Plant Cell Wall Polysaccharide O-Acetyltransferases
Abstract
1. Introduction
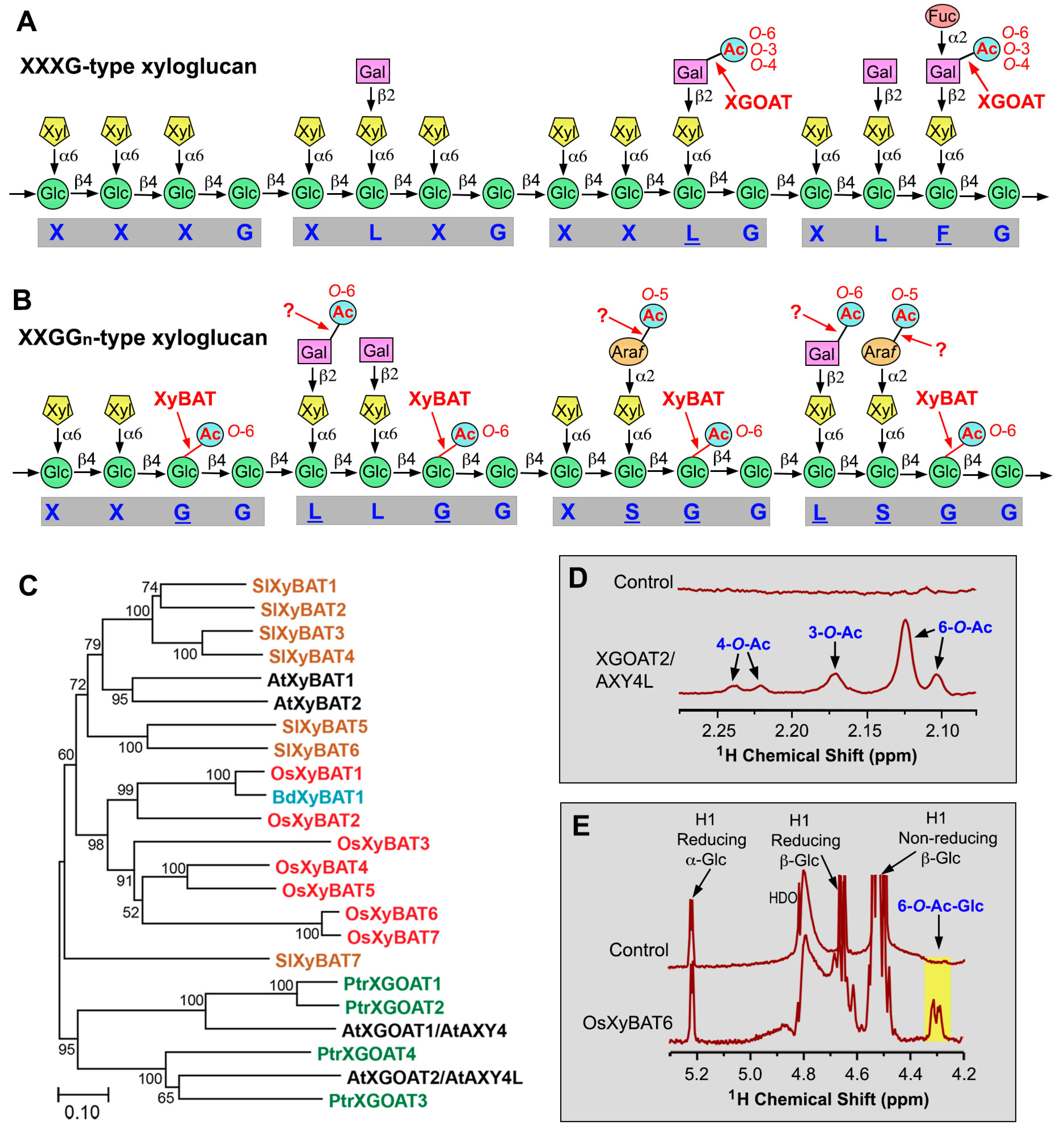
2. Xyloglucan O-Acetyltransferases
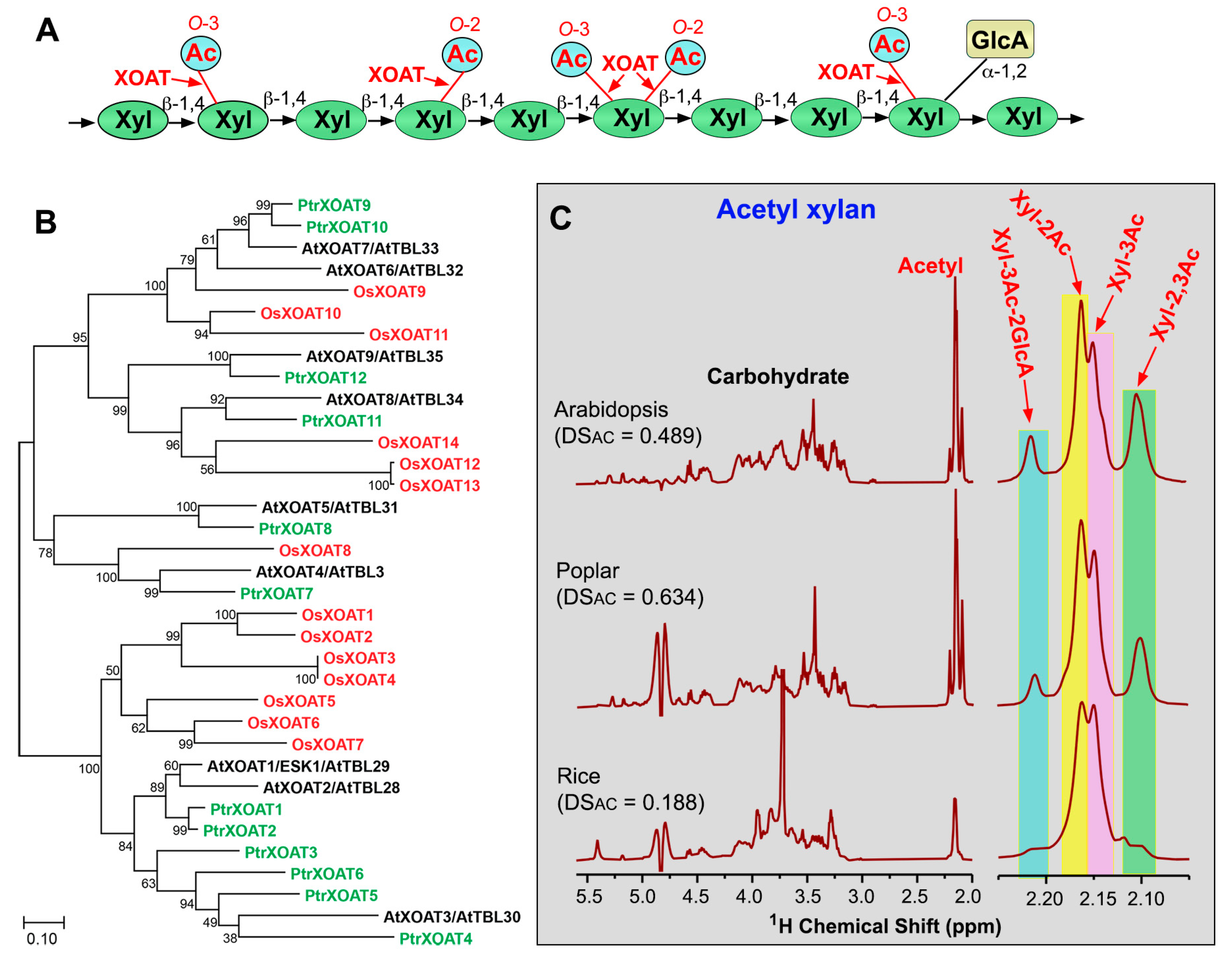
3. Xylan O-Acetyltransferases
4. Mannan O-Acetyltransferases
5. Pectin O-Acetyltransferases
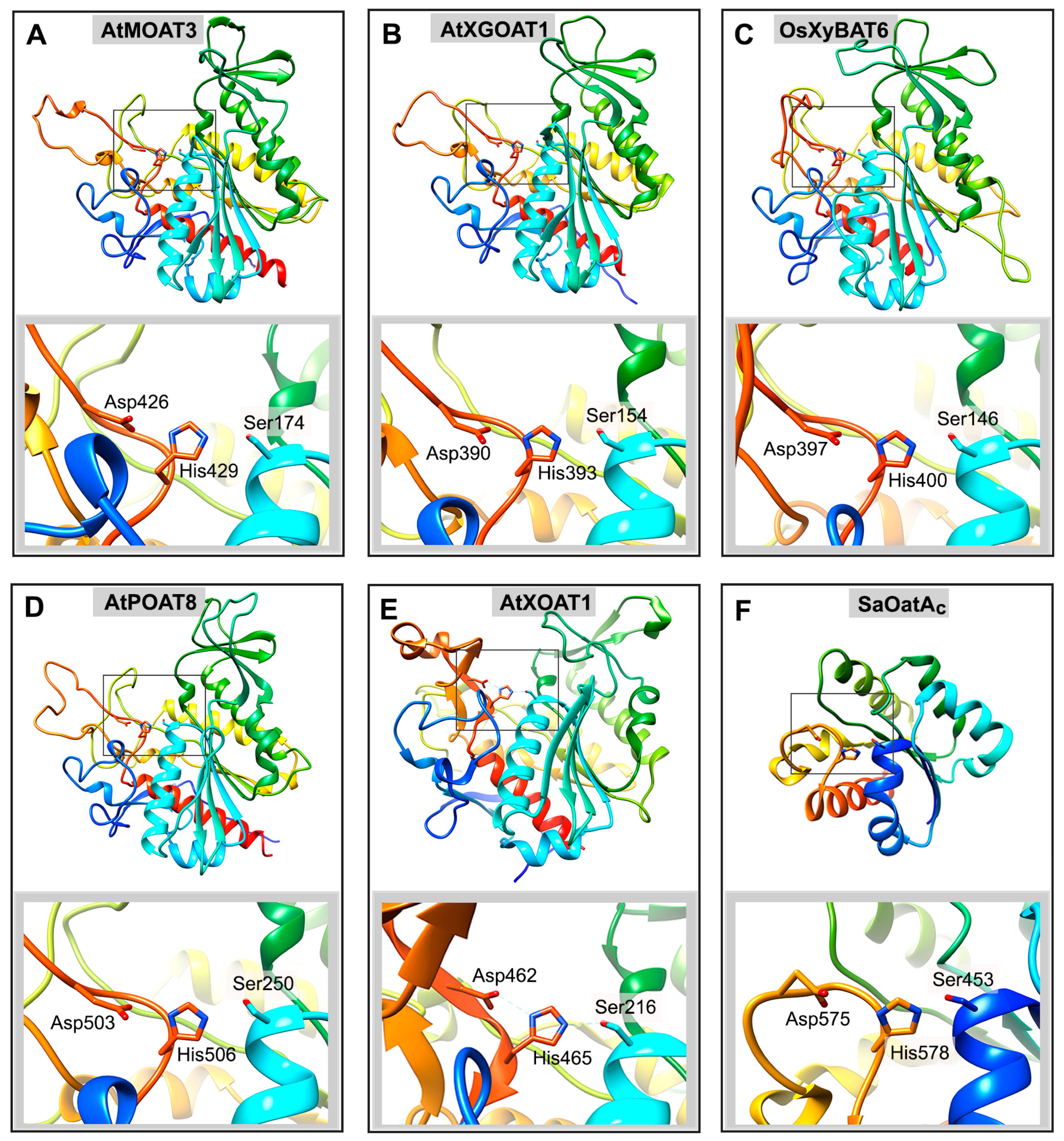
6. Structure and Mechanism of Action of Plant Cell Wall Polysaccharide O-Acetyltransferases
7. Roles of RWAs and AXY9 in Plant Cell Wall Polysaccharide O-Acetylation
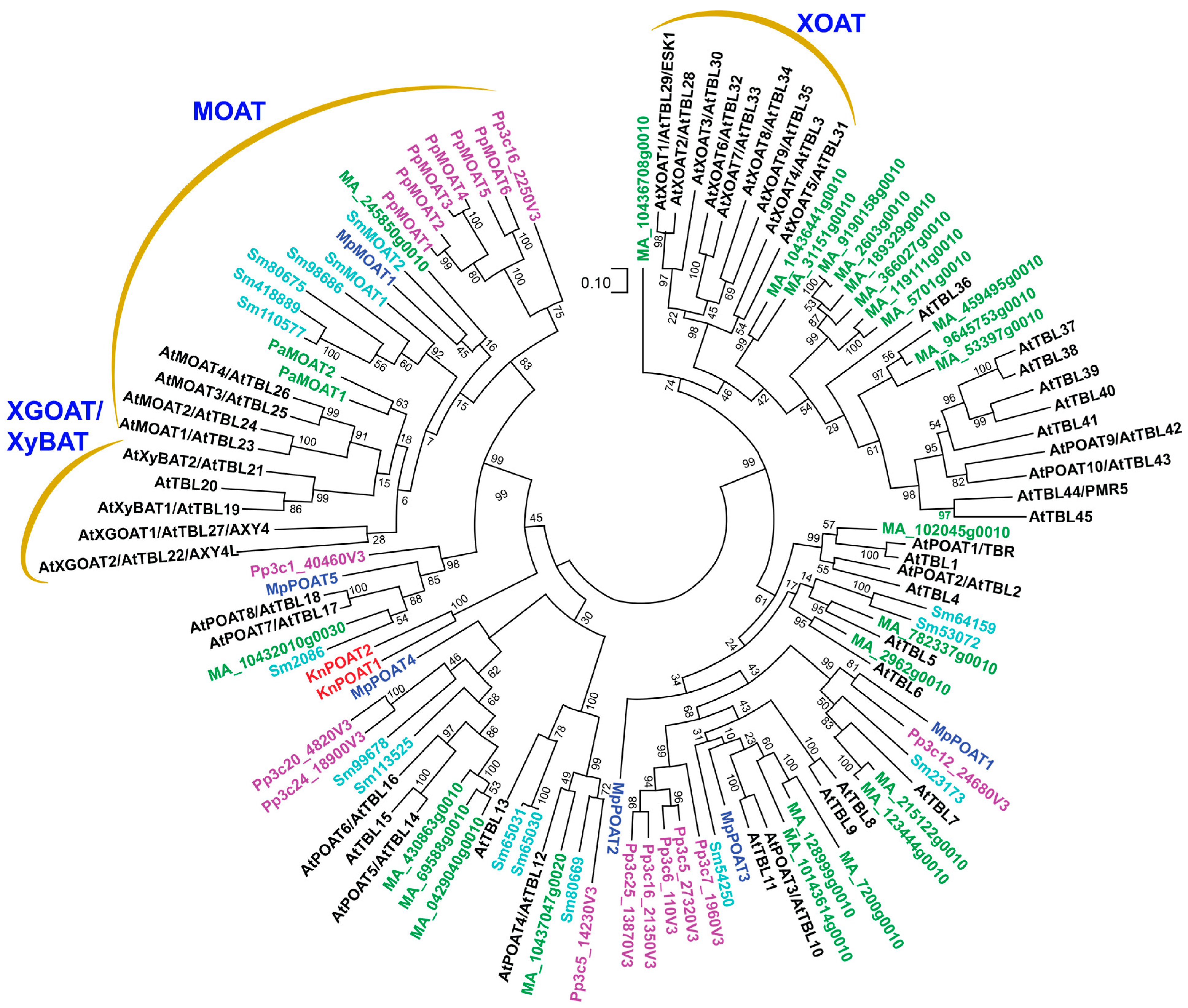
8. Evolutionary Origins of TBLs, RWAs and AXY9
9. Plant Cell Wall Polysaccharide O-Acetylesterases
10. Biological Functions of Plant Cell Wall Polysaccharide O-Acetylation
11. Biotechnological Applications of Manipulations of Plant Cell Wall Polysaccharide O-Acetylation
12. Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Gille, S.; Pauly, M. O-acetylation of plant cell wall polysaccharides. Front. Plant Sci. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Pauly, M.; Ramírez, V. New insights into wall polysaccharide O-acetylation. Front. Plant Sci. 2018, 9, 1210. [Google Scholar] [CrossRef] [PubMed]
- Pawar, P.M.; Koutaniemi, S.; Tenkanen, M.; Mellerowicz, E.J. Acetylation of woody lignocellulose: Significance and regulation. Front. Plant Sci. 2013, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, M.F.; Wu, A.M. Balanced xylan acetylation is the key regulator of plant growth development, and cell wall structure and for industrial utilization. Int. J. Mol. Sci. 2020, 21, 7875. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Cheng, K.; Pauly, M. Xylan O-acetylation impacts xylem development and enzymatic recalcitrance as indicated by the Arabidopsis mutant tbl29. Mol. Plant 2013, 6, 1373–1375. [Google Scholar] [CrossRef]
- Yuan, Y.; Teng, Q.; Zhong, R.; Haghighat, M.; Richardson, E.A.; Ye, Z.-H. Mutations of Arabidopsis TBL32 and TBL33 affect xylan acetylation and secondary wall deposition. PLoS ONE 2016, 11, e0146460. [Google Scholar] [CrossRef]
- Yuan, Y.; Teng, Q.; Zhong, R.; Ye, Z.-H. The Arabidopsis DUF231 domain-containing protein ESK1 mediates 2-O- and 3-O-acetylation of xylosyl residues in xylan. Plant Cell Physiol. 2013, 54, 1186–1199. [Google Scholar] [CrossRef]
- Chiniquy, D.; Underwood, W.; Corwin, J.; Ryan, A.; Szemenyei, H.; Lim, C.C.; Stonebloom, S.H.; Birdseye, D.S.; Vogel, J.; Kliebenstein, D.; et al. PMR5, an acetylation protein at the intersection of pectin biosynthesis and defense against fungal pathogens. Plant J. 2019, 100, 1022–1035. [Google Scholar] [CrossRef]
- Gou, J.Y.; Miller, L.M.; Hou, G.; Yu, X.H.; Chen, X.Y.; Liu, C.J. Acetylesterase-mediated deacetylation of pectin impairs cell elongation, pollen germination, and plant reproduction. Plant Cell 2012, 24, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.P.; Raab, T.K.; Somerville, C.R.; Somerville, S.C. Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. Plant J. 2004, 40, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Del Río, J.C.; Marques, G.; Rencoret, J.; Martínez, A.T.; Gutiérrez, A. Occurrence of naturally acetylated lignin units. J. Agric. Food Chem. 2007, 55, 5461–5468. [Google Scholar] [CrossRef] [PubMed]
- Schultink, A.; Liu, L.; Zhu, L.; Pauly, M. Structural diversity and function of xyloglucan sidechain substituents. Plants 2014, 3, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.C.; York, W.S.; Albersheim, P.; Darvill, A.; Hayashi, T.; Joseleau, J.P.; Kato, Y.; Lorences, E.P.; Maclachlan, G.A.; McNeil, M.; et al. An unambiguous nomenclature for xyloglucan-derived oligosaccharides. Physiol. Plant. 1993, 89, 1–3. [Google Scholar] [CrossRef]
- York, W.S.; Oates, J.E.; van Halbeek, H.; Darvill, A.G.; Albersheim, P.; Tiller, P.R.; Dell, A. Location of the O-acetyl substituents on a nonasaccharide repeating unit of sycamore extracellular xyloglucan. Carbohydr. Res. 1988, 173, 113–132. [Google Scholar] [CrossRef]
