A Comparative Study on the Antidiabetic Activity, Cytotoxicity and Lipid Profile of Trichilia emetica Oils
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yield from Seed and Aril
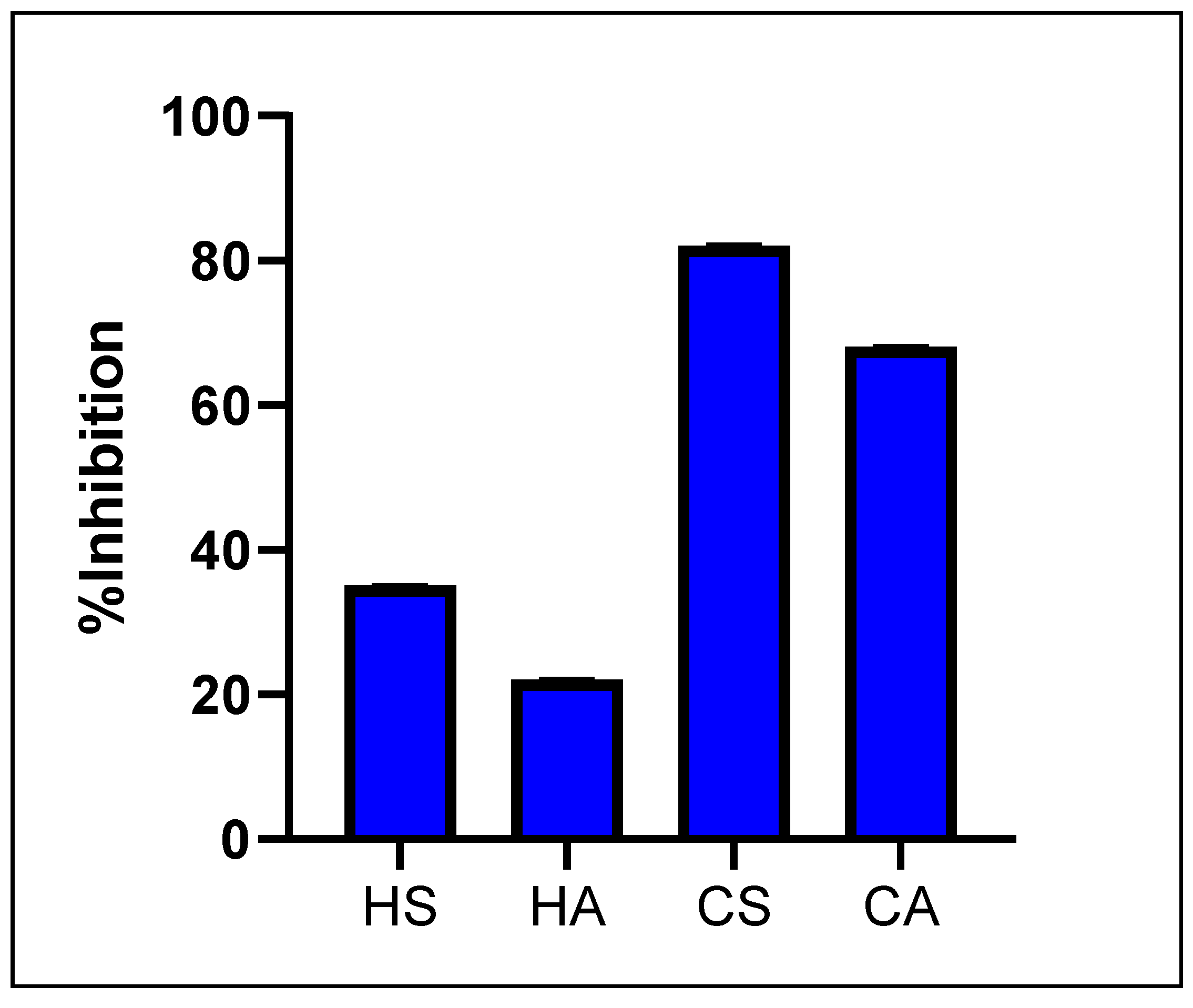
2.2. Antidiabetic Activity T. emetica Oils
