The Critical Role of Soil Ecological Stoichiometric Ratios: How Does Reforestation Improve Soil Nitrogen and Phosphorus Availability?
Abstract
:1. Introduction
2. Results
2.1. Effects of Reforestation Age, Vegetation Type, and Soil Depth on Soil Carbon, Nitrogen, Phosphorus, and Soil Stoichiometric Ratios
2.2. The Distribution of Soil C:N Stoichiometric Ratio
2.3. The Distribution of Soil C:P Stoichiometric Ratio
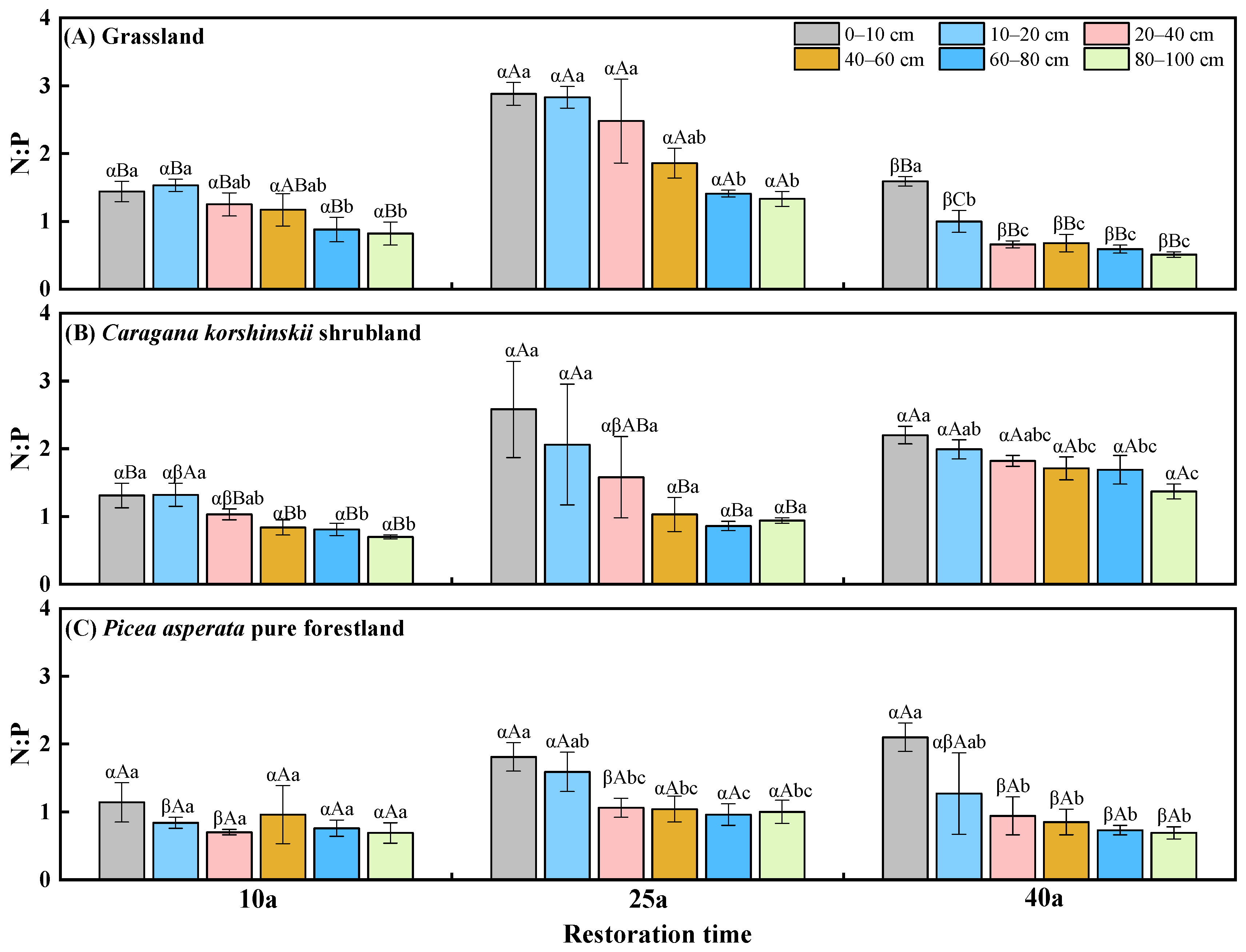
2.4. The Distribution of Soil N:P Stoichiometric Ratio
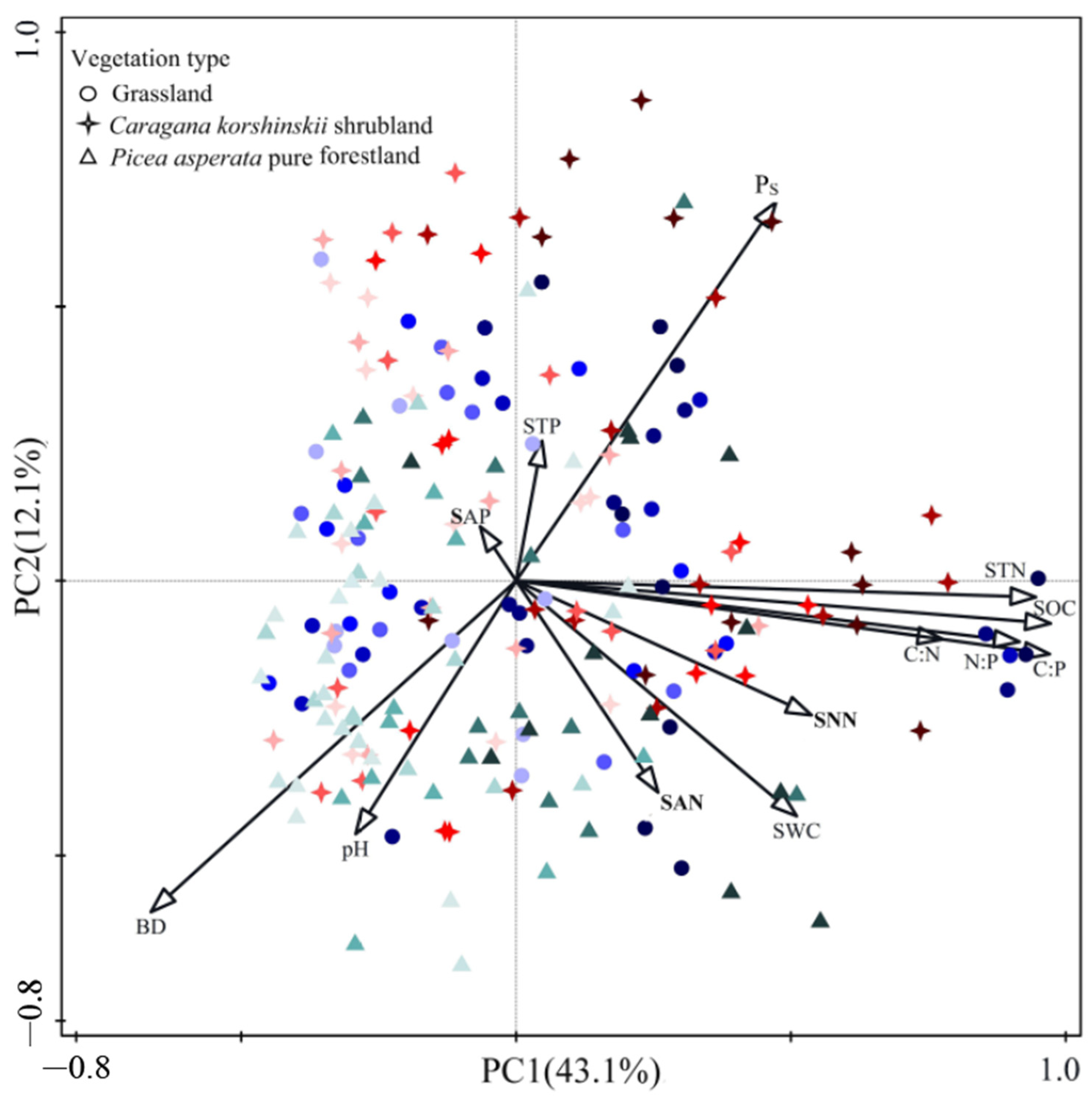
2.5. The Relationship between Soil C, N, and P Stoichiometric Ratios and Soil Physico-Chemical Properties across Soil Profile with Reforestation
2.6. The Relationship of Soil C, N, and P Stoichiometric Ratios and Plant Diversity
3. Materials and Methods
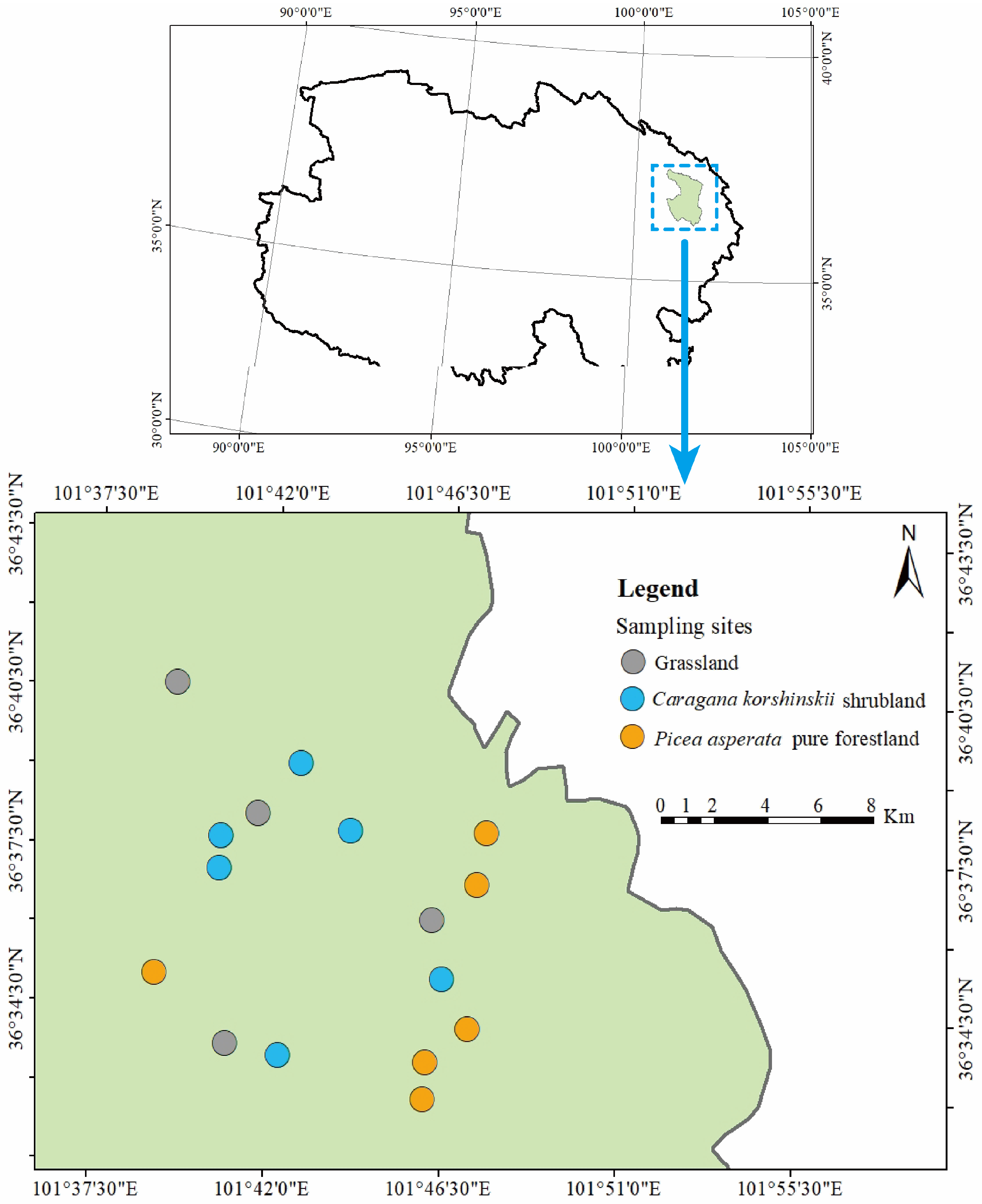
3.1. Study Area
3.2. Soil Sample Collection and Plant Species Diversity Calculation
3.3. The Determination of Soil Properties
3.4. Statistical Analysis
4. Discussion
4.1. Effects of Reforestation Type and Reforestation Age on Soil C, N, and P Stoichiometric Ratios
4.2. Soil C, N, and P Stoichiometric Ratios Significantly Influence Nutrient Availability
4.3. Plant Diversity Affected Soil C, N, and P Stoichiometric Ratios in Upper Soil Layer
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Yu, G. Ecological stoichiometry characteristics of ecosystem carbon, nitrogen and phosphorus elements. Acta Ecol. Sin. 2008, 28, 3937–3947. [Google Scholar]
- Joshi, R.K.; Garkoti, S.C. Influence of vegetation types on soil physical and chemical properties, microbial biomass and stoichiometry in the central Himalaya. Catena 2023, 222, 106835. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Duan, Y.; Wang, L.; Wang, X.; Yao, C.; Chen, Y.; Cao, W.; Niu, Y. Patterns and driving factors of soil ecological stoichiometry in typical ecologically fragile areas of China. Catena 2022, 219, 106628. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.; Yang, C.; Du, J.; Xu, W.; Xie, M. Seasonal Variation of Soil Carbon, Nitrogen and Phosphorus Stoichiometry Under Different Vegetation Types in Loess Hilly Region. J. Soil Water Conserv. 2021, 35, 343–349. [Google Scholar] [CrossRef]
