The Influence of Chitosan Derivatives in Combination with Bacillus subtilis Bacteria on the Development of Systemic Resistance in Potato Plants with Viral Infection and Drought
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant, Microbe, and Virus
2.2. Obtaining Conjugates of Chitosan with Hydroxycinnamic Acids
2.3. Detection of PVY by ELISA
2.4. Proline Content
2.5. Water Deficiency Application
2.6. Quantitative Real-Time PCR (qPCR) to Investigate Virus Accumulation
2.7. RNA Extraction and Real-Time PCR
2.8. Measuring of Protein Content
2.9. Two-Dimensional Electrophoresis
2.10. Mass Spectrometry
2.11. Statistical Processing
3. Results
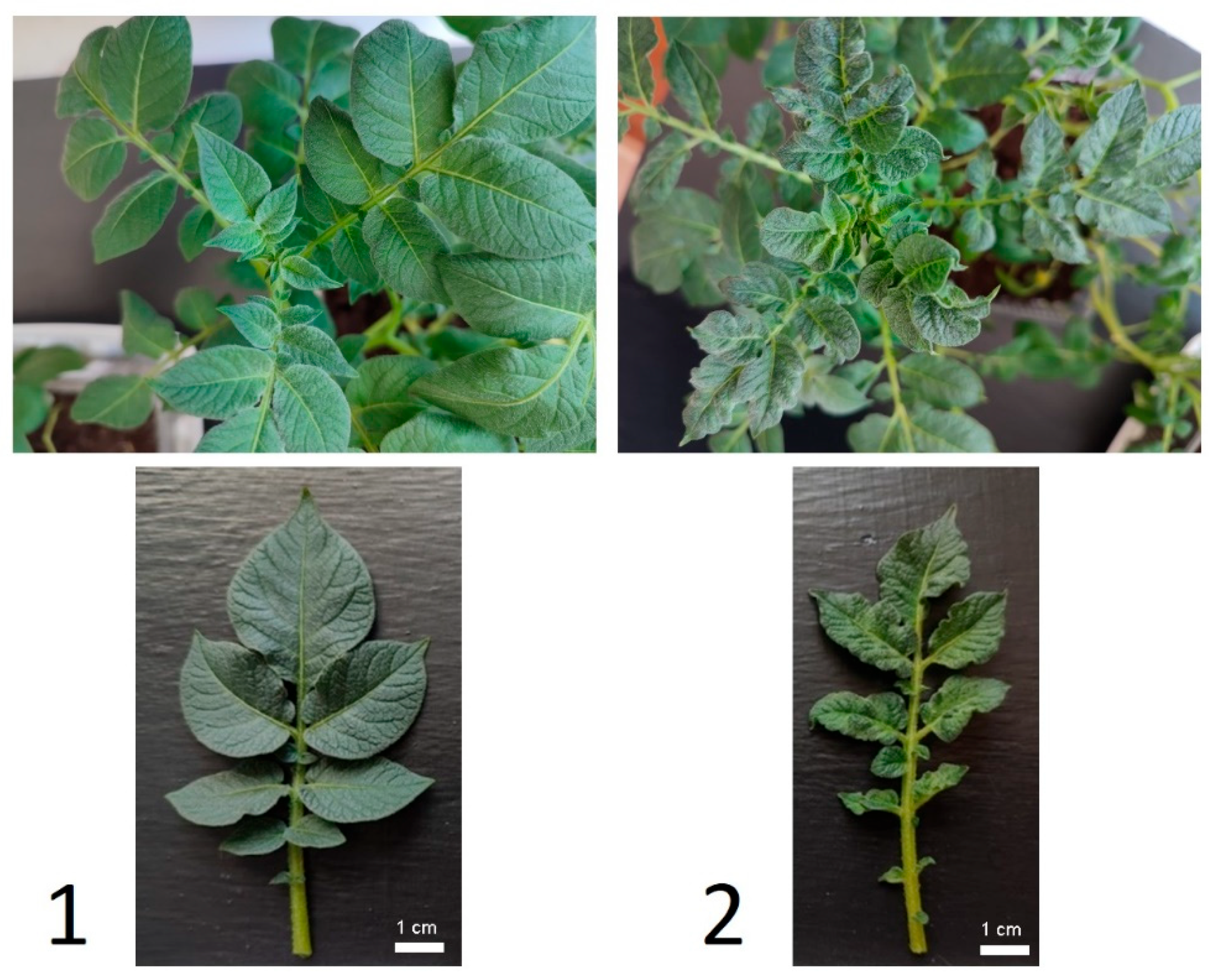
3.1. Susceptibility of Leaves to PVY
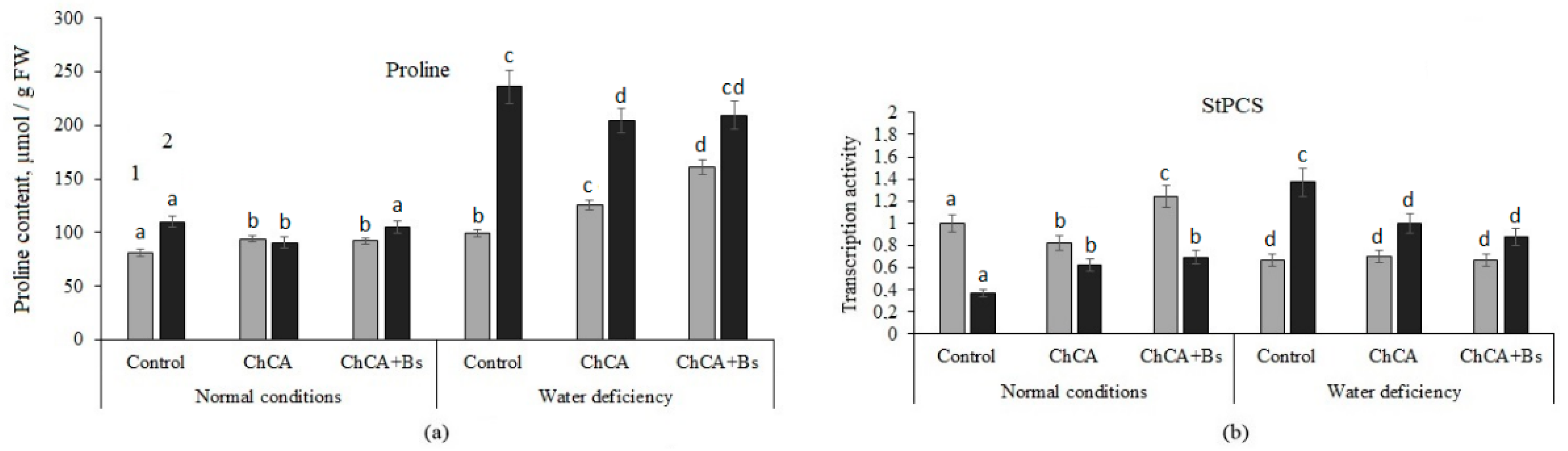
3.2. Content of Proline and Proline Synthase Activity
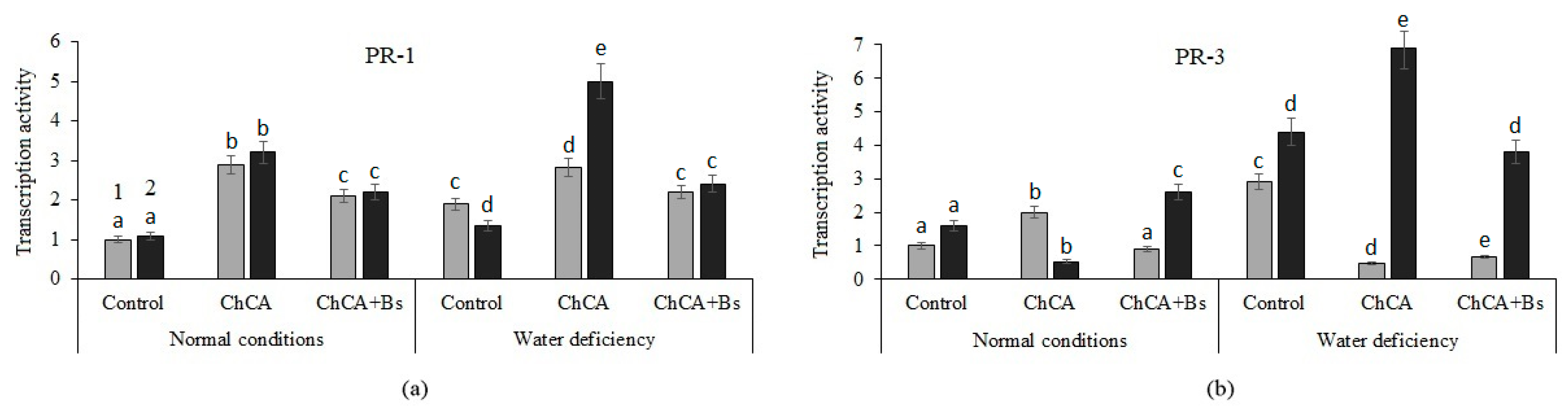
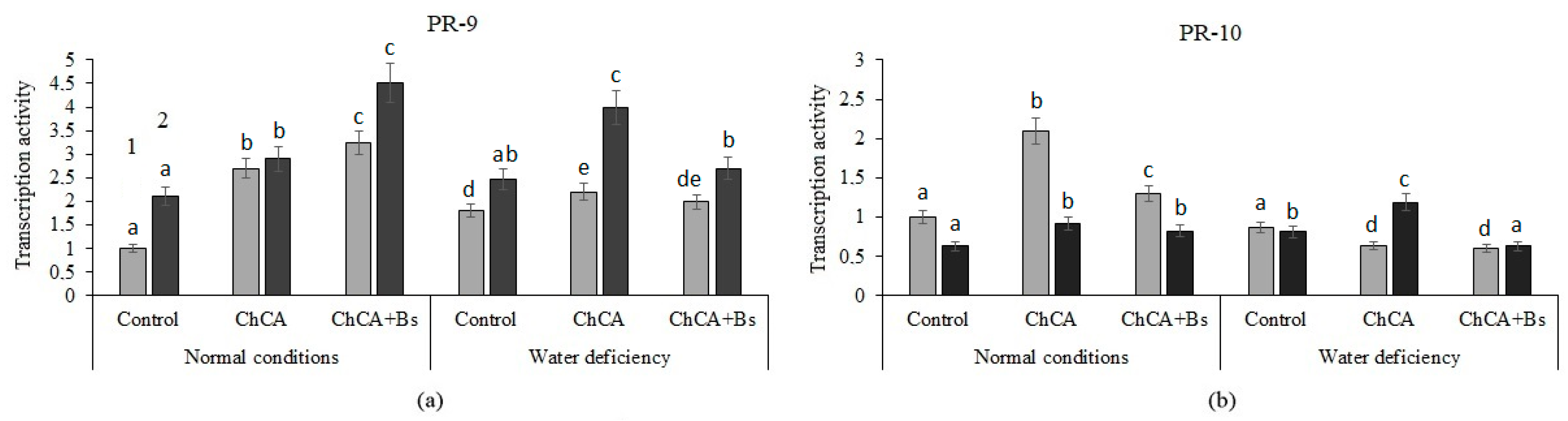
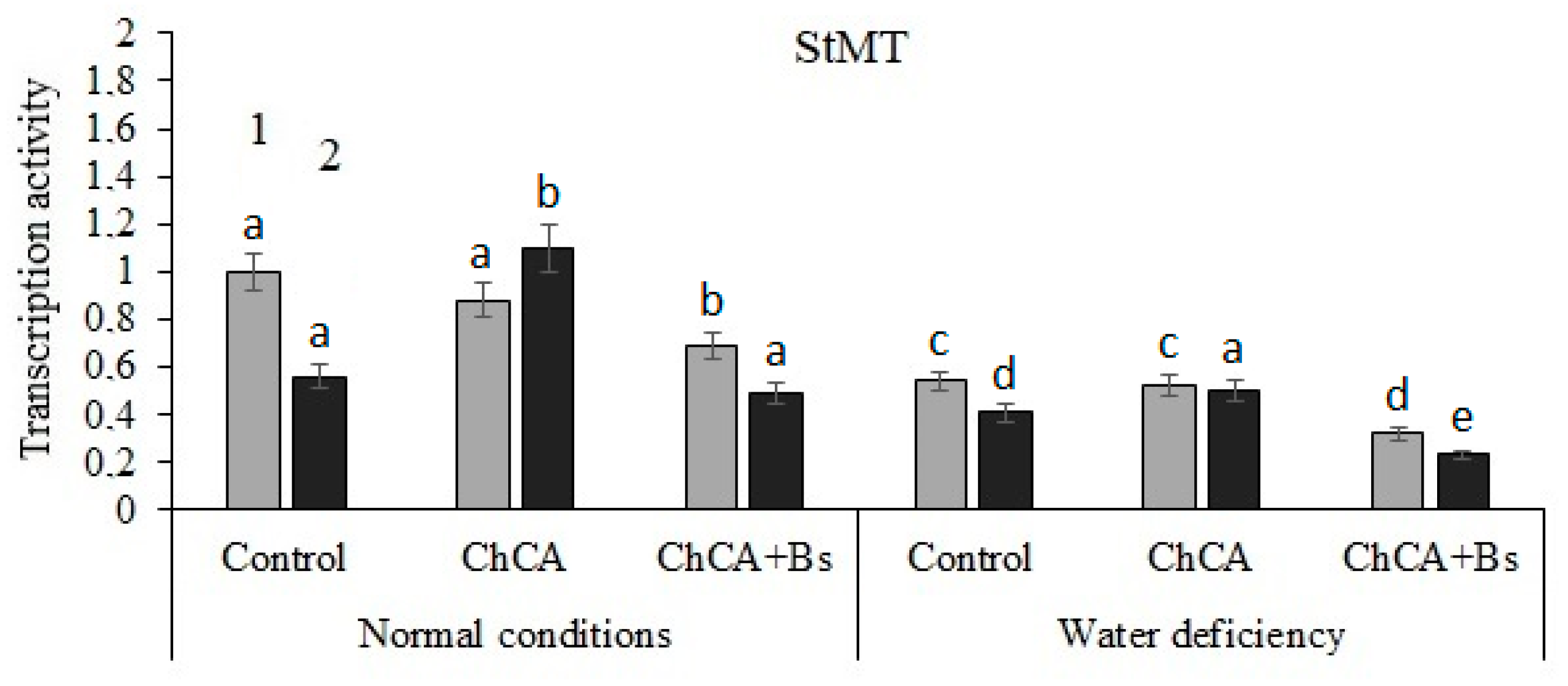
3.3. Transcriptional Activity of PR-Genes
3.4. Proteome
4. Discussion
4.1. Susceptibility of Leaves to PVY
4.2. Content of Proline and Proline Synthase Activity
4.3. Transcriptional Activity of PR-Genes
4.4. Proteome
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Maksimov, I.V.; Abizgil’dina, R.R.; Pusenkova, L.I. Plant growth promoting rhizobacteria as alternative to chemical crop protectors from pathogens (review). Appl. Biochem. Microbiol. 2011, 47, 333–345. [Google Scholar] [CrossRef]
- Rkhaila, A.; Chtouki, T.; Erguig, H.; El Haloui, N.; Ounine, K. Chemical Proprieties of Biopolymers (Chitin/Chitosan) and Their Synergic Effects with Endophytic Bacillus Species: Unlimited Applications in Agriculture. Molecules 2021, 26, 1117. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Razdan, N.; Saharan, K. Rhizospheric microbiome: Bio-based emerging strategies for sustainable agriculture development and future perspectives. Microbiol. Res. 2022, 254, 126901. [Google Scholar] [CrossRef] [PubMed]
- Pinski, A.; Betekhtin, A.; Hupert-Kocurek, K.; Mur, L.A.J.; Hasterok, R. Defining the Genetic Basis of Plant-Endophytic Bacteria Interactions. Int. J. Mol. Sci. 2019, 20, 1947. [Google Scholar] [CrossRef]
- Novikova, I.I.; Popova, E.V.; Krasnobaeva, I.L.; Kovalenko, N.M. Biological background to using chitosan inducers to increase the efficiency of biofungicides. Sel’skokhozyaistvennaya Biol. Agric. Biol. 2021, 56, 511–522. [Google Scholar] [CrossRef]
