Profiling and Improvement of Grain Quality Traits for Consumer Preferable Basmati Rice in the United States
Abstract
:1. Introduction
2. Consumer Preferences for Grain Quality Traits in Basmati Rice
3. Influencing Factors for Grain Quality Traits in Basmati Rice
3.1. Genes Related to Grain Quality Traits in Rice and Basmati Rice
3.2. Compounds Related to Grain Quality Traits in Basmati Rice
Regulation of Compounds Related to Grain Quality Traits in Basmati Rice
3.3. Environmental Factors Related to Grain Quality Traits in Basmati Rice
3.3.1. Temperature
3.3.2. Humidity
3.3.3. Light
3.3.4. Other Factors Influencing Grain Quality Traits in Basmati Rice
4. Breeding for High-Quality Basmati-Type Rice in the USA
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hori, K.; Sun, J. Rice grain size and quality. Rice 2022, 15, 33. [Google Scholar] [PubMed]
- Cheng, J.; Lin, X.; Long, Y.; Zeng, Q.; Zhao, K.; Hu, P.; Peng, J. Rice grain quality: Where we are and where to go? Adv. Agron. 2022, 172, 211–252. [Google Scholar]
- Rao, Y.; Li, Y.; Qian, Q. Recent progress on molecular breeding of rice in China. Plant Cell Rep. 2014, 33, 551–564. [Google Scholar]
- Gong, D.; Zhang, X.; He, F.; Chen, Y.; Li, R.; Yao, J.; Zhang, M.; Zheng, W.; Yu, G. Genetic improvements in rice grain quality: A review of elite genes and their applications in molecular breeding. Agronomy 2023, 13, 1375. [Google Scholar] [CrossRef]
- Cuevas, R.P.; de Guia, A.; Demont, M. Developing a framework of gastronomic systems research to unravel drivers of food choice. Int. J. Gastron. Food Sci. 2017, 9, 88–99. [Google Scholar]
- Liu, Q.; Chen, S.; Zhou, L.; Tao, Y.; Tian, J.; Xing, Z.; Wei, H.; Zhang, H. Characteristics of population quality and rice quality of semi-waxy japonica rice varieties with different grain yields. Agriculture 2022, 12, 241. [Google Scholar] [CrossRef]
- Demont, M.; Ndour, M. Upgrading rice value chains: Experimental evidence from 11 African markets. Glob. Food Secur. 2015, 5, 70–76. [Google Scholar]
- Custodio, M.C.; Cuevas, R.P.; Ynion, J.; Laborte, A.G.; Velasco, M.L.; Demont, M. Rice quality: How is it defined by consumers, industry, food scientists, and geneticists? Trends Food Sci. Technol. 2019, 92, 122–137. [Google Scholar] [PubMed]
- Chen, Y.; Wang, M.; Ouwerkerk, P.B. Molecular and environmental factors determining grain quality in rice. Food Energy Secur. 2012, 1, 111–132. [Google Scholar]
- Bao, J. Genes and QTLs for Rice Grain Quality Improvement; BoD—Books on Demand: Norderstedt, Germany, 2014; pp. 239–278. [Google Scholar]
- Li, Y.; Yang, Z.; Yang, C.; Liu, Z.; Shen, S.; Zhan, C.; Lyu, Y.; Zhang, F.; Li, K.; Shi, Y. The NET locus determines the food taste, cooking and nutrition quality of rice. Sci. Bull. 2022, 67, 2045–2049. [Google Scholar]
- Mouritsen, O.G.; Styrbæk, K. Mouthfeel: How Texture Makes Taste; Columbia University Press: New York, NY, USA; Chichester, UK, 2017. [Google Scholar]
- Samantara, K.; Reyes, V.P.; Mondal, K.; Raigar, O.P.; Priyadarshini, P.; Wani, S.H. Genetic improvement of rice grain quality. In QTL Mapping in Crop Improvement; Elsevier: Amsterdam, The Netherlands, 2023; pp. 235–256. [Google Scholar]
- Singh, R.; Singh, U.; Khush, G.; Rohilla, R.; Singh, J.; Singh, G.; Shekhar, K. Small and medium grained aromatic rices of India. In Aromatic Rices; Singh, R., Singh, U., Khush, G., Eds.; International Rice Research Institute: Oxford, UK; IBH: New Delhi, India, 2000; pp. 155–177. [Google Scholar]
- Arikit, S. Rapid Identification of Quantitative Trait Loci (QTL) and Development of DNA Markers for Grain Elongation in Rice (Oryza sativa) towards the Breeding for Basmati-Type Varieties for Non-Jasmine Rice Market; Thailand Science Research and Innovation: Bangkok, Thailand, 2018. [Google Scholar]
- Suwannaporn, P.; Linnemann, A. Rice-eating quality among consumers in different rice grain preference countries. J. Sens. Stud. 2008, 23, 1–13. [Google Scholar]
- Childs, N. Rice Outlook; US Department of Agriculture: Washington, DC, USA, 2017; Volume 22. [Google Scholar]
- Suwannaporn, P.; Linnemann, A.; Chaveesuk, R. Consumer preference mapping for rice product concepts. Br. Food J. 2008, 110, 595–606. [Google Scholar]
- Childs, N.; LeBeau, B. Rice Outlook: September 2022; US Department of Agriculture: Washington, DC, USA, 2022; Volume 23. [Google Scholar]
- Childs, N.W.; Livezey, J. Rice Backgrounder; US Department of Agriculture, Economic Research Service: Washington, DC, USA, 2006. [Google Scholar]
- Calingacion, M.; Laborte, A.; Nelson, A.; Resurreccion, A.; Concepcion, J.C.; Daygon, V.D.; Mumm, R.; Reinke, R.; Dipti, S.; Bassinello, P.Z. Diversity of global rice markets and the science required for consumer-targeted rice breeding. PLoS ONE 2014, 9, e85106. [Google Scholar]
- Zhao, X.; Fitzgerald, M. Climate change: Implications for the yield of edible rice. PLoS ONE 2013, 8, e66218. [Google Scholar]
- Fitzgerald, M.; Sackville, H.I.N.; Calingacion, M. Is there a second gene for fragrance in rice? Plant Biotechnol. J. 2008, 6, 416–423. [Google Scholar]
- Shi, W.; Yang, Y.; Chen, S.; Xu, M. Discovery of a new fragrance allele and the development of functional markers for the breeding of fragrant rice varieties. Mol. Breed. 2008, 22, 185–192. [Google Scholar]
- Fitzgerald, M.A.; McCouch, S.R.; Hall, R.D. Not just a grain of rice: The quest for quality. Trends Plant Sci. 2009, 14, 133–139. [Google Scholar] [PubMed]
- Supakornchuwong, C.; Suwannaporn, P. Attitudes toward rice compared with potatoes and pasta among british, french, dutch and belgian consumers. J. Sens. Stud. 2012, 27, 71–77. [Google Scholar]
- Hardke, J.; Sha, X.; Bateman, N. BR Wells Arkansas Rice Research Studies 2021; University of Arkansas: Fayetteville, AR, USA, 2022. [Google Scholar]
- Vashista, A. United States Basmati Rice Market Size, Growth, Forecast 2024–2032; Expert Market Research (EMR Inc.): Sheridan, WY, USA, 2023. [Google Scholar]
- TatahMentan, M.; Nyachoti, S.; Scott, L.; Phan, N.; Okwori, F.O.; Felemban, N.; Godebo, T.R. Toxic and essential elements in rice and other grains from the United States and other countries. Int. J. Environ. Res. Public Health 2020, 17, 8128. [Google Scholar] [CrossRef]
- Malabadi, R.B.; Kolkar, K.; Chalannavar, R. White, and Brown rice-Nutritional value and Health benefits: Arsenic Toxicity in Rice plants. Int. J. Innov. Sci. Res. Rev. 2022, 4, 3065–3082. [Google Scholar]
- Schenker, S. An overview of the role of rice in the UK diet. Nutr. Bull. 2012, 37, 309–323. [Google Scholar]
- Gunathilaka, M.; Ekanayake, S. Effect of Different Cooking Methods on Glycaemic Index of Indian and Pakistani Basmati Rice Varieties. 2015. Available online: http://dr.lib.sjp.ac.lk/handle/123456789/6631 (accessed on 1 June 2024).