- Ke, X.; Xiao, H.; Peng, Y.; Xia, X.; Wang, X. Nitrogen deficiency modulates carbon allocation to promote nodule nitrogen fixation capacity in soybean. Exploration 2023, 4, 20230104. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Cao, D.; Zhu, Z.; Ge, T. Stoichiometry mechanism of microbial utilization efficiency of soil organic carbon: A review. Soil Fertil. Sci. China 2024, 4, 228–234. [Google Scholar] [CrossRef]
- Parrotta, J.A. Productivity, nutrient cycling, and succession in single-and mixed-species plantations of Casuarina equisetifolia, Eucalyptus robusta, and Leucaena leucocephala in Puerto Rico. For. Ecol. Manag. 1999, 124, 45–77. [Google Scholar] [CrossRef]
- Paquette, A.; Messier, C. The role of plantations in managing the world’s forests in the Anthropocene. Front. Ecol. Environ. 2010, 8, 27–34. [Google Scholar] [CrossRef]
- Qiu, Z.; Shi, C.; Zhao, M.; Wang, K.; Zhang, M.; Wang, T.; Shi, F. Improving Effects of Afforestation with Different Forest Types on Soil Nutrients and Bacterial Community in Barren Hills of North China. Sustainability 2022, 14, 1202. [Google Scholar] [CrossRef]
- Deng, J.; Sun, P.; Zhao, F.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Soil C, N, P and its stratification ratio affected by artificial vegetation in subsoil, Loess Plateau China. PLoS ONE 2016, 11, e0151446. [Google Scholar] [CrossRef]
- Korkanç, S.Y. Effects of afforestation on soil organic carbon and other soil properties. Catena 2014, 123, 62–69. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, P.; Li, P.; Li, Z.; Min, Z.; Sun, J.; Cui, L.; Niu, H.; Zu, P.; Cao, M. Effects of vegetation restoration on soil organic carbon in the Loess Plateau: A meta-analysis. Land Degrad. Dev. 2023, 34, 2088–2097. [Google Scholar] [CrossRef]
- Duan, C.; Xu, G.; Shen, Y.; Luo, Y.; Li, Y.; Zhang, D.; Sun, Y.; He, C. Ecological Stoichiometry Characteristics of Soils in Eucalyptus Plantations with Different Ages in North Guangxi. For. Resour. Manag. 2018, 117–124. [Google Scholar] [CrossRef]
- Lou, B.; Wang, Y.; Yan, J.; Ai Si Kai Er, A. Characteristics of soil ecological stoichiometry of different tree spcies in sub-frigid desert steppe. Arid Zone Res. 2021, 38, 1385–1392. [Google Scholar] [CrossRef]
