Effects of Gamma Irradiation on Changes in Chemical Composition and Antioxidant Activity of Euphorbia maculata Callus
Abstract
:1. Introduction
2. Results
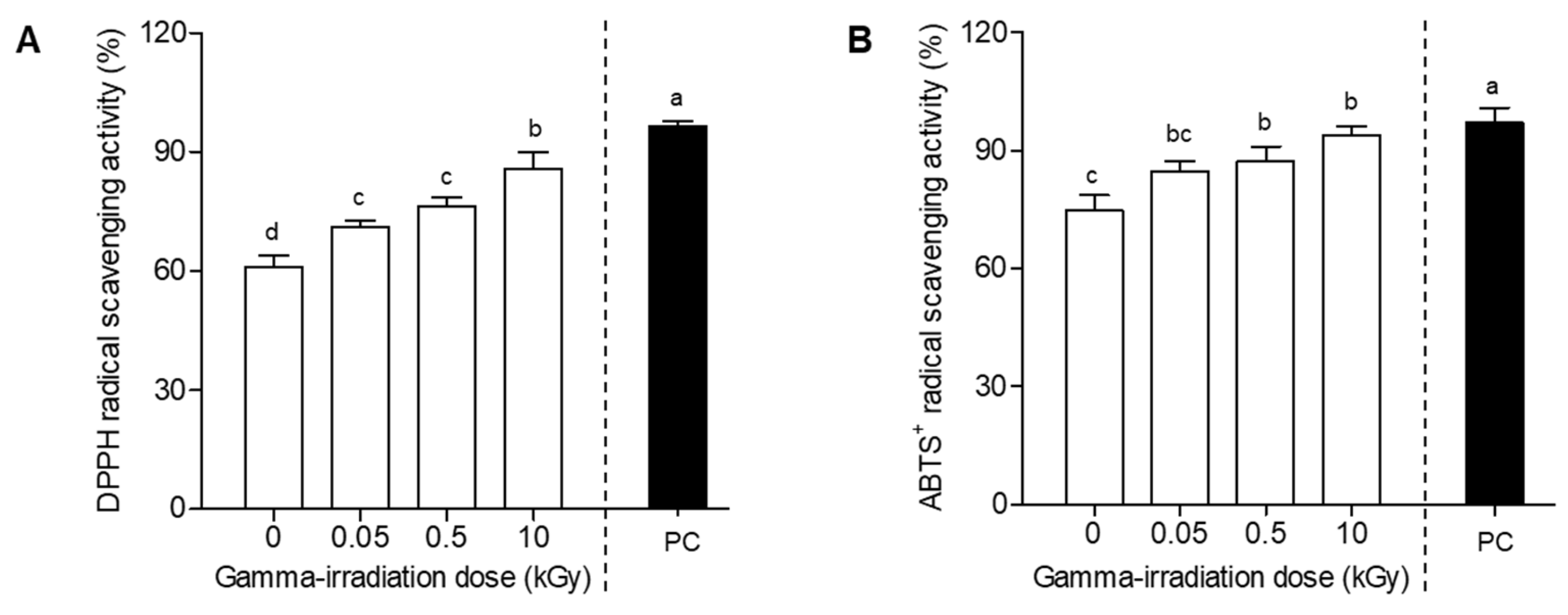
2.1. Radical Scavenging Ability of Plant-Derived Callus by Gamma Irradiation
2.2. Radical Scavenging Ability of E. maculata Callus after Different Doses of Gamma Irradiation
2.3. High-Performance Liquid Chromatography (HPLC) Analysis of E. maculata Callus by Gamma Irradiation
2.4. Identification of Compounds Produced in E. maculata Callus by Gamma Irradiation
2.5. Statistical Analysis of Changes in E. maculata Calli after Gamma Irradiation
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Gamma Irradiation of Plant-Derived Calli
4.3. Measurement of Radical Scavenging Activity
4.4. High-Performance Liquid Chromatography (HPLC) Analysis
4.5. Ultra-Performance Liquid Chromatography-Quadrupole Time-of-Flight/Mass Spectrometry (UPLC-QTOF/MS) Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hrazdina, G.; Jensen, R.A. Spatial organization of enzymes in plant metabolic pathways. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 241–267. [Google Scholar] [CrossRef]
- Real, A.; Sundell-Bergman, S.; Knowles, J.F.; Woodhead, D.S.; Zinger, I. Effects of ionising radiation exposure on plants, fish and mammals: Relevant data for environmental radiation protection. J. Radiol. Prot. 2004, 24, A123–A137. [Google Scholar] [CrossRef] [PubMed]
- Wi, S.G.; Chung, B.Y.; Kim, J.S.; Kim, J.H.; Baek, M.H.; Lee, J.W.; Kim, Y.S. Effects of gamma irradiation on morphological changes and biological responses in plants. Micron 2007, 38, 553–564. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; López-Saucedo, F.; Bucio, E. Gamma-irradiation applied in the synthesis of metallic and organic nanoparticles: A short review. Radiat. Phys. Chem. 2020, 169, 107962. [Google Scholar] [CrossRef]
- Choi, H.I.; Han, S.M.; Jo, Y.D.; Hong, M.J.; Kim, S.H.; Kim, J.B. Effects of acute and chronic gamma irradiation on the cell biology and physiology of rice plants. Plants 2021, 10, 439. [Google Scholar] [CrossRef]