- Srinivasa, D.; Raman, A.; Meena, P.; Chitale, G.; Marwaha, A.; Jainani, K.J. Glycaemic index (GI) of an Indian branded thermally treated basmati rice variety: A multi centric study. J. Assoc. Phys. India 2013, 61, 716–720. [Google Scholar]
- Siddiq, F.; Muralidharan, K.; Shobha, R. Basmati rice. In Directorate of Rice Research; IARI: New Delhi, India, 1997; pp. 1–14. [Google Scholar]
- Bhattacharjee, P.; Singhal, R.S.; Kulkarni, P.R. Basmati rice: A review. Int. J. Food Sci. Technol. 2002, 37, 1–12. [Google Scholar]
- Sodhi, N.S.; Singh, N.; Arora, M.; Singh, J. Changes in physico-chemical, thermal, cooking and textural properties of rice during aging. J. Food Process. Preserv. 2003, 27, 387–400. [Google Scholar]
- Faruq, G.; Prodhan, Z.H.; Nezhadahmadi, A. Effects of ageing on selected cooking quality parameters of rice. Int. J. Food Prop. 2015, 18, 922–933. [Google Scholar]
- Thakare, S.S.; Awana, M.; Warwate, S.I.; Mandal, S.; Rudra, S.G.; Bollinedi, H.; Ray, M.; Solanke, A.U.; Nair, L.S.; Kumar, S. Dynamics of physico-chemical properties towards understanding the optimum ageing of basmati and non-basmati rice (Oryza sativa L.) for consumer preferences. J. Cereal Sci. 2023, 112, 103714. [Google Scholar]
- Dutta, C.; Nath, D.J.; Phyllei, D. Aromatic rice and factors affecting aroma in rice. Int. J. Environ. Clim. Chang. 2022, 12, 1773–1779. [Google Scholar]
- Butardo, V.M.; Sreenivasulu, N.; Juliano, B.O. Improving rice grain quality: State-of-the-art and future prospects. In Rice Grain Quality: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2019; pp. 19–55. [Google Scholar]
- Brooker, D.B.; Bakker-Arkema, F.W.; Hall, C.W. Drying and Storage of Grains and Oilseeds; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1992; p. 450. [Google Scholar]
- Butt, M.; Anjum, F.; Salim-ur-Rehman; Tahir-Nadeem, M.; Sharif, M.; Anwer, M. Selected quality attributes of fine basmati rice: Effect of storage history and varieties. Int. J. Food Prop. 2008, 11, 698–711. [Google Scholar]
- Faruq, G.; Khalid, N.; Jennifer, A.; Subha, B.; Zulqarnain, M.; Osman, M.; Nazia, A.; Mohammad, O. Evaluation of kernel elongation ratio and aroma association in global popular aromatic rice cultivars in tropical environment. Afr. J. Agric. Res. 2010, 5, 1515–1522. [Google Scholar]
- Sood, B.; Siddiq, E. A rapid technique for scent determination in rice. Indian J. Genet. Plant Breed. 1978, 38, 268–275. [Google Scholar]
- Lu, L.; Shao, D.; Qiu, X.; Sun, L.; Yan, W.; Zhou, X.; Yang, L.; He, Y.; Yu, S.; Xing, Y. Natural variation and artificial selection in four genes determine grain shape in rice. New Phytol. 2013, 200, 1269–1280. [Google Scholar] [PubMed]
- Wang, S.; Wu, K.; Yuan, Q.; Liu, X.; Liu, Z.; Lin, X.; Zeng, R.; Zhu, H.; Dong, G.; Qian, Q. Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 2012, 44, 950–954. [Google Scholar] [PubMed]
- Zhang, W.; Sun, P.; He, Q.; Shu, F.; Wang, J.; Deng, H. Fine mapping of GS2, a dominant gene for big grain rice. Crop J. 2013, 1, 160–165. [Google Scholar]
- Hu, J.; Wang, Y.; Fang, Y.; Zeng, L.; Xu, J.; Yu, H.; Shi, Z.; Pan, J.; Zhang, D.; Kang, S. A rare allele of GS2 enhances grain size and grain yield in rice. Mol. Plant 2015, 8, 1455–1465. [Google Scholar]
- Li, Y.; Fan, C.; Xing, Y.; Yun, P.; Luo, L.; Yan, B.; Peng, B.; Xie, W.; Wang, G.; Li, X. Chalk5 encodes a vacuolar H+-translocating pyrophosphatase influencing grain chalkiness in rice. Nat. Genet. 2014, 46, 398–404. [Google Scholar]
- Wu, B.; Yun, P.; Zhou, H.; Xia, D.; Gu, Y.; Li, P.; Yao, J.; Zhou, Z.; Chen, J.; Liu, R. Natural variation in WHITE-CORE RATE 1 regulates redox homeostasis in rice endosperm to affect grain quality. Plant Cell 2022, 34, 1912–1932. [Google Scholar]
- Guo, T.; Liu, X.; Wan, X.; Weng, J.; Liu, S.; Liu, X.; Chen, M.; Li, J.; Su, N.; Wu, F. Identification of a stable quantitative trait locus for percentage grains with white chalkiness in rice (Oryza sativa). J. Integr. Plant Biol. 2011, 53, 598–607. [Google Scholar]
- Qiu, X.; Yang, J.; Zhang, F.; Niu, Y.; Zhao, X.; Shen, C.; Chen, K.; Teng, S.; Xu, J. Genetic dissection of rice appearance quality and cooked rice elongation by genome-wide association study. Crop J. 2021, 9, 1470–1480. [Google Scholar]
- Hao, Y.; Wang, Y.; Wu, M.; Zhu, X.; Teng, X.; Sun, Y.; Zhu, J.; Zhang, Y.; Jing, R.; Lei, J. The nuclear-localized PPR protein OsNPPR1 is important for mitochondrial function and endosperm development in rice. J. Exp. Bot. 2019, 70, 4705–4720. [Google Scholar] [PubMed]
- Zhao, D.-S.; Li, Q.-F.; Zhang, C.-Q.; Zhang, C.; Yang, Q.-Q.; Pan, L.-X.; Ren, X.-Y.; Lu, J.; Gu, M.-H.; Liu, Q.-Q. GS9 acts as a transcriptional activator to regulate rice grain shape and appearance quality. Nat. Commun. 2018, 9, 1240. [Google Scholar]
- Li, Y.; Fan, C.; Xing, Y.; Jiang, Y.; Luo, L.; Sun, L.; Shao, D.; Xu, C.; Li, X.; Xiao, J. Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat. Genet. 2011, 43, 1266–1269. [Google Scholar] [PubMed]
- Huang, R.; Jiang, L.; Zheng, J.; Wang, T.; Wang, H.; Huang, Y.; Hong, Z. Genetic bases of rice grain shape: So many genes, so little known. Trends Plant Sci. 2013, 18, 218–226. [Google Scholar] [PubMed]
- Jiang, L.; Zhong, H.; Jiang, X.; Zhang, J.; Huang, R.; Liao, F.; Deng, Y.; Tao, Y.; Zheng, J. Identification and pleiotropic effect analysis of GSE5 on rice chalkiness and grain shape. Front. Plant Sci. 2022, 12, 814928. [Google Scholar]
- Liu, Q.; Han, R.; Wu, K.; Zhang, J.; Ye, Y.; Wang, S.; Chen, J.; Pan, Y.; Li, Q.; Xu, X. G-protein βγ subunits determine grain size through interaction with MADS-domain transcription factors in rice. Nat. Commun. 2018, 9, 852. [Google Scholar]
