. Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study. Molecules 2017, 22, 2098. [Google Scholar] [CrossRef] [PubMed]
- Ielo, L.; Deri, B.; Germanò, M.P.; Vittorio, S.; Mirabile, S.; Gitto, R.; Rapisarda, A.; Ronsisvalle, S.; Floris, S.; Pazy, Y.; et al. Exploiting the 1-(4-fluorobenzyl)piperazine fragment for the development of novel tyrosinase inhibitors as anti-melanogenic agents: Design, synthesis, structural insights and biological profile. Eur. J. Med. Chem. 2019, 178, 380–389. [Google Scholar] [CrossRef]
- Omer, H.A.A.; Caprioli, G.; Abouelenein, D.; Mustafa, A.M.; Uba, A.I.; Ak, G.; Ozturk, R.B.; Zengin, G.; Yagi, S. Phenolic Profile, Antioxidant and Enzyme Inhibitory Activities of Leaves from Two Cassia and Two Senna Species. Molecules 2022, 27, 5590. [Google Scholar] [CrossRef]
- Karade, S.S.; Hill, M.L.; Kiappes, J.L.; Manne, R.; Aakula, B.; Zitzmann, N.; Warfield, K.L.; Treston, A.M.; Mariuzza, R.A. N-Substituted Valiolamine Derivatives as Potent Inhibitors of Endoplasmic Reticulum α-Glucosidases I and II with Antiviral Activity. J. Med. Chem. 2021, 64, 18010–18024. [Google Scholar] [CrossRef]
- Birgül, K.; Yıldırım, Y.; Karasulu, H.Y.; Karasulu, E.; Uba, A.I.; Yelekçi, K.; Bekçi, H.; Cumaoğlu, A.; Kabasakal, L.; Yılmaz, Ö.; et al. Synthesis, molecular modeling, in vivo study and anticancer activity against prostate cancer of (+) (S)-naproxen derivatives. Eur. J. Med. Chem. 2020, 208, 112841. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Han, M.İ.; Bekçi, H.; Uba, A.I.; Yıldırım, Y.; Karasulu, E.; Cumaoğlu, A.; Karasulu, H.Y.; Yelekçi, K.; Yılmaz, Ö.; Küçükgüzel, Ş.G. Synthesis, molecular modeling, in vivo study, and anticancer activity of 1,2,4-triazole containing hydrazide–hydrazones derived from (S)-naproxen. Arch. Der Pharm. 2019, 352, 1800365. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]

| Parts | Methods | Solvent | TPC (mg GAE/g) | TFC (mg RE/g) |
|---|---|---|---|---|
| Leaves | HAE | MeOH | 112.01 ± 1.08 a | 51.91 ± 0.15 a |
| MAC | MeOH | 100.24 ± 0.86 c | 48.09 ± 0.20 b | |
| HAE | Water | 49.26 ± 0.10 d | 20.80 ± 0.45 c | |
| MAC | Water | 34.57 ± 0.53 g | 11.52 ± 0.40 c | |
| Bark | HAE | MeOH | 105.99 ± 0.92 b | 10.33 ± 0.06 d |
| MAC | MeOH | 101.02 ± 1.20 c | 6.95 ± 0.09 e | |
| HAE | Water | 45.48 ± 0.57 e | 4.35 ± 0.06 f | |
| MAC | Water | 42.78 ± 0.28 f | 2.23 ± 0.32 g |
| No. | Rt | MS | Formula | Name | Stem Bark Methanol | Stem Bark Water | Leaves Methanol | Leaves Water |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.56 | 173.0806 | C8H12O4 | octene dioic acid | x | x | x | x |
| 2 | 0.65 | 127.0752 | C7H13NO | Norhygrine | x | x | x | x |
| 3 | 2.65 | 289.1762 | C10H20N6O4 | Asparagylarginin | x | x | x | x |
| 4 | 3.35 | 609.1811 | C28H33O15 | Spinosin | x | x | ||