- Ahuja, S.; Kumar, M.; Kumar, P.; Gupta, V.K.; Singhal, R.K.; Yadav, A.; Singh, B. Metabolic and biochemical changes caused by gamma irradiation in plants. J. Radioanal. Nucl. Chem. 2014, 300, 199–212. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, M.H.; Moon, Y.R.; Kim, J.S.; Wi, S.G.; Kim, T.H.; Chung, B.Y. Characterization of metabolic disturbances closely linked to the delayed senescence of Arabidopsis leaves after γ irradiation. Environ. Exp. Bot. 2009, 67, 363–371. [Google Scholar] [CrossRef]
- Lee, K.B.; Mahlberg, P.G. Ultrastructure and development of nonarticulated laticifers in seedlings of Euphorbia maculata L. J. Plant Biol. 1999, 42, 57–62. [Google Scholar] [CrossRef]
- Luyen, B.T.T.; Tai, B.H.; Thao, N.P.; Lee, S.H.; Jang, H.D.; Lee, Y.M.; Kim, Y.H. Evaluation of the anti-osteoporosis and antioxidant activities of phenolic compounds from Euphorbia maculata. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 573–579. [Google Scholar] [CrossRef]
- Rakotondrabe, T.F.; Fan, M.X.; Zhang, Y.L.; Guo, M.Q. Simultaneous screening and analysis of anti-inflammatory and antiproliferative compounds from Euphorbia maculata combining bio-affinity ultrafiltration with multiple drug targets. J. Anal. Test. 2022, 6, 98–110. [Google Scholar] [CrossRef]
- Matsunaga, S.; Tanaka, R.; Akagi, M. Triterpenoids from Euphorbia maculata. Phytochemistry 1988, 27, 535–537. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, L.L.; Tang, M.Y.; Feng, B.M.; Pei, Y.H.; Yasukawa, K. Triterpenoids from Euphorbia maculata and their anti-inflammatory effects. Molecules 2018, 23, 2112. [Google Scholar] [CrossRef]
- Ernst, M.; Grace, O.M.; Saslis-Lagoudakis, C.H.; Nilsson, N.; Simonsen, H.T.; Rønsted, N. Global medicinal uses of Euphorbia L. (Euphorbiaceae). J. Ethnopharmacol. 2015, 176, 90–101. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- de Oliveira, M.E.B.; Sartoratto, A.; Carlos Cardoso, J. In Vitro Calli Production Resulted in Different Profiles of Plant-Derived Medicinal Compounds in Phyllanthus amarus. Molecules 2020, 25, 5895. [Google Scholar] [CrossRef]
- Asyakina, L.; Ivanova, S.; Prosekov, A.; Dyshlyuk, L.; Chupakhin, E.; Ulrikh, E.; Babich, O.; Sukhikh, S. Determination of the qualitative composition of biologically active substances of extracts of in vitro callus, cell suspension, and root cultures of the medicinal plant Rhaponticum carthamoides. Appl. Sci. 2021, 11, 2555. [Google Scholar] [CrossRef]