- Jia, Z.; Cash, M.; Darvill, A.G.; York, W.S. NMR characterization of endogenously O-acetylated oligosaccharides isolated from tomato (Lycopersicon esculentum) xyloglucan. Carbohydr. Res. 2005, 340, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Sims, I.M.; Munro, S.L.; Currie, G.; Craik, D.; Bacic, A. Structural characterisation of xyloglucan secreted by suspension-cultured cells of Nicotiana plumbaginifolia. Carbohydr. Res. 1996, 293, 147–172. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.Y.; Harris, P.J. Structures of xyloglucans in primary cell walls of gymnosperms, monilophytes (ferns sensu lato) and lycophytes. Phytochemistry 2012, 79, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.J.; Darvill, A.G.; Eberhard, S.; York, W.S.; O’Neill, M.A. Moss and liverwort xyloglucans contain galacturonic acid and are structurally distinct from the xyloglucans synthesized by hornworts and vascular plants. Glycobiology 2008, 18, 891–904. [Google Scholar] [CrossRef]
- Gille, S.; de Souza, A.; Xiong, G.; Benz, M.; Cheng, K.; Schultink, A.; Reca, I.B.; Pauly, M. O-acetylation of Arabidopsis hemicellulose xyloglucan requires AXY4 or AXY4L, proteins with a TBL and DUF231 domain. Plant Cell 2011, 23, 4041–4053. [Google Scholar] [CrossRef]
- Liu, L.; Paulitz, J.; Pauly, M. The presence of fucogalactoxyloglucan and its synthesis in rice indicates conserved functional importance in plants. Plant Physiol. 2015, 168, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Cui, D.; Ye, Z.-H. Xyloglucan O-acetyltransferases from Arabidopsis thaliana and Populus trichocarpa catalyze acetylation of fucosylated galactose residues on xyloglucan side chains. Planta 2018, 248, 1159–1171. [Google Scholar] [CrossRef]
- Perrin, R.M.; Jia, Z.; Wagner, T.A.; O’Neill, M.A.; Sarria, R.; York, W.S.; Raikhel, N.V.; Keegstra, K. Analysis of xyloglucan fucosylation in Arabidopsis. Plant Physiol. 2003, 132, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Günl, M.; Neumetzler, L.; Kraemer, F.; de Souza, A.; Schultink, A.; Pena, M.; York, W.S.; Pauly, M. AXY8 encodes an α-fucosidase, underscoring the importance of apoplastic metabolism on the fine structure of Arabidopsis cell wall polysaccharides. Plant Cell 2011, 23, 4025–4040. [Google Scholar] [CrossRef]
- Zhong, R.; Cui, D.; Ye, Z.-H. Evolutionary origin of O-acetyltransferases responsible for glucomannan acetylation in land plants. New Phytol. 2019, 224, 466–479. [Google Scholar] [CrossRef]
- Zhong, R.; Cui, D.; Phillips, D.R.; Richardson, E.A.; Ye, Z.-H. A group of O-acetyltransferases catalyze xyloglucan backbone acetylation and can alter xyloglucan xylosylation pattern and plant growth when expressed in Arabidopsis. Plant Cell Physiol. 2020, 61, 1064–1079. [Google Scholar] [CrossRef]
- Liu, L.; Hsia, M.M.; Dama, M.; Vogel, J.; Pauly, M. A Xyloglucan backbone, 6-O-acetyltransferase from Brachypodium distachyon modulates xyloglucan xylosylation. Mol. Plant 2016, 9, 615–617. [Google Scholar] [CrossRef][Green Version]
- Ye, Z.-H.; Zhong, R. Outstanding questions on xylan biosynthesis. Plant Sci. 2022, 325, 111476. [Google Scholar] [CrossRef]
- Busse-Wicher, M.; Li, A.; Silveira, R.L.; Pereira, C.S.; Tryfona, T.; Gomes, T.C.; Skaf, M.S.; Dupree, P. Evolution of xylan substitution patterns in gymnosperms and angiosperms: Implications for xylan interaction with cellulose. Plant Physiol. 2016, 171, 2418–3241. [Google Scholar] [CrossRef]
- Evtuguin, D.V.; Tomás, J.L.; Silva, A.M.; Neto, C.P. Characterization of an acetylated heteroxylan from Eucalyptus globulus Labill. Carbohydr. Res. 2003, 338, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, M.; Teng, Q.; Zhong, R.; Ye, Z.-H. Evolutionary conservation of xylan biosynthetic genes in Selaginella moellendorffii and Physcomitrella patens. Plant Cell Physiol. 2016, 57, 1707–1719. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, C.; Teng, Q.; Zhong, R.; Ye, Z.-H. The four Arabidopsis REDUCED WALL ACETYLATION genes are expressed in secondary wall-containing cells and required for the acetylation of xylan. Plant Cell Physiol. 2011, 52, 1289–1301. [Google Scholar] [CrossRef]
- Teleman, A.; Lundqvist, J.; Tjerneld, F.; Stålbrand, H.; Dahlman, O. Characterization of acetylated 4-O-methylglucuronoxylan isolated from aspen employing 1H and 13C NMR spectroscopy. Carbohydr. Res. 2000, 329, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Teleman, A.; Tenkanen, M.; Jacobs, A.; Dahlman, O. Characterization of O-acetyl-(4-O-methylglucurono)xylan isolated from birch and beech. Carbohydr. Res. 2002, 337, 373–377. [Google Scholar] [CrossRef]