- Varlamov, V.P.; Ilyina, A.V.; Shagdarova, B.T.; Lunkov, A.P.; Mysyakina, I.S. Chitin/Chitosan and Its Derivatives: Fundamental Problems and Practical Approaches. Biochemistry 2020, 85, 154–176. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Preparation, characterization and antioxidant property of water-soluble ferulic acid grafted chitosan. Carbohydr. Polym. 2013, 96, 495–502. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.F.; Kan, J.; Tang, Y.Q.; Jin, C.H. Preparation, characterization and antioxidant activity of phenolic acids grafted carboxymethyl chitosan. Int. J. Biol. Macromol. 2013, 62, 85–93. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Sharma, M.; Mallubhotla, S. Diversity, Antimicrobial Activity, and Antibiotic Susceptibility Pattern of Endophytic Bacteria Sourced from Cordia dichotoma L. Front. Microbiol. 2022, 13, 879386. [Google Scholar] [CrossRef] [PubMed]
- Ilyasoglu, H.; Guo, Z. Characterization of different phenolic acids grafted chitosan and their application for Japanese sea bass (Lateolabrax japonicus) fillets preservation. Food Biosci. 2019, 29, 118–125. [Google Scholar] [CrossRef]
- Canton, H.; Food and Agriculture Organization of the United Nations—FAO. The Europa Directory of International Organizations; Routledge: Milton Park, UK, 2021; pp. 297–305. [Google Scholar]
- Vilvert, E.; Stridh, L.; Andersson, B.; Olson, Å.; Aldén, L.; Berlin, A. Evidence based disease control methods in potato production: A systematic map protocol. Environ. Evid. 2022, 11, 6. [Google Scholar] [CrossRef]
- Ramegowda, V.; Senthil-Kumar, M. The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 2015, 176, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sonnewald, U. Differences and commonalities of plant responses to single and combined stresses. Plant J. 2017, 90, 839–855. [Google Scholar] [CrossRef]
- Hamooh, B.T.; Sattar, F.A.; Wellman, G.; Mousa, M.A.A. Metabolomic and Biochemical Analysis of Two Potato (Solanum tuberosum L.) Cultivars Exposed to In Vitro Osmotic and Salt Stresses. Plants 2021, 10, 98. [Google Scholar] [CrossRef]
- Palazzini, J.; Reynoso, A.; Yerkovich, N.; Zachetti, V.; Ramírez, M.; Chulze, S. Combination of Bacillus velezensis RC218 and Chitosan to Control Fusarium Head Blight on Bread and Durum Wheat under Greenhouse and Field Conditions. Toxins 2022, 14, 499. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Ezziyyani, M.; Sánchez, C.P.; Candela, M.E. Effect of chitin on biological control activity of Bacillus spp. and Trichoderma harzianum against root rot disease in pepper (Capsicum annuum) plants. Eur. J. Plant Pathol. 2003, 109, 633–637. [Google Scholar] [CrossRef]
- Qi, J.; Song, C.P.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.K.; Gong, Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef]
- Samaras, A.; Kamou, N.; Tzelepis, G.; Karamanoli, K.; Menkissoglu-Spiroudi, U.; Karaoglanidis, G.S. Root Transcriptional and Metabolic Dynamics Induced by the Plant Growth Promoting Rhizobacterium (PGPR) Bacillus subtilis Mbi600 on Cucumber Plants. Plants 2022, 11, 1218. [Google Scholar] [CrossRef]