- Zhang, S.; Li, A.; Wang, H.; Wang, J.; Xu, X. Ecological stoichiometry of soil carbon, nitrogen and phosphorus in Cunninghamia lanceolata plantation across an elevation gradient. Ecol. Environ. Sci. 2020, 29, 97–104. [Google Scholar]
- Liu, J. Ecological Stoichiometry Characteristics Andseasonal Variation in Continuous Planted Eucalyptus Plantations. Master’s Thesis, Guangxi University, Nanning, China, 2017. [Google Scholar]
- Lucas-Borja, M.E.; Hedo, J.; Cerdá, A.; Candel-Pérez, D.; Viñegla, B. Unravelling the importance of forest age stand and forest structure driving microbiological soil properties, enzymatic activities and soil nutrients content in Mediterranean Spanish black pine (Pinus nigra Ar. ssp. salzmannii) Forest. Sci. Total Environ. 2016, 562, 145–154. [Google Scholar] [CrossRef]
- Hume, A.; Chen, H.Y.H.; Taylor, A.R.; Kayahara, G.J.; Man, R. Soil C:N:P dynamics during secondary succession following fire in the boreal forest of central Canada. For. Ecol. Manag. 2016, 369, 1–9. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Delgado-Baquerizo, M. Plant diversity and soil stoichiometry regulates the changes in multifunctionality during pine temperate forest secondary succession. Sci. Total Environ. 2019, 697, 134204. [Google Scholar] [CrossRef]
- Zethof, J.H.T.; Cammeraat, E.L.H.; Nadal-Romero, E. The enhancing effect of afforestation over secondary succession on soil quality under semiarid climate conditions. Sci. Total Environ. 2019, 652, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Ren, L.; Song, L.; Duan, Y.; Huang, T.; Han, X.; Hao, W. Plant stoichiometry characteristics and relationships with soil nutrients in Robinia pseudoacacia communities of different planting ages. Acta Ecol. Sin. 2017, 37, 355–362. [Google Scholar] [CrossRef]
- Cao, J.; Pan, H.; Chen, Z.; Shang, H. Dynamics in stoichiometric traits and carbon, nitrogen, and phosphorus pools across three different-aged Picea asperata Mast. plantations on the Eastern Tibet Plateau. Forests 2020, 11, 1346. [Google Scholar] [CrossRef]
- Xu, C.; Hu, C.; Zhang, Z.; Peng, X.; Hu, G. Ecological stoichiometric characteristics of leaf, litter, and soil in eucalyptus plantations with different ages in subtropical, South China. Appl. Ecol. Environ. Res 2023, 21, 3755–3765. [Google Scholar] [CrossRef]
