The Invasive Plant Amaranthus spinosus L. Exhibits a Stronger Resistance to Drought than the Native Plant A. tricolor L. under Co-Cultivation Conditions When Treated with Light Drought
Abstract
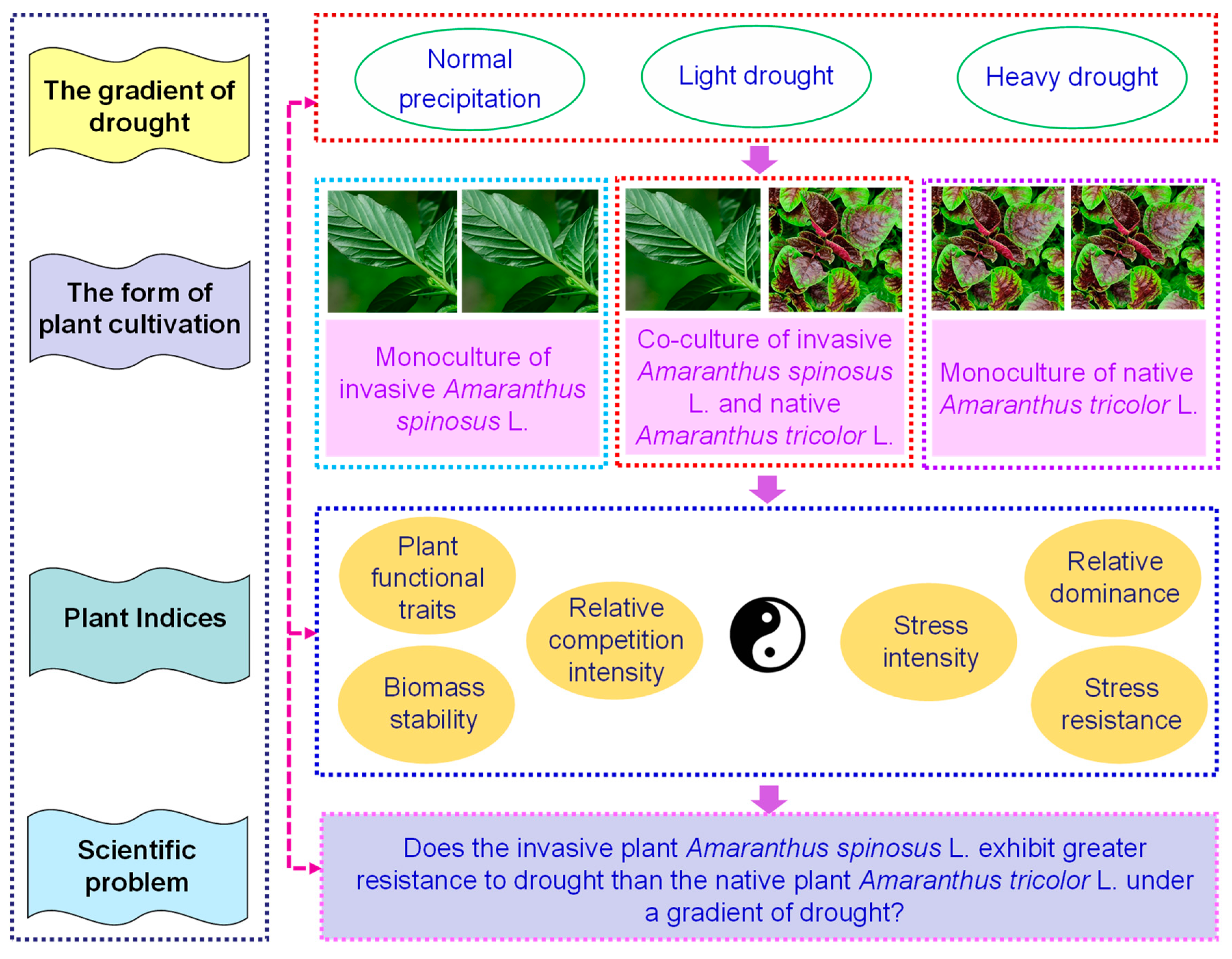
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
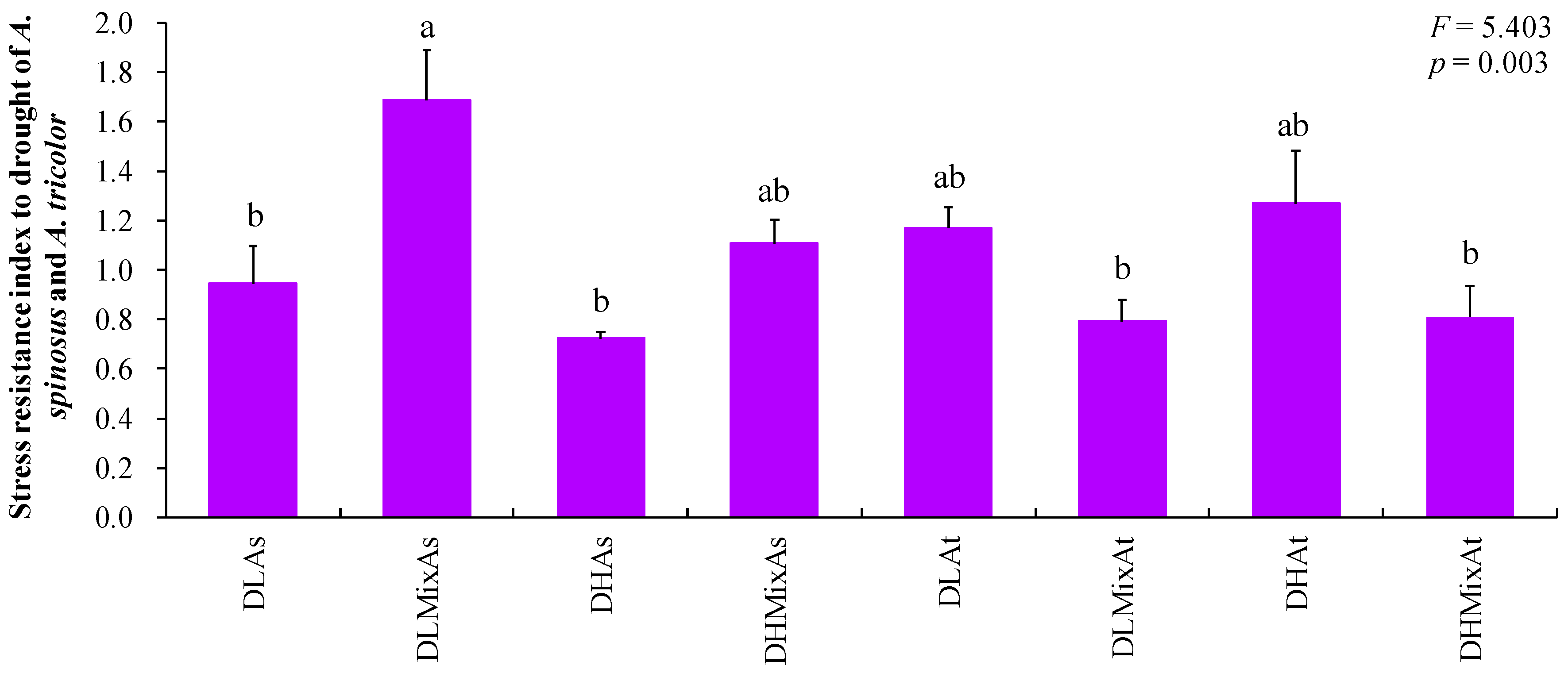
2.2. Method for Determining the Stress Resistance Index
2.3. Statistical Analysis
3. Results and Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IPS | Invasive plants |
| SRI | Stress resistance index |
| PCA | Principal component analysis |
| DLAs | Monocultural A. spinosus treated with a light drought |
| DLMixAs | Co-cultivated A. spinosus treated with a light drought |
| DHAs | Monocultural A. spinosus treated with a heavy drought |
| DHMixAs | Co-cultivated A. spinosus treated with a heavy drought |
| DLAt | Monocultural A. tricolor treated with a light drought |
| DLMixAt | Co-cultivated A. tricolor treated with a light drought |
| DHAt | Monocultural A. tricolor treated with a heavy drought |
| DHMixAt | Co-cultivated A. tricolor treated with a heavy drought |
References
- Rudolph, J.; Gornish, E.S.; Barberán, A. Plant–plant and plant–soil interactions under drought and the presence of invasive buffelgrass (Cenchrus ciliaris). Biol. Invasions 2024, 26, 1281–1293. [Google Scholar] [CrossRef]
- Esperon-Rodriguez, M.; Power, S.A.; Tjoelker, M.G.; Marchin, R.M.; Rymer, P.D. Contrasting heat tolerance of urban trees to extreme temperatures during heatwaves. Urban For. Urban Green. 2021, 66, 127387. [Google Scholar] [CrossRef]
- Zhong, S.S.; Xu, Z.L.; Cheng, H.Y.; Wang, Y.Y.; Yu, Y.L.; Du, D.L.; Wang, C.Y. Does drought stress intensify the allelopathy of invasive woody species Rhus typhina L.? Trees 2023, 37, 811–819. [Google Scholar] [CrossRef]