- Fabro, G.; Kovács, I.; Pavel, V.; Szabados, L.; Alvarez, M.E. Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. Mol. Plant Microb. Intrract. 2004, 17, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Seifikalhor, M.; Aliniaeifard, S.; Baymiev, A.; Pusenkova, L.; Garipova, S.; Kulabuhova, D.; Maksimov, I. Bacillus spp.: Efficient Biotic Strategy to Control Postharvest Diseases of Fruits and Vegetables. Plants 2019, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Harish Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; Mandzhieva, S. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Daphne Désiré, A.-L.; Elodie Rosette, M.A.-L. Chapter 2.2-Catalase. In Antioxidants Effects in Health; Elsevier: Amsterdam, The Netherlands, 2022; pp. 81–90. [Google Scholar] [CrossRef]
- Bakalova, S.; Nikolova, A.; Wedera, D. Isoenzyme profiles of peroxidase, catalase and superoxide dismutase as affected by dehydration stress and ABA during germination of wheat seeds. J. Plant Physiol. 2004, 30, 64–77. [Google Scholar] [CrossRef]
- Luna, C.M.; Pastori, G.M.; Driscoll, S.; Groten, K.; Bernard, S.; Foyer, C.H. Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J. Exp. Bot. 2005, 56, 417–423. [Google Scholar] [CrossRef]
- Haider, S.; Iqbal, J.; Naseer, S.; Yaseen, T.; Shaukat, M.; Bibi, H.; Ahmad, Y.; Daud, H.; Abbasi, N.L.; Mahmood, T. Molecular Mechanisms of Plant Tolerance to Heat Stress: Current Landscape and Future Perspectives. Plant Cell Rep. 2021, 40, 2247–2271. [Google Scholar] [CrossRef]
- Tarchevsky, I.A.; Egorova, A.M. Participation of Proline in Plant Adaptation to Stress Factors and Its Application in Agrobiotechnology (Review). Appl. Biochem. Microbiol. 2022, 58, 347–360. [Google Scholar] [CrossRef]
- Choi, D.S.; Hwang, I.S.; Hwang, B.K. The hypersensitive induced reaction and leucine-rich repeat proteins regulate plant cell death associated with disease and plant immunity. Plant Cell 2012, 24, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- Yarullina, L.G.; Burkhanova, G.F.; Cherepanova, E.A.; Sorokan, A.V.; Zaikina, E.A.; Tsvetkov, V.O.; Mardanshin, I.S.; Kalatskaya, J.N.; Balyuk, N.V. Effect of Bacillus subtilis and signaling molecules on the state of the pro/antioxidant system and the expression of protective protein genes in potato plants upon phytophthorosis and a moisture deficit. Appl. Biochem. Microbiol. 2021, 57, 760–769. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive methods for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.-A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting ready for battle. Mol. Plant Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, I.V.; Singh, B.P.; Cherepanova, E.A.; Burkhanova, G.F.; Khairullin, R.M. Prospects and Applications of Lipopeptide-Producing Bacteria for Plant Protection (Review). Appl. Biochem. Microbiol. 2020, 56, 15–28. [Google Scholar] [CrossRef]
