Genome-Wide Characterization of Glyceraldehyde-3-Phosphate Dehydrogenase Genes and Their Expression Profile under Drought Stress in Quercus rubra
Abstract
1. Introduction
2. Results
2.1. Identification of the Proteins Coded by the GAPDH Gene Family and Physicochemical Characterization of QrGAPDH
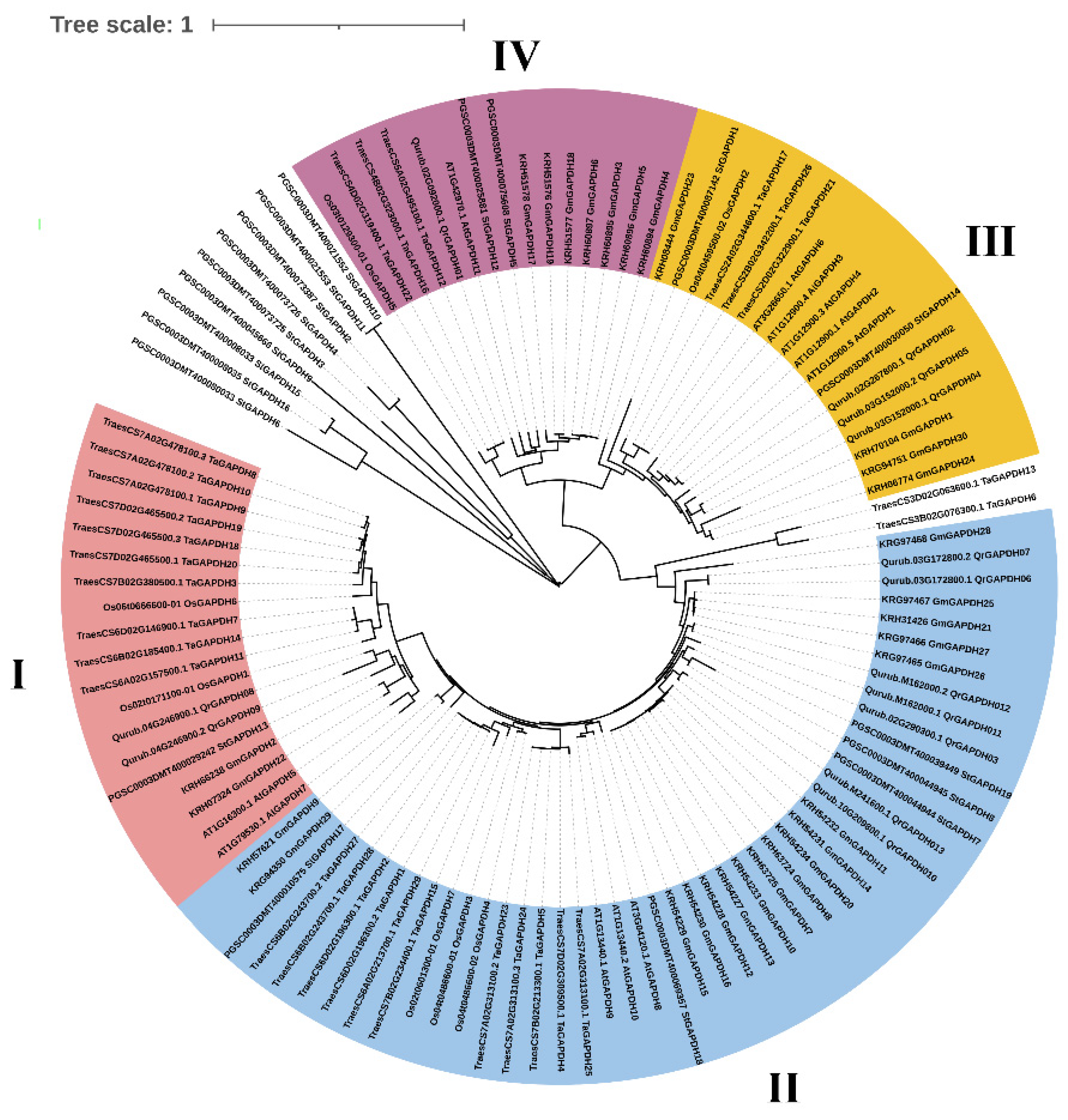
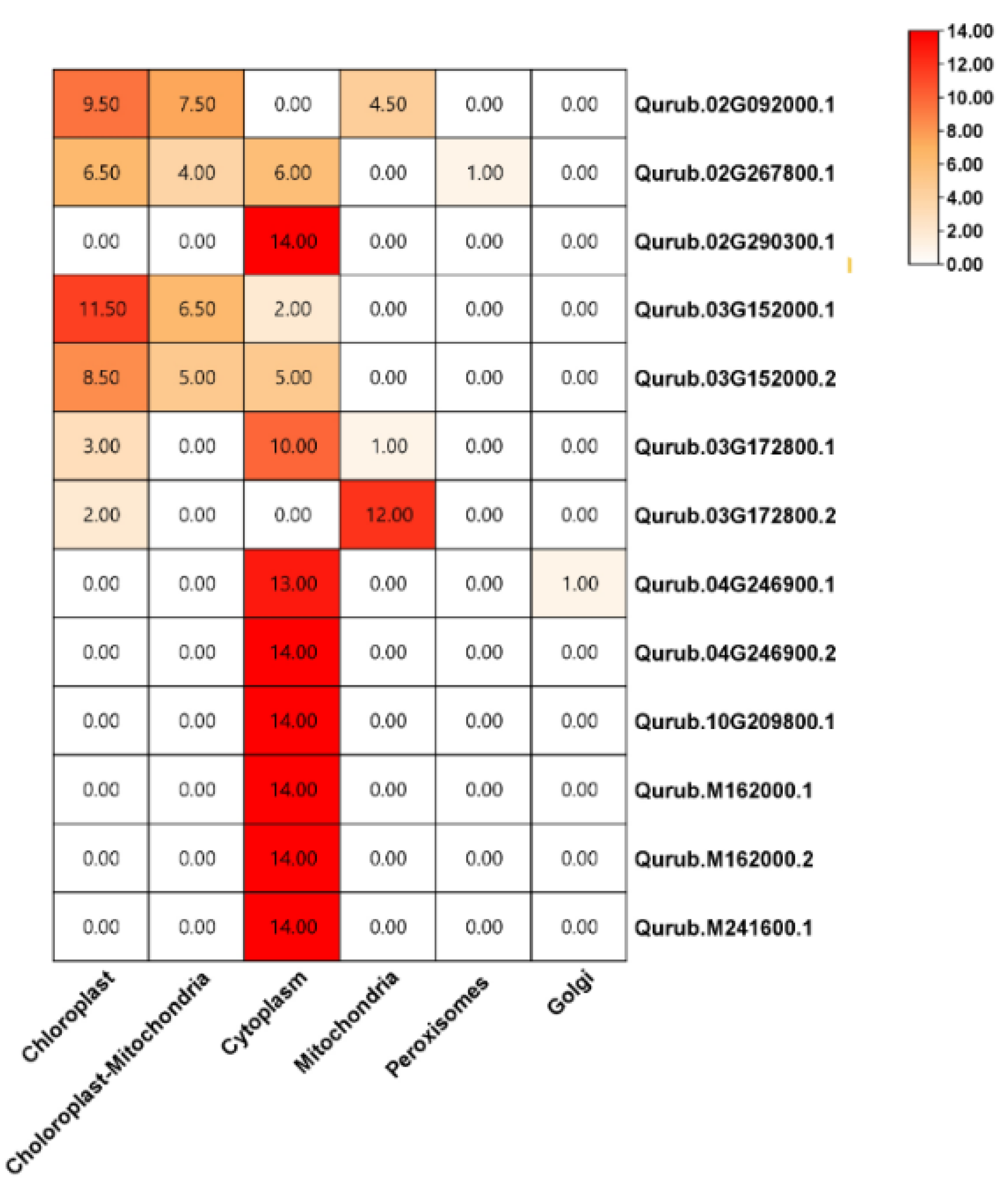
2.2. Phylogenetic Tree and Subcellular Localization of GAPDH Genes
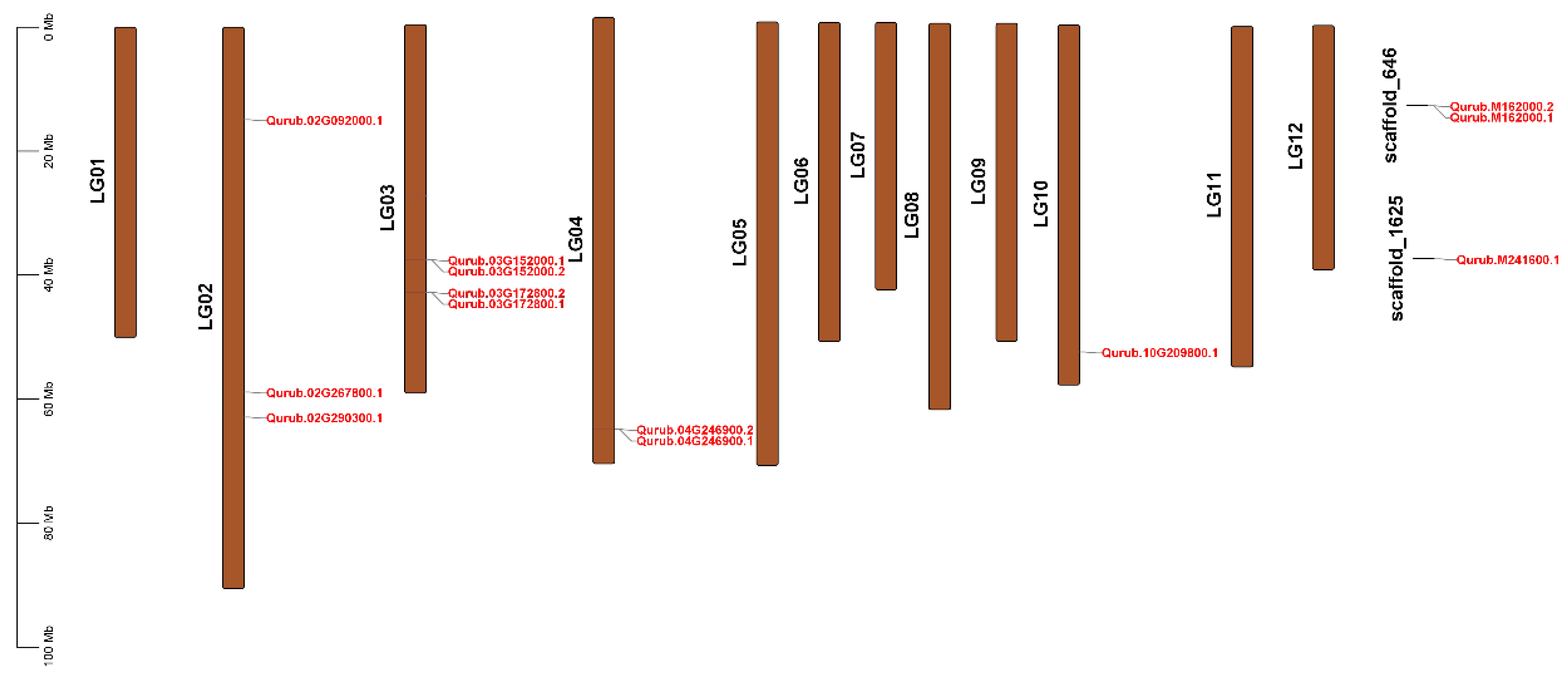
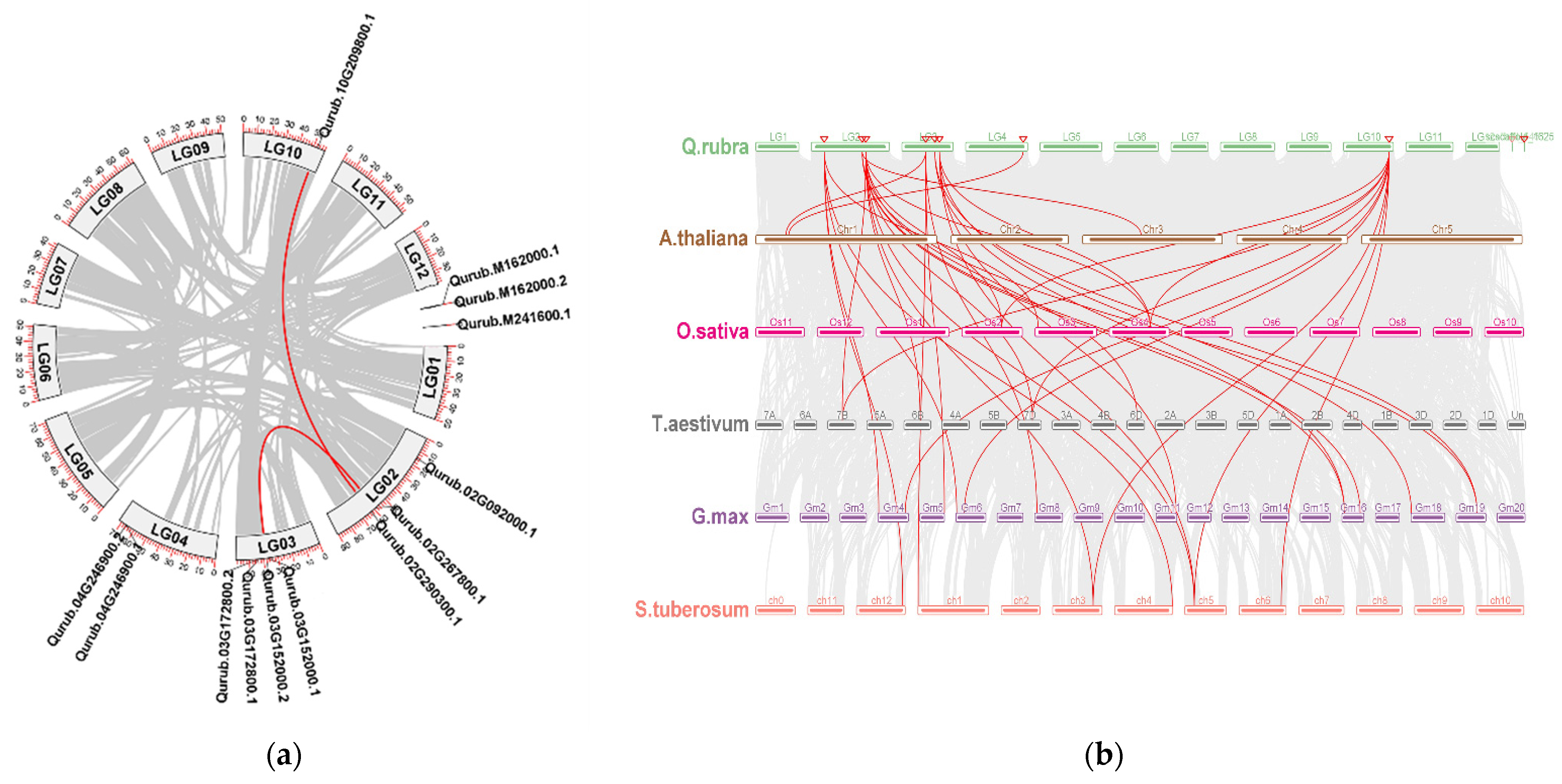
2.3. Chromosomal Location, Gene Duplication, Collinearity, and Synteny Analysis
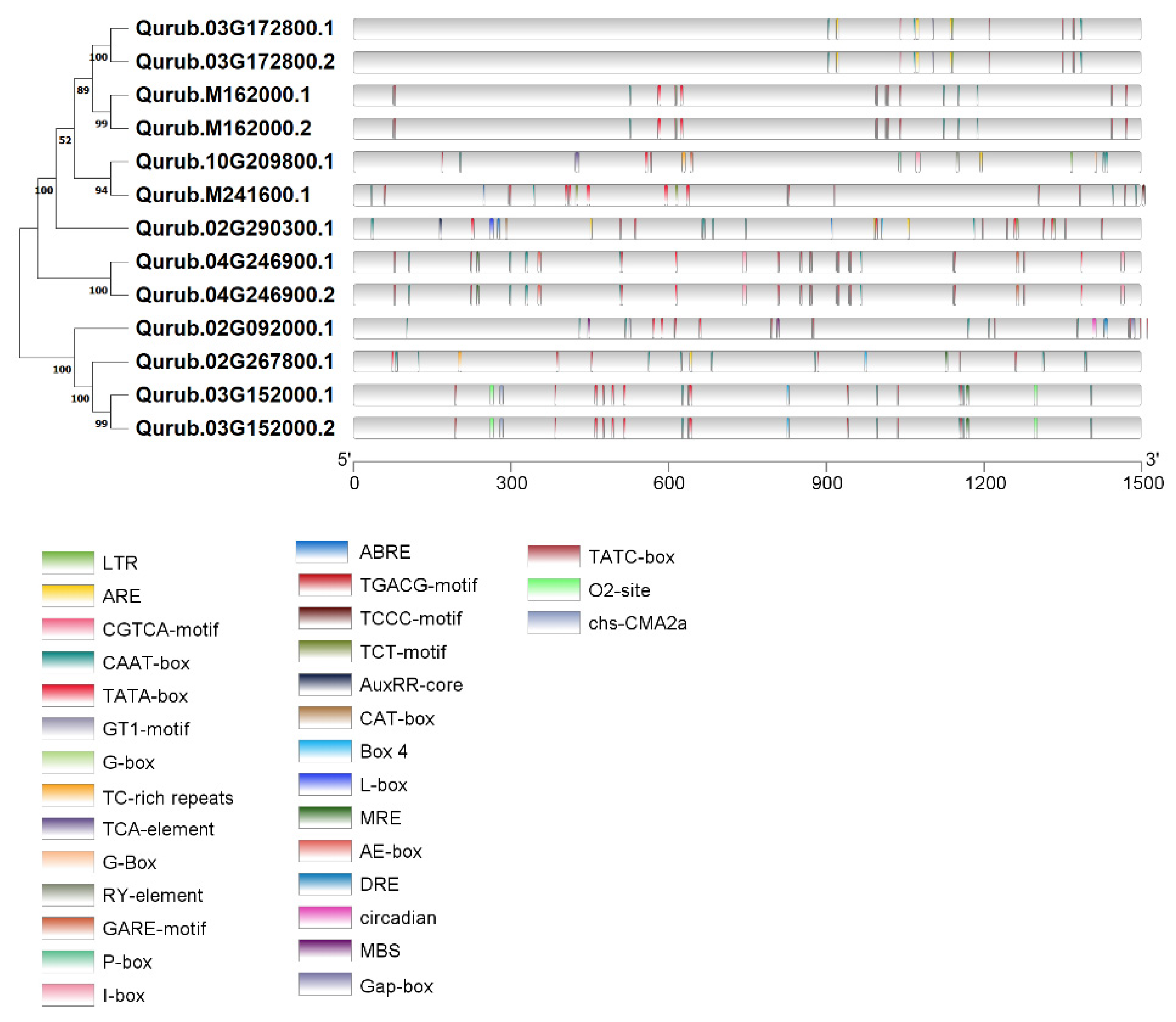
2.4. Gene Structure and Conserved Motifs Analysis of QrGAPDH Genes
2.5. GO Annotation and Cis-Element Analyses of GAPDH Genes in Q. rubra
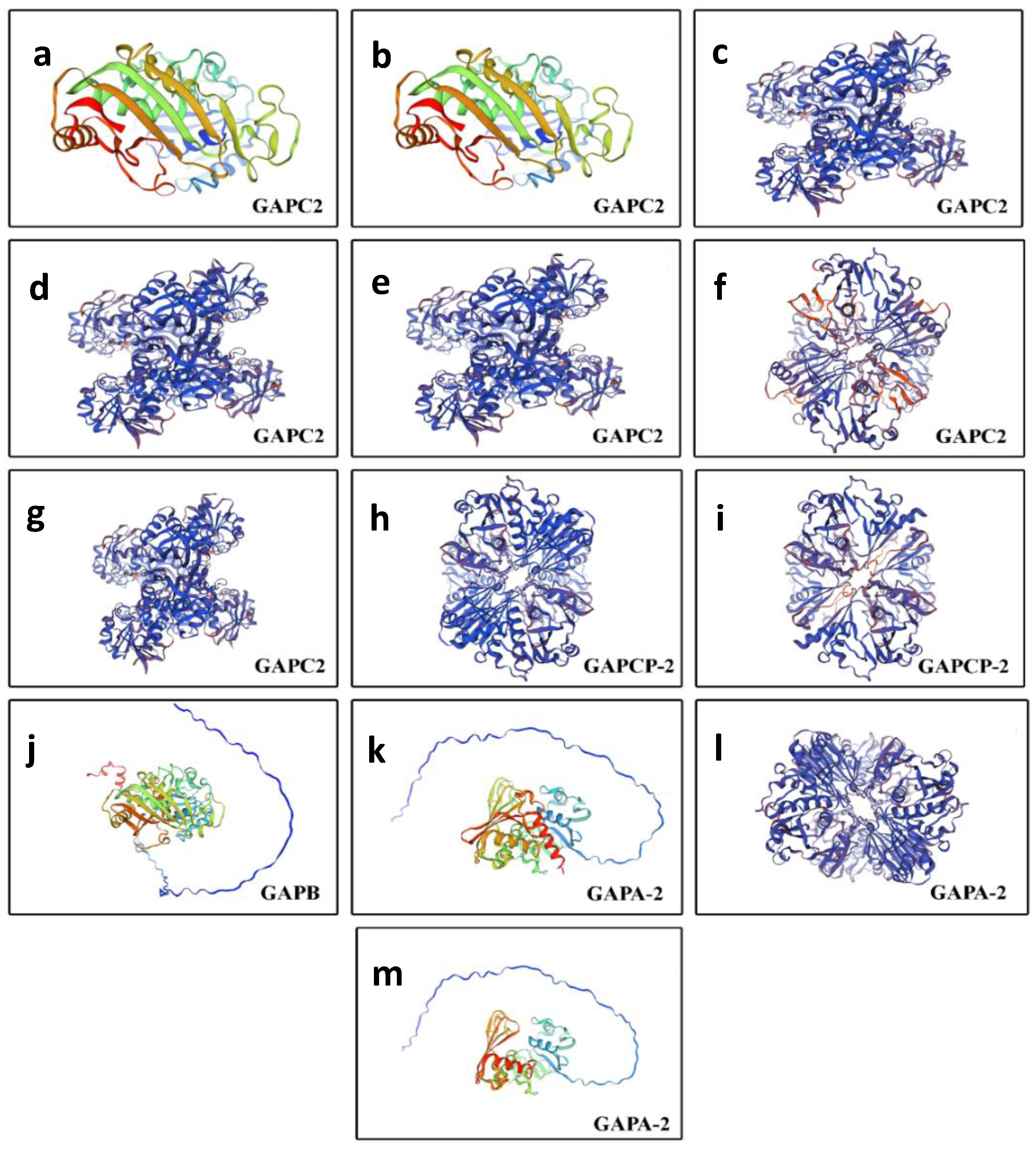
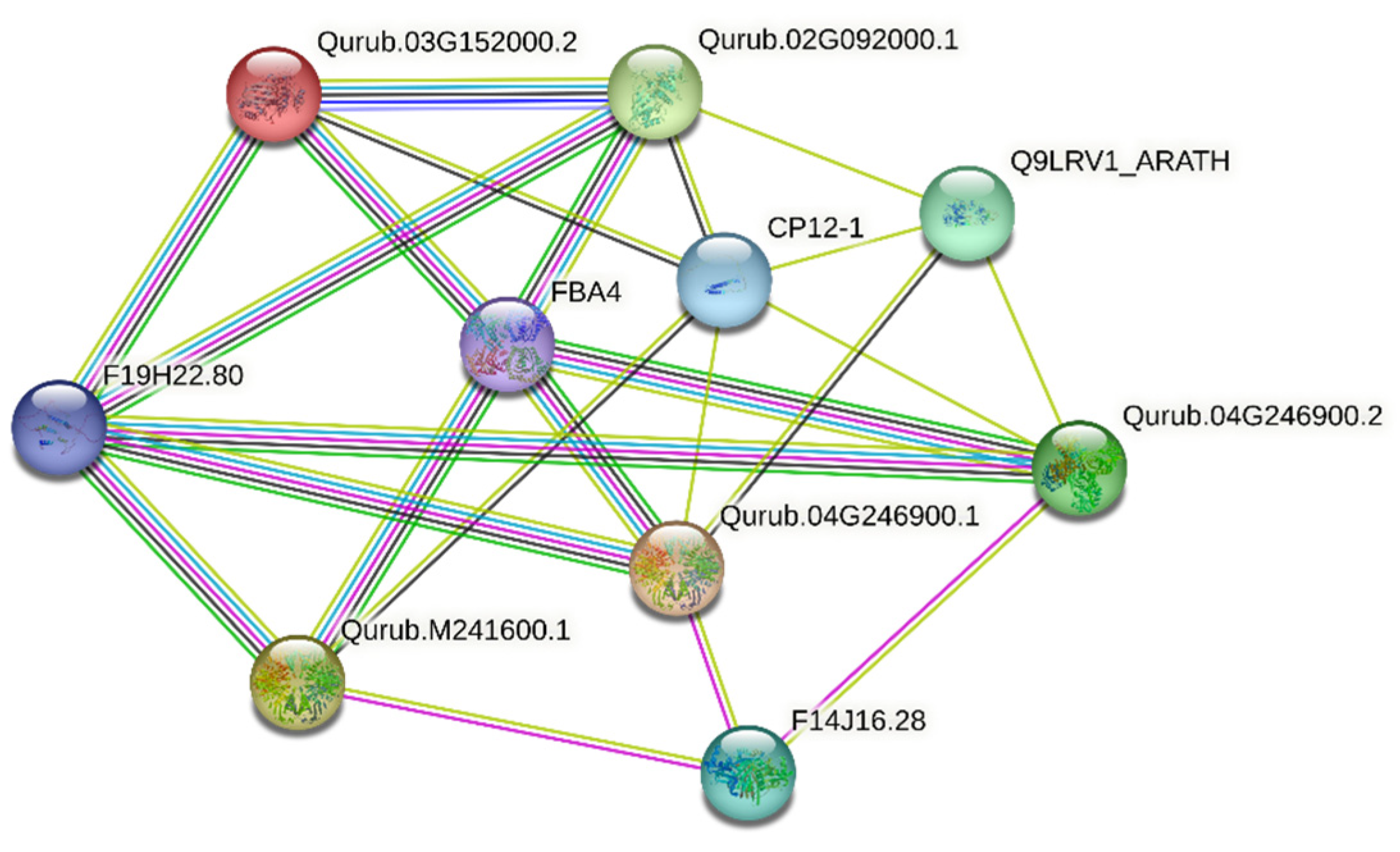
2.6. Three-Dimensional Structure Modeling and Protein–Protein Interaction Network Analysis
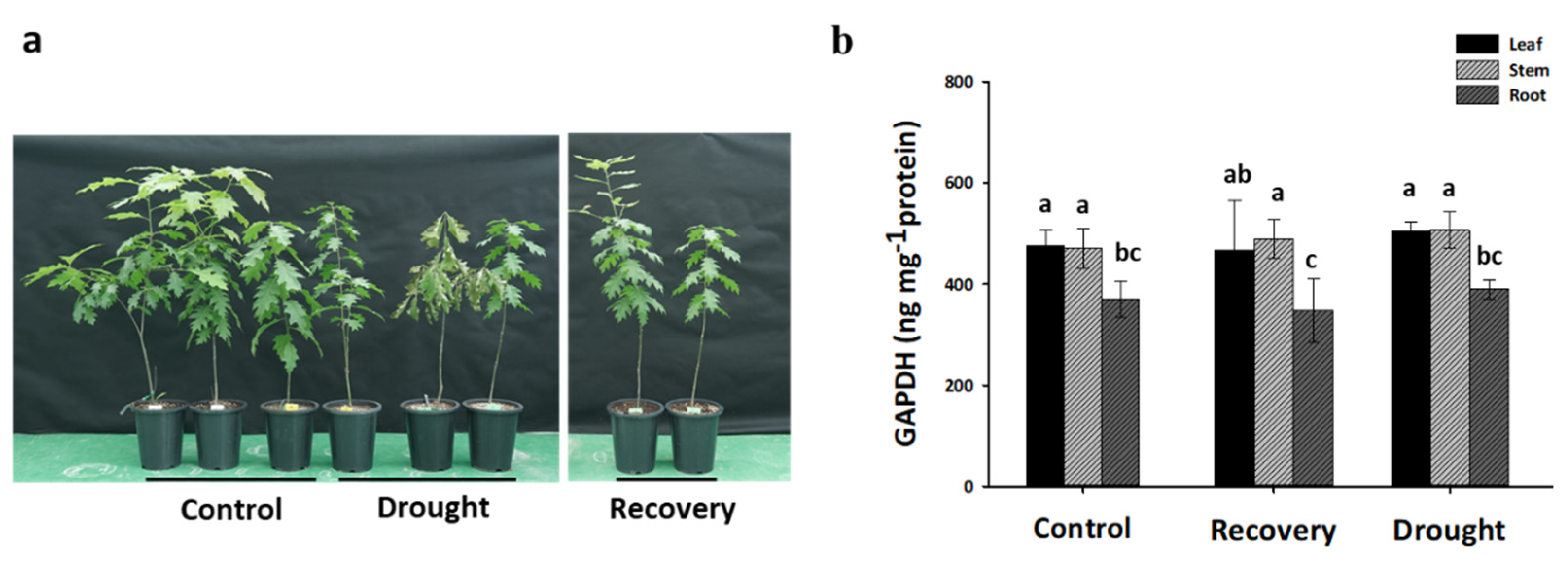
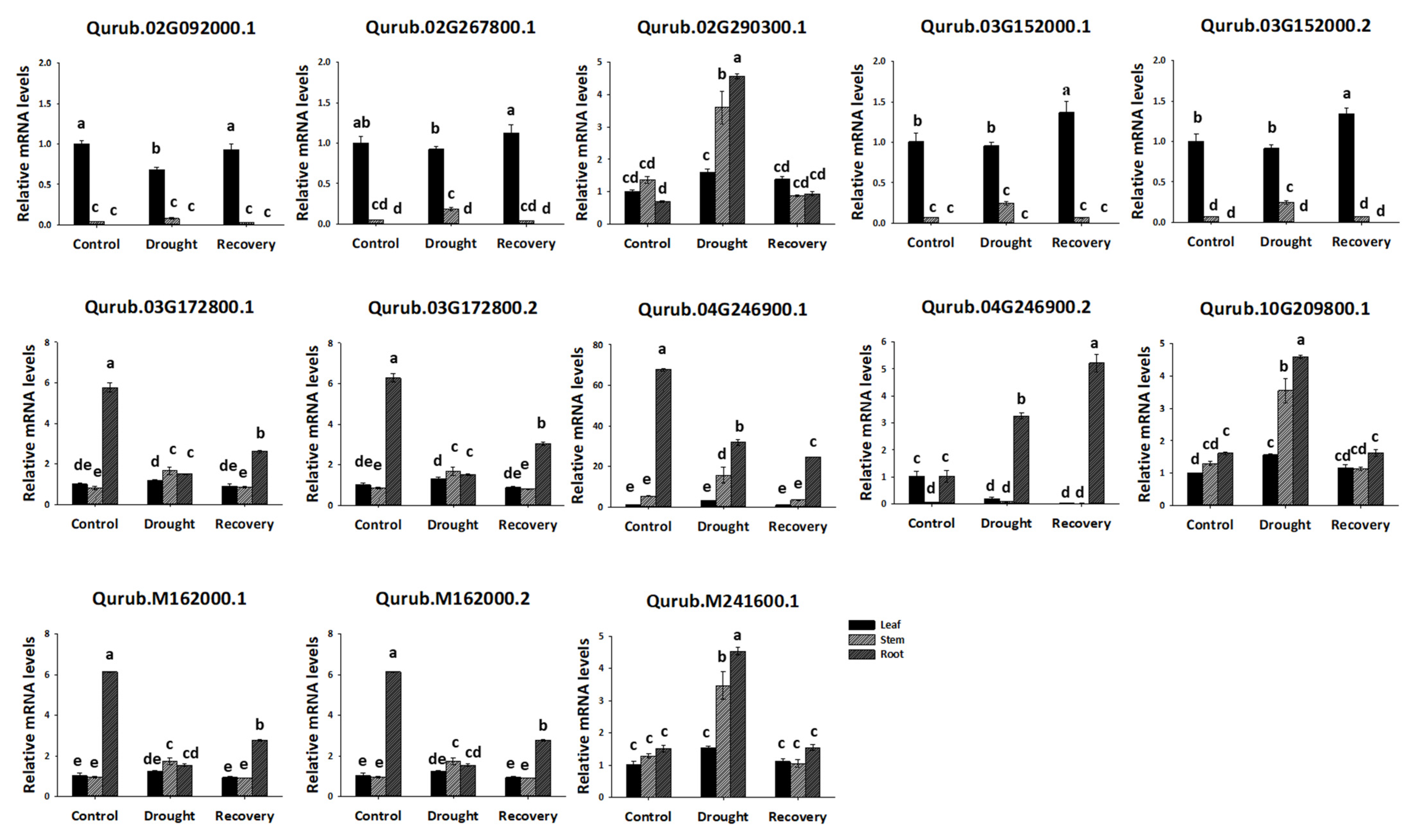
2.7. Expression Profiles of QrGAPDH Genes under Drought Stress and Re-Watering in Various Tissues
3. Discussion
4. Materials and Methods
4.1. Identification of the GAPDH Genes in Q. rubra
4.2. Phylogenetic Relationships, Gene Structure, and Conserved Motif Analyses of the GAPDH Genes
4.3. Chromosomal Distribution, Syntenic Analysis, Homology Modeling, and Predicting the Protein–Protein Interaction Network of the GAPDH Genes
4.4. GO, Nr Annotation, and Analyses of Cis-Regulatory Elements of the QrGAPDHs
4.5. Plant Materials and Drought Stress Treatment
4.6. Measurement of GAPDH Contents
4.7. RNA Extraction and Quantitative Real-Time PCR (qPCR)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plaxton, W.C. The organization and regulation of plant glycolysis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 185–214. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-L.; Denison, M.I.J.; Lim, H.; Chung, H.; Oh, C. Genome-wide analysis, identification, and characterization of the PFK gene family members of Populus deltoides. Forests 2023, 14, 1104. [Google Scholar] [CrossRef]