- Huang, X.; Qian, Q.; Liu, Z.; Sun, H.; He, S.; Luo, D.; Xia, G.; Chu, C.; Li, J.; Fu, X. Natural variation at the DEP1 locus enhances grain yield in rice. Nat. Genet. 2009, 41, 494–497. [Google Scholar] [PubMed]
- Zhang, X.; Wang, J.; Huang, J.; Lan, H.; Wang, C.; Yin, C.; Wu, Y.; Tang, H.; Qian, Q.; Li, J. Rare allele of OsPPKL1 associated with grain length causes extra-large grain and a significant yield increase in rice. Proc. Natl. Acad. Sci. USA 2012, 109, 21534–21539. [Google Scholar]
- Wang, S.; Li, S.; Liu, Q.; Wu, K.; Zhang, J.; Wang, S.; Wang, Y.; Chen, X.; Zhang, Y.; Gao, C. The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat. Genet. 2015, 47, 949–954. [Google Scholar]
- Nan, J.; Feng, X.; Wang, C.; Zhang, X.; Wang, R.; Liu, J.; Yuan, Q.; Jiang, G.; Lin, S. Improving rice grain length through updating the GS3 locus of an elite variety Kongyu 131. Rice 2018, 11, 1–9. [Google Scholar]
- Song, X.-J.; Huang, W.; Shi, M.; Zhu, M.-Z.; Lin, H.-X. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat. Genet. 2007, 39, 623–630. [Google Scholar]
- Miura, K.; Ashikari, M.; Matsuoka, M. The role of QTLs in the breeding of high-yielding rice. Trends Plant Sci. 2011, 16, 319–326. [Google Scholar]
- Weng, J.; Gu, S.; Wan, X.; Gao, H.; Guo, T.; Su, N.; Lei, C.; Zhang, X.; Cheng, Z.; Guo, X. Isolation and initial characterization of GW5, a major QTL associated with rice grain width and weight. Cell Res. 2008, 18, 1199–1209. [Google Scholar] [PubMed]
- Shomura, A.; Izawa, T.; Ebana, K.; Ebitani, T.; Kanegae, H.; Konishi, S.; Yano, M. Deletion in a gene associated with grain size increased yields during rice domestication. Nat. Genet. 2008, 40, 1023–1028. [Google Scholar] [PubMed]
- Wang, C.; Zhang, Y.; Zhu, Z.; Chen, T.; Zhao, L.; Lin, J.; Zhou, L. Development of a new japonica rice variety Nan-jing 46 with good eating quality by marker assisted selection. Rice Genom. Genet. 2010, 1. [Google Scholar] [CrossRef]
- Zhou, P.; Tan, Y.; He, Y.; Xu, C.; Zhang, Q. Simultaneous improvement for four quality traits of Zhenshan 97, an elite parent of hybrid rice, by molecular marker-assisted selection. Theor. Appl. Genet. 2003, 106, 326–331. [Google Scholar] [PubMed]
- Ni, D.; Zhang, S.; Chen, S.; Xu, Y.; Li, L.; Li, H.; Wang, Z.; Cai, X.; Li, Z.; Yang, J. Improving cooking and eating quality of Xieyou57, an elite indica hybrid rice, by marker-assisted selection of the Wx locus. Euphytica 2011, 179, 355–362. [Google Scholar]
- Zhang, C.; Yang, Y.; Chen, S.; Liu, X.; Zhu, J.; Zhou, L.; Lu, Y.; Li, Q.; Fan, X.; Tang, S. A rare Waxy allele coordinately improves rice eating and cooking quality and grain transparency. J. Integr. Plant Biol. 2021, 63, 889–901. [Google Scholar] [PubMed]
- Jin, L.; Lu, Y.; Shao, Y.; Zhang, G.; Xiao, P.; Shen, S.; Corke, H.; Bao, J. Molecular marker assisted selection for improvement of the eating, cooking and sensory quality of rice (Oryza sativa L.). J. Cereal Sci. 2010, 51, 159–164. [Google Scholar]
- Tan, Y.; Li, J.; Yu, S.; Xing, Y.; Xu, C.; Zhang, Q. The three important traits for cooking and eating quality of rice grains are controlled by a single locus in an elite rice hybrid, Shanyou 63. Theor. Appl. Genet. 1999, 99, 642–648. [Google Scholar] [PubMed]
- Sattari, A.; Mahdinezhad, N.; Fakheri, B.; Noroozi, M.; Beheshtizadeh, H. Improvement of the eating and cooking qualities of rice: A review. Int. J. Farming Allied Sci. 2015, 4, 153–160. [Google Scholar]
- Ashokkumar, S.; Jaganathan, D.; Ramanathan, V.; Rahman, H.; Palaniswamy, R.; Kambale, R.; Muthurajan, R. Creation of novel alleles of fragrance gene OsBADH2 in rice through CRISPR/Cas9 mediated gene editing. PLoS ONE 2020, 15, e0237018. [Google Scholar]
- Hinge, V.R.; Patil, H.B.; Nadaf, A.B. Aroma volatile analyses and 2AP characterization at various developmental stages in Basmati and Non-Basmati scented rice (Oryza sativa L.) cultivars. Rice 2016, 9, 1–22. [Google Scholar]
- Pradhan, S.; Pandit, E.; Pawar, S.; Bharati, B.; Chatopadhyay, K.; Singh, S.; Dash, P.; Reddy, J. Association mapping reveals multiple QTLs for grain protein content in rice useful for biofortification. Mol. Genet. Genom. 2019, 294, 963–983. [Google Scholar]
- Yang, Y.; Guo, M.; Sun, S.; Zou, Y.; Yin, S.; Liu, Y.; Tang, S.; Gu, M.; Yang, Z.; Yan, C. Natural variation of OsGluA2 is involved in grain protein content regulation in rice. Nat. Commun. 2019, 10, 1949. [Google Scholar] [PubMed]
- Ahn, S.; Bollich, C.; Tanksley, S. RFLP tagging of a gene for aroma in rice. Theor. Appl. Genet. 1992, 84, 825–828. [Google Scholar] [PubMed]
- Bradbury, L.M.; Fitzgerald, T.L.; Henry, R.J.; Jin, Q.; Waters, D.L. The gene for fragrance in rice. Plant Biotechnol. J. 2005, 3, 363–370. [Google Scholar] [PubMed]
- Amarawathi, Y.; Singh, R.; Singh, A.K.; Singh, V.P.; Mohapatra, T.; Sharma, T.R.; Singh, N.K. Mapping of quantitative trait loci for basmati quality traits in rice (Oryza sativa L.). Mol. Breed. 2008, 21, 49–65. [Google Scholar]
- Fan, C.; Xing, Y.; Mao, H.; Lu, T.; Han, B.; Xu, C.; Li, X.; Zhang, Q. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 2006, 112, 1164–1171. [Google Scholar] [PubMed]
- Takano-Kai, N.; Jiang, H.; Kubo, T.; Sweeney, M.; Matsumoto, T.; Kanamori, H.; Padhukasahasram, B.; Bustamante, C.; Yoshimura, A.; Doi, K. Evolutionary history of GS3, a gene conferring grain length in rice. Genetics 2009, 182, 1323–1334. [Google Scholar]
- Qi, P.; Lin, Y.-S.; Song, X.-J.; Shen, J.-B.; Huang, W.; Shan, J.-X.; Zhu, M.-Z.; Jiang, L.; Gao, J.-P.; Lin, H.-X. The novel quantitative trait locus GL3.1 controls rice grain size and yield by regulating Cyclin-T1; 3. Cell Res. 2012, 22, 1666–1680. [Google Scholar]