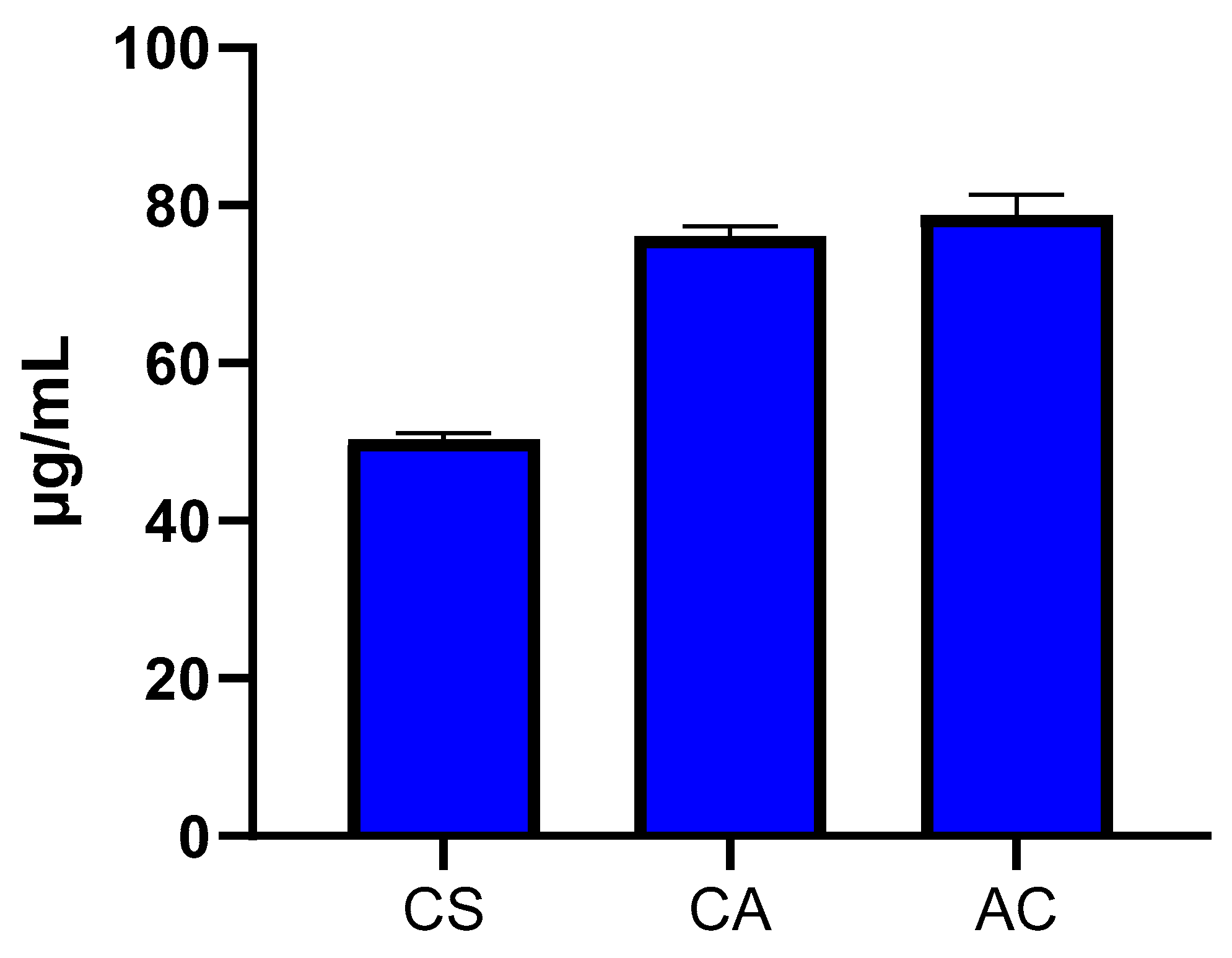
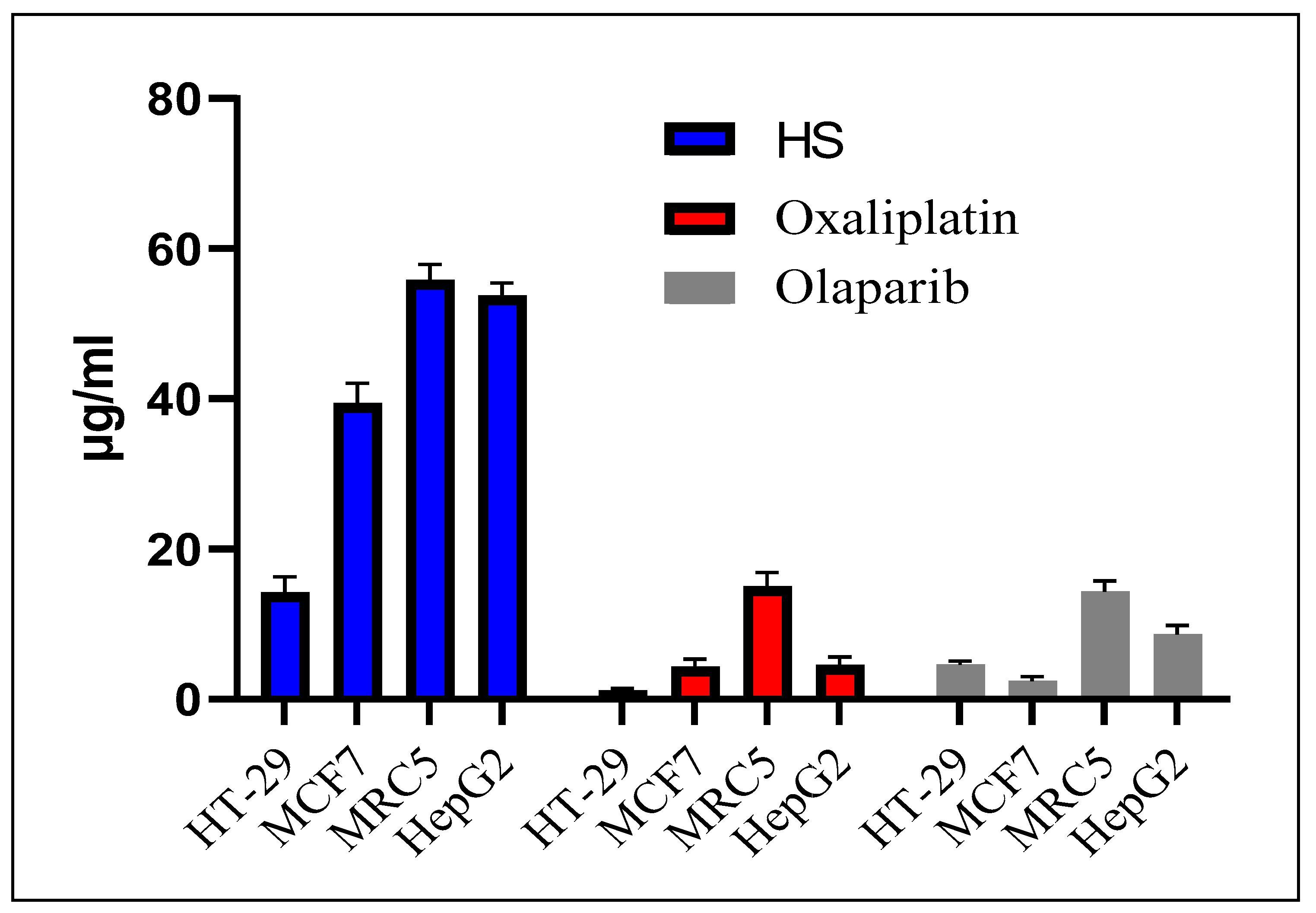
2.3. Cytotoxicity of T. emetica Oils
2.4. Fatty Acid Derivatization and GC-MS Analysis
2.5. Content of OA and PA in T. emetica Oils
2.6. Descriptive Statistics
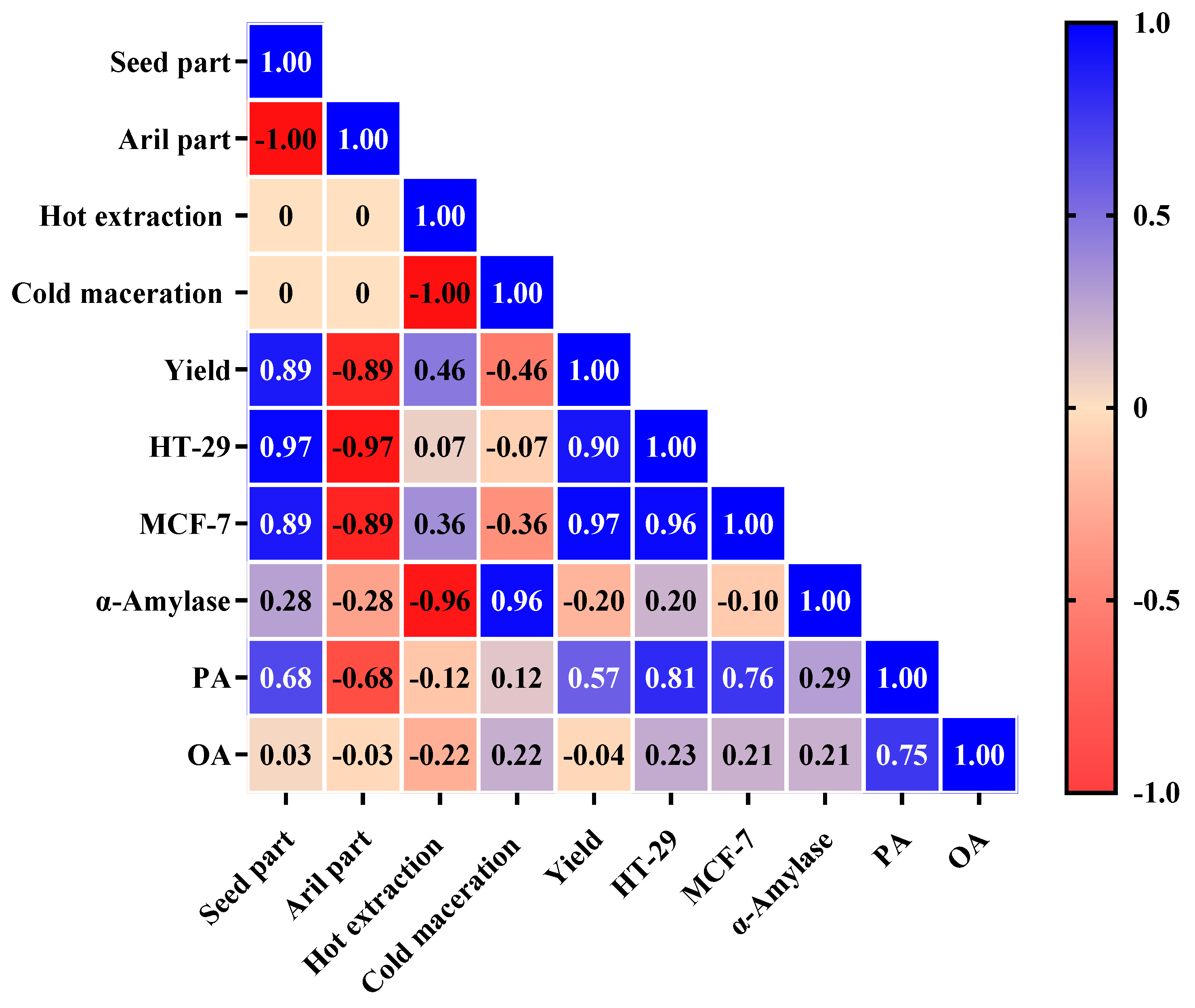
2.7. Pearson’s Analysis
3. Materials and Methods
3.1. Materials and Plant Preparation
3.2. Extraction and Determination of Oil Yield
3.3. Antidiabetic Activity
3.4. Cell Culture and Cytotoxicity
3.5. Samples Preparation and Derivatization
3.6. GC-MS Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bussmann, R.W.; Paniagua-Zambrana, N.Y.; Njoroge, G.N. Trichilia emetica Vahl Meliaceae; Springer: Cham, Switzerland, 2020; pp. 1–4. ISBN 978-3-319-77086-4. [Google Scholar]
- POWO. Plants of the World Online. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:579419-1 (accessed on 14 December 2023).