- Nicholls, C.; Li, H.; Liu, J.P. GAPDH: A common enzyme with uncommon functions. Clin. Exp. Pharmacol. Physiol. 2012, 39, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.J.; Park, S.C.; Kwon, H.B.; Byun, M.O. Isolation and characterization of the gene encoding glyceraldehyde-3-phosphate dehydrogenase. Biochem. Biophys. Res. Commun. 2000, 278, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Kappachery, S.; Baniekal-Hiremath, G.; Yu, J.W.; Park, S.W. Effect of over- and under-expression of glyceraldehyde 3-phosphate dehydrogenase on tolerance of plants to water-deficit stress. Plant Cell Tissue Organ Cult. 2015, 121, 97–107. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Yang, S. Cytosolic TaGAPC2 enhances tolerance to drought stress in transgenic Arabidopsis plants. Int. J. Mol. Sci. 2020, 21, 7499. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.J.; Park, S.C.; Byun, M.O. Improvement of salt tolerance in transgenic potato plants by glyceraldehyde-3 phosphate dehydrogenase gene transfer. Mol. Cells 2001, 12, 185–189. [Google Scholar] [CrossRef]
- Lim, H.; Hwang, H.; Kim, T.; Kim, S.; Chung, H.; Lee, D.; Kim, S.; Park, S.; Cho, W.; Ji, H.; et al. Transcriptomic Analysis of Rice Plants Overexpressing PsGAPDH in Response to Salinity Stress. Genes 2021, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Kosová, K.; Prášil, I.T.; Vítámvás, P. Protein contribution to plant salinity response and tolerance acquisition. Int. J. Mol. Sci. 2013, 14, 6757–6789. [Google Scholar] [CrossRef]