- Wen, J. Current Situation of Forest Resources in Xining City and Suggestions for Conservation and Utilization. Mod. Agric. Res. 2020, 26, 85–86. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bao, S. Study of Analysis of Soil and Agrochemistry, 3rd ed.; China Agriculture Press: Beijing, China, 2008. [Google Scholar]
- LY/T1215-1999; Forest Soil Moisture-Determination of Physical Properties. State Forestry Administration of People’s Republic of China: Beijing, China; Standards Press of China: Beijing, China, 1999.
- Zhang, Y.; Wu, H.; Liu, J.; Liu, D.; Lin, Y.; Kang, Y.; Guan, Q.; Zhang, Z. Vertical characteristics of soil carbon, nitrogen and phosphorus contents and ecological stoichiometry in the Changbai Mountains. Environ. Ecol. 2023, 5, 7–15+81. [Google Scholar]
- Guo, C.; Sheng, M.; He, Y.; Wang, L.; Shi, Q.; Luo, N. Effects of Land Use Types on Soil Carbon, Nitrogen, Phosphorus Stoichiometric Characteristics and Enzyme Activities in the Karst Area of Southwest China. Chin. J. Soil Sci. 2023, 54, 382–391. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J. Influencing factors of soil carbon and effects of litter on soil carbon storage. Agric. Technol. 2023, 43, 52–55. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, X.; Dang, H.; Ye, C.; Zhang, Y.; Zhang, Q. Linking litter production, quality and decomposition to vegetation succession following agricultural abandonment. Soil Biol. Biochem. 2013, 57, 803–813. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; He, H.; Zhao, W.; Kou, Y. Changes in structure and function of soil prokaryotic communities in subalpine Picea asperata plantations. J. Appl. Ecol. 2023, 34, 3279–3290. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, Q.; Yang, L.; Elrys, A.S.; Sun, J.; Ni, K.; Meng, L.; Zhu, T.; Müller, C. Afforestation increases soil inorganic N supply capacity and lowers plant N limitation in subtropical karst areas. Geoderma 2024, 443, 116848. [Google Scholar] [CrossRef]
- Xie, M.; Yuan, J.; Liu, S.; Xu, G.; Lu, Y.; Yan, L.; Li, G. Soil carbon and nitrogen pools and their storage characteristics under different vegetation restoration types on the Loess Plateau of Longzhong, China. Forests 2024, 15, 173. [Google Scholar] [CrossRef]