- Duan, P.; Xu, J.; Zeng, D.; Zhang, B.; Geng, M.; Zhang, G.; Huang, K.; Huang, L.; Xu, R.; Ge, S. Natural variation in the promoter of GSE5 contributes to grain size diversity in rice. Mol. Plant 2017, 10, 685–694. [Google Scholar]
- Zhao, D.-S.; Liu, J.-Y.; Ding, A.-Q.; Zhang, T.; Ren, X.-Y.; Zhang, L.; Li, Q.-F.; Fan, X.-L.; Zhang, C.-Q.; Liu, Q.-Q. Improving grain appearance of erect-panicle japonica rice cultivars by introgression of the null gs9 allele. J. Integr. Agric. 2021, 20, 2032–2042. [Google Scholar]
- Lisle, A.; Martin, M.; Fitzgerald, M. Chalky and translucent rice grains differ in starch composition and structure and cooking properties. Cereal Chem. 2000, 77, 627–632. [Google Scholar]
- Wang, J.; Chen, Z.; Zhang, Q.; Meng, S.; Wei, C. The NAC transcription factors OsNAC20 and OsNAC26 regulate starch and storage protein synthesis. Plant Physiol. 2020, 184, 1775–1791. [Google Scholar] [PubMed]
- Morrison, W.R. Lipids in cereal starches: A review. J. Cereal Sci. 1988, 8, 1–15. [Google Scholar]
- Duan, M.; Sun, S.S. Profiling the expression of genes controlling rice grain quality. Plant Mol. Biol. 2005, 59, 165–178. [Google Scholar]
- Zhang, C.; Chen, S.; Ren, X.; Lu, Y.; Liu, D.; Cai, X.; Li, Q.; Gao, J.; Liu, Q. Molecular structure and physicochemical properties of starches from rice with different amylose contents resulting from modification of OsGBSSI activity. J. Agric. Food Chem. 2017, 65, 2222–2232. [Google Scholar] [PubMed]
- Li, X.; Wu, L.; Geng, X.; Xia, X.; Wang, X.; Xu, Z.; Xu, Q. Deciphering the environmental impacts on rice quality for different rice cultivated areas. Rice 2018, 11, 1–10. [Google Scholar]
- Jeon, J.-S.; Ryoo, N.; Hahn, T.-R.; Walia, H.; Nakamura, Y. Starch biosynthesis in cereal endosperm. Plant Physiol. Biochem. 2010, 48, 383–392. [Google Scholar]
- Pfister, B.; Zeeman, S.C. Formation of starch in plant cells. Cell. Mol. Life Sci. 2016, 73, 2781–2807. [Google Scholar]
- Zhou, H.; Xia, D.; Li, P.; Ao, Y.; Xu, X.; Wan, S.; Li, Y.; Wu, B.; Shi, H.; Wang, K. Genetic architecture and key genes controlling the diversity of oil composition in rice grains. Mol. Plant 2021, 14, 456–469. [Google Scholar] [PubMed]
- Zhang, C.; Zhu, J.; Chen, S.; Fan, X.; Li, Q.; Lu, Y.; Wang, M.; Yu, H.; Yi, C.; Tang, S. Wxlv, the ancestral allele of rice Waxy gene. Mol. Plant 2019, 12, 1157–1166. [Google Scholar] [PubMed]
- Umemoto, T.; Yano, M.; Satoh, H.; Shomura, A.; Nakamura, Y. Mapping of a gene responsible for the difference in amylopectin structure between japonica-type and indica-type rice varieties. Theor. Appl. Genet. 2002, 104, 1–8. [Google Scholar] [PubMed]
- Zhou, H.; Wang, L.; Liu, G.; Meng, X.; Jing, Y.; Shu, X.; Kong, X.; Sun, J.; Yu, H.; Smith, S.M. Critical roles of soluble starch synthase SSIIIa and granule-bound starch synthase Waxy in synthesizing resistant starch in rice. Proc. Natl. Acad. Sci. USA 2016, 113, 12844–12849. [Google Scholar] [PubMed]
- Sun, X.; Ling, S.; Lu, Z.; Ouyang, Y.-d.; Liu, S.; Yao, J. OsNF-YB1, a rice endosperm-specific gene, is essential for cell proliferation in endosperm development. Gene 2014, 551, 214–221. [Google Scholar] [PubMed]
- Bello, B.K.; Hou, Y.; Zhao, J.; Jiao, G.; Wu, Y.; Li, Z.; Wang, Y.; Tong, X.; Wang, W.; Yuan, W. NF-YB 1-YC 12-bHLH 144 complex directly activates Wx to regulate grain quality in rice (Oryza sativa L.). Plant Biotechnol. J. 2019, 17, 1222–1235. [Google Scholar]
- Xu, X.; E, Z.; Zhang, D.; Yun, Q.; Zhou, Y.; Niu, B.; Chen, C. OsYUC11-mediated auxin biosynthesis is essential for endosperm development of rice. Plant Physiol. 2021, 185, 934–950. [Google Scholar]
- Zhang, J.; Nallamilli, B.R.; Mujahid, H.; Peng, Z. OsMADS6 plays an essential role in endosperm nutrient accumulation and is subject to epigenetic regulation in rice (Oryza sativa). Plant J. 2010, 64, 604–617. [Google Scholar] [PubMed]
- Peng, C.; Wang, Y.; Liu, F.; Ren, Y.; Zhou, K.; Lv, J.; Zheng, M.; Zhao, S.; Zhang, L.; Wang, C. FLOURY ENDOSPERM 6 encodes a CBM 48 domain-containing protein involved in compound granule formation and starch synthesis in rice endosperm. Plant J. 2014, 77, 917–930. [Google Scholar]
- Wu, M.; Ren, Y.; Cai, M.; Wang, Y.; Zhu, S.; Zhu, J.; Hao, Y.; Teng, X.; Zhu, X.; Jing, R. Rice FLOURY ENDOSPERM 10 encodes a pentatricopeptide repeat protein that is essential for the trans-splicing of mitochondrial nad1 intron 1 and endosperm development. New Phytol. 2019, 223, 736–750. [Google Scholar]
- Xue, M.; Liu, L.; Yu, Y.; Zhu, J.; Gao, H.; Wang, Y.; Wan, J. Lose-of-function of a rice nucleolus-localized pentatricopeptide repeat protein is responsible for the floury endosperm14 mutant phenotypes. Rice 2019, 12, 1–15. [Google Scholar]
- Yu, M.; Wu, M.; Ren, Y.; Wang, Y.; Li, J.; Lei, C.; Sun, Y.; Bao, X.; Wu, H.; Yang, H. Rice FLOURY ENDOSPERM 18 encodes a pentatricopeptide repeat protein required for 5′ processing of mitochondrial nad5 messenger RNA and endosperm development. J. Integr. Plant Biol. 2021, 63, 834–847. [Google Scholar] [PubMed]
- Cai, Y.; Li, S.; Jiao, G.; Sheng, Z.; Wu, Y.; Shao, G.; Xie, L.; Peng, C.; Xu, J.; Tang, S. OsPK2 encodes a plastidic pyruvate kinase involved in rice endosperm starch synthesis, compound granule formation and grain filling. Plant Biotechnol. J. 2018, 16, 1878–1891. [Google Scholar] [PubMed]
- Zhou, L.; Liu, Q.; Zhang, C.; Xu, Y.; Tang, S.; Gu, M. Variation and distribution of seed storage protein content and composition among different rice varieties. Acta Agron. Sin. 2009, 35, 884–891. [Google Scholar]
- Xinkang, L.; Chunmin, G.; Lin, W.; Liting, J.; Xiangjin, F.; Qinlu, L.; Zhengyu, H.; Chun, L. Rice storage proteins: Focus on composition, distribution, genetic improvement and effects on rice quality. Rice Sci. 2023, 30, 207–221. [Google Scholar]