- Zhong, R.; Cui, D.; Dasher, R.L.; Ye, Z.-H. Biochemical characterization of rice xylan O-acetyltransferases. Planta 2018, 247, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Cui, D.; Ye, Z.-H. A group of Populus trichocarpa DUF231 proteins exhibit differential O-acetyltransferase activities toward xylan. PLoS ONE 2018, 13, e0194532. [Google Scholar] [CrossRef] [PubMed]
- Timell, T.E. Recent progress in the chemistry of wood hemicelluloses. Wood Sci. Technol. 1967, 1, 45–70. [Google Scholar] [CrossRef]
- Neumuller, K.G.; de Souza, A.C.; van Rijn, J.H.; Streekstra, H.; Gruppen, H.; Schols, H.A. Positional preferences of acetyl esterases from different CE families towards acetylated, 4-O-methyl glucuronic acid-substituted xylo-oligosaccharides. Biotechnol. Biofuels 2015, 8, 7. [Google Scholar] [CrossRef]
- Goncalves, V.M.; Evtuguin, D.V.; Domingues, M.R. Structural characterization of the acetylated heteroxylan from the natural hybrid Paulownia elongata/Paulownia fortunei. Carbohydr. Res. 2008, 343, 256–266. [Google Scholar] [CrossRef]
- Carvalho, D.M.; Martínez-Abad, A.; Evtuguin, D.V.; Colodette, J.L.; Lindström, M.E.; Vilaplana, F.; Sevastyanova, O. Isolation and characterization of acetylated glucuronoarabinoxylan from sugarcane bagasse and straw. Carbohydr. Polym. 2017, 156, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Naran, R.; Black, S.; Decker, S.R.; Azadi, P. Extraction and characterization of native heteroxylans from delignified corn stover and aspen. Cellulose 2009, 16, 661–675. [Google Scholar] [CrossRef]
- Chong, S.L.; Virkki, L.; Maaheimo, H.; Juvonen, M.; Derba-Maceluch, M.; Koutaniemi, S.; Roach, M.; Sundberg, B.; Tuomainen, P.; Mellerowicz, E.J.; et al. O-acetylation of glucuronoxylan in Arabidopsis thaliana wild type and its change in xylan biosynthesis mutants. Glycobiology 2014, 24, 494–506. [Google Scholar] [CrossRef]
- Grantham, N.J.; Wurman-Rodrich, J.; Terrett, O.M.; Lyczakowski, J.J.; Stott, K.; Iuga, D.; Simmons, T.J.; Durand-Tardif, M.; Brown, S.P.; Dupree, R.; et al. An even pattern of xylan substitution is critical for interaction with cellulose in plant cell walls. Nat. Plants 2017, 3, 859–865. [Google Scholar] [CrossRef]
- Yuan, Y.; Teng, Q.; Zhong, R.; Ye, Z.-H. TBL3 and TBL31, two Arabidopsis DUF231 domain proteins, are required for 3-O-monoacetylation of xylan. Plant Cell Physiol. 2016, 57, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Teng, Q.; Zhong, R.; Ye, Z.-H. Roles of Arabidopsis TBL34 and TBL35 in xylan acetylation and plant growth. Plant Sci. 2016, 243, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Teng, Q.; Zhong, R.; Ye, Z.-H. Alterations of the degree of xylan acetylation in Arabidopsis xylan mutants. Plant Signal. Behav. 2014, 9, e27797. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Cui, D.; Ye, Z.-H. Regiospecific acetylation of xylan is mediated by a group of DUF231-containing O-acetyltransferases. Plant Cell Physiol. 2017, 58, 2126–2138. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, B.R.; Peña, M.J.; Moniz, H.A.; Moremen, K.W.; York, W.S. Two Arabidopsis proteins synthesize acetylated xylan in vitro. Plant J. 2014, 80, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Lunin, V.V.; Wang, H.T.; Bharadwaj, V.S.; Alahuhta, M.; Peña, M.J.; Yang, J.Y.; Archer-Hartmann, S.A.; Azadi, P.; Himmel, M.E.; Moremen, K.W.; et al. Molecular mechanism of polysaccharide acetylation by the Arabidopsis xylan O-acetyltransferase XOAT1. Plant Cell 2020, 32, 2367–2382. [Google Scholar] [CrossRef]
- Popper, Z.A.; Fry, S.C. Primary cell wall composition of bryophytes and charophytes. Ann. Bot. 2003, 91, 1–12. [Google Scholar] [CrossRef]
- Popper, Z.A.; Fry, S.C. Primary cell wall composition of pteridophytes and spermatophytes. New Phytol. 2004, 164, 165–174. [Google Scholar] [CrossRef]