- Gonzalez-Gallegos, E.; Laredo-Alcala, E.; Ascacio-Valdes, J.; de Rodriguez, D.; Hernandez-Castillo, F. Changes in the production of salicylic and jasmonic acid in potato plants (Solanum tuberosum) as response to foliar application of biotic and abiotic inductors. Am. J. Plant Sci. 2015, 6, 1785–1791. [Google Scholar] [CrossRef]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Application of Developmental Regulators for Enhancing Plant Regeneration and Genetic Transformation. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Babaki, S.A.; Barka, E.A. Chitosan as potential natural compound to manage plant diseases. Int. J. Biol. Macromol. 2022, 220, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Fernandez, M.; Marhuenda-Egea, F.C.; Lopez-Moya, F.; Arnao, M.B.; Cabrera-Escribano, F.; Nueda, M.J.; Gunsé, B.; Lopez-Llorca, L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Front. Plant Sci. 2020, 11, 572087. [Google Scholar] [CrossRef]
- Chakraborty, M.; Hasanuzzaman, M.; Rahman, M.; Khan, A.R.; Bhowmik, P.; Mahmud, N.U.; Tanveer, M.; Islam, T. Varietal specificity of opto-biological properties and phytometric parameters of winter wheat crops. Agriculture 2020, 10, 624. [Google Scholar] [CrossRef]
- Bordiec, S.; Paquis, S.; Lacroix, H.; Dhondt, S.; Barka, E.A.; Kauffmann, S.; Jeandet, P.; Mazeyrat-Gourbeyre, F.; Clément, C.; Baillieul, F.; et al. Comparative analysis of defence responses induced by the endophytic plant growth-promoting rhizobacterium Burkholderia phytofirmans strain PsJN and the non-host bacterium Pseudomonas syringae pv. pisi in grapevine cell suspensions. J. Exp. Bot. 2011, 62, 595–603. [Google Scholar] [CrossRef]
- Pfannschmidt, T.; Brautigam, K.; Wagner, R.; Dietzel, L.; Schroter, Y.; Steiner, S.; Nykytenko, A. Photosynthetic redox control of nuclear gene expression. Ann. Bot. 2009, 103, 599–607. [Google Scholar] [CrossRef]
- Chen, F.; Wang, M.; Zhang, Y.; Luo, J.; Yang, X.; Wang, X. Quantitative changes of plant defense enzymes and phytohormone in biocontrol of cucumber Fusarium wilt by Bacillus subtilis B579. World J. Microbiol. Biotechnol. 2010, 26, 675–684. [Google Scholar] [CrossRef]
- Vasyukova, N.I.; Ozeretskovskaya, O.L. Jasmonate-dependent defense signaling in plant tissues. Russ. J. Plant Physiol. 2009, 56, 581–590. [Google Scholar] [CrossRef]
