The Effects of Soil Acidity and Aluminium on the Root Systems and Shoot Growth of Lotus pedunculatus and Lupinus polyphyllus
Abstract
:1. Introduction
2. Results
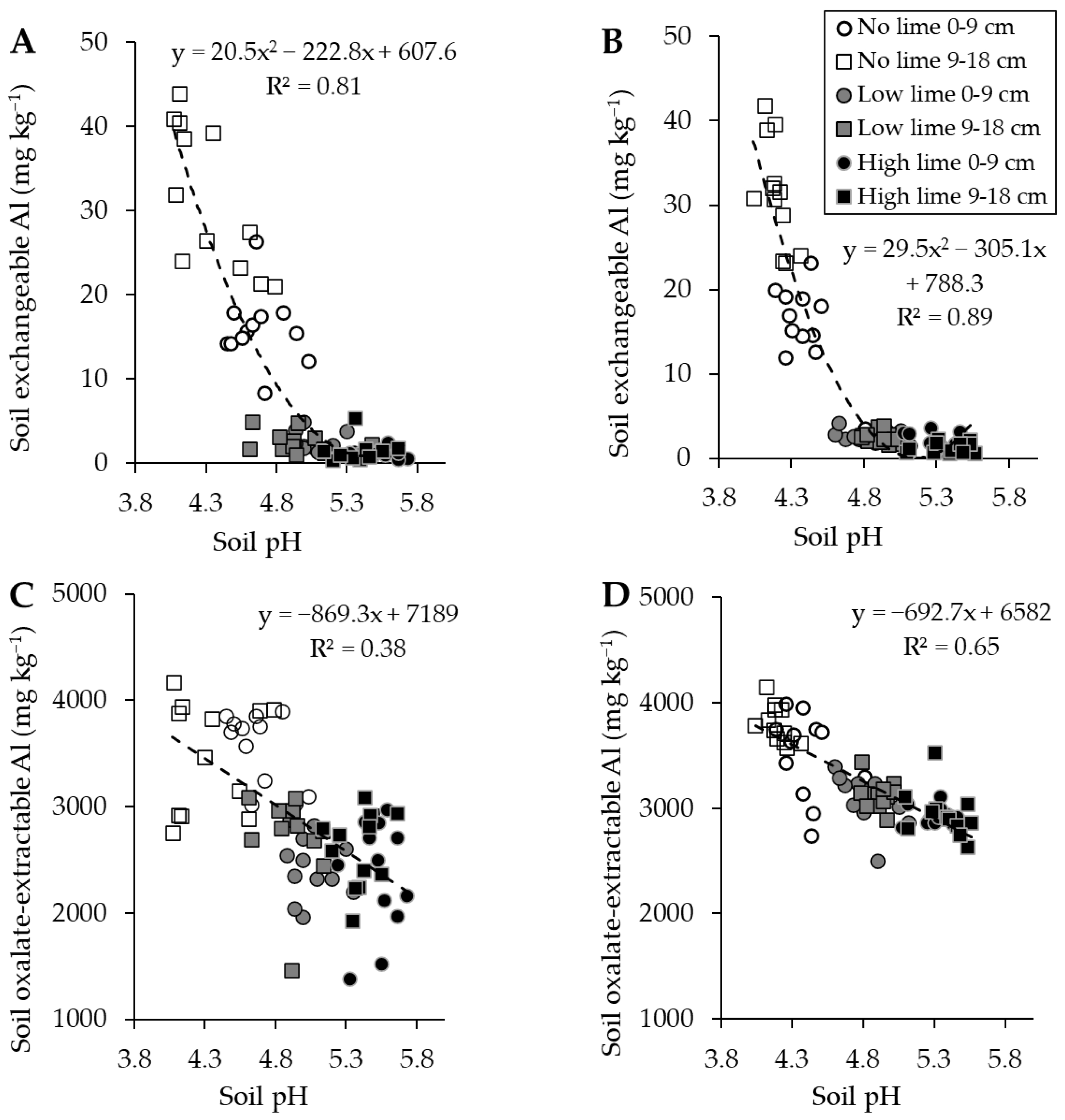
2.1. Soil pH, Exchangeable Al, and Oxalate-Extractable Al
2.2. Shoot Biomass and Shoot Nitrogen
2.3. Root Morphology
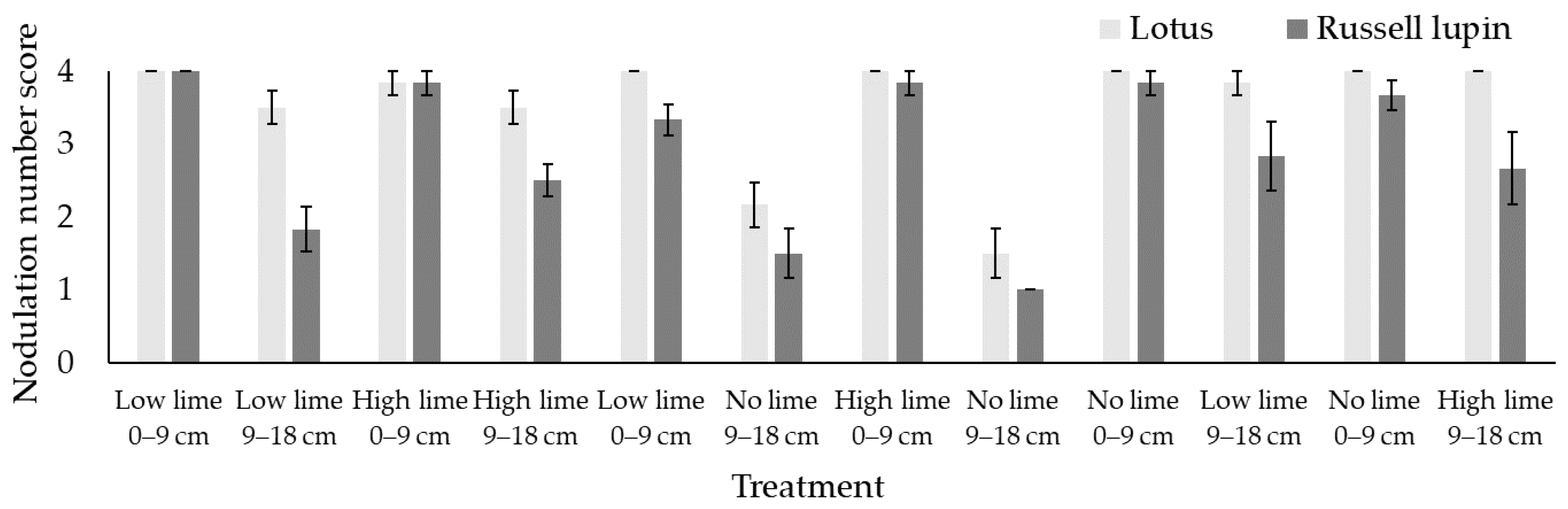
2.4. Nodulation
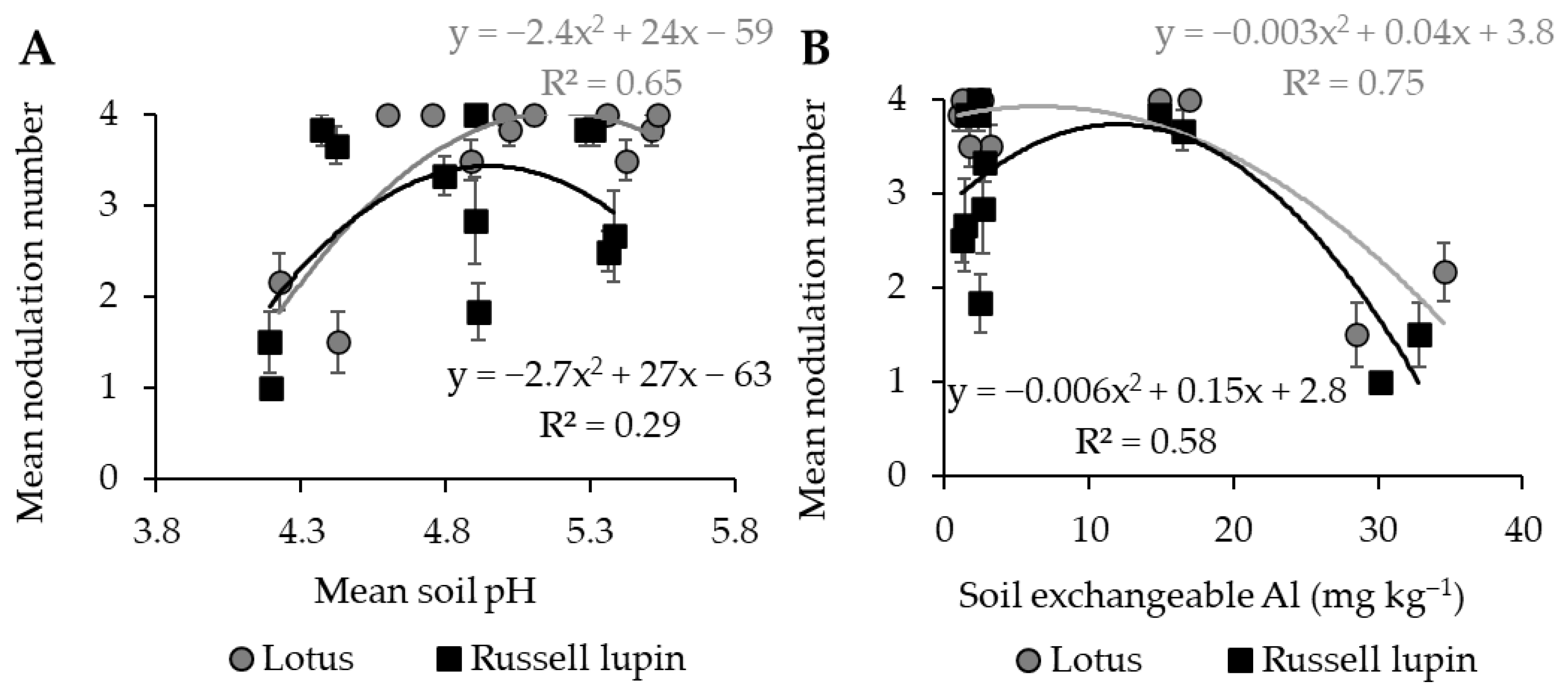
2.5. Relationships
3. Discussion
4. Materials and Methods
4.1. Soil Collection and Soil Chemical Analysis
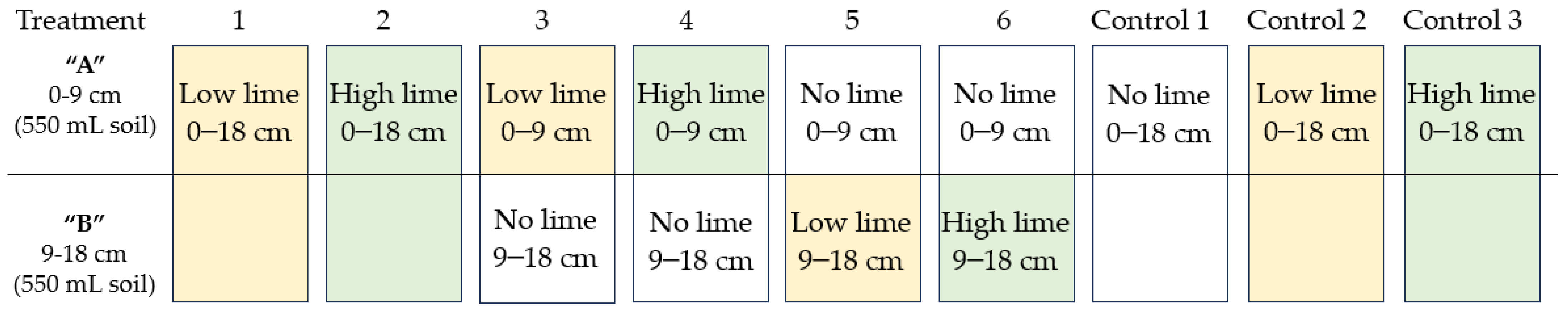
4.2. Experimental Design and Treatments
4.3. Trial Management
4.4. Measurements and Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von Uexküll, H.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Rout, G.; Samantaray, S.; Das, P. Aluminium toxicity in plants: A review. Agronomie 2001, 21, 3–21. [Google Scholar] [CrossRef]
- Kochian, L.V. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Biol. 1995, 46, 237–260. [Google Scholar] [CrossRef]
- Rahman, R.; Upadhyaya, H. Aluminium Toxicity and Its Tolerance in Plant: A Review. J. Plant Biol. 2021, 64, 101–121. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, D.K.; Singh, S.; Sharma, S.; Dubey, N.K.; Chauhan, D.K.; Vaculík, M. Toxicity of aluminium on various levels of plant cells and organism: A review. Environ. Exp. Bot. 2017, 137, 177–193. [Google Scholar] [CrossRef]
- Ruíz-Herrera, L.F.; López-Bucio, J. Aluminum induces low phosphate adaptive responses and modulates primary and lateral root growth by differentially affecting auxin signaling in Arabidopsis seedlings. Plant Soil 2013, 371, 593–609. [Google Scholar] [CrossRef]