- Silva, G.B.; Ionashiro, M.; Carrara, T.B.; Crivellari, A.C.; Tiné, M.A.S.; Prado, J.; Carpita, N.C.; Buckeridge, M.S. Cell wall polysaccharides from fern leaves: Evidence for a mannan-rich Type III cell wall in Adiantum raddianum. Phytochemistry 2011, 72, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Willför, S.; Sundberg, A.; Hemming, J.; Holmbom, B. Polysaccharides in some industrially important softwood species. Wood Sci. Technol. 2005, 39, 245–257. [Google Scholar] [CrossRef]
- Melton, L.D.; Smith, B.G.; Ibrahim, R.; Schröder, R. Mannans in primary and secondary plant cell walls New Zeal. J. For. Sci. 2009, 39, 153–160. [Google Scholar]
- Voiniciuc, C. Modern mannan: A hemicellulose’s journey. New Phytol. 2022, 234, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Cui, S.W.; Phillips, G.O.; Goff, H.D.; Wang, Q. Study on Dendrobium officinale O-acetyl-glucomannan (Dendronan®): Part II. Fine structures of O-acetylated residues. Carbohydr. Polym. 2015, 117, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Capek, P.; Alföldi, J.; Lišková, D. An acetylated galactoglucomannan from Picea abies L. Karst. Carbohydr. Res. 2002, 337, 1033–1037. [Google Scholar] [CrossRef]
- Hazendonk, J.M.; Reinerink, E.J.M.; Waard, P.; Dam, J.E.G. Structural analysis of acetylated hemicellulose polysaccharides from fibre flax (Linum usitatissimum L.). Carbohydr. Res. 1996, 291, 141–154. [Google Scholar] [CrossRef]
- Lundqvist, J.; Teleman, A.; Junel, L.; Zacchi, G.; Dahlman, O.; Tjerneld, F.; Stålbrand, H. Isolation and characterization of galactoglucomannan from spruce (Picea abies). Carbohydr. Polym. 2002, 48, 29–39. [Google Scholar] [CrossRef]
- Teleman, A.; Nordström, M.; Tenkanen, M.; Jacobs, A.; Dahlman, O. Isolation and characterization of O-acetylated glucomannans from aspen and birch wood. Carbohydr. Res. 2003, 338, 525–534. [Google Scholar] [CrossRef]
- Zhong, R.; Cui, D.; Ye, Z.-H. Members of the DUF231 family are O-acetyltransferases catalyzing, 2-O- and 3-O-acetylation of mannan. Plant Cell Physiol. 2018, 59, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Adams, E.R.; Ye, Z.-H. Ancient origin of acetyltransferases catalyzing O-acetylation of plant cell wall polysaccharides. Plant Cell Physiol. 2024, pcae070. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.T. We be jammin’: An update on pectin biosynthesis trafficking dynamics. J. Exp. Bot. 2016, 67, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Ishii, T. O-acetylated oligosaccharides from pectins of potato tuber cell walls. Plant Physiol. 1997, 113, 1265–1272. [Google Scholar] [CrossRef]
- Komalavilas, P.; Mort, A.J. The acetylation at O-3 of galacturonic acid in the rhamnose-rich portion of pectins. Carbohydr. Res. 1989, 189, 261–272. [Google Scholar] [CrossRef]
- Lerouge, P.; O’Neill, M.A.; Darvill, A.G.; Albersheim, P. Structural characterization of endo-glycanase-generated oligoglycosyl side chains of rhamnogalacturonan I. Carbohydr. Res. 1993, 243, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Perrone, P.; Hewage, C.M.; Thomson, A.R.; Bailey, K.; Sadler, I.H.; Fry, S.C. Patterns of methyl and O-acetyl esterification in spinach pectins: New complexity. Phytochemistry 2002, 60, 67–77. [Google Scholar] [CrossRef]
- Quéméner, B.; Pino, J.C.C.; Ralet, M.-C.; Bonnin, E.; Thibault, J.-F. Assignment of acetyl groups to O-2 and/or O-3 of pectic oligogalacturonides using negative electrospray ionization ion trap mass spectrometry. J. Mass. Spectrom. 2003, 38, 641–648. [Google Scholar] [CrossRef]
- Ralet, M.C.; Cabrera, J.C.; Bonnin, E.; Quéméner, B.; Hellìn, P.; Thibault, J.F. Mapping sugar beet pectin acetylation pattern. Phytochemistry 2005, 66, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Sengkhamparn, N.; Bakx, E.J.; Verhoef, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G.J. Okra pectin contains an unusual substitution of its rhamnosyl residues with acetyl and alpha-linked galactosyl groups. Carbohydr. Res. 2009, 344, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Whitcombe, A.J.; O’Neill, M.A.; Steffan, W.; Albersheim, P.; Darvill, A.G. Structural characterization of the pectic polysaccharide rhamnogalacturonan II. Carbohydr. Res. 1995, 271, 15–29. [Google Scholar] [CrossRef]