- He, M.; Xu, Y.; Cao, J. Subcellular localization and functional analyses of a PR10 protein gene from Vitis pseudoreticulata in response to Plasmopara viticola infection. Protoplasma 2013, 250, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Gagné-Bourque, F.; Mayer, B.F. Alleviation of drought stress and metabolic changes in timothy (Phleum pratense L.) colonized with Bacillus subtilis B26. PLoS ONE 2015, 10, e0130456. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, C. Chloroplast Chaperonin: An Intricate Protein Folding Machine for Photosynthesis. Front. Mol. Biosci. 2018, 4, 98. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Mueller-Cajar, O.; Saschenbrecker, S.; Hartl, F.U.; Hayer-Hartl, M. Chaperonin cofactors, Cpn10 and Cpn20, of green algae and plants function as hetero-oligomeric ring complexes. J. Biol. Chem. 2012, 287, 20471. [Google Scholar] [CrossRef] [PubMed]
- Ranford, J.C.; Henderson, B. Chaperonins in disease: Mechanisms, models, and treatments. Mol. Pathol. 2002, 55, 209. [Google Scholar] [CrossRef] [PubMed]
- Peart, J.; Mestre, P.; Lu, R.; Malcuit, I.; Baulcombe, D. NRG1, a CC-NB-LRR Protein, together with N, a TIR-NB-LRR Protein, Mediates Resistance against Tobacco Mosaic Virus. Curr. Biol. 2005, 15, 968–973. [Google Scholar] [CrossRef]
- Dinesh-Kumar, S.P.; Tham, W.H.; Baker, B.J. Structure-function analysis of the tobacco mosaic virus resistance gene N. Proc. Natl. Acad. Sci. USA 2000, 97, 14789–14794. [Google Scholar] [CrossRef] [PubMed]
- Marathe, R.; Anandalakshmi, R.; Liu, Y.; Dinesh-Kumar, S.P. The tobacco mosaic virus resistance gene, N. Mol. Plant Pathol. 2002, 3, 167–172. [Google Scholar] [CrossRef]
- Do, H.; Kim, I.S.; Jeon, B. Structural understanding of the recycling of oxidized ascorbate by dehydroascorbate reductase (OsDHAR) from Oryza sativa L. japonica. Sci. Rep. 2016, 6, 19498. [Google Scholar] [CrossRef]
- Loi, M.; Leonardis, S.; Mulè, G.; Logrieco, A.F.; Paciolla, C. A Novel and Potentially Multifaceted Dehydroascorbate Reductase Increasing theAntioxidant Systems Is Induced by Beauvericinin Tomato. Antioxidants 2020, 9, 435. [Google Scholar] [CrossRef]
- Ding, H.; Wang, B.; Han, Y.; Li, S. The pivotal function of dehydroascorbate reductase in glutathione homeostasis in plants. J. Exp. Bot. 2020, 71, 3405–3416. [Google Scholar] [CrossRef] [PubMed]
- Lingmo, C.; Huan, S.; Hua, Y.; Xuehua, W.; Zhizhe, S.; Fang, C.; Wei, W. Over-expression of dehydroascorbate reductase enhances oxidative stress tolerance in tobacco. Electron. J. Biotechnol. 2017, 25, 1–8. [Google Scholar] [CrossRef]
- Chavan, S.N.; De Kesel, J.; Desmedt, W.; Degroote, E.; Singh, R.R.; Nguyen, G.T.; Demeestere, K.; De Meyer, T.; Kyndt, T. Dehydroascorbate induces plant resistance in rice against root-knot nematode Meloidogyne graminicola. Mol. Plant Pathol. 2022, 23, 1303–1319. [Google Scholar] [CrossRef]