- Peng, B.; Kong, H.; Li, Y.; Wang, L.; Zhong, M.; Sun, L.; Gao, G.; Zhang, Q.; Luo, L.; Wang, G. OsAAP6 functions as an important regulator of grain protein content and nutritional quality in rice. Nat. Commun. 2014, 5, 4847. [Google Scholar]
- Li, P.; Chen, Y.-H.; Lu, J.; Zhang, C.-Q.; Liu, Q.-Q.; Li, Q.-F. Genes and their molecular functions determining seed structure, components, and quality of rice. Rice 2022, 15, 18. [Google Scholar]
- Hong, J.; Rosental, L.; Xu, Y.; Xu, D.; Orf, I.; Wang, W.; Hu, Z.; Su, S.; Bai, S.; Ashraf, M. Genetic architecture of seed glycerolipids in Asian cultivated rice. Plant Cell Environ. 2023, 46, 1278–1294. [Google Scholar]
- Liu, H.L.; Yin, Z.J.; Xiao, L.; Xu, Y.N.; Qu, L.Q. Identification and evaluation of ω-3 fatty acid desaturase genes for hyperfortifying α-linolenic acid in transgenic rice seed. J. Exp. Bot. 2012, 63, 3279–3287. [Google Scholar]
- Shi, J.; Cao, Y.; Fan, X.; Li, M.; Wang, Y.; Ming, F. A rice microsomal delta-12 fatty acid desaturase can enhance resistance to cold stress in yeast and Oryza sativa. Mol. Breed. 2012, 29, 743–757. [Google Scholar]
- Ding, W.; Lin, L.; Zhang, B.; Xiang, X.; Wu, J.; Pan, Z.; Zhu, S. OsKASI, a β-ketoacyl-[acyl carrier protein] synthase I, is involved in root development in rice (Oryza sativa L.). Planta 2015, 242, 203–213. [Google Scholar]
- Cho, D.-H.; Lim, S.-T. Germinated brown rice and its bio-functional compounds. Food Chem. 2016, 196, 259–271. [Google Scholar] [PubMed]
- Long, Q.; Zhang, W.; Wang, P.; Shen, W.; Zhou, T.; Liu, N.; Wang, R.; Jiang, L.; Huang, J.; Wang, Y. Molecular genetic characterization of rice seed lipoxygenase 3 and assessment of its effects on seed longevity. J. Plant Biol. 2013, 56, 232–242. [Google Scholar]
- Huang, J.; Cai, M.; Long, Q.; Liu, L.; Lin, Q.; Jiang, L.; Chen, S.; Wan, J. OsLOX2, a rice type I lipoxygenase, confers opposite effects on seed germination and longevity. Transgenic Res. 2014, 23, 643–655. [Google Scholar]
- Zhou, G.; Ren, N.; Qi, J.; Lu, J.; Xiang, C.; Ju, H.; Cheng, J.; Lou, Y. The 9-lipoxygenase Osr9-LOX1 interacts with the 13-lipoxygenase-mediated pathway to regulate resistance to chewing and piercing-sucking herbivores in rice. Physiol. Plant. 2014, 152, 59–69. [Google Scholar]
- Khan, M.S.S.; Basnet, R.; Islam, S.A.; Shu, Q. Mutational analysis of OsPLDα1 reveals its involvement in phytic acid biosynthesis in rice grains. J. Agric. Food Chem. 2019, 67, 11436–11443. [Google Scholar]
- Kovach, M.J.; Calingacion, M.N.; Fitzgerald, M.A.; McCouch, S.R. The origin and evolution of fragrance in rice (Oryza sativa L.). Proc. Natl. Acad. Sci. USA 2009, 106, 14444–14449. [Google Scholar]
- Chen, S.; Yang, Y.; Shi, W.; Ji, Q.; He, F.; Zhang, Z.; Cheng, Z.; Liu, X.; Xu, M. Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell 2008, 20, 1850–1861. [Google Scholar]
- Hashemi, F.G.; Rafii, M.; Ismail, M.; Mahmud, T.; Rahim, H.; Asfaliza, R.; Malek, M.; Latif, M. Biochemical, genetic and molecular advances of fragrance characteristics in rice. Crit. Rev. Plant Sci. 2013, 32, 445–457. [Google Scholar]
- Wakte, K.; Zanan, R.; Hinge, V.; Khandagale, K.; Nadaf, A.; Henry, R. Thirty-three years of 2-acetyl-1-pyrroline, a principal basmati aroma compound in scented rice (Oryza sativa L.): A status review. J. Sci. Food Agric. 2017, 97, 384–395. [Google Scholar] [PubMed]
- Buttery, R.G.; Ling, L.C.; Juliano, B.O.; Turnbaugh, J.G. Cooked rice aroma and 2-acetyl-1-pyrroline. J. Agric. Food Chem. 1983, 31, 823–826. [Google Scholar]
- Paule, C.M.; Powers, J. Sensory and chemical examination of aromatic and nonaromatic rices. J. Food Sci. 1989, 54, 343–346. [Google Scholar]
- Roy, S.; Banerjee, A.; Basak, N.; Kumar, J.; Mandal, N. The future of rice demand: Quality beyond productivity. In Aromatic Rice; Costa de Oliveira, A., Pegoraro, C., Ebeling Viana, V., Eds.; Springer: Cham, Switzerland, 2020; pp. 251–282. [Google Scholar]
- Buttery, R.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar]
- Jezussek, M.; Juliano, B.O.; Schieberle, P. Comparison of key aroma compounds in cooked brown rice varieties based on aroma extract dilution analyses. J. Agric. Food Chem. 2002, 50, 1101–1105. [Google Scholar]
- Hussain, A.; Mujtaba Naqvi, S.; Hammerschmidt, F. Isolation and identification of volatile components from basmati rice (Oryza sativa L.). In Proceedings of the Flavour Science and Technology: Proceedings of the 5th Weurman Flavour Research Symposium, Sara Hotel, Voksenasen, Oslo, Norway, 23–25 March 1987. [Google Scholar]
- Petrov, M.; Danzart, M.; Giampaoli, P.; Faure, J.; Richard, H. Rice aroma analysis: Discrimination between a scented and a non-scented rice. Sci. Des Aliment. 1996, 16, 347–360. [Google Scholar]
- Widjaja, R.; Craske, J.D.; Wootton, M. Comparative studies on volatile components of non-fragrant and fragrant rices. J. Sci. Food Agric. 1996, 70, 151–161. [Google Scholar]
- Tava, A.; Bocchi, S. Aroma of cooked rice (Oryza sativa): Comparison between commercial Basmati and Italian line B5-3. Cereal Chem. 1999, 76, 526–529. [Google Scholar]
- Yang, D.S.; Shewfelt, R.L.; Lee, K.-S.; Kays, S.J. Comparison of odor-active compounds from six distinctly different rice flavor types. J. Agric. Food Chem. 2008, 56, 2780–2787. [Google Scholar] [PubMed]
- Bett-Garber, K.L.; Bryant, R.J.; Grimm, C.C.; Chen, M.H.; Lea, J.M.; McClung, A.M. Physicochemical and sensory analysis of US rice varieties developed for the basmati and jasmine markets. Cereal Chem. 2017, 94, 602–610. [Google Scholar]
