Transcriptome Analysis Revealed the Paternal Importance to Vegetative Growth Heterosis in Populus
Abstract
:1. Introduction
2. Results
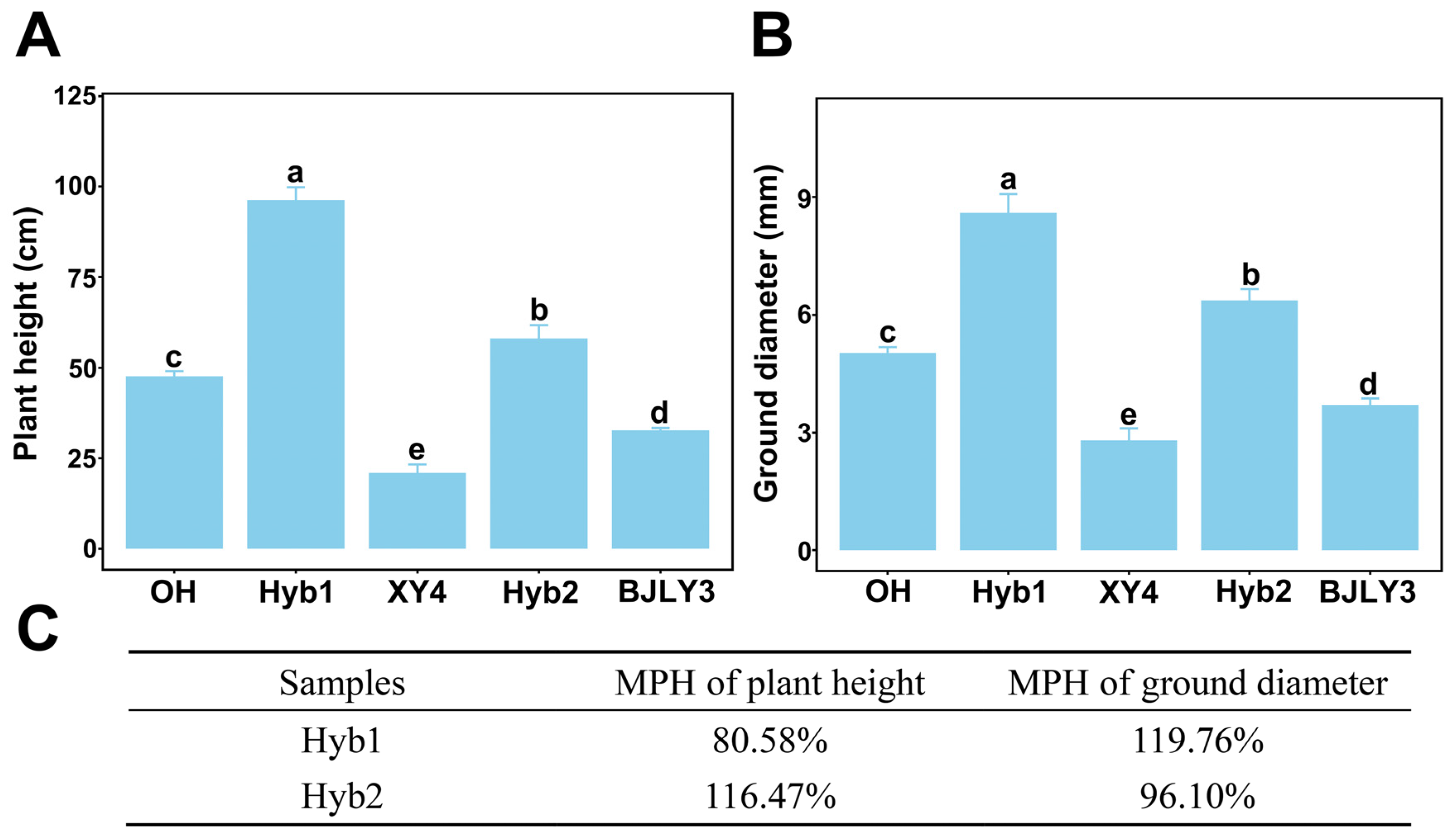
2.1. Heterosis in Plant Height and Ground Diameter
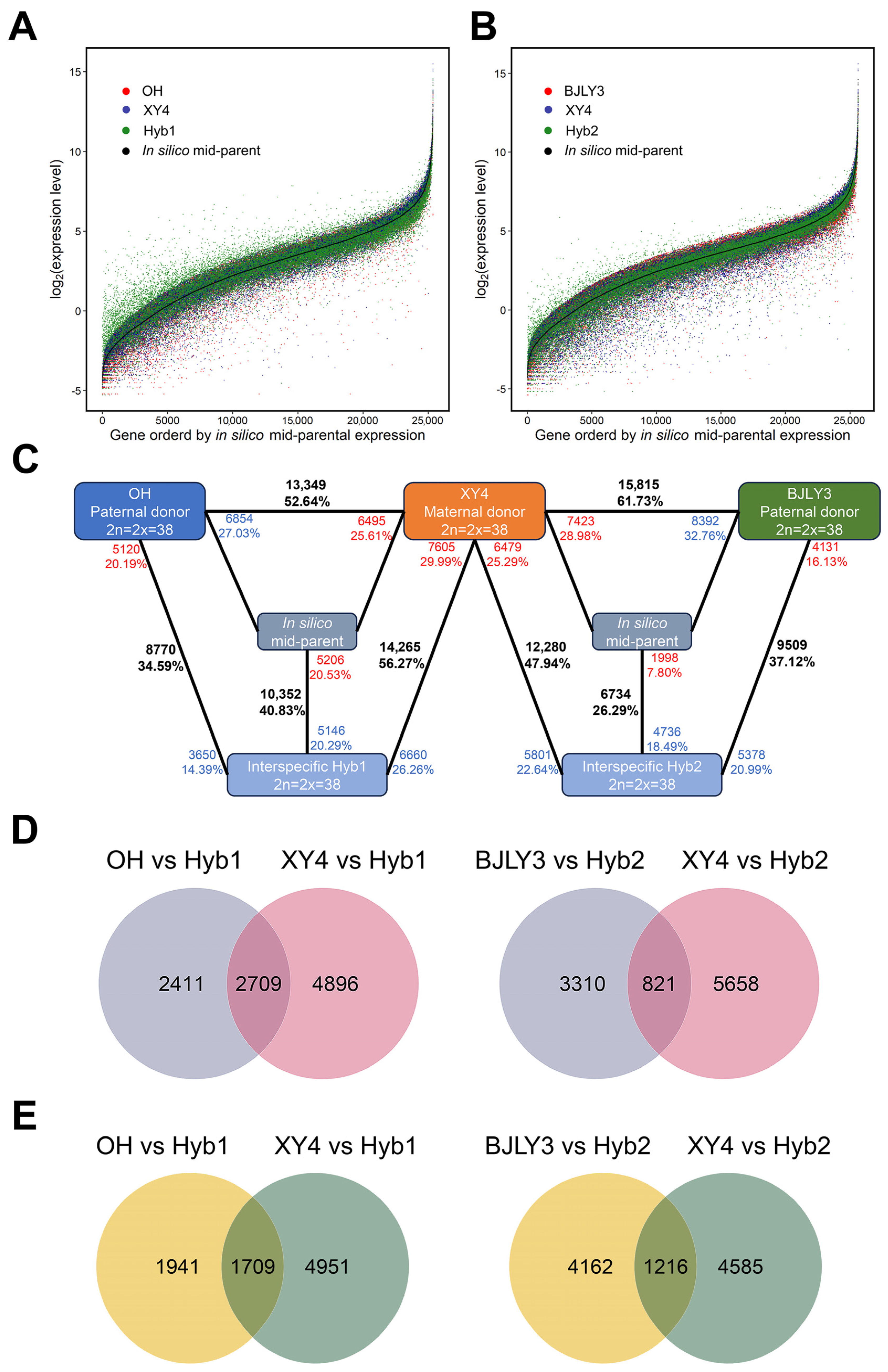
2.2. RNA Sequencing Data Analysis and qPCR Validation
2.3. Identification of DEGs between Hybrids and Parents
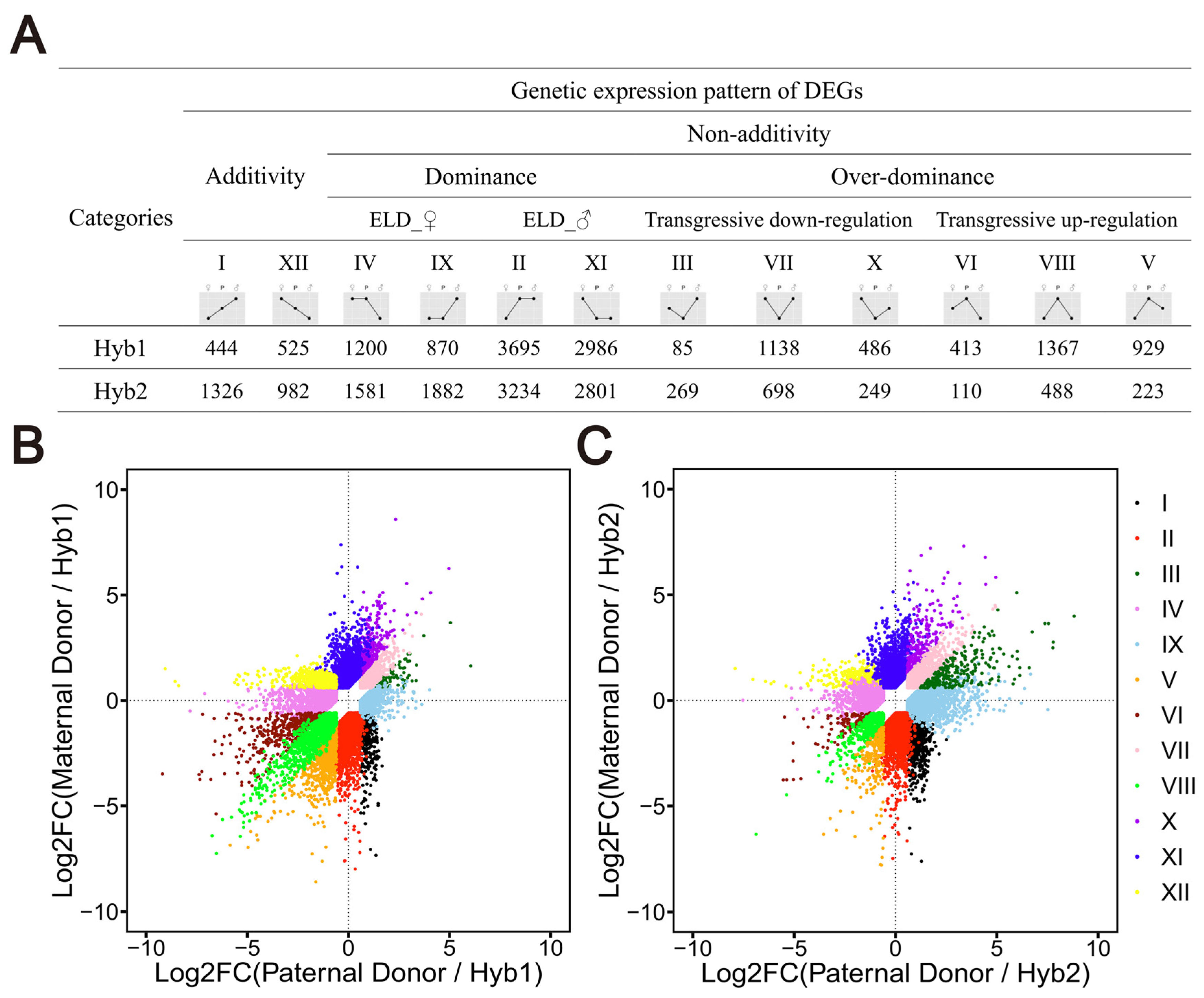
2.4. Genetic Pattern Analysis of Gene Expression
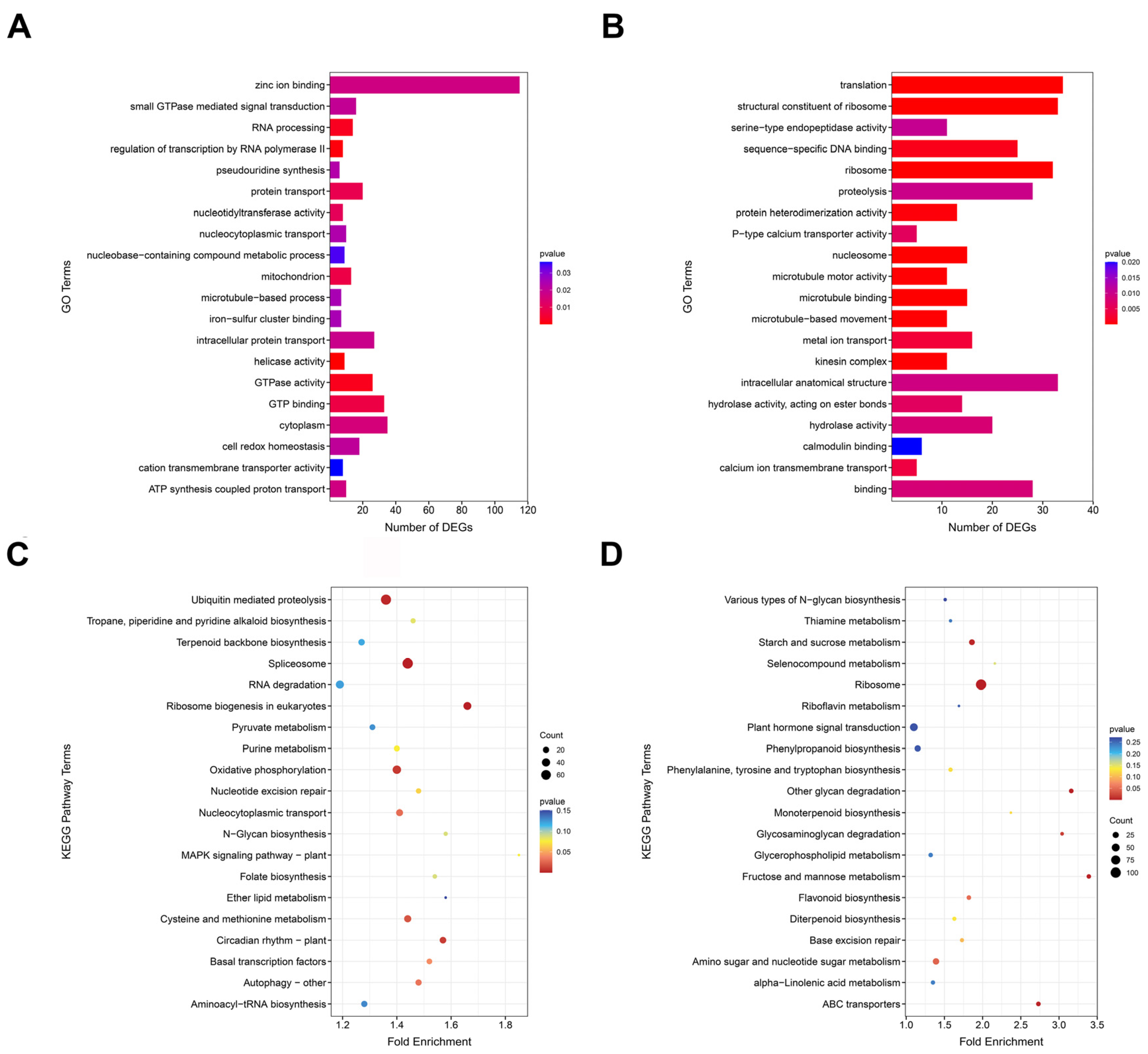
2.5. GO and KEGG Enrichment Analysis of Non-Additively Expressed Genes in Hybrids
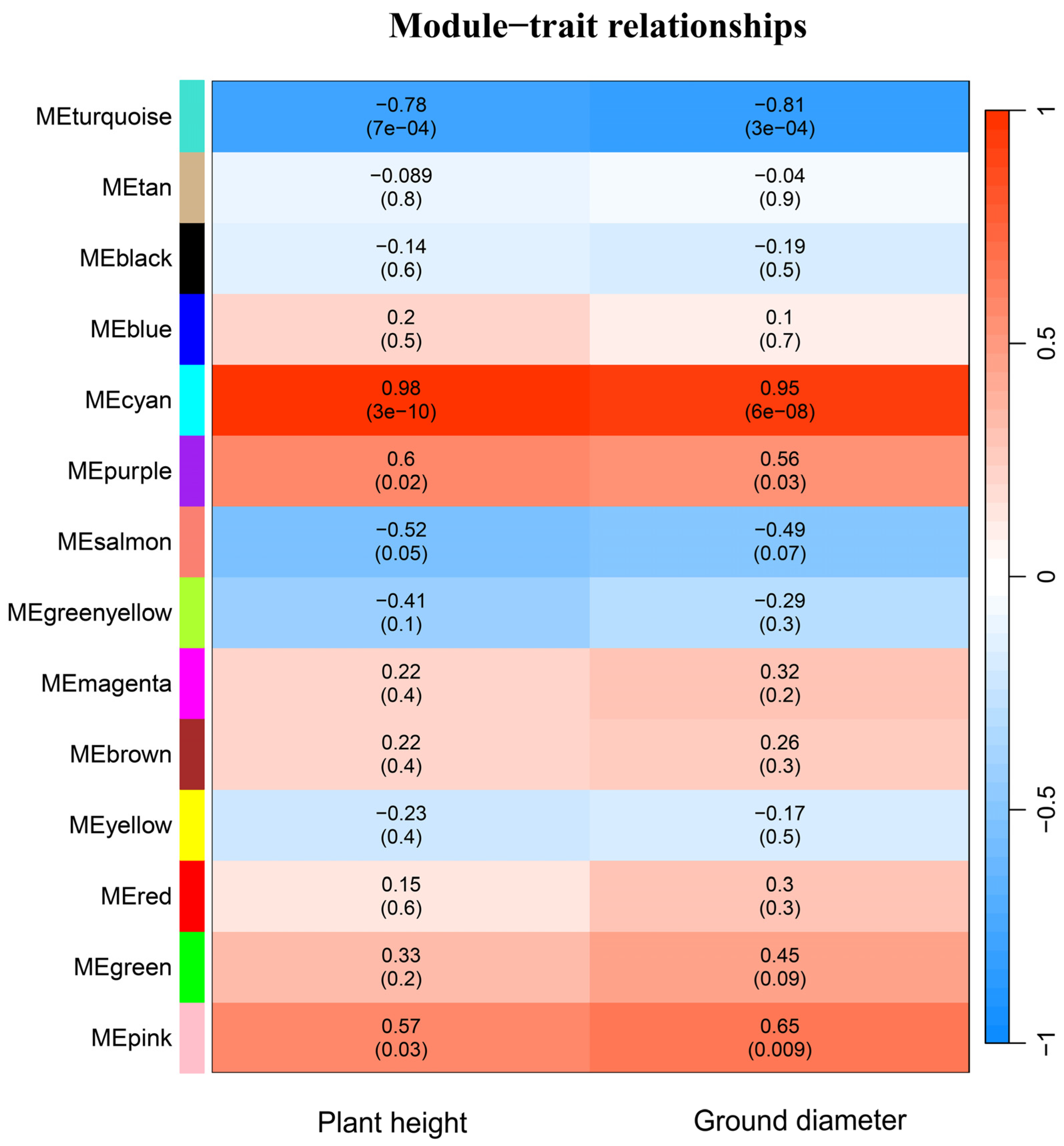
2.6. Weighted Co-Expression Network Analysis
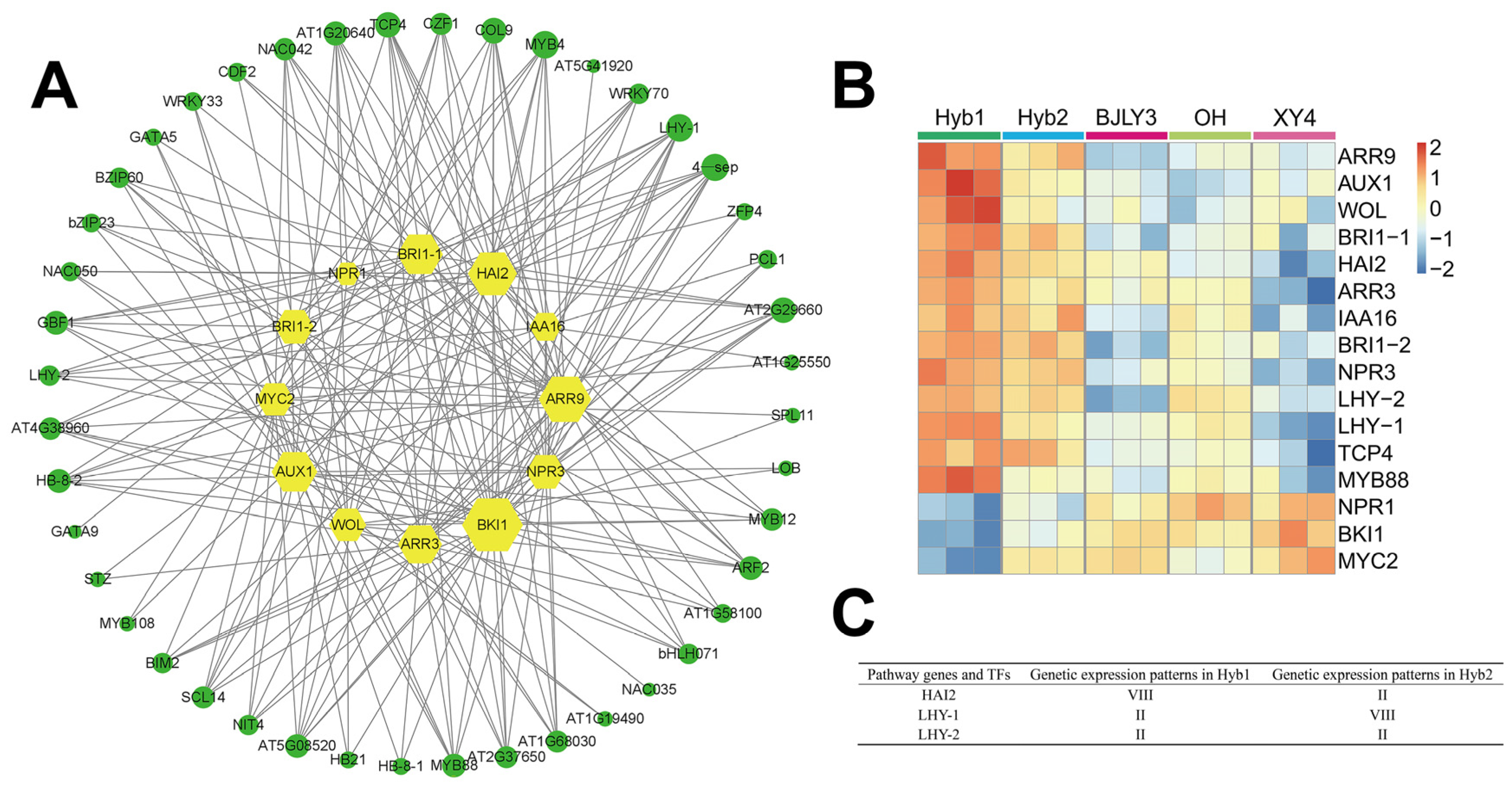
2.7. Identification of Regulators of Plant Hormone Signal Transduction Pathway
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Calculation of Mid-Parent Heterosis
4.3. cDNA Preparation and Ion Porton Sequencing
4.4. Quality Control and Mapping of RNA-Seq
4.5. Gene Expression Analysis
4.6. Identification of Differentially Expressed Genes (DEGs) and Gene Inheritance Patterns
4.7. Functional Annotation of DEGs
4.8. Gene Co-Expression Network Analysis and Visualization
4.9. Quantitative Real-Time PCR (qPCR) Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| BKI1 | BRI1 kinase inhibitor 1 |
| BR | Brassinosteroid |
| BRI1 | Brassinosteroid insensitive 1 |
| COI1 | Coronatine insensitive 1 |
| DEG | Differentially expressed gene |
| ELD_♀ | Maternal expression dominant effect |
| ELD_♂ | Paternal expression dominant effect |
| FDR | False discovery rate |
| GO | Gene ontology |
| PsbQ-2 | photosystem II subunit Q-2 |
| HAI2 | Highly ABA-induced PP2C gene 2 |
| JA | Jasmonic acid |
| KEGG | Kyoto encyclopedia of genes and genomes |
| NCED3 | 9-cis-epoxycarotenoid dioxygenase 3 |
| TF | Transcription factor |
| WGCNA | Weighted gene co-expression network analysis |
References