- Ferguson, B.; Lin, M.-H.; Gresshoff, P.M. Regulation of legume nodulation by acidic growth conditions. Plant Signal. Behav. 2013, 8, e23426. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.D.; Moir, J.L. Soil aluminium toxicity in New Zealand pastoral farming—A review. J. N. Z. Grassl. 2018, 80, 129–136. [Google Scholar] [CrossRef]
- Peoples, M.; Brockwell, J.; Hunt, J.; Swan, A.; Watson, L.; Hayes, R.; Li, G.; Hackney, B.; Nuttall, J.; Davies, S. Factors affecting the potential contributions of N2 fixation by legumes in Australian pasture systems. Crop Pasture Sci. 2012, 63, 759–786. [Google Scholar] [CrossRef]
- Matthews, P.; Hodgson, J.; White, J. Livestock farming systems in New Zealand. In New Zealand Pasture Crop Science; White, J., Ed.; Oxford University Press: Auckland, New Zealand, 1999; pp. 147–151. [Google Scholar]
- Brown, C.; Green, R. The challenges facing legumes in a dryland environment-a consultant’s view. NZGA Res. Pract. Ser. 2003, 11, 7–12. [Google Scholar] [CrossRef]
- Scott, D. Dryland legumes: Perspectives and problems. NZGA Res. Pract. Ser. 2003, 11, 27–36. [Google Scholar] [CrossRef]
- Morrison, C.A.; Westbrooke, V.C.; Moir, J.L. Potential profit gains from improving pasture productivity on New Zealand South Island high-country farms. J. N. Z. Grassl. 2020, 82, 183–189. [Google Scholar] [CrossRef]
- Whitley, A.E.; Moir, J.L.; Almond, P.C. A meta-analysis of exchangeable aluminium in New Zealand soils using the National Soils Database. Soil Res. 2019, 57, 113–123. [Google Scholar] [CrossRef]