- Cui, G.; Bai, Y.; Li, W.; Gao, Z.; Chen, S.; Qiu, N.; Satoh, T.; Kakuchi, T.; Duan, Q. Synthesis and characterization of Eu(III) complexes of modified D-glucosamine and poly(N-isopropylacrylamide). Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 78, 603–608. [Google Scholar] [CrossRef]
- Zeng, L.; Deng, R.; Guo, Z.; Yang, S.; Deng, X. Genome-wide identification and characterization of glyceraldehyde-3-phosphate dehydrogenase genes family in wheat (Triticum aestivum). BMC Genom. 2016, 17, 240. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, M.; Nisar, M.; Khan, N.; Hazrat, A.; Khan, A.H.; Hayat, K.; Fahad, S.; Khan, A.; Ullah, A. Drought tolerance strategies in plants: A mechanistic approach. J. Plant Growth Regul. 2021, 40, 926–944. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-L.; Lim, H.; Chung, H.; Veerappan, K.; Oh, C. Elevated CO2 alters the physiological and transcriptome responses of Pinus densiflora to long-term CO2 exposure. Plants 2022, 11, 3530. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ma, F.; Wei, F.; Fanella, B.; Allen, D.K.; Wang, X. Cytosolic phosphorylating glyceraldehyde-3-phosphate dehydrogenases affect Arabidopsis cellular metabolism and promote seed oil accumulation. Plant Cell 2014, 26, 3023–3035. [Google Scholar] [CrossRef] [PubMed]
- Bustos, D.M.; Bustamante, C.A.; Iglesias, A.A. Involvement of nonphosphorylating glyceraldehyde-3-phosphate dehydrogenase in response to oxidative stress. J. Plant Physiol. 2007, 165, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, J.; Xia, N.; Qu, Y.; Zhan, Y.; Teng, W.; Li, H.; Li, W.; Li, Y.; Zhao, X.; et al. Genome-wide identification and analysis of glyceraldehyde-3-phosphate dehydrogenase family reveals the role of GmGAPDH14 to improve salt tolerance in soybean (Glycine max L.). Front. Plant Sci. 2023, 14, 1193044. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Chen, C.; Yao, L.; Tran, J.; Zhang, H. Genome-wide identification, characterization, interaction network and expression profile of GAPDH gene family in sweet orange (Citrus sinensis). Peer J. 2019, 7, e7934. [Google Scholar] [CrossRef]