- Bhavsar, R.B.; Makley, L.N.; Tsonis, P.A. The other lives of ribosomal proteins. Hum. Genom. 2010, 4, 327. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.; Quan, Y.; Arrivé, M.; Waltz, F.; Da Silva, N.; Jomat, L.; Cohen, M.; Jupin, I.; Giegé, P. Towards plant resistance to viruses using protein-only RNase P. Nat. Commun. 2021, 12, 1007. [Google Scholar] [CrossRef]
- Li, W.; Xiong, Y.; Lai, L.B.; Zhang, K.; Li, Z.; Kang, H.; Dai, L.; Gopalan, V.; Wang, G.L.; Liu, W. The rice RNase P protein subunit Rpp30 confers broad-spectrum resistance to fungal and bacterial pathogens. Plant Biotechnol. J. 2021, 19, 1988–1999. [Google Scholar] [CrossRef] [PubMed]
- Arrivé, M.; Bruggeman, M.; Skaltsogiannis, V.; Coudray, L.; Quan, Y.F.; Schelcher, C.; Cognat, V.; Hammann, P.; Chicher, J.; Wolff, P.; et al. tRNA-modifying enzyme facilitates RNase P activity in Arabidopsis nuclei. Nat. Plants 2023, 9, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.; Kaitany, k.; Kelly, A.; Yacoub, M.; Koutmos, M. The protein-only RNase Ps, endonucleases that cleave pre-tRNA: Biological relevance, molecular architectures, substrate recognition and specificity, and protein interactomes. WIREs RNA 2024, 15, e1836. [Google Scholar] [CrossRef]
- Sugawara, T.; Trifonova, E.A.; Kochetov, A.V. Expression of an extracellular ribonuclease gene increases resistance to Cucumber mosaic virus in tobacco. BMC Plant Biol. 2024, 16 (Suppl. S3), 246. [Google Scholar] [CrossRef]
- Possa, K.F.; Silva, J.A.G.; Resende, M.L.V.; Tenente, R.; Pinheiro, C.; Chaves, I.; Planchon, S.; Monteiro, A.C.A.; Renaut, J.; Carvalho, M.A.F.; et al. Primary Metabolism Is Distinctly Modulated by Plant Resistance Inducers in Coffea arabica Leaves Infected by Hemileia vastatrix. Front. Plant Sci. 2020, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Rego, A.; Mora-Ocampo, I.; Pirovani, C.; Luz, E.; Corrêa, R. Protein Level Defense Responses of Theobroma cacao Interaction with Phytophthora palmivora. Front. Agron. 2022, 4, 836360. [Google Scholar] [CrossRef]
- Peng, X.; Yu, D.; Yan, J.; Zhang, N.; Lin, J.; Wang, J. Physiological and Proteomic Analyses Reveal Adaptive Mechanisms of Ryegrass (Annual vs. Perennial) Seedlings to Salt Stress. Agronomy 2019, 9, 843. [Google Scholar] [CrossRef]