- Hendrie, D.L.; Moir, J.L.; Stevens, E.J.; Black, A.D.; Moot, D.J. Soil pH, exchangeable aluminium and legume yield responses to deep-placed lime at Omarama Station. J. N. Z. Grassl. 2018, 80, 137–144. [Google Scholar] [CrossRef]
- Moir, J.L.; Jordan, P.; Moot, D.J.; Lucas, R. Phosphorus response and optimum pH ranges of twelve pasture legumes grown in an acid upland New Zealand soil under glasshouse conditions. J. Soil Sci. Plant Nutr. 2016, 16, 438–460. [Google Scholar] [CrossRef]
- Stevens, D.; Casey, M.; Bennett, C.; Thompson, B.; Garden, N.; Garden, P. Responses of Lotus pedunculatus to sulphur fertiliser and defoliation regime in acid soils at low temperature. J. N. Z. Grassl. 2022, 84, 49–56. [Google Scholar]
- Sheath, G. Lotus pedunculatus-an agricultural plant? Proc. N. Z. Grassl. Assoc. 1981, 42, 160–168. [Google Scholar] [CrossRef]
- Armstrong, C.S. ‘Grasslands Maku’ tetraploid lotus (Lotus pedunculatus Cav.). N. Z. J. Exp. Agric. 1974, 2, 333–336. [Google Scholar] [CrossRef]
- Scott, D. Perennial or Russell lupin: A potential high country pasture legume. Proc. N. Z. Grassl. Assoc. 1989, 50, 203–206. [Google Scholar] [CrossRef]
- Eckstein, R.L.; Welk, E.; Klinger, Y.P.; Lennartsson, T.; Wissman, J.; Ludewig, K.; Hansen, W.; Ramula, S. Biological Flora of Central Europe–Lupinus polyphyllus Lindl. Perspect. Plant Ecol. Evol. Syst. 2023, 58, 125715. [Google Scholar] [CrossRef]
- Läuchli, A.; Grattan, S.R. Soil pH extremes. In Plant Stress Physiology; CABI: Wallingford, UK, 2012; pp. 194–209. [Google Scholar]
- Edmeades, D.; Smart, C.; Wheeler, D. Aluminium toxicity in New Zealand soils: Preliminary results on the development of diagnostic criteria. N. Z. J. Agric. Res. 1983, 26, 493–501. [Google Scholar] [CrossRef]