- Zhong, R.; Cui, D.; Richardson, E.A.; Ye, Z.-H. Acetylation of homogalacturonan and rhamnogalacturonan-I is catalyzed by a suite of trichome birefringence-like proteins. Plant J. 2024, 117, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Potikha, T.; Delmer, D.P. A mutant of Arabidopsis thaliana displaying altered patterns of cellulose deposition. Plant J. 1995, 7, 453–460. [Google Scholar] [CrossRef]
- Bischoff, V.; Nita, S.; Neumetzler, L.; Schindelasch, D.; Urbain, A.; Eshed, R.; Persson, S.; Delmer, D.; Scheible, W.R. TRICHOME BIREFRINGENCE and its homolog AT5G01360 encode plant-specific DUF231 proteins required for cellulose biosynthesis in Arabidopsis. Plant Physiol. 2010, 153, 590–602. [Google Scholar] [CrossRef]
- Sinclair, S.A.; Larue, C.; Bonk, L.; Khan, A.; Castillo-Michel, H.; Stein, R.J.; Grolimund, D.; Begerow, D.; Neumann, U.; Haydon, M.J.; et al. Etiolated seedling development requires repression of photomorphogenesis by a small cell-wall-derived dark signal. Curr. Biol. 2017, 27, 3403–3418. [Google Scholar] [CrossRef]
- Stranne, M.; Ren, Y.; Fimognari, L.; Birdseye, D.; Yan, J.; Bardor, M.; Mollet, J.C.; Komatsu, T.; Kikuchi, J.; Scheller, H.V.; et al. TBL10 is required for O-acetylation of pectic rhamnogalacturonan-I in Arabidopsis thaliana. Plant J. 2018, 96, 772–785. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, V.; Selbig, J.; Scheible, W.R. Involvement of TBL/DUF231 proteins into cell wall biology. Plant Signal. Behav. 2010, 5, 1057–1059. [Google Scholar] [CrossRef]
- Anderson, A.C.; Stangherlin, S.; Pimentel, K.N.; Weadge, J.T.; Clarke, A.J. The SGNH hydrolase family: A template for carbohydrate diversity. Glycobiology 2022, 32, 826–848. [Google Scholar] [CrossRef] [PubMed]
- Mølgaard, A.; Kauppinen, S.; Larsen, S. Rhamnogalacturonan acetylesterase elucidates the structure and function of a new family of hydrolases. Structure 2000, 8, 373–383. [Google Scholar] [CrossRef]
- Jones, C.S.; Sychantha, D.; Howell, P.L.; Clarke, A.J. Structural basis for the O-acetyltransferase function of the extracytoplasmic domain of OatA from Staphylococcus aureus. J. Biol. Chem. 2020, 295, 8204–8213. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, P.J.; Clarke, A.J. Mechanism of action of peptidoglycan O-acetyltransferase, B. involves a Ser-His-Asp catalytic triad. Biochemistry 2014, 53, 6243–6251. [Google Scholar] [CrossRef] [PubMed]
- Sychantha, D.; Clarke, A.J. Peptidoglycan modification by the catalytic domain of Streptococcus pneumoniae OatA follows a ping-pong Bi-Bi mechanism of action. Biochemistry 2018, 57, 2394–2401. [Google Scholar] [CrossRef] [PubMed]
- Sychantha, D.; Brott, A.S.; Jones, C.S.; Clarke, A.J. Mechanistic pathways for peptidoglycan O-acetylation and de-O-acetylation. Front. Microbiol. 2018, 9, 2332. [Google Scholar] [CrossRef]
- Janbon, G.; Himmelreich, U.; Moyrand, F.; Improvisi, L.; Dromer, F. Cas1p is a membrane protein necessary for the O-acetylation of the Cryptococcus neoformans capsular polysaccharide. Mol. Microbiol. 2001, 42, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.; Nafisi, M.; Verhertbruggen, Y.; Orfila, C.; Gille, S.; Rautengarten, C.; Cherk, C.; Marcus, S.E.; Somerville, S.; Pauly, M.; et al. Loss-of-function mutation of REDUCED WALL ACETYLATION2 in Arabidopsis leads to reduced cell wall acetylation and increased resistance to Botrytis cinerea. Plant Physiol. 2011, 155, 1068–1078. [Google Scholar] [CrossRef]
- Manabe, Y.; Verhertbruggen, Y.; Gille, S.; Harholt, J.; Chong, S.L.; Pawar, P.M.; Mellerowicz, E.J.; Tenkanen, M.; Cheng, K.; Pauly, M.; et al. Reduced Wall Acetylation proteins play vital and distinct roles in cell wall O-acetylation in Arabidopsis. Plant Physiol. 2013, 163, 1107–1117. [Google Scholar] [CrossRef]
- Pawar, P.M.; Ratke, C.; Balasubramanian, V.K.; Chong, S.L.; Gandla, M.L.; Adriasola, M.; Sparrman, T.; Hedenström, M.; Szwaj, K.; Derba-Maceluch, M.; et al. Downregulation of RWA genes in hybrid aspen affects xylan acetylation and wood saccharification. New Phytol. 2017, 214, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Pauly, M.; Scheller, H.V. O-Acetylation of plant cell wall polysaccharides: Identification and partial characterization of a rhamnogalacturonan O-acetyl-transferase from potato suspension-cultured cells. Planta 2000, 210, 659–667. [Google Scholar] [CrossRef]