- Abbas, A.M.; Al-Kahtani, M.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Badry, M.O. Floristic diversity and phytogeography of JABAL Fayfa: A subtropical dry zone, South-West Saudi Arabia. Diversity 2020, 12, 345. [Google Scholar] [CrossRef]
- Alfarhan, A.; Al-Turky, T.; Basahy, A. Flora of Jazan Region; King Abdulaziz City for Science and Technology: Riyadh, Saudi Arabia, 2005. [Google Scholar]
- Komane, B.M.; Olivier, E.I.; Viljoen, A.M. Trichilia emetica (Meliaceae)—A review of traditional uses, biological activities and phytochemistry. Phytochem. Lett. 2011, 4, 1–9. [Google Scholar] [CrossRef]
- Van Wyk, B.; Van Wyk, P.; Van Wyk, B.E. Photographic Guide to Trees of Southern Africa, 1st ed.; Briza Pubns: Johannesburg, South Africa, 2000; ISBN 9781875093243. [Google Scholar]
- Uamusse, A.; Yeboah, S.O. A comparative study of the oils from the seed arils of Trichilia emetica from Mozambique. Int. J. Agric. Sci. 2016, 6, 1172–1177. [Google Scholar]
- Usman, A. Phytochemical Investigation of Trichilia emetica (Natal mahogany); Bangor University: Bangor, UK, 2015. [Google Scholar]
- Diallo, D.; Paulsen, B.S.; Liljebäck, T.H.A.; Michaelsen, T.E. The malian medicinal plant Trichilia emetica; studies on polysaccharides with complement fixing ability. J. Ethnopharmacol. 2003, 84, 279–287. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.L.; de Almeida, R.; e Silva, F.T.; Verícimo, M.A. Review on the therapeutic activities of the genus Trichilia. Res. Soc. Dev. 2021, 10, e29610514916. [Google Scholar] [CrossRef]
- Passos, M.S.; Nogueira, T.S.R.; Azevedo, O.d.A.; Vieira, M.G.C.; Terra, W.d.S.; Braz-Filho, R.; Vieira, I.J.C. Limonoids from the genus Trichilia and biological activities: Review. Phytochem. Rev. 2021, 20, 1055–1086. [Google Scholar] [CrossRef]
- Brigitte, K.; Flaurant, T.; Emmanuel, T. Antimicrobial, Antioxidant and Protective Effect of Methanol Extract of Trichilia emetica (Meliaceae) Stem and Root Bark against Free Radical-induced Oxidative Haemolysis. European J. Med. Plants 2017, 19, 1–14. [Google Scholar] [CrossRef]
- Usman, A.; Thoss, V.; Nur-E-Alam, M. Isolation of Taxifolin from Trichilia emetica Whole Seeds. Am. Sci. Res. J. Eng. 2016, 21, 77–82. [Google Scholar]
- Usman, A.; Thoss, V.; Nur-e-Alam, M. Isolation and Identification of Flavonoids Components from Trichilia emetica Whole Seeds. J. Nat. Prod. Resour. 2018, 4, 179–181. [Google Scholar] [CrossRef]
- Adinew, B. Minerals and fatty acid composition analysis of Trichilia emetica seed oil and the possibility of its use in cosmetic preparation. World J. Pharm. Sci. 2015, 3, 2185–2191. [Google Scholar]
- Usman, A.; Udoji Itodo, A.; Liman Usman, N.; Haruna, M. Fatty Acid Methyl Esters Composition of Trichilia Emetica Shell Oil. Am. Sci. Res. J. Eng. 2016, 21, 83–90. [Google Scholar]
- Germanò, M.P.; D’Angelo, V.; Sanogo, R.; Catania, S.; Alma, R.; De Pasquale, R.; Bisignano, G. Hepatoprotective and antibacterial effects of extracts from Trichilia emetica Vahl. (Meliaceae). J. Ethnopharmacol. 2005, 96, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Shai, L.J.; McGaw, L.J.; Masoko, P.; Eloff, J.N. Antifungal and antibacterial activity of seven traditionally used South African plant species active against Candida albicans. S. Afr. J. Bot. 2008, 74, 677–684. [Google Scholar] [CrossRef]
- Babalola, I.T.; Adelakun, E.A.; Ibrahim Babalola, C.T. Phytochemical analysis and antimicrobial activity of Trichilia emetica Vahl (Meliaceae). J. Pharmacogn. Phytochem. 2018, 7, 1980–1982. [Google Scholar]
- Perumal, A.; Krishna, S.B.N.; Sershen; Pillay, K.; Govender, P. Phytochemical composition and biological investigation of Trichilia emetica Vahl. seed extracts. Lett. Appl. NanoBioScience 2020, 9, 1111–1116. [Google Scholar] [CrossRef]