- Ou, Y.; Wang, X.; Li, J.; Jia, H.; Zhao, Y.; Huang, Z.; Hong, M. Content and ecological stoichiometry characteristics of soil carbon, nitrogen, and phosphorus in artificial grassland under different restoration years. Chin. J. Appl. Environ. Biol. 2019, 25, 0038–0045. [Google Scholar] [CrossRef]
- Wu, W.; Chen, G.; Meng, T.; Li, C.; Feng, H.; Si, B.; Siddique, K.H.M. Effect of different vegetation restoration on soil properties in the semi-arid Loess Plateau of China. Catena 2023, 220, 106630. [Google Scholar] [CrossRef]
- Pan, J.; Song, N.; Wu, X.; Yang, X.; Chen, L.; Qu, W.; Zhai, D. Effects of different planting-years of artificial Caragana korshinskii shrubland on soil organic carbon, nitrogen and phosphorus stoichiometry characteristics in desert steppe. J. Zhejiang Univ. Agric. Life Sci. 2015, 41, 160–168. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, D.; Zhang, W.; Ren, C.; Hao, W.; Liu, C.; Han, X.; Yang, G. Carbon, nitrogen and phosphorus stoichiometry and homeostasis characteristics of leaves, soil, and microbial biomass of Robinia pseudoacacia forests in the Loess Hilly Region of China. Acta Ecol. Sin. 2019, 39, 5527–5535. [Google Scholar] [CrossRef]
- Wen, W. OC, TN and TP Content and Their Stoichiometric Characteristics in Tree Leaves, Litter and Soil of Four Typical Plantation Tree Species in the Alpine Zone of the Loess Plateau in Qinghai. Master’s Thesis, Beijing Forestry University, Beijing, China, 2023. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, B.; Han, F.; Zhang, X. Ecological Stoichiometric Characteristics of Soils in Robinia pseudoacacia Forests of Different Ages on the Loess Plateau. J. Soil Water Conserv. 2017, 31, 339–344. [Google Scholar] [CrossRef]
- Da, Q.; Cui, D.; Zhang, Y.; Zhao, Y.; Nijatkasim; Liu, S. Ecostoichiometric characteristics of soil carbon, nitrogen and phosphorus in wild apple trees of different ages in Yili, Xinjiang. Ecol. Sci. 2022, 41, 98–104. [Google Scholar] [CrossRef]
- Chadwick, O.A.; Derry, L.A.; Vitousek, P.M.; Huebert, B.J.; Hedin, L.O. Changing sources of nutrients during four million years of ecosystem development. Nature 1999, 397, 491–497. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, G.; Zhang, D.; Liu, S.; Chu, G.; Yan, J. N and P stoichiometry of plant and soil in lower subtropical forest successional series in southern China. Chin. J. Plant Ecol. 2010, 34, 64–71. [Google Scholar] [CrossRef]