- Kim, T.-L.; Lim, H.; Denison, M.I.J.; Natarajan, S.; Oh, C. Genome-wide identification of the PFK gene family and their expression analysis in Quercus rubra. Front. Genet. 2023, 14, 1289557. [Google Scholar] [CrossRef]
- Konar, A.; Choudhury, O.; Bullis, R.; Fiedler, L.; Kruser, J.M.; Stephens, M.T.; Gailing, O.; Schlarbaum, S.; Coggeshall, M.V.; Staton, M.E.; et al. High-quality genetic mapping with ddRADseq in the non-model tree Quercus rubra. BMC Genom. 2017, 18, 417. [Google Scholar] [CrossRef]
- Kim, S.C.; Guo, L.; Wang, X. Nuclear moonlighting of cytosolic glyceraldehyde-3-phosphate dehydrogenase regulates Arabidopsis response to heat stress. Nat. Commun. 2020, 11, 3439. [Google Scholar] [CrossRef]
- Li, Y.M.; Sun, S.R.; Wang, Y.; Cai, X.X.; Yao, J.X.; Zhu, L. Identification of the GAPDH gene family in Citrullus lanatus and functional characteristics of ClGAPC2 in Arabidopsis thaliana. Plant Biol. 2023, 25, 334–342. [Google Scholar] [CrossRef]
- Ranjbar, M.; Khakdan, F.; Ghorbani, A.; Zargar, M.; Chen, M. The variations in gene expression of GAPDH in Ocimum basilicum cultivars under drought-induced stress conditions. Environ. Sci. Pollut. Res. Int. 2023, 30, 119187–119203. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Movahedi, A.; Yang, J.; Zhang, Y.; Liu, G.; Zhu, S.; Yu, C.; Chen, Y.; Zhong, F.; Zhang, J. Characteristics and molecular identification of glyceraldehyde-3-phosphate dehydrogenases in poplar. Int. J. Biol. Macromol. 2022, 219, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Sasvari, Z.; Gonzalez, P.A.; Friso, G.; Rowland, E.; Liu, X.M.; van Wijk, K.J.; Nagy, P.D.; Klessig, D.F. Salicylic acid inhibits the replication of tomato bushy stunt virus by directly targeting a host component in the replication complex. Mol. Plant Microbe Interact. 