- Bryant, R.; McClung, A. Volatile profiles of aromatic and non-aromatic rice cultivars using SPME/GC–MS. Food Chem. 2011, 124, 501–513. [Google Scholar]
- Mohanty, K.; Bose, L.; Sujata, V.; Chaudhary, D.; Nagaraju, M. Detection of aroma in different stages and plant parts of scented rice varieties. Oryza 1991, 28, 395–397. [Google Scholar]
- Suvarnalatha, G.; Narayan, M.; Ravishankar, G.; Venkataraman, L. Flavour production in plant cell cultures of basmati rice (Oryza sativa L). J. Sci. Food Agric. 1994, 66, 439–442. [Google Scholar]
- Komes, D.; Ulrich, D.; Lovric, T. Characterization of odor-active compounds in Croatian Rhine Riesling wine, subregion Zagorje. Eur. Food Res. Technol. 2006, 222, 1–7. [Google Scholar]
- Sheung, K.; Min, S.; Sastry, S. Dynamic head space analyses of orange juice flavor compounds and their absorption into packaging materials. J. Food Sci. 2004, 69, 549–556. [Google Scholar]
- Djojoputro, H.; Ismadji, S. Density and viscosity correlation for several common fragrance and flavor esters. J. Chem. Eng. Data 2005, 50, 727–731. [Google Scholar]
- Cha-Um, S.; Supaibulwatana, K.; Kirdmanee, C. Glycinebetaine accumulation, physiological characterizations and growth efficiency in salt-tolerant and salt-sensitive lines of indica rice (Oryza sativa L. ssp. indica) in response to salt stress. J. Agron. Crop Sci. 2007, 193, 157–166. [Google Scholar]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar]
- Kinnersley, A.M.; Turano, F.J. Gamma aminobutyric acid (GABA) and plant responses to stress. Crit. Rev. Plant Sci. 2000, 19, 479–509. [Google Scholar]
- Yoshihashi, T.; Huong, N.T.T.; Inatomi, H. Precursors of 2-acetyl-1-pyrroline, a potent flavor compound of an aromatic rice variety. J. Agric. Food Chem. 2002, 50, 2001–2004. [Google Scholar]
- Gay, F.; Maraval, I.; Roques, S.; Gunata, Z.; Boulanger, R.; Audebert, A.; Mestres, C. Effect of salinity on yield and 2-acetyl-1-pyrroline content in the grains of three fragrant rice cultivars (Oryza sativa L.) in Camargue (France). Field Crops Res. 2010, 117, 154–160. [Google Scholar]
- Yoshihashi, T.; Huong, N.T.T.; Surojanametakul, V.; Tungtrakul, P.; Varanyanond, W. Effect of storage conditions on 2–Acetyl-1–pyrroline content in aromatic rice variety, khao dawk mali 105. J. Food Sci. 2005, 70, S34–S37. [Google Scholar]
- Golestan Hashemi, F.S.; Ismail, M.R.; Rafii, M.Y.; Aslani, F.; Miah, G.; Muharam, F.M. Critical multifunctional role of the betaine aldehyde dehydrogenase gene in plants. Biotechnol. Biotechnol. Equip. 2018, 32, 815–829. [Google Scholar]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Zarei, A.; Deyman, K.L.; Brikis, C.J. Hypothesis/review: Contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 2012, 193, 130–135. [Google Scholar] [PubMed]
- Niu, X.; Tang, W.; Huang, W.; Ren, G.; Wang, Q.; Luo, D.; Xiao, Y.; Yang, S.; Wang, F.; Lu, B.-R. RNAi-directed downregulation of OsBADH2 results in aroma (2-acetyl-1-pyrroline) production in rice (Oryza sativa L.). BMC Plant Biol. 2008, 8, 100. [Google Scholar]
- Fitzgerald, T.L.; Waters, D.L.E.; Brooks, L.O.; Henry, R.J. Fragrance in rice (Oryza sativa) is associated with reduced yield under salt treatment. Environ. Exp. Bot. 2010, 68, 292–300. [Google Scholar]
- Wijerathna, Y.; Kottearachchi, N.; Gimhani, D.; Sirisena, D. Exploration of relationship between fragrant gene and growth performances of fragrant rice (Oryza sativa L.) seedlings under salinity stress. J. Exp. Biol. Agric. Sci. 2014, 2, 7–12. [Google Scholar]
- Mahajan, G.; Matloob, A.; Singh, R.; Singh, V.P.; Chauhan, B.S. Basmati rice in the Indian subcontinent: Strategies to boost production and quality traits. Adv. Agron. 2018, 151, 159–213. [Google Scholar]
- Shi, W.; Yin, X.; Struik, P.C.; Xie, F.; Schmidt, R.C.; Jagadish, K.S. Grain yield and quality responses of tropical hybrid rice to high night-time temperature. Field Crops Res. 2016, 190, 18–25. [Google Scholar]
- Liu, X.; Guo, T.; Wan, X.; Wang, H.; Zhu, M.; Li, A.; Su, N.; Shen, Y.; Mao, B.; Zhai, H. Transcriptome analysis of grain-filling caryopses reveals involvement of multiple regulatory pathways in chalky grain formation in rice. BMC Genom. 2010, 11, 730. [Google Scholar]
- Siebenmorgen, T.J.; Grigg, B.C.; Lanning, S.B. Impacts of preharvest factors during kernel development on rice quality and functionality. Annu. Rev. Food Sci. Technol. 2013, 4, 101–115. [Google Scholar]
- Latif, T.; Haider, Z.; Ramzan, M.; Akhter, M.; Gull, S.; Mahmood, A.; Riaz, A.; Khan, R.A.R. Quantifying effects of photoperiod, temperature and humidity on flowering initiation in Basmati Rice lines. Am. J. Plant Sci. 2019, 10, 893–903. [Google Scholar]
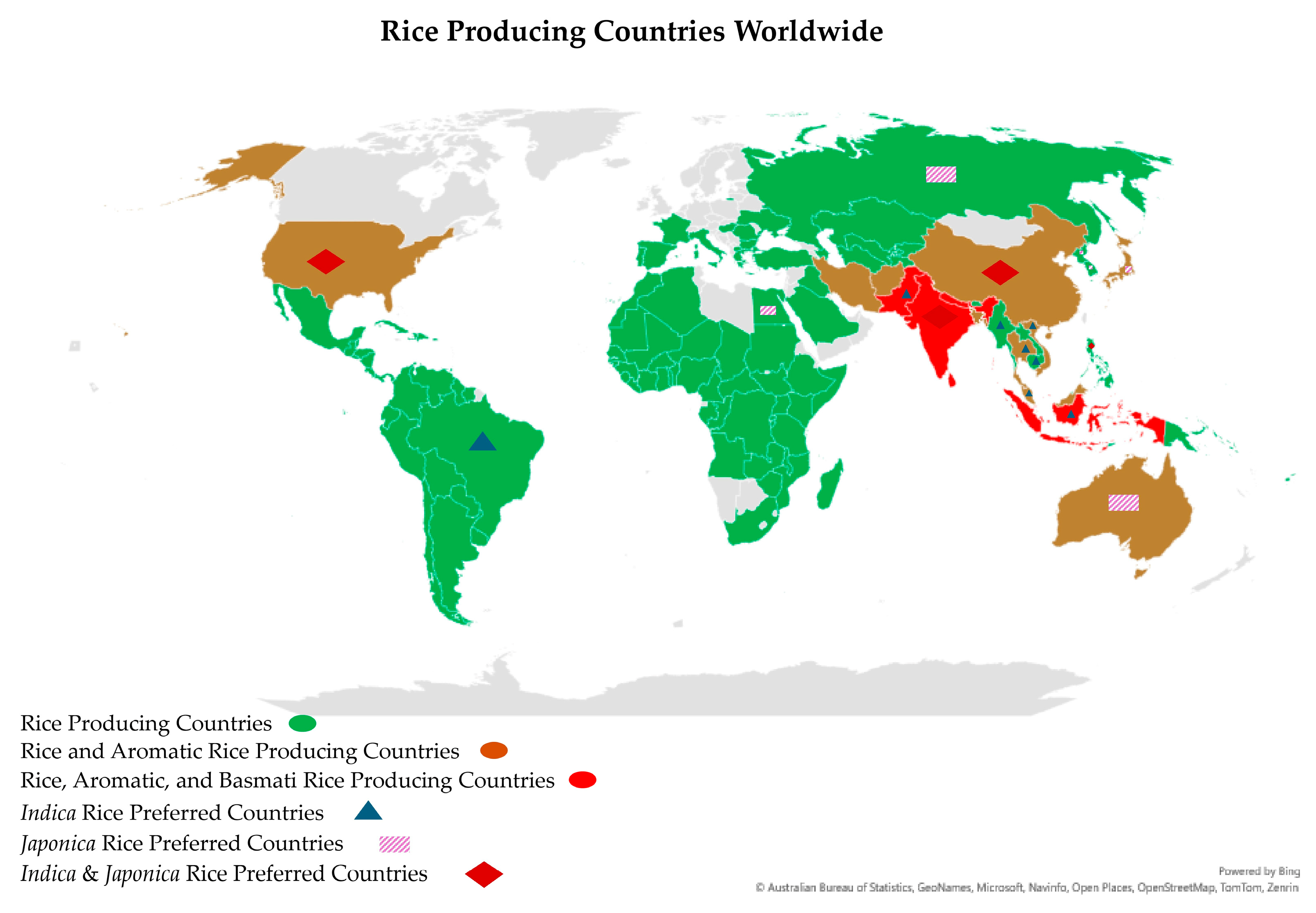
- Sarwar, N.; Ahmad, S.; Khan, M.A.; Hasanuzzaman, M. World rice production: An overview. In Modern Techniques of Rice Crop Production, 1st ed.; Sarwar, N., Ahmad, S., Khan, M.A., Hasanuzzaman, M., Eds.; Springer: Singapore, 2022; pp. 3–12. [Google Scholar]
- FAOSTAT. Rice Production Indices; FAOSTAT: Rome, Italy, 2024. [Google Scholar]
- Khush, G.S.; Paule, C.; Cruz, N.d.l. Rice grain quality evaluation and improvement at IRRI. In Proceedings of the Workshop on Chemical Aspects of Rice Grain Quality. Rice Grain Research, Los Banos, Laguna, Philippines, 1 January 1981; pp. 21–31. [Google Scholar]
- Juliano, B. The chemical basis of rice grain quality. Chem. Asp. Rice Grain Qual. 1979, 69, 90. [Google Scholar]
- Ali, A.; Karim, M.; Ali, S.; Ali, L.; Majid, A. Relationship of transplanting time to grain quality in Basmati 385. Int. Rice Res. Newsl. 1991, 16, 11. [Google Scholar]
- Ferrero, A. Constraints and opportunities for the sustainable development of rice-based production systems in Europe. In Proceedings of the the International Conference on Sustainable Rice Systems; FAO: Rome, Italy, 2004. [Google Scholar]