| 5 | 3.46 | 121.0508 | C7H7NO | Benzamide | x | x | x | x |
| 6 | 3.63 | 609.1811 | C28H33O15 | Isospinosin | x | x | x | |
| 7 | 3.81 | 579.1952 | C30H27O12 | Procyanidin B2 * | x | x | x | x |
| 8 | 3.9 | 465.1833 | C21H21O12 | Quercetin-3-O-glucoside * | x | x | ||
| 9 | 4.03 | 429.152 | C23H32N4O4 | Nummularine F | x | x | x | x |
| 10 | 4.08 | 319.117 | C17H18O6 | dimethyl catechin | x | x | x | x |
| 11 | 4.17 | 449.1952 | C21H21O11 | Quercetin-3-O-rhamnoside * | x | x | ||
| 12 | 4.29 | 593.123 | C32H41N5O6 | Nummularine B | x | x | x | x |
| 13 | 4.44 | 433.1402 | C18H24O12 | Apiosylglucosyl 4-hydroxybenzoate | x | x | ||
| 14 | 4.81 | 291.243 | C15H15O6 | Catechin * | x | x | x | x |
| 15 | 5.19 | 291.243 | C15H15O6 | Epicatechin * | x | x | x | x |
| 16 | 5.22 | 465.139 | C21H21O12 | Myricetin-3-O-rhamnoside * | x | x | ||
| 17 | 5.33 | 495.1501 | C23H26O12 | Pimentol | x | x | x | |
| 18 | 5.47 | 337.198 | C12H24N4O7 | Fructopyranosil arginine | x | x | x | |
| 19 | 5.93 | 489.2703 | C24H40O10 | alpha-Ionol O-[arabinosyl-(1->6)-glucoside] | x | x | ||
| 20 | 6.35 | 559.1371 | C30H38O10 | Secoisolariciresinol-sesquilignan | x | x | ||
| 21 | 6.48 | 471.2995 | C26H39N4O4 | Nummularine U | x | x | x | x |
| 22 | 6.61 | 559.1371 | C30H38O10 | Secoisolariciresinol-sesquilignan | x | x | ||
| 23 | 6.89 | 559.1371 | C30H38O10 | Secoisolariciresinol-sesquilignan | x | x | ||
| 24 | 7.04 | 158.1545 | C11H11N | dimethylquinoline | x | x | x | |
| 25 | 7.07 | 313.0702 | C13H12O9 | Caftaric acid * | x | x | ||
| 26 | 7.09 | 279.0953 | C10H18N2O5S | Methionyl glutamate | x | x | ||
| 27 | 7.35 | 343.1978 | C20H24NO4 | Magnoflorine | x | x | x | x |
| 28 | 7.5 | 287.056 | C15H10O6 | Luteolin * | x | x | ||
| 29 | 7.63 | 465.0923 | C21H21O13 | quercetin-hexoside | x | x | ||
| 30 | 7.97 | 327.0421 | C15H18O8 | Coumaroyl hexoside | X | x | ||
| 31 | 8.06 | 469.3521 | C31H48O3 | Methyl 3-oxo-12-oleanen-28-oate | x | x | X | x |
| 32 | 8.26 | 559.2542 | C30H38O10 | Secoisolariciresinol-sesquilignan | x | |||
| 33 | 8.26 | 595.189 | C27H31O15 | Quercetin-dirhamnoside | X | x | ||
| 34 | 8.36 | 307.0821 | C15H15O7 | Gallocatechin * | x | x | x | x |
| 35 | 8.96 | 437.1445 | C21H24O10 | Phloridzin * | x | x | X | x |
| 36 | 9.21 | 337,1062 | C16H16O8 | Caffeoyl shikimic acid | x | x | ||
| 37 | 9.74 | 609.2123 | C28H33O15 | Isorhamnetin-diglucoside | x | x | ||
| 38 | 9.83 | 315.1792 | C16H26O6 | Carveol hexoside | x | x | ||
| 39 | 9.86 | 559.2542 | C30H38O10 | Secoisolariciresinol-sesquilignan | x | |||
| 40 | 10.1 | 327.0421 | C15H18O8 | Coumaroyl hexoside | x | x | ||
| 41 | 11.17 | 301.141 | C14H20O7 | Methoxy-benzyl-hexoside | x | x | x | |
| 42 | 11.18 | 149.0238 | C5H8O3S | 2-oxo-4-methylthiobutanoate | x | x | ||
| 43 | 11.51 | 299.1839 | C19H22O3 | Auraptene | x | x | ||