- Birchler, J.A.; Auger, D.L.; Riddle, N.C. In search of the molecular basis of heterosis. Plant Cell 2003, 15, 2236–2239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J. Genomic and epigenetic insights into the molecular bases of heterosis. Nat. Rev. Genet. 2013, 14, 471. [Google Scholar] [CrossRef]
- Paril, J.; Reif, J.; Fournier-Level, A.; Pourkheirandish, M. Heterosis in crop improvement. Plant J. 2024, 117, 23–32. [Google Scholar] [CrossRef]
- Cheng, S.H.; Zhuang, J.Y.; Fan, Y.Y.; Du, J.H.; Cao, L.Y. Progress in research and development on hybrid rice: A super-domesticate in China. Ann. Bot. 2007, 100, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lin, C.; Fu, F.; Zhong, X.; Peng, B.; Yan, H.; Zhang, J.; Zhang, W.; Wang, P.; Ding, X.; et al. Comparative transcriptome analysis of flower heterosis in two soybean F1 hybrids by RNA-seq. PLoS ONE 2017, 12, e0181061. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Du, J.; Ma, L.; Ding, C.; Zhang, P.; Kang, X. Regional testing of triploid hybrid clones of Populus tomentosa. BMC Plant Biol. 2023, 23, 277. [Google Scholar] [CrossRef]
- Muneera Parveen, A.B.; Muthupandi, M.; Kumar, N.; Singh Chauhan, S.; Vellaichamy, P.; Senthamilselvam, S.; Rajasugunasekar, D.; Nagarajan, B.; Mayavel, A.; Bachpai, V.K.W.; et al. Quantitative genetic analysis of wood property traits in biparental population of Eucalyptus camaldulensis × E. tereticornis. J. Genet. 2021, 100, 46. [Google Scholar] [CrossRef] [PubMed]
- Bertan, I.; de Carvalho, F.I.F.; Oliveira, A.C. Parental selection strategies in plant breeding programs. J. Crop Sci. Biotechnol. 2007, 10, 211–222. [Google Scholar]
- Carrodeguas-Gonzalez, A.; Zúñiga-Orozco, A. Methods used for parental selection in pre-breeding of plants. Cult. Trop. 2022, 43, e15. [Google Scholar]
- Pirnajmedin, F.; Majidi, M.M.; Taleb, M.H.; Rostami, D. Genetic parameters and selection in full-sib families of tall fescue using best linear unbiased prediction (BLUP) analysis. BMC Plant Biol. 2022, 22, 293. [Google Scholar] [CrossRef]
- Xing, N.; Fan, C.; Zhou, Y. Parental selection of hybrid breeding based on maternal and paternal inheritance of traits in rapeseed (Brassica napus L.). PLoS ONE 2014, 9, e103165. [Google Scholar] [CrossRef] [PubMed]