- Werner, C.M.; Harrison, S.P.; Safford, H.D.; Bohlman, G.N.; Serata, R. Extreme pre-fire drought decreases shrub regeneration on fertile soils. Ecol. Appl. 2021, 32, e02464. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wei, M.; Cheng, H.Y.; Wu, B.D.; Du, D.L.; Wang, C.Y. Indigenous plant species and invasive alien species tend to diverge functionally under heavy metal pollution and drought stress. Ecotoxicol. Environ. Saf. 2020, 205, 111160. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.L.; Cheng, H.Y.; Wang, S.; Wei, M.; Wang, C.Y.; Du, D.L. Drought may be beneficial to the competitive advantage of Amaranthus spinosus. J. Plant Ecol. 2022, 15, 494–508. [Google Scholar] [CrossRef]
- Ettinger, C.L.; Laforgia, M.L. Invasive plant species interact with drought to shift key functions and families in the native rhizosphere. Plant Soil 2024, 494, 567–588. [Google Scholar] [CrossRef]
- NeSmith, J.E.; Alba, C.; Flory, S.L. Experimental drought and plant invasion additively suppress primary pine species of southeastern US forests. For. Ecol. Manag. 2018, 411, 158–165. [Google Scholar] [CrossRef]
- Khanalizadeh, A.; Eshaghi Rad, J.; Amiri, G.Z.; Zare, H.; Schall, P.; Lexer, M.J. The relationship between plant diversity and aboveground biomass in managed and unmanaged temperate forests. Eur. J. For. Res. 2023, 142, 1167–1175. [Google Scholar] [CrossRef]
- Wuest, S.E.; Schulz, L.; Rana, S.; Frommelt, J.; Ehmig, M.; Pires, N.D.; Grossniklaus, U.; Hardtke, C.S.; Hammes, U.Z.; Schmid, B.; et al. Single-gene resolution of diversity-driven overyielding in plant genotype mixtures. Nat. Commun. 2023, 14, 3379. [Google Scholar] [CrossRef]