- Geyid, A.; Abebe, D.; Debella, A.; Makonnen, Z.; Aberra, F.; Teka, F.; Kebede, T.; Urga, K.; Yersaw, K.; Biza, T.; et al. Screening of some medicinal plants of Ethiopia for their anti-microbial properties and chemical profiles. J. Ethnopharmacol. 2005, 97, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Germanò, M.P.; D’Angelo, V.; Biasini, T.; Sanogo, R.; De Pasquale, R.; Catania, S. Evaluation of the antioxidant properties and bioavailability of free and bound phenolic acids from Trichilia emetica Vahl. J. Ethnopharmacol. 2006, 105, 368–373. [Google Scholar] [CrossRef]
- Sanogo, R.; Diallo, D.; Maiga, A.; De Tommasi, N.; De Pasquale, R. Analgesic and anti-inflammatory activities of the aqueous extracts of Maytenus senegalensis, Stereospermum kunthianum and Trichilia emetica used in the treatment of dysmenorrhoea in Mali. Planta Med. 2006, 72, 258. [Google Scholar] [CrossRef]
- Konaté, K.; Yomalan, K.; Sytar, O.; Zerbo, P.; Brestic, M.; Patrick, V.D.; Gagniuc, P.; Barro, N. Free Radicals Scavenging Capacity, Antidiabetic and Antihypertensive Activities of Flavonoid-Rich Fractions from Leaves of Trichilia emetica and Opilia amentacea in an Animal Model of Type 2 Diabetes Mellitus. Evid. -Based Complement. Altern. Med. 2014, 2014, 867075. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, V.; Nayak, S.K.; Wadhwa, P.; Kaur, P.; Sahu, S.K. Alpha-amylase as molecular target for treatment of diabetes mellitus: A comprehensive review. Chem. Biol. Drug Des. 2021, 98, 539–560. [Google Scholar] [CrossRef]
- Ogunyemi, O.M.; Gyebi, G.A.; Saheed, A.; Paul, J.; Nwaneri-Chidozie, V.; Olorundare, O.; Adebayo, J.; Koketsu, M.; Aljarba, N.; Alkahtani, S.; et al. Inhibition mechanism of alpha-amylase, a diabetes target, by a steroidal pregnane and pregnane glycosides derived from Gongronema latifolium Benth. Front. Mol. Biosci. 2022, 9, 866719. [Google Scholar] [CrossRef] [PubMed]
- Traore, M.; Zhai, L.; Chen, M.; Olsen, C.E.; Odile, N.; Pierre, G.; Bosco, O.; Robert, G.; Christensen, S.B. Cytotoxic kurubasch aldehyde from Trichilia emetica. Nat. Prod. Res. 2007, 21, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Nchimbi, H.Y. Quantitative and qualitative assessment on the suitability of seed oil from water plant (Trichilia emetica) for soap making. Saudi J. Biol. Sci. 2020, 27, 3161–3168. [Google Scholar] [CrossRef] [PubMed]
- Khumalo, L.W.; Majoko, L.; Read, J.S.; Ncube, I. Characterisation of some underutilised vegetable oils and their evaluation as starting materials for lipase-catalysed production of cocoa butter equivalents. Ind. Crop. Prod. 2002, 16, 237–244. [Google Scholar] [CrossRef]
- York, E.; Darko, G. Extraction and Characterization of oils from Trichilia Emetica Seed; Kwame Nkrumah University of Science & Technology: Kumasi, Ghana, 2013. [Google Scholar]
- Nikolić, N.Č.; Cakić, S.M.; Novaković, S.M.; Cvetković, M.D.; Stanković, M.Z. Effect of extraction techniques on yield and composition of soybean oil. Maced. J. Chem. Chem. Eng. 2009, 28, 173. [Google Scholar] [CrossRef]
- Sabadin Piva, G.; Weschenfelder, T.A.; Franceschi, E.; Cansian, R.L.; Paroul, N.; Steffens, C. Flaxseed (Linum usitatissimum) Oil Extraction Using Different Solvents. Food Technol. Biotechnol. 2018, 56, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Cesa, S.; Sisto, F.; Zengin, G.; Scaccabarozzi, D.; Kokolakis, A.K.; Scaltrito, M.M.; Grande, R.; Locatelli, M.; Cacciagrano, F.; Angiolella, L.; et al. Phytochemical analyses and pharmacological screening of Neem oil. S. Afr. J. Bot. 2019, 120, 331–337. [Google Scholar] [CrossRef]