- Reju, M.J.; Meenakumari, T.; John, A.; Gireesh, T. Role of parental selection in classical Hevea breeding in India. Rubber Sci. 2021, 34, 237–249. [Google Scholar]
- Shi, X.; Li, W.; Guo, Z.; Wu, M.; Zhang, X.; Yuan, L.; Qiu, X.; Xing, Y.; Sun, X.; Xie, H.; et al. Comparative transcriptomic analysis of maize ear heterosis during the inflorescence meristem differentiation stage. BMC Plant Biol. 2022, 22, 348. [Google Scholar] [CrossRef] [PubMed]
- Howlader, J.; Robin, A.H.K.; Natarajan, S.; Biswas, M.K.; Sumi, K.R.; Song, C.Y.; Park, J.; Nou, I. Transcriptome analysis by RNA-seq reveals genes related to plant height in two sets of parent-hybrid combinations in Easter lily (Lilium longiflorum). Sci. Rep. 2020, 10, 9082. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, P.; Chen, X.; Sun, Y.; Yue, C.; Ye, N. Transcriptome and metabolite profiling reveal novel insights into volatile heterosis in the tea plant (Camellia Sinensis). Molecules 2019, 24, 3380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Greaves, I.K.; Liu, P.C.; Wu, L.; Dennis, E.S.; Peacock, W.J. Early changes of gene activity in developing seedlings of Arabidopsis hybrids relative to parents may contribute to hybrid vigour. Plant J. 2016, 88, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Taliercio, E.; Eickholt, D.; Rouf, R.; Carter, T. Changes in gene expression between a soybean F1 hybrid and its parents are associated with agronomically valuable traits. PLoS ONE 2017, 12, e0177225. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, H.; Diao, X.; Liu, Z.; Li, K.; Wu, Y.; Liang, Q.; Wang, H.; Huang, C. Transcriptome profiling and comparison of maize ear heterosis during the spikelet and floret differentiation stage. BMC Genom. 2016, 17, 959. [Google Scholar] [CrossRef] [PubMed]
- Georgii, E.; Kugler, K.; Pfeifer, M.; Vanzo, E.; Block, K.; Domagalska, M.A.; Jud, W.; AbdElgawad, H.; Asard, H.; Reinhardt, R.; et al. The systems architecture of molecular memory in poplar after abiotic stress. Plant Cell. 2019, 31, 346–367. [Google Scholar] [CrossRef] [PubMed]