- Akhter, M.; Ahmad, M.; Ramzan, M. Effect of photoperiod sensitivity on yield and other economic traits of new strains of basmati rice (Oryza sativa L.). J. Anim. Plant Sci. 2007, 17, 79–82. [Google Scholar]
- Mann, R. Basmati rice: A wonder of Pakistan’s agriculture. Int. Rice Comm. Newsl. 1987, 36, 23–28. [Google Scholar]
- Chun, A.; Lee, H.-J.; Hamaker, B.R.; Janaswamy, S. Effects of ripening temperature on starch structure and gelatinization, pasting, and cooking properties in rice (Oryza sativa). J. Agric. Food Chem. 2015, 63, 3085–3093. [Google Scholar]
- Asaoka, M.; Okuno, K.; Fuwa, H. Effect of environmental temperature at the milky stage on amylose content and fine structure of amylopectin of waxy and nonwaxy endosperm starches of rice (Oryza sativa L.). Agric. Biol. Chem. 1985, 49, 373–379. [Google Scholar]
- Rohilla, R.; Singh, V.; Singh, U.; Singh, R.; Khush, G. Crop husbandry and environmental factors affecting aroma and other quality traits. In Aromatic Rices; Singh, R., Singh, U., Khush, G., Eds.; International Rice Research Institute: Oxford, UK; IBH: New Delhi, India, 2000; pp. 201–216. [Google Scholar]
- Su, Q.; Rohila, J.S.; Ranganathan, S.; Karthikeyan, R. Rice yield and quality in response to daytime and nighttime temperature increase—A meta-analysis perspective. Sci. Total Environ. 2023, 898, 165256. [Google Scholar] [PubMed]
- Reay, D.; Reay, D. Climate-Smart Rice. In Climate-Smart Food; Springer: Berlin/Heidelberg, Germany, 2019; pp. 121–133. [Google Scholar]
- Weerakoon, W.; Maruyama, A.; Ohba, K. Impact of humidity on temperature-induced grain sterility in rice (Oryza sativa L). J. Agron. Crop Sci. 2008, 194, 135–140. [Google Scholar]
- Yan, H.; Wang, C.; Liu, K.; Tian, X. Detrimental effects of heat stress on grain weight and quality in rice (Oryza sativa L.) are aggravated by decreased relative humidity. PeerJ 2021, 9, e11218. [Google Scholar] [PubMed]
- Thompson, J.; Mutters, R. Effect of weather and rice moisture at harvest on milling quality of California medium-grain rice. Trans. ASABE 2006, 49, 435–440. [Google Scholar]
- Guo, W.; Wang, X.; Sun, J.; Ding, A.; Zou, J. Comparison of land-atmosphere interaction at different surface types in the mid-to lower Yangzi River Valley. Atmos. Chem. Phys. 2016, 16, 9875–9890. [Google Scholar]
- Tian, X.; Matsui, T.; Li, S.; Yoshimoto, M.; Kobayasi, K.; Hasegawa, T. Heat-induced floret sterility of hybrid rice (Oryza sativa L.) cultivars under humid and low wind conditionsin the field of Jianghan Basin, China. Plant Prod. Sci. 2010, 13, 243–251. [Google Scholar]
- Wada, H.; Nonami, H.; Yabuoshi, Y.; Maruyama, A.; Tanaka, A.; Wakamatsu, K.; Sumi, T.; Wakiyama, Y.; Ohuchida, M.; Morita, S. Increased ring-shaped chalkiness and osmotic adjustment when growing rice grains under foehn-induced dry wind condition. Crop Sci. 2011, 51, 1703–1715. [Google Scholar]
- Prodhan, Z.H.; Qingyao, S. Rice aroma: A natural gift comes with price and the way forward. Rice Sci. 2020, 27, 86–100. [Google Scholar]
- Chen, H.; Li, Q.-P.; Zeng, Y.-L.; Deng, F.; Ren, W.-J. Effect of different shading materials on grain yield and quality of rice. Sci. Rep. 2019, 9, 9992. [Google Scholar]
- Liu, Q.-H.; Xiu, W.; Chen, B.-C.; Jie, G. Effects of low light on agronomic and physiological characteristics of rice including grain yield and quality. Rice Sci. 2014, 21, 243–251. [Google Scholar]
- Mo, Z.; Li, W.; Pan, S.; Fitzgerald, T.L.; Xiao, F.; Tang, Y.; Wang, Y.; Duan, M.; Tian, H.; Tang, X. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice 2015, 8, 1–10. [Google Scholar]
- Li, Y.; Liang, L.; Fu, X.; Gao, Z.; Liu, H.; Tan, J.; Potcho, M.P.; Pan, S.; Tian, H.; Duan, M. Light and water treatment during the early grain filling stage regulates yield and aroma formation in aromatic rice. Sci. Rep. 2020, 10, 14830. [Google Scholar]
- Liu, Q.; Li, T.; Zhang, J. Effect of shading at the early stage on the growth of function leaves at the grain filling stage and quality in rice. Chin. J. Ecol. 2006, 20, 1167–1172. [Google Scholar]
- Fu, G.-F.; Li, H.; Tao, L.-X.; Zhang, X.-F.; Wang, D.-Y. Effects of shading at grain-filling stage on the growth and Q enzyme activity of rice grain. Chin. J. Ecol. 2009, 28, 438. [Google Scholar]
- Ren, W.-J.; Yang, W.-Y.; Xu, J.-W. Effect of low light on grains growth and quality in rice. Acta Agron. Sin. 2003, 29, 785–790. [Google Scholar]
- Tu, Z.; Lin, X.; Huang, Q.; Cai, W.; Feng, H.; Ye, L. Photosynthetic characterisation of rice varieties in relation to growth irradiance. Funct. Plant Biol. 1988, 15, 277–286. [Google Scholar]
- Okpala, N.E.; Potcho, M.P.; An, T.; Ahator, S.D.; Duan, L.; Tang, X. Low temperature increased the biosynthesis of 2-AP, cooked rice elongation percentage and amylose content percentage in rice. J. Cereal Sci. 2020, 93, 102980. [Google Scholar]
- Xie, H.; Xie, W.; Pan, S.; Liu, X.; Tian, H.; Duan, M.; Wang, S.; Tang, X.; Mo, Z. Effects of light quality treatments during the grain filling period on yield, quality, and fragrance in fragrant rice. Agronomy 2021, 11, 531. [Google Scholar] [CrossRef]
- Ishfaq, M.; Wang, Y.; Xu, J.; Hassan, M.U.; Yuan, H.; Liu, L.; He, B.; Ejaz, I.; White, P.J.; Cakmak, I. Improvement of nutritional quality of food crops with fertilizer: A global meta-analysis. Agron. Sustain. Dev. 2023, 43, 74. [Google Scholar]
- Xu, Y.; Guan, X.; Han, Z.; Zhou, L.; Zhang, Y.; Asad, M.A.; Wang, Z.; Jin, R.; Pan, G.; Cheng, F. Combined effect of nitrogen fertilizer application and high temperature on grain quality properties of cooked rice. Front. Plant Sci. 2022, 13, 874033. [Google Scholar]