2015, 28, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, H.; Yang, H.; Pu, X.; Li, C.; Huo, H.; Chu, Z.; Chang, Y.; Lin, Y.; Liu, L. Translocation of drought-responsive proteins from the chloroplasts. Cells 2020, 9, 259. [Google Scholar] [CrossRef]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell Signal. 2011, 23, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.; Brunnemann, R.; Selbig, J. The regulatory code for transcriptional response diversity and its relation to genome structural properties in A. thaliana. PLoS Genet. 2007, 3, e11. [Google Scholar] [CrossRef]
- Li, B.; Fan, R.; Sun, G.; Sun, T.; Fan, Y.; Bai, S.; Guo, S.; Huang, S.; Liu, J.; Zhang, H.; et al. Flavonoids improve drought tolerance of maize seedlings by regulating the homeostasis of reactive oxygen species. Plant Soil 2021, 461, 389–405. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Kasuga, M.; Liu Qiang, L.Q.; Nakashima, K.; Sakuma, Y.; Abe, H.; Shinwari, Z.K.; Seki, M.; Shinozaki, K. Development of drought-resistant and water stress-tolerant crops through biotechnology. In Proceedings of the 8th JIRCAS International Symposium, Tsukuba, Japan, 27–28 November 2001; JIRCAS: Tsukuba, Japan, 2002; pp. 23–34. [Google Scholar]
- Xu, Z.; Wang, M.; Guo, Z.; Zhu, X.; Xia, Z. Identification of a 119-bp promoter of the maize sulfite oxidase gene (ZmSO) that confers high-level gene expression and ABA or drought inducibility in transgenic plants. Int. J. Mol. Sci. 2019, 20, 3326. [Google Scholar] [CrossRef]
- Baldoni, E.; Genga, A.; Cominelli, E. Plant MYB Transcription Factors: Their Role in Drought Response Mechanisms. Int. J. Mol. Sci. 2015, 16, 15811–15851. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Dogra, V.; Joshi, R.; Kumar, S. Stress-responsive cis-regulatory elements underline podophyllotoxin biosynthesis and better performance of Sinopodophyllum hexandrum under water deficit conditions. Front. Plant Sci. 2022, 12, 751846. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, H.; Sun, L.; Fan, G.; Ye, M.; Jiang, L.; Liu, X.; Ma, K.; Shi, C.; Bao, F.; et al. The genetic architecture of floral traits in the woody plant Prunus mume. Nat. Commun. 2018, 9, 1702. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhu, C.; Fu, H.; Li, X.; Chen, L.; Lin, Y.; Lai, Z.; Guo, Y. Genome-wide investigation of superoxide dismutase (SOD) gene family and their regulatory miRNAs reveal the involvement in abiotic stress and hormone response in tea plant (Camellia sinensis). PLoS ONE 2019, 14, e0223609. [Google Scholar] [CrossRef] [PubMed]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 15, 3674–3676. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Makela, M.; Michael, P.; Theriault, G.; Nkongolo, K. High genetic variation among closely related red oak (Quercus rubra) populations in an ecosystem under metal stress: Analysis of gene regulation. Genes Genom. 2016, 38, 967–976. [Google Scholar] [CrossRef]
- Migueal, P.; Nkongolo, K.; Michael, P.; Djeukam, C. Differential gene transcription in red oak (Quercus rubra) genotypes resistant to copper toxicity. Am. J. Biochem. Biotechnol. 2017, 13, 215–225. [Google Scholar] [CrossRef]

| Gene ID | Gene Symbol | Database ID | Number of AAs z | MW y (KDa) | Theoretical pI x | Total Number of Negatively Charged Residues (Asp + Glu) | Total Number of Positively Charged Residues (Arg + Lys) | Instability Index | Aliphatic Index | GRAVY w |
|---|---|---|---|---|---|---|---|---|---|---|
| QrGAPDH01 | GAPB | Qurub.02G092000.1 | 452 | 48,239.24 | 8.82 | 29 | 33 | 27.65 | 91.44 | −0.031 |
| QrGAPDH02 | GAPA-2 | Qurub.02G267800.1 | 402 | 42,859.98 | 8.60 | 32 | 37 | 24.91 | 96.77 | −0.001 |
| QrGAPDH03 | GAPC2 | Qurub.02G290300.1 | 337 | 36,689.02 | 7.02 | 34 | 41 | 24.78 | 90.24 | −0.103 |