- Xing, S.; Poirier, Y. The protein acetylome and the regulation of metabolism. Trend Plant Sci. 2012, 17, 423–430. [Google Scholar] [CrossRef]
- Zhong, R.; Cui, D.; Richardson, E.A.; Phillips, D.R.; Azadi, P.; Lu, G.; Ye, Z.-H. Cytosolic acetyl-CoA generated by ATP-citrate lyase is essential for acetylation of cell wall polysaccharides. Plant Cell Physiol. 2020, 61, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Schultink, A.; Naylor, D.; Dama, M.; Pauly, M. The role of the plant-specific ALTERED XYLOGLUCAN9 protein in Arabidopsis cell wall polysaccharide O-acetylation. Plant Physiol. 2015, 167, 1271–1283. [Google Scholar] [CrossRef]
- Baker, P.; Ricer, T.; Moynihan, P.J.; Kitova, E.N.; Walvoort, M.T.; Little, D.J.; Whitney, J.C.; Dawson, K.; Weadge, J.T.; Robinson, H.; et al. P. aeruginosa SGNH hydrolase-like proteins AlgJ and AlgX have similar topology but separate and distinct roles in alginate acetylation. PLoS Pathog. 2014, 10, e1004334. [Google Scholar] [CrossRef]
- Riley, L.M.; Weadge, J.T.; Baker, P.; Robinson, H.; Codée, J.D.; Tipton, P.A.; Ohman, D.E.; Howell, P.L. Structural and functional characterization of Pseudomonas aeruginosa AlgX: Role of AlgX in alginate acetylation. J. Biol. Chem. 2013, 288, 22299–22314. [Google Scholar] [CrossRef]
- Niklas, K.J.; Kutschera, U. The evolution of the land plant life cycle. New Phytol. 2010, 185, 27–41. [Google Scholar] [CrossRef]
- Hori, K.; Maruyama, F.; Fujisawa, T.; Togashi, T.; Yamamoto, N.; Seo, M.; Sato, S.; Yamada, T.; Mori, H.; Tajima, N.; et al. Klebsormidium flaccidum genome reveals primary factors for plant terrestrial adaptation. Nat. Commun. 2014, 5, 3978. [Google Scholar] [CrossRef]
- Ishizaki, K.; Nishihama, R.; Yamato, K.T.; Kohchi, T. Molecular genetic tools and techniques for Marchantia polymorpha research. Plant Cell Physiol. 2016, 57, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Bordenave, M.; Goldberg, R.; Huet, J.C.; Pernollet, J.C. A novel protein from mung bean hypocotyl cell walls with acetyl esterase activity. Phytochemistry 1995, 38, 315–319. [Google Scholar] [CrossRef]
- Williamson, G. Purification and characterization of pectin acetylesterase from orange peel. Phytochemistry 1991, 30, 445–449. [Google Scholar] [CrossRef]
- de Souza, A.J.; Pauly, M. Comparative genomics of pectinacetylesterases: Insight on function and biology. Plant Signal. Behav. 2015, 10, e1055434. [Google Scholar] [CrossRef]
- Liu, J.J.; Schoettle, A.W.; Sniezko, R.A.; Waring, K.M.; Williams, H.; Zamany, A.; Johnson, J.S.; Kegley, A. Comparative association mapping reveals conservation of major gene resistance to white pine blister rust in southwestern white pine (Pinus strobiformis) and limber pine (P. flexilis). Phytopathology 2022, 112, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Philippe, F.; Pelloux, J.; Rayon, C. Plant pectin acetylesterase structure and function: New insights from bioinformatic analysis. BMC Genom. 2017, 18, 456. [Google Scholar] [CrossRef]
- de Souza, A.; Hull, P.A.; Gille, S.; Pauly, M. Identification and functional characterization of the distinct plant pectin esterases PAE8 and PAE9 and their deletion mutants. Planta 2014, 240, 1123–1138. [Google Scholar] [CrossRef]
- Dauphin, B.G.; Ropartz, D.; Ranocha, P.; Rouffle, M.; Carton, C.; Le Ru, A.; Martinez, Y.; Fourquaux, I.; Ollivier, S.; Mac-Bear, J.; et al. TBL38 atypical homogalacturonan-acetylesterase activity and cell wall microdomain localization in Arabidopsis seed mucilage secretory cells. iScience 2024, 27, 109666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, L.; Li, F.; Zhang, D.; Liu, X.; Wang, H.; Xu, Z.; Chu, C.; Zhou, Y. Control of secondary cell wall patterning involves xylan deacetylation by a GDSL esterase. Nat. Plants 2017, 3, 17017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, C.; Mentink-Vigier, F.; Tang, L.; Zhang, D.; Wang, S.; Cao, S.; Xu, Z.; Liu, X.; Wang, T.; et al. Arabinosyl deacetylase modulates the arabinoxylan acetylation profile and secondary wall formation. Plant Cell 2019, 31, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Bouchabke-Coussa, O.; Quashie, M.L.; Seoane-Redondo, J.; Fortabat, M.N.; Gery, C.; Yu, A.; Linderme, D.; Trouverie, J.; Granier, F.; Téoulé, E.; et al. ESKIMO1 is a key gene involved in water economy as well as cold acclimation and salt tolerance. BMC Plant Biol. 2008, 8, 125. [Google Scholar] [CrossRef]