- Marschner, H. Mechanisms of adaptation of plants to acid soils. Plant Soil 1991, 134, 1–20. [Google Scholar] [CrossRef]
- Vives-Peris, V.; De Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Chen, Z.C.; Liao, H. Organic acid anions: An effective defensive weapon for plants against aluminum toxicity and phosphorus deficiency in acidic soils. J. Genet. Genom. 2016, 43, 631–638. [Google Scholar] [CrossRef]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Kidd, D.R.; Ryan, M.H.; Colmer, T.D.; Simpson, R.J. Root growth response of serradella species to aluminium in solution culture and soil. Grass Forage Sci. 2021, 76, 57–71. [Google Scholar] [CrossRef]
- Kidd, D.; Premaratne, M.; Wisdom, J.; Nicol, D.; Ryan, M. An agronomic study of legacy effects from annual legume pastures in acid soils. J. Agron. Crop Sci. 2023, 209, 439–458. [Google Scholar] [CrossRef]
- Bouray, M.; Moir, J.L.; Condron, L.M.; Lehto, N.J. Lime-induced pH elevation influences phosphorus biochemical processes and dynamics in the rhizosphere of Lupinus polyphyllus and Lupinus angustifolius. J. Soil Sci. Plant Nutr. 2021, 21, 1978–1992. [Google Scholar] [CrossRef]
- Stoutjesdijk, P.A.; Sale, P.W.; Larkin, P.J. Possible involvement of condensed tannins in aluminium tolerance of Lotus pedunculatus. Funct. Plant Biol. 2001, 28, 1063–1074. [Google Scholar] [CrossRef]
- Poirier, V.; Roumet, C.; Munson, A.D. The root of the matter: Linking root traits and soil organic matter stabilization processes. Soil Biol. Biochem. 2018, 120, 246–259. [Google Scholar] [CrossRef]