- Porth, I.; El-Kassaby, Y.A. Using Populus as a lignocellulosic feedstock for bioethanol. Biotechnol. J. 2015, 10, 510–524. [Google Scholar] [CrossRef]
- Bannoud, F.; Bellini, C. Adventitious rooting in Populus species: Update and perspectives. Front. Plant Sci. 2021, 12, 668837. [Google Scholar] [CrossRef]
- Ren, J.; Ji, X.; Wang, C.; Hu, J.; Nervo, G.; Li, J. Variation and genetic parameters of leaf morphological traits of eight families from Populus simonii× P. nigra. Forests 2020, 11, 1319. [Google Scholar] [CrossRef]
- Pearce, D.W.; Rood, S.B.; Wu, R. Phytohormones and shoot growth in a three-generation hybrid poplar family. Tree Physiol. 2004, 24, 217–224. [Google Scholar] [CrossRef]
- Azad, A.K.; Sarker, U.; Ercisli, S.; Assouguem, A.; Ullah, R.; Almeer, R.; Sayed, A.A.; Peluso, I. Evaluation of combining ability and heterosis of popular restorer and male sterile lines for the development of superior rice hybrids. Agronomy 2022, 12, 965. [Google Scholar] [CrossRef]
- Ren, X.; Chen, L.; Deng, L.; Zhao, Q.; Yao, D.; Li, X.; Cong, W.; Zang, Z.; Zhao, D.; Zhang, M.; et al. Comparative transcriptomic analysis reveals the molecular mechanism underlying seedling heterosis and its relationship with hybrid contemporary seeds DNA methylation in soybean. Front. Plant Sci. 2024, 15, 1364284. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Yi, Z. Heterosis for biomass yield and quality traits in a reciprocal cross population between Miscanthus sinensis and Miscanthus lutarioriparius. Ind. Crops Prod. 2023, 205, 117451. [Google Scholar] [CrossRef]
- Würschum, T.; Zhu, X.; Zhao, Y.; Jiang, Y.; Reif, J.C.; Maurer, H.P. Maximization through optimization? On the relationship between hybrid performance and parental genetic distance. Theor. Appl. Genet. 2023, 136, 186. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, H.; Ye, J.; Shi, L. Relationship between parental genetic distance and offspring’s heterosis for early growth traits in Liriodendron: Implication for parent pair selection in cross breeding. New For. 2016, 47, 163–177. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, Y.; Yan, T.; Li, Y.; Jiang, N.; Zhou, Y.; Zhou, Q.; Qin, P.; Fu, C.; Lin, H.; et al. Transcriptome profiling of two super hybrid rice provides insights into the genetic basis of heterosis. BMC Plant Biol. 2022, 22, 314. [Google Scholar] [CrossRef]
- Li, D.; Zeng, R.; Li, Y.; Zhao, M.; Chao, J.; Li, Y.; Wang, K.; Zhu, L.; Tian, W.-M.; Liang, C. Gene expression analysis and SNP/InDel discovery to investigate yield heterosis of two rubber tree F1 hybrids. Sci. Rep. 2016, 6, 24984. [Google Scholar] [CrossRef]
- Bao, J.; Lee, S.; Chen, C.; Zhang, X.; Zhang, Y.; Liu, S.; Clark, T.; Wang, J.; Cao, M.; Yang, H.; et al. Serial analysis of gene expression study of a hybrid rice strain (LYP9) and its parental cultivars. Plant Physiol. 2005, 138, 1216–1231. [Google Scholar] [CrossRef]
- Xiong, J.; Hu, K.; Shalby, N.; Zhuo, C.; Wen, J.; Yi, B.; Shen, J.; Ma, C.; Fu, T.; Tu, J. Comparative transcriptomic analysis reveals the molecular mechanism underlying seedling biomass heterosis in Brassica napus. BMC Plant Biol. 2022, 22, 283. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; Lin, Y.; Dang, K.; Wan, J.; Meng, S.; Qiu, X.; Wang, Q.; Mu, L.; Ding, D.; et al. Comparative transcriptomics analysis at the key stage of maize ear development dissect heterosis. Plant Genome 2023, 16, e20293. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, K.; Zhang, X.; Guo, L.; Qi, T.; Tang, H.; Zhang, M.; Zhang, B.; Wang, H.; Qiao, X.; Feng, J.; et al. Comparative transcriptome analysis of inbred lines and contrasting hybrids reveals overdominance mediate early biomass vigor in hybrid cotton. BMC Genom. 2020, 21, 140. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Y.; Zhang, H.; He, C.; Wang, X.; Cui, Y.; Heng, Y.; Lin, Y.; Gu, R.; Wang, J.; et al. Integrated multi-omics reveals significant roles of non-additively expressed small RNAs in heterosis for maize plant height. Int. J. Mol. Sci. 2023, 24, 9150. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, L.; Wang, Y.; Zhang, W.; Zhang, X.; Dong, J.; Ying, J.; Wang, L.; Ma, Y.; Liu, L. Integration of transcriptome and DNA methylome analysis reveals the molecular mechanism of taproot yield heterosis in radish (Raphanus sativus L.). Hortic. Plant J. 2023. [Google Scholar] [CrossRef]
- Chen, L.; Bian, J.; Shi, S.; Yu, J.; Khanzada, H.; Wassan, G.M.; Zhu, C.; Luo, X.; Tong, S.; Yang, X.; et al. Genetic analysis for the grain number heterosis of a super-hybrid rice WFYT025 combination using RAN-Seq. Rice 2018, 11, 37. [Google Scholar] [CrossRef]
- Li, R.; Nie, S.; Zhang, N.; Tian, M.; Zhang, L. Transcriptome Analysis Reveals a Major Gene Expression Pattern and Important Metabolic Pathways in the Control of Heterosis in Chinese Cabbage. Plants 2023, 12, 1195. [Google Scholar] [CrossRef] [PubMed]
- Végvári, G.; Vidéki, E. Plant hormones, plant growth regulators. Orvosi Hetil. 2014, 155, 1011–1018. [Google Scholar] [CrossRef]
- Hu, S.; Wang, C.; Sanchez, D.L.; Lipka, A.E.; Liu, P.; Yin, Y.; Blanco, M.; Lübberstedt, T. Gibberellins promote brassinosteroids action and both increase heterosis for plant height in maize (Zea mays L.). Front. Plant Sci. 2017, 8, 1039. [Google Scholar] [CrossRef]
- Clouse, S.D. Brassinosteroid signal transduction: From receptor kinase activation to transcriptional networks regulating plant development. Plant Cell 2011, 23, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Jaillais, Y.; Chory, J. Unraveling the paradoxes of plant hormone signaling integration. Nat. Struct. Mol. Biol. 2010, 17, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Buttò, V.; Deslauriers, A.; Rossi, S.; Rozenberg, P.; Shishov, V.; Morin, H. The role of plant hormones in tree-ring formation. Trees 2020, 34, 315–335. [Google Scholar] [CrossRef]
- Sehr, E.M.; Agusti, J.; Lehner, R.; Farmer, E.E.; Schwarz, M.; Greb, T. Analysis of secondary growth in the Arabidopsis shoot reveals a positive role of jasmonate signalling in cambium formation. Plant J. 2010, 63, 811–822. [Google Scholar] [CrossRef]