- Ma, H.; Xie, M.; Hu, H.; Guo, Y.; Ren, C.; Zhao, F. Effects of stoichiometric characteristics of soil-plant-litter on soil nitrogen components in different forests along an elevational gradient of Qinling Mountains. Chin. J. Ecol. 2020, 39, 749–757. [Google Scholar]
- Gao, H.; Xiao, L.; Niu, D.; Ni, Y.; Huang, Y. Effects of Converting Farmland into Forest and Grassland on Soil Nitrogen Component and Conversion Enzyme Activity in the Mountainous Area of Southern Ningxia. Environ. Sci. 2019, 40, 3825–3832. [Google Scholar]
- Chu, H.; Grogan, P. Soil microbial biomass, nutrient availability and nitrogen mineralization potential among vegetation-types in a low arctic tundra landscape. Plant Soil 2010, 329, 411–420. [Google Scholar] [CrossRef]
- Gundersen, P.; Callesen, I.; de Vriesb, W. Nitrate leaching in forest ecosystems is related to forest floor C/N ratios. Environ. Pollut. 1998, 102, 403–407. [Google Scholar] [CrossRef]
- Rowe, E.C.; Evans, C.D.; Emmett, B.A.; Reynolds, B.; Helliwell, R.C.; Coull, M.C.; Curtis, C.J. Vegetation type affects the relationship between soil carbon to nitrogen ratio and nitrogen leaching. Water Air Soil Pollut. 2006, 177, 335–347. [Google Scholar] [CrossRef]
- Yang, Y.; Dou, Y.; Wang, B.; Xue, Z.; Wang, Y.; An, S.; Chang, S.X. Deciphering factors driving soil microbial life-history strategies in restored grasslands. iMeta 2023, 2, e66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gong, X.; Lü, G.; Zhang, X.; Jiang, L.; Qie, Y. Soil Nitrogen Content and Components Under Different Halophyte Communities in Saline Desert. Soils 2019, 51, 871–878. [Google Scholar]
- Chen, R.; Lin, X. Availability of soil carbon and nitrogen determines excitation effect: Nitrogen extraction theory coexists with microbiometrics theory. In Proceedings of the 7th National Symposium on Soil Biology and Biochemistry, Wuhan, China, 15 May 2014. [Google Scholar]
- Zhang, R.; Yuan, D.; Qin, S.; Hu, C. Effects of carbon, nitrogen, and phosphorus stoichiometry on the priming of soil carbon mineralization. Chin. J. Eco-Agric. 2023, 31, 1311–1321. [Google Scholar] [CrossRef]