- Liang, M.; Baiser, B.; Hallett, L.M.; Hautier, Y.; Jiang, L.; Loreau, M.; Record, S.; Sokol, E.R.; Zarnetske, P.L.; Wang, S. Consistent stabilizing effects of plant diversity across spatial scales and climatic gradients. Nat. Ecol. Evol. 2022, 6, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Shumi, G.; Rodrigues, P.; Hanspach, J.; Härdtle, W.; Hylander, K.; Senbeta, F.; Fischer, J.; Schultner, J. Woody plant species diversity as a predictor of ecosystem services in a social–ecological system of southwestern Ethiopia. Landsc. Ecol. 2021, 36, 373–391. [Google Scholar] [CrossRef]
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate extremes: Observations, modeling, and impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Liu, K.; Jiang, D.B. Projected changes in the dry/wet climate of China under the RCP4.5 scenario. Chin. J. Atmos. Sci. 2015, 39, 489–502. [Google Scholar]
- Jiang, J.; Jiang, D.B.; Lin, Y.H. Changes and projection of dry/wet areas over China. J. Atmos. Sci. 2017, 41, 43–56. [Google Scholar]
- Reynolds, J.F.; Smith, D.M.S.; Lambin, E.F.; Turner, B.L.I.; Walker, B. Global desertification: Building a science for dryland development. Science 2007, 316, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Pointing, S.B.; Belnap, J. Microbial colonization and controls in dryland systems. Nat. Rev. Microbiol. 2012, 10, 551–562. [Google Scholar] [CrossRef]
- Leal, R.P.; Silveira, M.J.; Petsch, D.K.; Mormul, R.P.; Thomaz, S.M. The success of an invasive Poaceae explained by drought resilience but not by higher competitive ability. Environ. Exp. Bot. 2022, 194, 104717. [Google Scholar] [CrossRef]