- Yi, X.; Hargett, S.R.; Frankel, L.K.; Bricker, T.M. The PsbQ protein is required in Arabidopsis for photosystem II assembly/stability and photoautotrophy under low light conditions. J. Biol. Chem. 2006, 281, 26260–26267. [Google Scholar] [CrossRef]
- Xie, Z.; Li, D.; Wang, L.; Sack, F.D.; Grotewold, E. Role of the stomatal development regulators FLP/MYB88 in abiotic stress responses. Plant J. 2010, 64, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Bu, T.; Cheng, Q.; Dong, L.; Su, T.; Chen, Z.; Kong, F.; Gong, Z.; Liu, B.; Li, M. Two homologous LHY pairs negatively control soybean drought tolerance by repressing the abscisic acid responses. New Phytol. 2021, 229, 2660–2675. [Google Scholar] [CrossRef]
- Belbin, F.E. Dodd ANABA signalling is regulated by the circadian clock component LHY. New Phytol. 2018, 220, 661–663. [Google Scholar] [CrossRef]
- Adams, S.; Grundy, J.; Veflingstad, S.R.; Dyer, N.P.; Hannah, M.A.; Ott, S.; Carré, I.A. Circadian control of abscisic acid biosynthesis and signalling pathways revealed by genome-wide analysis of LHY binding targets. New Phytol. 2018, 220, 893–907. [Google Scholar] [CrossRef]
- Antoni, R.; Gonzalez-Guzman, M.; Rodriguez, L.; Rodrigues, A.; Pizzio, G.A.; Rodriguez, P.L. Selective inhibition of clade A phosphatases type 2C by PYR/PYL/RCAR abscisic acid receptors. Plant Physiol. 2012, 158, 970–980. [Google Scholar] [CrossRef]
- Challa, K.R.; Aggarwal, P.; Nath, U. Activation of YUCCA5 by the transcription factor TCP4 integrates developmental and environmental signals to promote hypocotyl elongation in Arabidopsis. Plant Cell. 2016, 28, 2117–2130. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Wei, H. Breeding polyploid Populus: Progress and perspective. For. Res. 2022, 2, 4–13. [Google Scholar] [CrossRef]
- Li, D.; Tian, J.; Xue, Y.; Chen, H.; Wang, J. Triploid production via heat-induced diploidisation of megaspores in Populus pseudo-simonii. Euphytica 2019, 215, 1–9. [Google Scholar] [CrossRef]