- Zadražnik, T.; Moen, A.; Šuštar-Vozlič, J. Chloroplast proteins involved in drought stress response in selected cultivars of common bean (Phaseolus vulgaris L.). 3 Biotech 2019, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, K.; Lin, B.; Mauch, F. The chloroplast protein RPH1 plays a role in the immune response of Arabidopsis to Phytophthora brassicae. Plant J. 2009, 58, 278. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Goswami, S.; Kumar, R.R.; Singh, K.; Singh, J.P.; Kumar, A.; Sakhrey, A.; Ra, G.K.; Praveen, S. Wheat Oxygen Evolving Enhancer Protein: Identification and Characterization of Mn-Binding Metalloprotein of Photosynthetic Pathway Involved in Regulating Photosytem II Integrity and Network of Antioxidant Enzymes under Heat Stress. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 177. [Google Scholar] [CrossRef]
- Battle, D.; Doudna, J. The stem-loop binding protein forms a highly stable and specific complex with the 3′ stem-loop of histone mRNAs. RNA 2001, 7, 123–132. [Google Scholar] [CrossRef]
- Zheng, L.; Dominski, Z.; Yang, X.C.; Elms, P.; Raska, C.S.; Borchers, C.H.; Marzluff, W.F. Phosphorylation of stem-loop binding protein (SLBP) on two threonines triggers degradation of SLBP, the sole cell cycle-regulated factor required for regulation of histone mRNA processing, at the end of S phase. Mol. Cell. Biol. 2003, 23, 1590–1601. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.J.; Hoyle, J.; Valdivia, L.E.; Cerveny, K.L.; Hart, W.; Mangoli, M.; Geisler, R.; Rees, M.; Houart, C.; Poole, R.J.; et al. Abrogation of Stem Loop Binding Protein (Slbp) function leads to a failure of cells to transition from proliferation to differentiation, retinal coloboma and midline axon guidance deficits. PLoS ONE 2019, 14, e0211073. [Google Scholar] [CrossRef]
- Berka, M.; Kopecká, R.; Berková, V.; Brzobohatý, B.; Černý, M. Regulation of heat shock proteins 70 and their role in plant immunity. J. Exp. Bot. 2022, 73, 1894–1909. [Google Scholar] [CrossRef]


| Product | Gene | NCBI Number | Direct Primer | Reverse Primer |
|---|---|---|---|---|
| Actin | StAct | X55749 | gat-ggt-gtc-agc-cac-ac | att-cca-gca-gct-tcc-att-cc |
| PR-1 | StPR1 | AY050221 | tgg-gtg-gtg-gtt-cat-ttc-ttg-t | cat-tta-att-cct-tac-aca-tca-taa-g |
| Chitinase, PR-3 | StPR3 | U49970 | ttc-tgg-atg-aca-gca-cag-gat | ggc-gtc-cat-tgc-cca-at |
| Thaumatin-like protein, PR-5 | StPR5 | AY737317 | ccc-gtt-tga-cat-tga-cct-ttg | cga-ata-cgg-tgg-aac-atg-ga |
| Proteinase inhibitor, PR-6 | StPR6 | JX683427 | ggg-aaa-gaa-tat-gct-caa-gtt-at | aat-tct-cca-tca-tct-tcc-act-g |
| Peroxidase, PR-9 | StPR9 | M21334 | gta-atc-ctg-ccg-cac-aac-t | gca-gca-aaa-tct-cca-agg-aa |
| Ribonuclease, PR-10 | StPR10 | AF500589 | ctc-gct-aac-cct-tct-gtc-tat-g | caa-cac-gtc-ctg-atc-atc-tct-c |
| Methyl transferase | StMT | XM_006356514 | ggc-aat-gga-cat-taa-ccg | tca-aga-aga-ggc-aaa-gca-g |
| Proline carboxylate synthase | StPCS | XM015308529 | tta-aag-agg-acg-gag-ctt-gc | cag-tgc-atc-agg-tcg-tga-ct |