- Takahashi, T.; Dahlgren, R.A. Nature, properties and function of aluminum–humus complexes in volcanic soils. Geoderma 2016, 263, 110–121. [Google Scholar] [CrossRef]
- Whitley, A.E. Lime, Phosphorus and Sulphur Response of Six Forage Legume Species on an Acidic High Country Soil under Glasshouse Conditions. Bachelor Dissertation, Lincoln University, Lincoln, New Zealand, 2013; p. 93. [Google Scholar]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.; Ludecke, T. Yield, root morphology and chemical composition of two pasture legumes as affected by lime and phosphorus applications to an acid soil. Plant Soil 1981, 62, 241–254. [Google Scholar] [CrossRef]
- Keyser, H.; Munns, D. Effects of calcium, manganese, and aluminum on growth of rhizobia in acid media. Soil Sci. Soc. Am. J. 1979, 43, 500–503. [Google Scholar] [CrossRef]
- Wood, M.; Cooper, J.; Bjourson, A. Response of Lotus rhizobia to acidity and aluminium in liquid culture and in soil. Plant Soil 1988, 107, 227–231. [Google Scholar] [CrossRef]
- Lowther, W.; Hay, R.; Ryan, D. Effect of strain of rhizobia, lime, and phosphorus on dry matter yield of three lotus species in differing environments in Otago and Southland. N. Z. J. Exp. Agric. 1987, 15, 135–142. [Google Scholar] [CrossRef]
- Hewitt, A. New Zealand Soil Classification, 3rd ed.; Manaaki Whenua Press: Lincoln, New Zealand, 2010. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 13th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2022.
- Blakemore, L.C.; Searle, P.L.; Daly, B.K. Methods for Chemical Analysis of Soils; New Zealand Soil Bureau: Lower Hutt, New Zealand, 1987; Volume 80. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954.