- Huang, X.Y.; Shang, J.; Zhong, Y.H.; Li, D.L.; Song, L.J.; Wang, J. Disaggregation of ploidy, gender, and genotype effects on wood and fiber traits in a diploid and triploid hybrid poplar family. Front. Plant Sci. 2022, 13, 866296. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Shang, F.; Kang, X. High temperature-induced production of unreduced pollen and its cytological effects in Populus. Sci. Rep. 2017, 7, 5281. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Feng, Y.; Wang, H.; Zhan, X.; Shen, X.; Wu, W.; Zhang, Y.; Chen, D.; Dai, G.; Yang, Z.; et al. Transcriptome analysis of rice root heterosis by RNA-Seq. BMC Genom. 2013, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Tuskan, G.A.; DiFazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A.; et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar] [PubMed]
- Wang, K.; Singh, D.; Zeng, Z.; Coleman, S.J.; Huang, Y.; Savich, G.L.; He, X.; Mieczkowski, P.; Grimm, S.A.; Perou, C.M.; et al. MapSplice: Accurate mapping of RNA-seq reads for splice junction discovery. Nucleic Acids Res. 2010, 38, e178. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Wang, X.; Lin, X.; Sun, S.; Shen, K.; Wang, J.; Jiang, T.; Zhong, S.; Xu, C.; et al. Transcriptome shock in an interspecific F1 triploid hybrid of Oryza revealed by RNA sequencing. J. Integr. Plant Biol. 2016, 58, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Wu, L.; Zhong, Y.; Zhao, X.; Xu, M.; Wang, J. Transcriptome Analysis Revealed the Paternal Importance to Vegetative Growth Heterosis in Populus. Plants 2024, 13, 2278. https://doi.org/10.3390/plants13162278
Ren Y, Wu L, Zhong Y, Zhao X, Xu M, Wang J. Transcriptome Analysis Revealed the Paternal Importance to Vegetative Growth Heterosis in Populus. Plants. 2024; 13(16):2278. https://doi.org/10.3390/plants13162278
Chicago/Turabian StyleRen, Yuxin, Lixia Wu, Yuhang Zhong, Xinwen Zhao, Meng Xu, and Jun Wang. 2024. "Transcriptome Analysis Revealed the Paternal Importance to Vegetative Growth Heterosis in Populus" Plants 13, no. 16: 2278. https://doi.org/10.3390/plants13162278