- Augusto, L.; Boča, A. Tree functional traits, forest biomass, and tree species diversity interact with site properties to drive forest soil carbon. Nat. Commun. 2022, 13, 1097. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Va’zquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 2707. [Google Scholar] [CrossRef]




| Factor | SOC | STN | STP | SAP | SAN | SNN | C:N | C:P | N:P |
|---|---|---|---|---|---|---|---|---|---|
| Restoration time | 26.486 *** | 27.223 *** | 15.993 *** | 1.284 | 6.206 ** | 22.666 *** | 3.9000 * | 30.487 *** | 28.726 *** |
| Vegetation type | 26.163 *** | 15.411 *** | 1.108 | 0.431 | 2.087 | 23.078 *** | 22.005 *** | 23.359 *** | 11.84 *** |
| Soil depth | 38.792 *** | 32.527 *** | 0.736 | 0.755 | 1.054 | 2.378 * | 14.688 *** | 33.352 *** | 22.704 *** |
| Restoration time × Vegetation type | 26.992 *** | 28.071 *** | 2.129 | 5.185 *** | 11.491 *** | 5.096 *** | 6.095 *** | 22.641 *** | 20.032 *** |
| Restoration time × Soil depth | 2.806 ** | 2.278 * | 0.194 | 1.008 | 1.285 | 0.935 | 0.884 | 2.919 ** | 1.987 * |
| Vegetation type × Soil depth | 1.078 | 0.900 | 0.461 | 0.852 | 0.883 | 0.729 | 0.939 | 0.889 | 0.556 |
| Restoration time × Vegetation type × Soil depth | 1.054 | 0.824 | 0.74 | 0.555 | 0.864 | 0.790 | 0.839 | 0.711 | 0.619 |
| SAP | SAN | SNN | BD | PS | pH | |
|---|---|---|---|---|---|---|
| Soil C:N | −0.103 | 0.157 * | 0.474 *** | −0.425 *** | 0.274 *** | −0.152 * |
| Soil C:P | −0.063 | 0.227 ** | 0.486 *** | −0.539 *** | 0.339 *** | −0.217 ** |
| Soil N:P | −0.085 | 0.218 ** | 0.406 *** | −0.527 *** | 0.333 *** | −0.229 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Xu, X.; Wen, Y.; Cheng, M.; Wang, X. The Critical Role of Soil Ecological Stoichiometric Ratios: How Does Reforestation Improve Soil Nitrogen and Phosphorus Availability? Plants 2024, 13, 2320. https://doi.org/10.3390/plants13162320
Chen Z, Xu X, Wen Y, Cheng M, Wang X. The Critical Role of Soil Ecological Stoichiometric Ratios: How Does Reforestation Improve Soil Nitrogen and Phosphorus Availability? Plants. 2024; 13(16):2320. https://doi.org/10.3390/plants13162320
Chicago/Turabian StyleChen, Zhixuan, Xia Xu, Yongli Wen, Man Cheng, and Xiao Wang. 2024. "The Critical Role of Soil Ecological Stoichiometric Ratios: How Does Reforestation Improve Soil Nitrogen and Phosphorus Availability?" Plants 13, no. 16: 2320. https://doi.org/10.3390/plants13162320