- Watkinson, J.; Kear, M. Sulfate and mineralisable organic sulfur in pastoral soils of New Zealand. I. A quasi equilibrium between sulfate and mineralisable organic sulfur. Soil Res. 1996, 34, 385–403. [Google Scholar] [CrossRef]
- Rayment, G.; Higginson, F.R. Australian Laboratory Handbook of Soil and Water Chemical Methods; Inkata Press Pty Ltd.: Melbourne, Australia, 1992. [Google Scholar]
- Brown, I.C. A rapid method of determining exchangeable hydrogen and total exchangeable bases of soils. Soil Sci. 1943, 56, 353–358. [Google Scholar] [CrossRef]
- Hoyt, P.B.; Nyborg, M. Toxic metals in acid soil: I. Estimation of plant-available aluminium. Soil Sci. Soc. Am. Proc. 1971, 35, 163–167. [Google Scholar] [CrossRef]
- Brodie, C.I.P. Response of Gland clover (Trifolium gladulierum) and Cupped clover (Trifolium cherleri) to Liming, Phosphorus and Sulphur additions on an Acidic High Country Soil under Glasshouse Conditions. Bachelor Dissertation, Lincoln University, Lincoln, New Zealand, 2020; p. 63. [Google Scholar]
- Watson, K.J. The response of falcata lucerne (Medicago falcata) to phosphorus, sulphur and lime application in an acidic high country soil under glasshouse conditions. Bachelor Dissertation, Lincoln University, Lincoln, New Zealand, 2022; p. 82. [Google Scholar]
- Davis, M. The comparative phosphorus requirements of some temperate perennial legumes. Plant Soil 1991, 133, 17–30. [Google Scholar] [CrossRef]
- Jarvis; Lucas, R.; White, J. Sulphur and phosphorus responses of Russell lupin in rangeland. IGC Proc. 1997, 11. Available online: https://uknowledge.uky.edu/igc/1997/session10/11/ (accessed on 8 August 2024).