- Beshai, R.A.; Truong, D.A.; Henry, A.K.; Sorte, C.J.B. Biotic resistance or invasional meltdown? Diversity reduces invasibility but not exotic dominance in southern California epibenthic communities. Biol. Invasions 2023, 25, 533–549. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, A.; Kohli, R.K.; Singh, H.P.; Batish, D.R. Bidens pilosa (Asteraceae) invasion reshapes the pattern of plant communities and edaphic properties across the north-western Himalayan landscape. Ecol. Inform. 2023, 77, 102281. [Google Scholar] [CrossRef]
- Savage, C.; Savage, K.; Keller, K.R. Effect of Carpobrotus edulis invasion history on plant communities. West. N. Am. Nat. 2023, 83, 484–497. [Google Scholar] [CrossRef]
- Yan, J.; Yan, X.L.; Li, H.R.; Du, C.; Ma, J.S. Composition, time of introduction and spatial-temporal distribution of naturalized plants in East China. Biodivers. Sci. 2021, 29, 428–438. [Google Scholar] [CrossRef]
- Yan, X.L.; Liu, Q.R.; Shou, H.Y.; Zeng, X.F.; Zhang, Y.; Chen, L.; Liu, Y.; Ma, H.Y.; Qi, S.Y.; Ma, J.S. The categorization and analysis on the geographic distribution patterns of Chinese alien invasive plants. Biodivers. Sci. 2014, 22, 667–676. [Google Scholar]
- Wang, C.Y.; Liu, J.; Xiao, H.G.; Zhou, J.W.; Du, D.L. Floristic characteristics of alien invasive seed plant species in China. An. Acad. Bras. Cienc. 2016, 88, 1791–1797. [Google Scholar] [CrossRef]
- Mandák, B.; Zákravský, P.; Dostál, P.; Plačková, I. Population genetic structure of the noxious weed Amaranthus retroflexus in Central Europe. Flora 2011, 206, 697–703. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. Zhenjiang Statistical Yearbook 2023; China Statistics Press: Beijing, China, 2023. [Google Scholar]
- GB/T 20481-2017; Grades of Meteorological Drought. National Standard of China. State Administration for Market Regulation of China and Standardization Administration of China: Beijing, China, 2017.