| 44 | 12.41 | 801.598 | C44H81O10P | PG(18:3(9Z,12Z,15Z)/20:0 Phosphatidyl glycerol | x | |||
| 45 | 13.5 | 771.465 | C45H89NO8 | Phospholipid | x | x | x | |
| 46 | 13.6 | 850.6961 | C50H92NO7P | PC(o-22:1(13Z)/20:4(8Z,11Z,14Z,17Z)) | x | |||
| 47 | 13.61 | 299.1839 | C19H22O3 | Geranyl umbelliferone | x | x | ||
| 48 | 13.8 | 329.111 | C18H16O6 | 7,8,4′-Trimethylisoscutellarein | x | x | x | x |
| Name | Stem Bark Methanol HAE (mg/g) | Stem Bark Methanol MAC (mg/g) | Stem Bark Water HAE (mg/g) | Stem Bark Water MAC (mg/g) | Leaves Methanol HAE (mg/g) | Leaves Methanol MAC (mg/g) | Leaves Water HAE (mg/g) | Leaves Water MAC (mg/g) |
|---|---|---|---|---|---|---|---|---|
| Spinosin | 8.42 ± 0.25 a | 7.88 b ± 0.50 | 7.25 ± 0.20 c | 7.02 d ± 0.30 | ||||
| Isospinosin | 0.25 ± 0.22 | 0.21 ± 0.12 | 6.85 ± 0.22 a | 6.35 ± 0.22 b | 6.19 ± 0.11 c | 5.89 ± 0.32 d | ||
| Procyanidin B2 * | 1.22 ± 0.04 a | 1.04 ± 0.06 b | 0.54 ± 0.03 | 0.48 ± 0.13 | 0.68 ± 0.13 c | 0.53 ± 0.10 d | 0.34 ± 0.05 | 0.31 ± 0.05 |
| Quercetin-3-O-glucoside * | 6.29 ± 0.20 a | 6.01 ± 0.20 b | 4.13 ± 0.22 a | 3.99 ± 0.12 b | ||||
| dimethyl catechin | 0.28 ± 0.03 | 0.29 ± 0.03 | 0.13 ± 0.01 | 0.15 ± 0.01 | 0.31 ± 0.13 | 0.25 ± 0.03 | 0.26 ± 0.01 | 0.22 ± 0.01 |
| Quercetin-3-O-rhamnoside | 1.46 ± 0.10 a | 1.10 ± 0.21 b | 1.41 ± 0.19 a | 0.99 ± 0.14 b | ||||
| Catechin * | 2.51 ± 0.18 a | 2.23 ± 0.18 b | 1.41 ± 0.11 c | 1.24 ± 0.09 d | 6.89 ± 0.15 c | 6.35 ± 0.15 d | 5.71 ± 0.15 e | 5.16 ± 0.19 f |
| Epicatechin * | 3.62 ± 0.16 a | 3.19 ± 0.16 b | 1.58 ± 0.15 c | 1.30 ± 0.22 d | 6.72 ± 0.15 e | 6.20 ± 0.15 f | 4.77 ± 0.22 g | 4.19 ± 0.22 h |
| Myricetin-3-O-rhamnoside | 3.25 ± 0.15 a | 3.11 ± 0.08 b | 2.85 ± 0.12 c | 2.35 ± 0.12 d | ||||
| Luteolin * | 0.34 ± 0.02 | 0.32 ± 0.02 | 0.12 ± 0.05 | 0.10 ± 0.05 | ||||
| Gallocatechin | 1.39 ± 0.11 a | 1.21 ± 0.10 b | 0.78 ± 0.10 | 0.64 ± 0.10 | 0.49 ± 0.05 c | 0.39 ± 0.02 d | 0.36 ± 0.05 | 0.31 ± 0.05 |
| Auraptene | 0.21 ± 0.05 a | 0.20 ± 0.09 a | 0.13 ± 0.03 b | 0.16 ± 0.06 b | ||||
| Geranyl umbelliferone | 0.13 ± 0.05 | 0.10 ± 0.05 | 0.08 ± 0.05 | 0.06 ± 0.05 | ||||
| 7,8,4′-Trimethylisoscutellarein | 0.15 ± 0.02 | 0.15 ± 0.02 | 0.07 ± 0.02 | 0.07 ± 0.02 | 0.34 ± 0.08 | 0.28 ± 0.07 | 0.07 ± 0.02 | 0.06 ± 0.02 |
| Parts | Methods | Solvent | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | PBD (mmol TE/g) | MCA (mg EDTAE/g) |
|---|---|---|---|---|---|---|---|---|
| Leaves | HAE | MeOH | 414.30 ± 4.73 a | 747.25 ± 5.34 a | 698.46 ± 4.48 b | 325.59 ± 2.99 a | 3.91 ± 0.25 a | 10.17 ± 0.62 d |
| MAC | MeOH | 331.83 ± 4.49 d | 545.97 ± 5.01 d | 693.98 ± 2.92 b | 265.05 ± 3.19 c | 3.16 ± 0.10 b | 11.79 ± 0.64 d | |
| HAE | Water | 92.32 ± 9.99 e | 226.51 ± 3.67 e | 262.89 ± 2.47 c | 119.78 ± 3.07 d | 1.29 ± 0.11 d | 15.18 ± 0.61 c | |