| QrGAPDH04 | GAPA-2 | Qurub.03G152000.1 | 404 | 43,237.28 | 8.46 | 34 | 42 | 26.59 | 95.54 | −0.018 |
| QrGAPDH05 | GAPA-2 | Qurub.03G152000.2 | 346 | 36,881.19 | 9.46 | 35 | 43 | 28.18 | 95.55 | −0.060 |
| QrGAPDH06 | GAPC2 | Qurub.03G172800.1 | 308 | 33,677.08 | 9.25 | 39 | 43 | 20.55 | 90.49 | −0.106 |
| QrGAPDH07 | GAPC2 | Qurub.03G172800.2 | 307 | 33,606.00 | 9.25 | 42 | 45 | 20.58 | 90.46 | −0.112 |
| QrGAPDH08 | GAPCP-2 | Qurub.04G246900.1 | 418 | 44,648.66 | 8.48 | 42 | 45 | 35.19 | 83.95 | −0.101 |
| QrGAPDH09 | GAPCP-2 | Qurub.04G246900.2 | 298 | 31,696.12 | 8.86 | 43 | 45 | 31.22 | 81.85 | −0.107 |
| QrGAPDH10 | GAPC2 | Qurub.10G209800.1 | 338 | 36,616.96 | 8.25 | 43 | 45 | 21.75 | 91.66 | −0.072 |
| QrGAPDH11 | GAPC2 | Qurub.M162000.1 | 340 | 37,073.58 | 8.22 | 43 | 47 | 22.51 | 90.82 | −0.113 |
| QrGAPDH12 | GAPC2 | Qurub.M162000.2 | 339 | 37,002.50 | 8.22 | 44 | 47 | 22.55 | 90.8 | −0.119 |
| QrGAPDH13 | GAPC2 | Qurub.M241600.1 | 279 | 30,216.82 | 9.03 | 46 | 53 | 24.85 | 92.22 | −0.147 |
| Gene 1 | Gene 2 | Ka | Ks | Ka_Ks | Divergence Time (Mya z) | Duplication Type |
|---|---|---|---|---|---|---|
| Qurub.02G267800.1 | Qurub.03G152000.2 | 0.076964373 | 1.304651722 | 0.058992275 | 95.09123335 | Tandem |
| Qurub.02G290300.1 | Qurub.10G209800.1 | 0.065133869 | 0.971411426 | 0.067050755 | 70.80258209 | Tandem |
| Cluster | Node 1 | Node 1 ID | Node 2 | Node 2 ID | Score |
|---|---|---|---|---|---|
| Cluster I | Qurub.02G092000.1 | 3702.P25857 | Qurub.03G152000.2 | 3702.Q9LPW0 | 0.983 |
| FBA4 | 3702.F4KGQ0 | 0.839 | |||
| Q9LRV1_ARATH | 3702.Q9LRV1 | 0.758 | |||
| CP12-1 | 3702.O22914 | 0.714 | |||
| F19H22.80 | 3702.Q9SVJ5 | 0.568 | |||
| Cluster II | Qurub.03G152000.2 | 3702.Q9LPW0 | Qurub.02G092000.1 | 3702.P25857 | 0.983 |
| FBA4 | 3702.F4KGQ0 | 0.839 | |||
| F19H22.80 | 3702.Q9SVJ5 | 0.568 | |||
| CP12-1 | 3702.O22914 | 0.432 | |||