- Kwon, S.U.; Cha, J.Y.; Lee, H.Y.; Xin, M.; Ji, S.J.; Kim, D.K.; Park, D.S.; Pyo, M.K.; Lee, Y.M. Chloroform fraction of Euphorbia maculata has antiplatelet activity via suppressing thromboxane B2 formation. Mol. Med. Rep. 2015, 11, 4255–4261. [Google Scholar] [CrossRef] [PubMed]
- Song, H.P.; Kim, D.H.; Jo, C.; Lee, C.H.; Kim, K.S.; Byun, M.W. Effect of gamma irradiation on the microbiological quality and antioxidant activity of fresh vegetable juice. Food Microbiol. 2006, 23, 372–378. [Google Scholar] [CrossRef]
- Jan, S.; Parween, T.; Siddiqi, T.O.; Mahmooduzzafar. Effect of gamma radiation on morphological, biochemical, and physiological aspects of plants and plant products. Environ. Rev. 2012, 20, 17–39. [Google Scholar] [CrossRef]
- Lee, S.S.; Lee, E.M.; An, B.C.; Kim, T.H.; Lee, K.S.; Cho, J.Y.; Yoo, S.H.; Bae, J.S.; Chung, B.Y. Effects of irradiation on decolourisation and biological activity in Schizandra chinensis extracts. Food Chem. 2011, 125, 214–220. [Google Scholar] [CrossRef]
- Azeez, H.; Ibrahim, K.; Pop, R.; Pamfil, D.; Hârţa, M.; Bobiș, O. Changes induced by gamma ray irradiation on biomass production and secondary metabolites accumulation in Hypericum triquetrifolium Turra callus cultures. Ind. Crop. Prod. 2017, 108, 183–189. [Google Scholar] [CrossRef]
- Jeong, G.H.; Lee, H.; Lee, H.K.; Choi, H.J.; Chung, B.Y.; Bai, H.W. Inhibitory effect of γ-ray-modified hydroxymethylated baicalins on NO production. Bioorg. Med. Chem. Lett. 2023, 96, 129491. [Google Scholar] [CrossRef] [PubMed]
- Colles, S.M.; Chisolm, G.M. Lysophosphatidylcholine-induced cellular injury in cultured fibroblasts involves oxidative events. J. Lipid Res. 2000, 41, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Rivera, R.; Chun, J. Biological effects of lysophospholipids. Rev. Physiol. Biochem. Pharmacol. 2008, 160, 25–46. [Google Scholar] [CrossRef] [PubMed]
- De Maria, L.; Vind, J.; Oxenbøll, K.M.; Svendsen, A.; Patkar, S. Phospholipases and their industrial applications. Appl. Microbiol. Biotechnol. 2007, 74, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.H.; Kim, T.H. Hydroxymethylation of rutin induced by radiolysis as novel α-glucosidase inhibitors. Chem. Pharm. Bull. 2017, 65, 678–682. [Google Scholar] [CrossRef]