- Martin-Hendrie, D.L. Liming Effects on Legume Production and Phosphorus Availability in Acid South Island Hill and High Country Soils. Ph.D. Thesis, Lincoln University, Lincoln, New Zealand, 2019. [Google Scholar]
- Rice, W.; Penney, D.; Nyborg, M. Effects of soil acidity on rhizobia numbers, nodulation and nitrogen fixation by alfalfa and red clover. Can. J. Soil Sci. 1977, 57, 197–203. [Google Scholar] [CrossRef]
- Whitley, A.E.; Moir, J.L.; Lehto, N.J.; Paramashivam, D. Extractable aluminium in New Zealand Andisols and Inceptisols. Geoderma Reg. 2020, 22, e00315. [Google Scholar] [CrossRef]
- McKeague, J.; Day, J. Dithionite-and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Can. J. Soil Sci. 1966, 46, 13–22. [Google Scholar] [CrossRef]

| Species | Significance | ||||
|---|---|---|---|---|---|
| Lotus | Russell Lupin | Species | Lime Rate | Sp*Lime | |
| Shoot DM biomass (g DM plant−1) | 11.1 ± 0.53 | 1.8 ± 0.19 | * | ns | ns |
| Shoot DM biomass (g DM m2−1) | 501 ± 24 | 73 ± 7.5 | * | ns | ns |
| Shoot %N | 2.4 ± 0.05 | 3.3 ± 0.13 | * | ns | ns |
| Total root DM biomass (g plant−1) | 2.0 ± 0.14 | 1.1 ± 0.23 | * | ns | ns |
| Total root length (cm plant−1) | 5687 ± 357 | 1473 ± 221 | * | ns | ns |
| Total surface area (cm2 plant−1) | 816 ± 51 | 265 ± 36 | * | ns | ns |
| Average root diameter (mm plant−1) | 0.49 ± 0.01 | 0.61 ± 0.02 | * | ns | ns |
| Shoot:root ratio | 5.6 ± 0.35 | 1.6 ± 0.19 | * | ns | ns |
| Total biomass (g plant−1) | 13.2 ± 0.62 | 2.9 ± 0.40 | * | ns | ns |
| Treatment | Root DM Biomass (g) | Root Length (cm) | Surface Area (cm2) | Average Diameter (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lotus | ||||||||||||
| 1A Low lime | 1.87 | ±0.17 | j | 2848 | ±326 | i | 429 | ±48 | k | 0.48 | ±0.01 | abcdefg |
| 1B Low lime | 0.53 | ±0.11 | def | 2723 | ±456 | i | 373 | ±69 | jk | 0.43 | ±0.01 | a |
| 2A High lime | 1.71 | ±0.24 | ij | 2896 | ±285 | i | 434 | ±53 | k | 0.47 | ±0.02 | abcdefg |
| 2B High lime | 0.60 | ±0.12 | defg | 3772 | ±728 | i | 483 | ±85 | k | 0.47 | ±0.06 | abcde |
| 3A Low lime | 1.57 | ±0.19 | ghij | 3584 | ±855 | i | 489 | ±106 | k | 0.58 | ±0.08 | eghijklm |
| 3B No lime | 0.53 | ±0.1 | def | 2853 | ±430 | i | 425 | ±68 | k | 0.47 | ±0.02 | abcdef |
| 4A High lime | 1.16 | ±0.24 | efghij | 2402 | ±321 | hi | 359 | ±48 | jk | 0.47 | ±0.02 | abcdefg |
| 4B No lime | 0.29 | ±0.06 | d | 2040 | ±381 | hi | 292 | ±60 | ijk | 0.44 | ±0.02 | ab |
| 5A No lime | 1.31 | ±0.23 | fgij | 2914 | ±759 | i | 426 | ±99 | k | 0.55 | ±0.07 | bcdefghij |
| 5B Low lime | 0.72 | ±0.23 | defgh | 3142 | ±447 | i | 474 | ±75 | k | 0.48 | ±0.02 | abcdefg |
| 6A No lime | 1.40 | ±0.32 | fghij | 2612 | ±738 | hi | 378 | ±95 | jk | 0.54 | ±0.06 | bdefgh |