| Protein Name in Uniprot | pI | MW | Protein Content, μg/g Fresh Weight | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal Conditions | Soil Moisture Deficiency | |||||||||||||
| C | ChCA | Bs ChCA | PVY | PVY ChCA | PVY Bs ChCA | C | ChCA | Bs ChCA | PVY | PVY ChCA | PVY Bs ChCA | |||
| 20 kDa chaperonin | 5 | 25 | 14.7 | 12 | 10.8 | 6.8 | 3.1 | 3.8 | 4.8 | 8.4 | 3.7 | 4.6 | 9.2 | 10 |
| TMV resistance protein N-like | 6 | 25 | 9.6 | 4.3 | 6.8 | 9.4 | 4.4 | 5 | 4.6 | 8.8 | 4.1 | 4.7 | 8.2 | 6 |
| Dehydroascorbate reductase | 6 | 25 | 8 | 10 | 4 | 5 | 4.2 | 4.2 | 3.3 | 3.7 | 4.5 | 4.3 | 4.6 | 4.2 |
| 50S ribosomal protein L4 | 6 | 30 | 18.3 | 7.4 | 7.4 | 7.4 | 12.4 | 9.4 | 12.4 | 7.6 | 7.1 | 7.1 | 8 | 15 |
| Proteinaceous RNase P 1 | 6.5 | 35 | 3.3 | 3.1 | 4.9 | 3 | 7.4 | - | 9 | 4 | 6.2 | - | 3.5 | 4.7 |
| 2-methylene-furan-3-one reductase | 6 | 40 | 11.6 | 11 | 11 | 3.7 | 12.8 | 13.4 | 18.5 | 19 | - | - | 3.8 | 3.1 |
| Oxygen-evolving enhancer protein 2 | 5 | 25 | 9.6 | 4.4 | 3.5 | 5 | 7.2 | 6.8 | 8.2 | 9 | - | - | - | - |
| Stem-loop binding protein of 41 kDa a | 6 | 40 | 8.4 | 12.1 | 13.5 | 4.5 | 6.6 | - | 4 | 11.4 | - | - | 4 | - |
| 70 kDa heat shock-related protein | 4 | 75 | 6.2 | 8.4 | - | 8.4 | - | 3.1 | 4.6 | 3.1 | - | - | 3.6 | - |
| Coat protein, partial [Potato virus Y]/Polyprotein | 7 | 30 | - | - | - | 17.7 | 11.8 | - | - | - | - | 4.1 | 3.2 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yarullina, L.; Kalatskaja, J.; Tsvetkov, V.; Burkhanova, G.; Yalouskaya, N.; Rybinskaya, K.; Zaikina, E.; Cherepanova, E.; Hileuskaya, K.; Nikalaichuk, V. The Influence of Chitosan Derivatives in Combination with Bacillus subtilis Bacteria on the Development of Systemic Resistance in Potato Plants with Viral Infection and Drought. Plants 2024, 13, 2210. https://doi.org/10.3390/plants13162210
Yarullina L, Kalatskaja J, Tsvetkov V, Burkhanova G, Yalouskaya N, Rybinskaya K, Zaikina E, Cherepanova E, Hileuskaya K, Nikalaichuk V. The Influence of Chitosan Derivatives in Combination with Bacillus subtilis Bacteria on the Development of Systemic Resistance in Potato Plants with Viral Infection and Drought. Plants. 2024; 13(16):2210. https://doi.org/10.3390/plants13162210
Chicago/Turabian StyleYarullina, Liubov, Joanna Kalatskaja, Vyacheslav Tsvetkov, Guzel Burkhanova, Ninel Yalouskaya, Katerina Rybinskaya, Evgenia Zaikina, Ekaterina Cherepanova, Kseniya Hileuskaya, and Viktoryia Nikalaichuk. 2024. "The Influence of Chitosan Derivatives in Combination with Bacillus subtilis Bacteria on the Development of Systemic Resistance in Potato Plants with Viral Infection and Drought" Plants 13, no. 16: 2210. https://doi.org/10.3390/plants13162210