- Xin, Z.; Browse, J. eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Proc. Natl. Acad. Sci. USA 1998, 95, 7799–7804. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Mandaokar, A.; Chen, J.; Last, R.L.; Browse, J. Arabidopsis ESK1 encodes a novel regulator of freezing tolerance. Plant J. 2007, 49, 786–799. [Google Scholar] [CrossRef]
- Lefebvre, V.; Fortabat, M.N.; Ducamp, A.; North, H.M.; Maia-Grondard, A.; Trouverie, J.; Boursiac, Y.; Mouille, G.; Durand-Tardif, M. ESKIMO1 disruption in Arabidopsis alters vascular tissue and impairs water transport. PLoS ONE 2011, 6, e16645. [Google Scholar] [CrossRef]
- Bensussan, M.; Lefebvre, V.; Ducamp, A.; Trouverie, J.; Gineau, E.; Fortabat, M.N.; Guillebaux, A.; Baldy, A.; Naquin, D.; Herbette, S.; et al. Suppression of dwarf and irregular xylem phenotypes generates low-acetylated biomass lines in Arabidopsis. Plant Physiol. 2015, 168, 452–463. [Google Scholar] [CrossRef]
- Ramírez, V.; Xiong, G.; Mashiguchi, K.; Yamaguchi, S.; Pauly, M. Growth- and stress-related defects associated with wall hypoacetylation are strigolactone-dependent. Plant Direct 2018, 2, e00062. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, J.C.; Miles, G.P.; Brown, D.M.; Zhang, Z.; Segura, M.P.; Weimar, T.; Yu, X.; Seffen, K.A.; Stephens, E.; Turner, S.R.; et al. Absence of branches from xylan in Arabidopsis gux mutants reveals potential for simplification of lignocellulosic biomass. Proc. Natl. Acad. Sci. USA 2010, 107, 17409–17414. [Google Scholar] [CrossRef] [PubMed]
- Orfila, C.; Degan, F.D.; Jorgensen, B.; Scheller, H.V.; Ray, P.M.; Ulvskov, P. Expression of mung bean pectin acetyl esterase in potato tubers: Effect on acetylation of cell wall polymers and tuber mechanical properties. Planta 2012, 236, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Pippen, E.L.; McCready, R.M.; Owens, H.S. Gelation properties of partially acetylated pectins. J. Am. Chem. Soc. 1950, 72, 813–816. [Google Scholar] [CrossRef]
- Ralet, M.C.; Crepeau, M.J.; Buchholt, H.C.; Thibault, J.F. Polyelectrolyte behaviour and calcium binding properties of sugar beet pectins differing in their degrees of methylation and acetylation. Biochem. Eng. J. 2003, 16, 191–201. [Google Scholar] [CrossRef]
- Williamson, G.; Faulds, C.B.; Matthew, J.A.; Archer, D.B.; Morris, V.J.; Brownsey, G.J.; Ridout, M.J. Gelation of sugarbeet and citrus pectins using enzymes extracted from orange peel. Carbohydr. Polym. 1990, 13, 387–397. [Google Scholar] [CrossRef]
- Yang, Y.; Anderson, C.T. Biosynthesis, localization, and function of pectins in plants. In Pectins: Technological and Physiological Properties; Kontogiorgos, V., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 1–15. [Google Scholar]
- Ellis, C.; Turner, J.G. The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell 2001, 13, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Blanco, C.; Feng, D.X.; Hu, J.; Sánchez-Vallet, A.; Deslandes, L.; Llorente, F.; Berrocal-Lobo, M.; Keller, H.; Barlet, X.; Sánchez-Rodríguez, C.; et al. Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell 2007, 19, 890–903. [Google Scholar] [CrossRef]
- Nishimura, M.T.; Stein, M.; Hou, B.H.; Vogel, J.P.; Edwards, H.; Somerville, S.C. Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 2003, 301, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Pogorelko, G.; Lionetti, V.; Fursova, O.; Sundaram, R.M.; Qi, M.; Whitham, S.A.; Bogdanove, A.J.; Bellincampi, D.; Zabotina, O.A. Arabidopsis and Brachypodium distachyon transgenic plants expressing Aspergillus nidulans acetylesterases have decreased degree of polysaccharide acetylation and increased resistance to pathogens. Plant Physiol. 2013, 162, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Sun, Y.; Zhang, B.C.; Mansoori, N.; Wan, J.X.; Liu, Y.; Wang, Z.W.; Shi, Y.Z.; Zhou, Y.H.; Zheng, S.J. TRICHOME BIREFRINGENCE-LIKE27 affects aluminum sensitivity by modulating the O-acetylation of xyloglucan and aluminum-binding capacity in Arabidopsis. Plant Physiol. 2014, 166, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Indrarti, L.; Azuma, J.-I. Affinity of hemicellulose for cellulose produced by Acetobacter xylinum. Cellulose 1998, 5, 215–228. [Google Scholar] [CrossRef]