- Jeong, G.H.; Cho, J.H.; Jo, C.; Lee, S.; Lee, S.S.; Bai, H.W.; Chung, B.Y.; Kim, T.H. Gamma irradiation-assisted degradation of rosmarinic acid and evaluation of structures and anti-adipogenic properties. Food Chem. 2018, 258, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.F.; Tsimidou, M.; Zhang, H.Y. Estimation of scavenging activity of phenolic compounds using the ABTS•+ assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef]


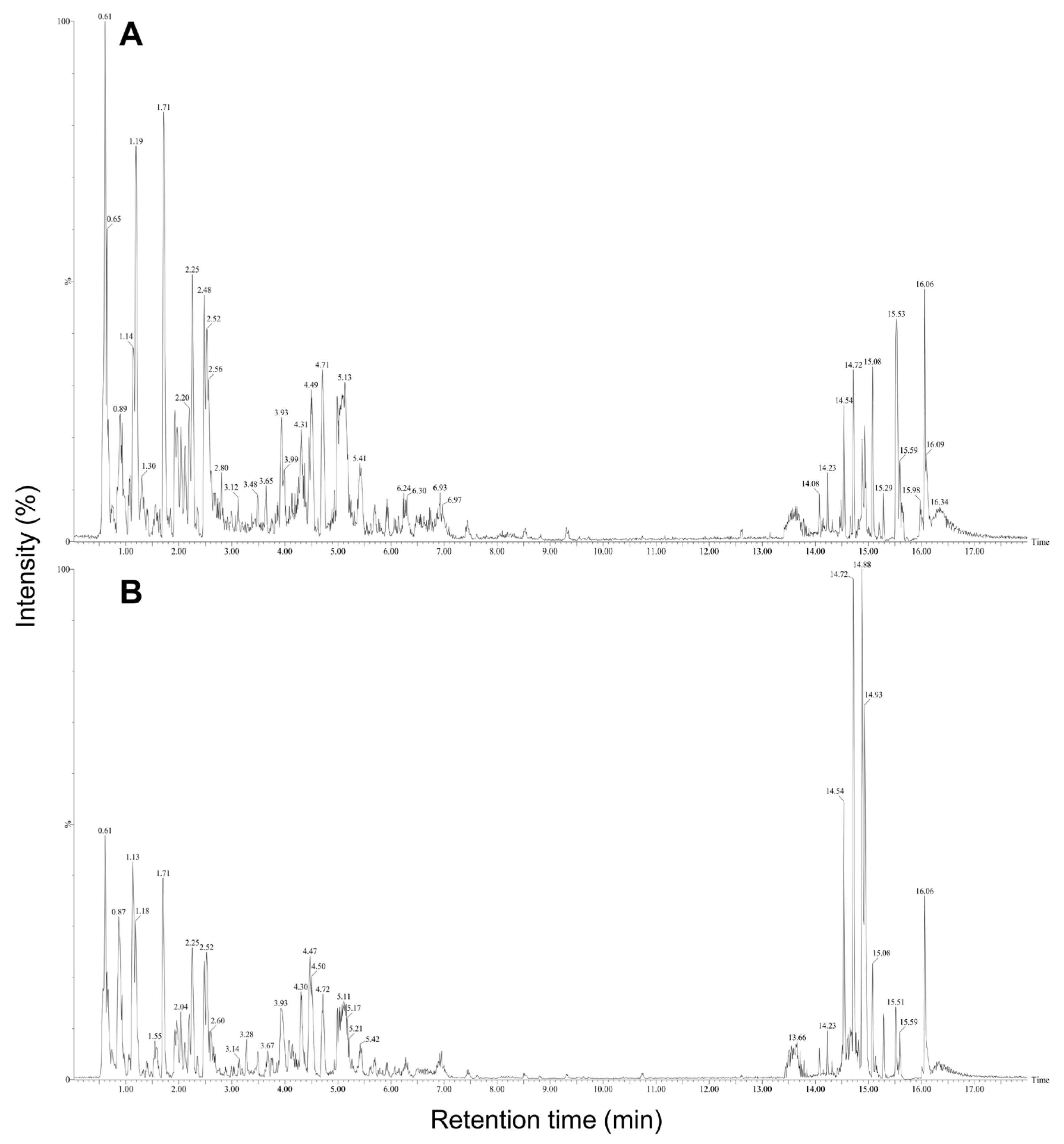


| Metabolite No. | RT (min) | Compounds | Exact Mass (m/z) | Fragment Ions (m/z) | Non- Irradiated | Irradiated |
|---|---|---|---|---|---|---|
| 1 | 0.61 | L-Arginine | 175.12 | 70 | + | + |
| 2 | 0.65 | Pteroside B | 381.08 | 365, 219, 175 | + | + |
| 3 | 0.74 | N-(1-deoxy-1-fructosyl)isoleucine | 294.15 | 276, 248 | + | + |
| 4 | 0.87 | γ-Glutamyl lysine | 276.14 | 258, 230, 212, 147, 86 | + | + |
| 5 | 1.08 | Epicatechin 3-glucuronide | 467.08 | 449, 393, 153 | + | + |
| 6 | 1.14 | N-(1-deoxy-1-fructosyl)phenylalanine | 328.14 | 310, 292, 264 | + | + |
| 7 | 1.19 | L-Phenylalanine | 166.09 | 120, 103, 91 | + | + |
| 8 | 1.30 | Sinapic acid | 225.04 | 207, 153 | + | + |
| 9 | 1.34 | Gallic acid 3-O-gallate | 323.04 | 277, 259, 171, 153, 127 | + | + |
| 10 | 1.40 | γ-Glutamyglutamic acid | 277.04 | 259, 231, 171 | + | − |
| 11 | 1.59 | N-(1-deoxy-1-fructosyl)tryptophan | 367.15 | 349, 188, 163 | + | + |
| 12 | 1.71 | L-Trytophan | 205.10 | 188, 170, 159, 146, 118, 115 | + | + |
| 13 | 1.93 | 2′,7-Dihydorxy-4′,5′-dimethoxyisoflavone | 315.07 | 297, 153 | + | + |
| 14 | 1.97 | Epicatechin 4′-glucuronide | 467.08 | 341, 291, 153 | + | + |
| 15 | 2.12 | Quinic acid | 193.09 | 149, 131, 115, 105, 103 | + | + |
| 16 | 2.20 | Chlorogenic acid | 355.10 | 193 | + | + |
| 17 | 2.25 | Methyl gallate | 158.04 | 153, 141, 123, 97 | + | + |
| 18 | 2.48 | Caffeic acid ethyl ester | 209.04 | 177 | + | + |
| 19 | 2.56 | Kaempferol 3-rhamnosyl-6″-(4″-acetylrhamnosyl)glucoside | 783.07 | 637, 619 | + | + |
| 20 | 2.60 | Acetylpterosin C | 277.04 | 259, 197, 171 | + | + |
| 21 | 2.80 | 1,2,3,4,6-Pentagallolyglucose | 941.09 | 619 | + | + |
| 22 | 2.99 | Phenylalanylglycine | 223.06 | 177, 120 | + | + |
| 23 | 3.48 | Leucocyanidin | 307.04 | 275, 247 | + | − |
| 24 | 3.57 | Kaempferol 3-rhamnosyl-(6″-acetyl)galactosyl-7-glucoside | 783.09 | 619 | + | + |
| 25 | 3.65 | 7-Glucosyl-4″glucuronoyl epigallocatechin gallate | 797.10 | 619 | + | + |