| MAC | Water | 33.90 ± 1.29 f | 67.96 ± 1.65 g | 102.33 ± 0.50 e | 36.00 ± 0.44 f | 0.81 ± 0.04 e | 31.71 ± 0.72 b | |
| Bark | HAE | MeOH | 365.67 ± 3.74 b | 630.63 ± 6.87 b | 761.14 ± 22.58 a | 279.64 ± 4.44 b | 3.11 ± 0.25 bc | 14.60 ± 0.72 c |
| MAC | MeOH | 348.11 ± 1.53 c | 601.00 ± 9.43 c | 747.38 ± 5.34 a | 277.69 ± 5.74 b | 2.67 ± 0.20 c | 14.90 ± 1.35 c | |
| HAE | Water | 103.25 ± 6.25 e | 189.52 ± 11.48 f | 259.43 ± 1.67 c | 114.96 ± 1.47 d | 1.07 ± 0.07 de | 30.71 ± 0.10 b | |
| MAC | Water | 13.92 ± 1.43 g | 65.58 ± 4.19 g | 203.86 ± 3.60 d | 88.11 ± 0.91 e | 0.84 ± 0.02 e | 35.23 ± 0.27 a |
| Parts | Methods | Solvent | AChE (mg GALAE/g) | BChE (mg GALAE/g) | Tyrosinase (mg KAE/g) | Amylase (mmol ACAE/g) | Glucosidase (mmol ACAE/g) |
|---|---|---|---|---|---|---|---|
| Leaves | HAE | MeOH | 2.55 ± 0.02 a | Na | 75.97 ± 0.73 a | 0.78 ± 0.01 a | 2.07 ± 0.01 b |
| MAC | MeOH | 2.14 ± 0.08 c | Na | 73.79 ± 0.44 a | 0.76 ± 0.01 a | 2.11 ± 0.01 a | |
| HAE | Water | 0.09 ± 0.01 d | Na | 47.53 ± 2.08 b | 0.25 ± 0.01 d | 2.11 ± 0.01 a | |
| MAC | Water | Na | Na | 33.47 ± 0.30 c | 0.15 ± 0.01 e | 0.20 ± 0.02 d | |
| Bark | HAE | MeOH | 2.39 ± 0.06 b | 1.57 ± 0.13 a | 75.48 ± 0.08 a | 0.72 ± 0.02 b | na |
| MAC | MeOH | 2.41 ± 0.02 b | 1.14 ± 0.36 b | 74.95 ± 0.74 a | 0.69 ± 0.02 c | na | |
| HAE | Water | Na | Na | 28.56 ± 3.02 d | 0.13 ± 0.01 e | 1.87 ± 0.01 c | |
| MAC | Water | Na | Na | 13.55 ± 0.42 e | 0.16 ± 0.01 e | 1.87 ± 0.01 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nilofar; Sinan, K.I.; Dall’Acqua, S.; Sut, S.; Uba, A.I.; Etienne, O.K.; Ferrante, C.; Ahmad, J.; Zengin, G. Ziziphus mauritiana Lam. Bark and Leaves: Extraction, Phytochemical Composition, In Vitro Bioassays and In Silico Studies. Plants 2024, 13, 2195. https://doi.org/10.3390/plants13162195
Nilofar, Sinan KI, Dall’Acqua S, Sut S, Uba AI, Etienne OK, Ferrante C, Ahmad J, Zengin G. Ziziphus mauritiana Lam. Bark and Leaves: Extraction, Phytochemical Composition, In Vitro Bioassays and In Silico Studies. Plants. 2024; 13(16):2195. https://doi.org/10.3390/plants13162195
Chicago/Turabian StyleNilofar, Kouadio Ibrahime Sinan, Stefano Dall’Acqua, Stefania Sut, Abdullahi Ibrahim Uba, Ouattara Katinan Etienne, Claudio Ferrante, Jamil Ahmad, and Gokhan Zengin. 2024. "Ziziphus mauritiana Lam. Bark and Leaves: Extraction, Phytochemical Composition, In Vitro Bioassays and In Silico Studies" Plants 13, no. 16: 2195. https://doi.org/10.3390/plants13162195
APA StyleNilofar, Sinan, K. I., Dall’Acqua, S., Sut, S., Uba, A. I., Etienne, O. K., Ferrante, C., Ahmad, J., & Zengin, G. (2024). Ziziphus mauritiana Lam. Bark and Leaves: Extraction, Phytochemical Composition, In Vitro Bioassays and In Silico Studies. Plants, 13(16), 2195. https://doi.org/10.3390/plants13162195