- Balezentiene, L.; Marozas, V.; Miksa, O. Comparison of the carbon and water fluxes of some aggressive invasive species in baltic grassland and shrub habitats. Atmos 2021, 12, 12080969. [Google Scholar]
- Shen, S.C.; Xu, G.F.; Li, D.Y.; Yang, S.S.; Jin, G.M.; Liu, S.F.; Clements, D.R.; Chen, A.D.; Rao, J.; Wen, L.L.; et al. Potential use of Helianthus tuberosus to suppress the invasive alien plant Ageratina adenophora under different shade levels. Bmc Ecol. Evol. 2021, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Xuan, S.L.; Muneer, M.A.; Sun, B.; Shi, C.L.; Liu, F.S.; Wu, R.Y. Response of dry matter partition and yield components to waterlogging and sunlight shortage in different growth stages of wheat. Nat. Hazards 2022, 110, 1133–1152. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kumar, S. Soil Physical Productivity and Plant Growth. In Soil Physical Environment and Plant Growth: Evaluation and Management; Sharma, P.K., Kumar, S., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–32. [Google Scholar]
- O’Leary, B.; Burd, M.; Venn, S.E.; Gleadow, R. Integrating the Passenger-Driver hypothesis and plant community functional traits to the restoration of lands degraded by invasive trees. For. Ecol. Manag. 2018, 408, 112–120. [Google Scholar] [CrossRef]
- Aan, A.; Hallik, L.; Kull, O. Photon flux partitioning among species along a productivity gradient of an herbaceous plant community. J. Ecol. 2006, 94, 1143–1155. [Google Scholar] [CrossRef]
- Gross, N.; Suding, K.N.; Lavorel, S.; Roumet, C. Complementarity as a mechanism of coexistence between functional groups of grasses. J. Ecol. 2007, 95, 1296–1305. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Plant height and evolutionary games. Trends Ecol. Evol. 2003, 18, 337–343. [Google Scholar] [CrossRef]
- Okubo, S.; Tomatsu, A.; Parikesit; Muhamad, D.; Harashina, K.; Takeuchi, K. Leaf functional traits and functional diversity of multistoried agroforests in West Java, Indonesia. Agric. Ecosyst. Environ. 2012, 149, 91–99. [Google Scholar] [CrossRef]
- Funk, J.L. Differences in plasticity between invasive and native plants from a low resource environment. J. Ecol. 2008, 96, 1162–1173. [Google Scholar] [CrossRef]
- Rijkers, T.; Pons, T.L.; Bongers, F. The effect of tree height and light availability on photosynthetic leaf traits of four neotropical species differing in shade tolerance. Funct. Ecol. 2000, 14, 77–86. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Traits Associated with Invasiveness in Alien Plants: Where Do we Stand? In Biological Invasions; Nentwig, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 97–125. [Google Scholar]

| Stress Resistance Index | ||
|---|---|---|
| Height | r | 0.534 ** |
| p | 0.007 | |
| Ground diameter | r | 0.347 |
| p | 0.097 | |
| Fresh weight | r | 0.769 *** |
| p | <0.0001 | |
| Dry weight | r | 0.892 *** |
| p | <0.0001 | |
| Water content | r | −0.146 |
| p | 0.495 | |
| Leaf length | r | 0.266 |
| p | 0.210 | |
| Leaf width | r | 0.019 |
| p | 0.929 | |
| Specific leaf area | r | −0.201 |
| p | 0.347 | |
| Leaf fresh weight | r | 0.029 |
| p | 0.893 | |
| Leaf dry weight | r | 0.210 |
| p | 0.324 | |
| Leaf water content | r | −0.076 |
| p | 0.726 | |
| Leaf chlorophyll content | r | 0.121 |
| p | 0.573 | |
| Leaf nitrogen content | r | 0.134 |
| p | 0.533 | |
| Malondialdehyde content | r | −0.309 |
| p | 0.142 | |
| Proline content | r | −0.123 |
| p | 0.567 | |
| Catalase activity | r | −0.160 |
| p | 0.454 | |
| Peroxidase activity | r | 0.410 * |
| p | 0.047 | |
| Superoxide dismutase activity | r | 0.315 |
| p | 0.133 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Liu, Y.; Li, C.; Li, Y.; Du, D. The Invasive Plant Amaranthus spinosus L. Exhibits a Stronger Resistance to Drought than the Native Plant A. tricolor L. under Co-Cultivation Conditions When Treated with Light Drought. Plants 2024, 13, 2251. https://doi.org/10.3390/plants13162251
Wang C, Liu Y, Li C, Li Y, Du D. The Invasive Plant Amaranthus spinosus L. Exhibits a Stronger Resistance to Drought than the Native Plant A. tricolor L. under Co-Cultivation Conditions When Treated with Light Drought. Plants. 2024; 13(16):2251. https://doi.org/10.3390/plants13162251
Chicago/Turabian StyleWang, Congyan, Yingsheng Liu, Chuang Li, Yue Li, and Daolin Du. 2024. "The Invasive Plant Amaranthus spinosus L. Exhibits a Stronger Resistance to Drought than the Native Plant A. tricolor L. under Co-Cultivation Conditions When Treated with Light Drought" Plants 13, no. 16: 2251. https://doi.org/10.3390/plants13162251