| 26 | 3.76 | Quercetin 3,7-diglucosyl-4″-galactoside | 789.11 | 771, 303 | + | + |
| 27 | 4.31 | Isoquercitrin | 465.10 | 303 | + | − |
| 28 | 4.37 | Quercetin 3-coumaroyl-triglucoside | 953.10 | 465, 303 | + | − |
| 29 | 4.45 | Quercetin 3-(2‴,6‴-digalloyl)galactoside | 769.09 | 617, 465, 303 | + | + |
| 30 | 4.71 | Quercetin 3-(2-galloyl)glucoside | 617.12 | 465, 315, 303, 297, 153 | + | + |
| 31 | 5.13 | Isorhamnetin 3-rutinoyl-4′-rhamnoside | 771.10 | 665, 287 | + | + |
| 32 | 5.31 | Kaempferol 3-(2″-rhamnosyl-6″-acetyl)galactosyl-7-rhamnoside | 785.09 | 767, 287 | + | − |
| 33 | 5.54 | Kaempferol 3-feruloyl-triglucoside | 967.12 | 619, 287, 177 | + | − |
| 34 | 5.69 | Isooreientin | 449.11 | 317, 287 | + | − |
| 35 | 14.23 | Dehydrophytosphingosine | 316.29 | 298, 280 | + | + |
| 36 | 14.48 | LysoPC(18:3) | 518.33 | 184 | + | + |
| 37 | 14.65 | LysoPC(18:2) | 520.34 | 184 | + | + |
| 38 | 14.89 | LysoPC(16:0) | 496.34 | 184 | + | + |
| 39 | 14.94 | LysoPC(18:1) | 522.35 | 184 | + | + |
| 40 | 15.08 | Phytosphingsine 1-phosphate | 398.23 | 280 | + | − |
| 41 | 15.20 | Methyl phaephoribide B | 621.27 | 531, 487 | + | − |
| 42 | 15.29 | Pheophorbide A | 593.28 | 531, 487 | + | + |
| 43 | 15.53 | Pheophorbide B | 607.29 | 547 | + | + |
| 44 | 16.06 | PC(18:3/18:3) | 778.54 | 184 | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, G.H.; Kaur, S.; Yoo, Y.; Ryu, Y.B.; Lee, S.J.; Jung, K.-W.; Chung, M.-S.; Bai, H.-W.; Kim, J.-H.; Lee, S.; et al. Effects of Gamma Irradiation on Changes in Chemical Composition and Antioxidant Activity of Euphorbia maculata Callus. Plants 2024, 13, 2306. https://doi.org/10.3390/plants13162306
Jeong GH, Kaur S, Yoo Y, Ryu YB, Lee SJ, Jung K-W, Chung M-S, Bai H-W, Kim J-H, Lee S, et al. Effects of Gamma Irradiation on Changes in Chemical Composition and Antioxidant Activity of Euphorbia maculata Callus. Plants. 2024; 13(16):2306. https://doi.org/10.3390/plants13162306
Chicago/Turabian StyleJeong, Gyeong Han, Shubhpreet Kaur, Youngchul Yoo, Young Bae Ryu, Seo Jun Lee, Kwang-Woo Jung, Moon-Soo Chung, Hyoung-Woo Bai, Jin-Hong Kim, Sungbeom Lee, and et al. 2024. "Effects of Gamma Irradiation on Changes in Chemical Composition and Antioxidant Activity of Euphorbia maculata Callus" Plants 13, no. 16: 2306. https://doi.org/10.3390/plants13162306