| 6B High lime | 0.46 | ±0.08 | de | 2337 | ±176 | hi | 332 | ±31 | jk | 0.45 | ±0.02 | abc |
| Russell lupin | ||||||||||||
| 1A Low lime | 0.99 | ±0.53 | defghi | 1042 | ±333 | fg | 191 | ±58 | fghi | 0.68 | ±0.09 | klmno |
| 1B Low lime | 0.05 | ±0.04 | ab | 417 | ±120 | abc | 61 | ±20 | abc | 0.46 | ±0.02 | abcd |
| 2A High lime | 0.35 | ±0.05 | d | 450 | ±114 | abcde | 90 | ±23 | acdef | 0.63 | ±0.01 | hjklmno |
| 2B High lime | 0.02 | ±0.006 | a | 302 | ±48 | ab | 50 | ±8 | ab | 0.54 | ±0.04 | bcdefghi |
| 3A Low lime | 1.42 | ±0.76 | defghij | 1173 | ±558 | cefg | 210 | ±94 | efghi | 0.70 | ±0.08 | no |
| 3B No lime | 0.15 | ±0.09 | bc | 663 | ±322 | abcd | 112 | ±57 | abcd | 0.51 | ±0.03 | abcdefg |
| 4A High lime | 0.83 | ±0.39 | defg | 671 | ±226 | bcdef | 138 | ±46 | defgh | 0.66 | ±0.02 | kmno |
| 4B No lime | 0.11 | ±0.08 | abc | 408 | ±164 | a | 74 | ±32 | a | 0.56 | ±0.03 | defghijkl |
| 5A No lime | 0.74 | ±0.22 | defghi | 929 | ±156 | fg | 177 | ±25 | ghij | 0.62 | ±0.02 | hijklmn |
| 5B Low lime | 0.05 | ±0.03 | ab | 432 | ±82 | abcde | 76 | ±18 | abcde | 0.54 | ±0.02 | cdefghijk |
| 6A No lime | 1.76 | ±0.68 | efghij | 1657 | ±683 | gh | 287 | ±89 | hijk | 0.80 | ±0.11 | o |
| 6B High lime | 0.17 | ±0.08 | c | 696 | ±166 | cdef | 125 | ±29 | defg | 0.57 | ±0.02 | ghijklmn |
| Species | * | * | * | * | ||||||||
| Treatment | * | * | * | * | ||||||||
| Species*Treatment | * | * | * | ns | ||||||||
| Soil Analysis | Value | By Method of |
|---|---|---|
| pHH2O | 4.7 | [43] |
| Olsen P (μg mL−1) | 13 | [44] |
| Sulphate Sulphur (μg g−1) | 17 | [45] |
| Potassium (me 100 g−1) | 0.59 | [46] |
| Calcium (me 100 g−1) | 2.1 | |
| Magnesium (me 100 g−1) | 0.94 | |
| Sodium (me 100 g−1) | 0.10 | |
| CEC (me 100 g−1) | 19 | [47] |
| Total Base Saturation (%) | 19.6 | |
| Exchangeable AlCaCl2 (mg kg−1) | 25 | [48] |
| Organic matter (%w w−1) | 11.5 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bell, L.E.; Moir, J.L.; Black, A.D. The Effects of Soil Acidity and Aluminium on the Root Systems and Shoot Growth of Lotus pedunculatus and Lupinus polyphyllus. Plants 2024, 13, 2268. https://doi.org/10.3390/plants13162268
Bell LE, Moir JL, Black AD. The Effects of Soil Acidity and Aluminium on the Root Systems and Shoot Growth of Lotus pedunculatus and Lupinus polyphyllus. Plants. 2024; 13(16):2268. https://doi.org/10.3390/plants13162268
Chicago/Turabian StyleBell, Lucy E., Jim L. Moir, and Alistair D. Black. 2024. "The Effects of Soil Acidity and Aluminium on the Root Systems and Shoot Growth of Lotus pedunculatus and Lupinus polyphyllus" Plants 13, no. 16: 2268. https://doi.org/10.3390/plants13162268