- Hannuksela, T.; Tenkanen, M.; Holmbom, B. Sorption of dissolved galactoglucomannans and galactomannans to bleached kraft pulp. Cellulose 2002, 9, 251–261. [Google Scholar] [CrossRef]
- Carroll, A.; Somerville, C. Cellulosic biofuels. Annu. Rev. Plant Biol. 2009, 60, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Selig, M.J.; Adney, W.S.; Himmel, M.E.; Decker, S.R. The impact of cell wall acetylation on corn stover hydrolysis by cellulolytic and xylanolytic enzymes. Cellulose 2009, 16, 711–722. [Google Scholar] [CrossRef]
- Helle, S.; Cameron, D.; Lam, J.; White, B.; Duff, S. Effect of inhibitory compounds found in biomass hydrolysates on growth and xylose fermentation by a genetically engineered strain of S. cerevisiae. Enzyme Microb. Technol. 2003, 33, 786–792. [Google Scholar] [CrossRef]
- Klein-Marcuschamer, D.; Oleskowicz-Popiel, P.; Simmons, B.A.; Blanch, H.W. Technoeconomic analysis of biofuels: A wiki-based platform for lignocellulosic biorefineries. Biomass Bioenergy 2010, 34, 1914–1921. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Sharma, A.M.; Rastogi, L.; Dewangan, B.P.; Sharma, R.; Singh, D.; Sah, R.K.; Das, S.; Bhattacharjee, S.; Mellerowicz, E.J.; et al. Modifying lignin composition and xylan O-acetylation induces changes in cell wall composition, extractability, and digestibility. Biotechnol. Biofuels Bioprod. 2024, 17, 73. [Google Scholar] [CrossRef]
- Pawar, P.M.; Derba-Maceluch, M.; Chong, S.L.; Gómez, L.D.; Miedes, E.; Banasiak, A.; Ratke, C.; Gaertner, C.; Mouille, G.; McQueen-Mason, S.J.; et al. Expression of fungal acetyl xylan esterase in Arabidopsis thaliana improves saccharification of stem lignocellulose. Plant Biotechnol. J. 2016, 14, 387–397. [Google Scholar] [CrossRef]
- Pawar, P.M.; Derba-Maceluch, M.; Chong, S.L.; Gandla, M.L.; Bashar, S.S.; Sparrman, T.; Ahvenainen, P.; Hedenström, M.; Özparpucu, M.; Rüggeberg, M.; et al. In muro deacetylation of xylan affects lignin properties and improves saccharification of aspen wood. Biotechnol. Biofuels 2017, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pawar, P.M.; Derba-Maceluch, M.; Hedenström, M.; Chong, S.L.; Tenkanen, M.; Jönsson, L.J.; Mellerowicz, E.J. Hybrid aspen expressing a carbohydrate esterase family 5 acetyl xylan esterase under control of a wood-specific promoter shows improved saccharification. Front. Plant Sci. 2020, 11, 380. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; He, C.; Zhang, D.; Liu, X.; Xu, Z.; Tian, Y.; Liu, X.H.; Zang, S.; Pauly, M.; Zhou, Y.; et al. Two Trichome Birefringence-Like proteins mediate xylan acetylation, which is essential for leaf blight resistance in rice. Plant Physiol. 2017, 173, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Wang, H.T.; Qiao, Z.; Yao, T.; Xie, M.; Urbanowicz, B.R.; Zeng, W.; Jawdy, S.S.; Gunter, L.E.; et al. Overexpression of REDUCED WALL ACETYLATION C increases xylan acetylation and biomass recalcitrance in Populus. Plant Physiol. 2023, 194, 243–257. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, R.; Zhou, D.; Chen, L.; Rose, J.P.; Wang, B.-C.; Ye, Z.-H. Plant Cell Wall Polysaccharide O-Acetyltransferases. Plants 2024, 13, 2304. https://doi.org/10.3390/plants13162304
Zhong R, Zhou D, Chen L, Rose JP, Wang B-C, Ye Z-H. Plant Cell Wall Polysaccharide O-Acetyltransferases. Plants. 2024; 13(16):2304. https://doi.org/10.3390/plants13162304
Chicago/Turabian StyleZhong, Ruiqin, Dayong Zhou, Lirong Chen, John P. Rose, Bi-Cheng Wang, and Zheng-Hua Ye. 2024. "Plant Cell Wall Polysaccharide O-Acetyltransferases" Plants 13, no. 16: 2304. https://doi.org/10.3390/plants13162304
APA StyleZhong, R., Zhou, D., Chen, L., Rose, J. P., Wang, B.-C., & Ye, Z.-H. (2024). Plant Cell Wall Polysaccharide O-Acetyltransferases. Plants, 13(16), 2304. https://doi.org/10.3390/plants13162304