| Cluster III | Qurub.04G246900.1 | 3702.Q9SAJ6 | FBA4 | 3702.F4KGQ0 | 0.879 |
| CP12-1 | 3702.O22914 | 0.857 | |||
| F14J16.28 | 3702.F4I3I1 | 0.559 | |||
| F19H22.80 | 3702.Q9SVJ5 | 0.512 | |||
| Q9LRV1_ARATH | 3702.Q9LRV1 | 0.439 | |||
| Cluster IV | Qurub.04G246900.2 | 3702.Q5E924 | FBA4 | 3702.F4KGQ0 | 0.880 |
| CP12-1 | 3702.O22914 | 0.857 | |||
| F14J16.28 | 3702.F4I3I1 | 0.559 | |||
| F19H22.80 | 3702.Q9SVJ5 | 0.512 | |||
| Q9LRV1_ARATH | 3702.Q9LRV1 | 0.400 | |||
| Cluster V | Qurub.M241600.1 | 3702.Q9FX54 | FBA4 | 3702.F4KGQ0 | 0.873 |
| CP12-1 | 3702.O22914 | 0.870 | |||
| F19H22.80 | 3702.Q9SVJ5 | 0.512 | |||
| F14J16.28 | 3702.F4I3I1 | 0.425 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.; Denison, M.I.J.; Lee, K.; Natarajan, S.; Kim, T.-L.; Oh, C. Genome-Wide Characterization of Glyceraldehyde-3-Phosphate Dehydrogenase Genes and Their Expression Profile under Drought Stress in Quercus rubra. Plants 2024, 13, 2312. https://doi.org/10.3390/plants13162312
Lim H, Denison MIJ, Lee K, Natarajan S, Kim T-L, Oh C. Genome-Wide Characterization of Glyceraldehyde-3-Phosphate Dehydrogenase Genes and Their Expression Profile under Drought Stress in Quercus rubra. Plants. 2024; 13(16):2312. https://doi.org/10.3390/plants13162312
Chicago/Turabian StyleLim, Hyemin, Michael Immanuel Jesse Denison, Kyungmi Lee, Sathishkumar Natarajan, Tae-Lim Kim, and Changyoung Oh. 2024. "Genome-Wide Characterization of Glyceraldehyde-3-Phosphate Dehydrogenase Genes and Their Expression Profile under Drought Stress in Quercus rubra" Plants 13, no. 16: 2312. https://doi.org/10.3390/plants13162312
APA StyleLim, H., Denison, M. I. J., Lee, K., Natarajan, S., Kim, T.-L., & Oh, C. (2024). Genome-Wide Characterization of Glyceraldehyde-3-Phosphate Dehydrogenase Genes and Their Expression Profile under Drought Stress in Quercus rubra. Plants, 13(16), 2312. https://doi.org/10.3390/plants13162312