- Kazeem, M.I.; Dansu, T.V.; Adeola, S.A. Inhibitory Effect of Azadirachta indica A. Juss Leaf Extract on the Activities of α-Amylase and α-Glucosidase. Pak. J. Biol. Sci. 2013, 16, 1358–1362. [Google Scholar] [CrossRef]
- Sinan, K.I.; Ferrarese, I.; Aktumsek, A.; Peron, G.; Glamocilja, J.; Sokovic, M.; Nenadić, M.; Dall’Acqua, S.; Zengin, G. NMR and LC-MSn coupled with pharmacological network analysis for the assessment of phytochemical content and biopharmaceutical potential of Carapa procera extracts. J. Pharm. Biomed. Anal. 2021, 203, 114184. [Google Scholar] [CrossRef]
- Jafari, S.; Saeidnia, S.; Hajimehdipoor, H.; Ardekani, M.R.S.; Faramarzi, M.A.; Hadjiakhoondi, A.; Khanavi, M. Cytotoxic evaluation of Melia azedarach in comparison with, Azadirachta indica and its phytochemical investigation. DARU J. Pharm. Sci. 2013, 21, 37. [Google Scholar] [CrossRef]
- Islas, J.F.; Acosta, E.; G-Buentello, Z.; Delgado-Gallegos, J.L.; Moreno-Treviño, M.G.; Escalante, B.; Moreno-Cuevas, J.E. An overview of Neem (Azadirachta indica) and its potential impact on health. J. Funct. Foods 2020, 74, 104171. [Google Scholar] [CrossRef]
- López-Bascón, M.A.; Luque de Castro, M.D. Soxhlet Extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 327–354. [Google Scholar]
- Nogoy, K.M.C.; Kim, H.J.; Lee, Y.; Zhang, Y.; Yu, J.; Lee, D.H.; Li, X.Z.; Smith, S.B.; Seong, H.A.; Choi, S.H. High dietary oleic acid in olive oil-supplemented diet enhanced omega-3 fatty acid in blood plasma of rats. Food Sci. Nutr. 2020, 8, 3617–3625. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Lee, A.Y.; Park, S.; Kim, J.-H.; Cho, M.-H. Multiple pathways are involved in palmitic acid-induced toxicity. Food Chem. Toxicol. 2014, 67, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002, 22, 2587–2590. [Google Scholar] [PubMed]
- Urso, C.J.; Zhou, H. Palmitic Acid Lipotoxicity in Microglia Cells Is Ameliorated by Unsaturated Fatty Acids. Int. J. Mol. Sci. 2021, 22, 9093. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.; Tran, H.-D.; Xuan, T.; Ahmad, A.; Dat, T.; Khanh, T.; Teschke, R. Momilactones A and B Are α-Amylase and α-Glucosidase Inhibitors. Molecules 2019, 24, 482. [Google Scholar] [CrossRef]
- Ahmad, R.; Alqathama, A.; Aldholmi, M.; Riaz, M.; Mukhtar, M.H.; Aljishi, F.; Althomali, E.; Alamer, M.A.; Alsulaiman, M.; Ayashy, A.; et al. Biological Screening of Glycyrrhiza glabra L. from Different Origins for Antidiabetic and Anticancer Activity. Pharmaceuticals 2022, 16, 7. [Google Scholar] [CrossRef]
- Kiefer, K. Derivatization of Corn Oil for Analysis by GC; Supelco: Bellefonte, PA, USA, 1997. [Google Scholar]


| Sample | Plant Material (g) | Oil Extracts (g) | Yield (Oil per g of Plant Material) | Yield (%) |
|---|---|---|---|---|
| HS | 24 | 9.9 | 0.4125 | 41 |
| HA | 24 | 6.8 | 0.2833 | 28 |
| CS | 24 | 8.2 | 0.3417 | 34 |
| CA | 24 | 5.3 | 0.2208 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldholmi, M.; Althomali, E.; Aljishi, F.; Ahmad, R.; Alqathama, A.; Alaswad, D. A Comparative Study on the Antidiabetic Activity, Cytotoxicity and Lipid Profile of Trichilia emetica Oils. Plants 2024, 13, 2234. https://doi.org/10.3390/plants13162234
Aldholmi M, Althomali E, Aljishi F, Ahmad R, Alqathama A, Alaswad D. A Comparative Study on the Antidiabetic Activity, Cytotoxicity and Lipid Profile of Trichilia emetica Oils. Plants. 2024; 13(16):2234. https://doi.org/10.3390/plants13162234
Chicago/Turabian StyleAldholmi, Mohammed, Ebtihal Althomali, Fatema Aljishi, Rizwan Ahmad, Aljawharah Alqathama, and Deema Alaswad. 2024. "A Comparative Study on the Antidiabetic Activity, Cytotoxicity and Lipid Profile of Trichilia emetica Oils" Plants 13, no. 16: 2234. https://doi.org/10.3390/plants13162234