- Hou, F. Effects of fertilizer on rice quality. Spec. Publ. Taichung Dist. Agric. Improv. Stn. 1988, 13, 242–248. [Google Scholar]
- Sarwar, N.; Maqsood, M.; Wajid, S.A.; Anwar-ul-Haq, M. Impact of nursery seeding density, nitrogen, and seedling age on yield and yield attributes of fine rice. Chil. J. Agric. Res. 2011, 71, 343. [Google Scholar]
- Virk, A.L.; Farooq, M.S.; Ahmad, A.; Khaliq, T.; Rehmani, M.I.A.; Haider, F.U.; Ejaz, I. Effect of seedling age on growth and yield of fine rice cultivars under alternate wetting and drying system. J. Plant Nutr. 2020, 44, 1–15. [Google Scholar]
- Mahajan, G.; Sharma, N.; Kaur, R.; Chauhan, B. Comparison of photoperiod-sensitive and photoperiod-insensitive basmati cultivars for grain yield, water productivity, and quality traits under varied transplanting dates in Northwest India. Crop Pasture Sci. 2015, 66, 793–801. [Google Scholar]
- Singh, N.; Paul, P.; Virdi, A.S.; Kaur, P.; Mahajan, G. Influence of early and delayed transplantation of paddy on physicochemical, pasting, cooking, textural, and protein characteristics of milled rice. Cereal Chem. 2014, 91, 389–397. [Google Scholar]
- Latchumanan, M.; Kandaswamy, P.; Ramaswami, P. Effect of biofertilizers on the quality of rice grain. Curr. Res. 1979, 8, 9–10. [Google Scholar]
- Quyen, N.; Sharma, S.; Gautam, R. Comparative study of organic and traditional farming for sustainable rice production. OmonRice 2002, 10, 74–78. [Google Scholar]
- Pooniya, V.; Shivay, Y.S. Influence of green manuring and zinc fertilization on quality parameters of basmati rice. Commun. Soil Sci. Plant Anal. 2015, 46, 382–392. [Google Scholar]
- Mahajan, G.; Gill, M.; Dogra, B. Performance of basmati rice (Oryza sativa) through organic source of nutrients. Indian J. Agric. Sci. 2012, 82, 459. [Google Scholar]
- Aulakh, C.; Kaur, P.; Walia, S.; Gill, R.; Sharma, S.; Buttar, G. Productivity and quality of basmati rice (Oryza sativa) in relation to nitrogen management. Indian J. Agron. 2016, 61, 467–473. [Google Scholar]
- Durand-Morat, A.; Bairagi, S. World and US Rice Baseline Outlook, 2022–2032; University of Arkansas System: Arkansas Agricultural Experiment Station: Fayetteville, AR, USA, 2023; p. 258. [Google Scholar]
- Wisdom, D.; Moldenhauer, K.; de Guzman, C.; Sha, X.; Bulloch, J.; Boyett, V.; Thompson, V.; Belmar, S.; Kelsey, C.; McCarty, D. ARoma 22, an Aromatic Jasmine-Type, Long-Grain Rice Variety; University of Arkansas System: Rice Research and Extension Center: Stuttgart, AR, USA, 2022; pp. 85–87. [Google Scholar]
- Marchetti, M.; Bollich, C.; Webb, B.; Jackson, B.; McClung, A.; Scott, J.; Hung, H. Registration of ‘Jasmine 85’ rice. Crop Sci. 1998, 38, 896. [Google Scholar]
- Jodon, N.E.; Sonnier, E.A. Registration of Della Rice 1 (Reg. No. 37). Crop Sci. 1973, 13, 773. [Google Scholar]
- Al-Khatib, K.; Linquist, B.; Swett, C.; Espino, L.; Leinfelder-Miles, M.; Brim-Deforest, W.; Mckenzie, K. California Rice Production Workshop; University of California Agriculture and Natural Resource: Davis, CA, USA, 2018. [Google Scholar]
- Mathure, S.; Shaikh, A.; Renuka, N.; Wakte, K.; Jawali, N.; Thengane, R.; Nadaf, A. Characterisation of aromatic rice (Oryza sativa L.) germplasm and correlation between their agronomic and quality traits. Euphytica 2011, 179, 237–246. [Google Scholar]
- Ahuja, U.; Ahuja, S.; Thakrar, R.; Rani, N.S. Scented rices of India. Asian Agri-Hist. 2008, 12, 267–283. [Google Scholar]
- Prasad, G.; Padmavathi, G.; Suneetha, K.; Madhav, M.; Muralidharan, K. Assessment of diversity of Indian aromatic rice germplasm collections for morphological, agronomical, quality traits and molecular characters to identify a core set for crop improvement. CABI Agric. Biosci. 2020, 1, 1–24. [Google Scholar]
- Prodhan, Z.H.; Faruq, G.; Rashid, K.A.; Taha, R.M. Effects of temperature on volatile profile and aroma quality in rice. Int. J. Agric. Biol. 2017, 19, 1065–1072. [Google Scholar]
- Rutger, J.N.; Mckenzie, K.S.; Moldenhauer, K.A.K.; Mcclung, A.M.; Gravois, K.A.; Linscombe, S.D.; Kanter, D.G. Rice quality breeding efforts in the US. In Proceedings of the Cahiers Options Méditerranéennes, Nottingham, UK, 24–27 November 1997. [Google Scholar]
- Siddiq, E.; Vemireddy, L.; Nagaraju, J. Basmati rices: Genetics, breeding and trade. Agric. Res. 2012, 1, 25–36. [Google Scholar]
- Rohila, J.S.; Jain, R.K.; Wu, R. Genetic improvement of Basmati rice for salt and drought tolerance by regulated expression of a barley Hva1 cDNA. Plant Sci. 2002, 163, 525–532. [Google Scholar]
- Patra, N.; Hariharan, S.; Gain, H.; Maiti, M.K.; Das, A.; Banerjee, J. TypiCal but DeliCate Ca++re: Dissecting the essence of calcium signaling network as a robust response coordinator of versatile abiotic and biotic stimuli in plants. Front. Plant Sci. 2021, 12, 752246. [Google Scholar]
- Pradhan, A.; Chary, G.; Chandrakar, T.; Mishra, V. Enhancing productivity through climate smart agriculture under changing weather of rainfed farming system. J. Crop Weed 2021, 17, 159–164. [Google Scholar]
- Pradhan, S.; Das, S.; Patra, B. Advances in Rice Breeding: Stress Tolerance, Climate Resilience, Quality & High Yield; ICAR-National Rice Research Institute: Cuttack, India, 2021; Volume 426. [Google Scholar]
- Sah, R.; Kumar, A.; Dash, S.; TP, M.A.; Pradhan, S. Speed breeding–A unique tool for higher genetic gain in rice. In Advances in Rice Breeding: Stress Tolerance, Climate Resilience, Quality & High Yield; Pradhan, S., Das, S., Patra, B., Eds.; ICAR-National Rice Research Institute: Cuttack, India, 2021; p. 316. [Google Scholar]
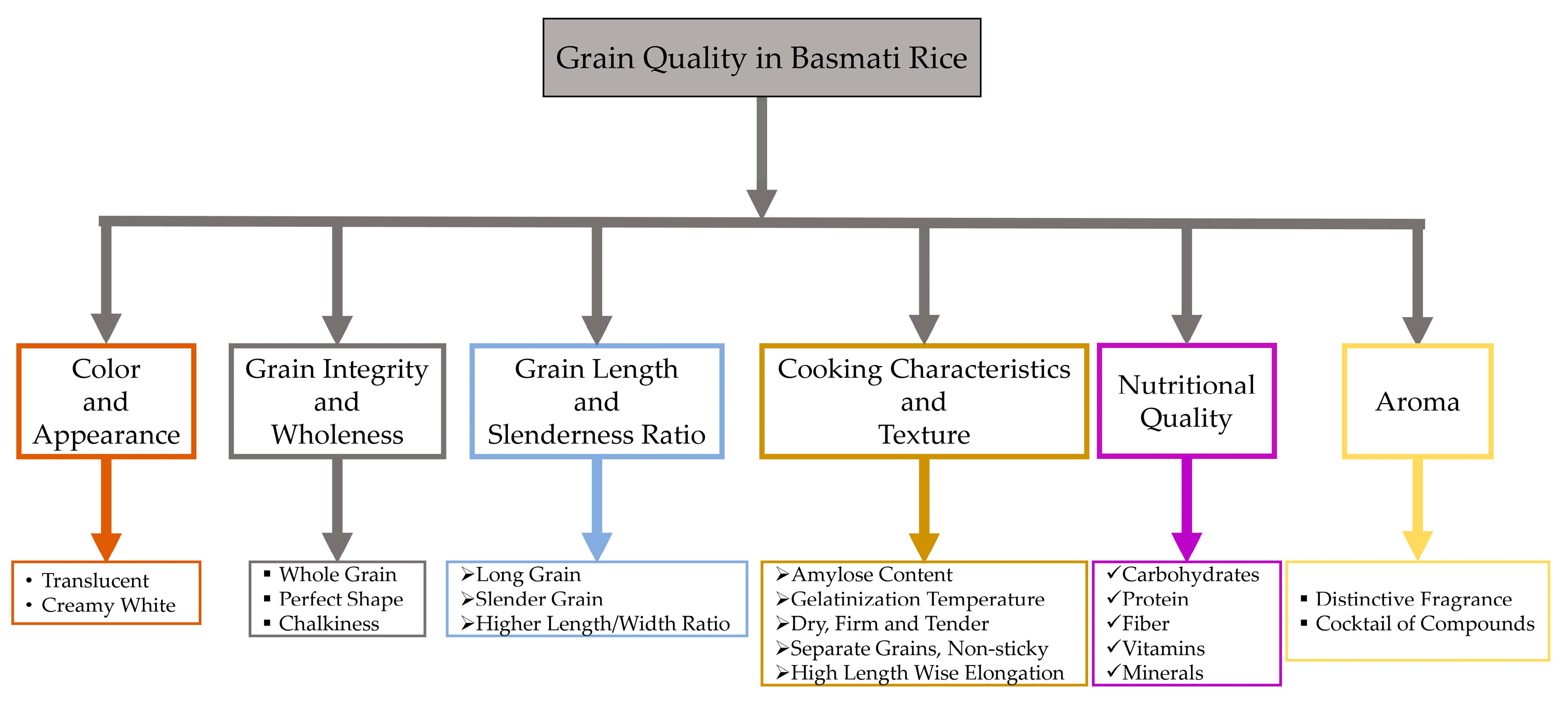
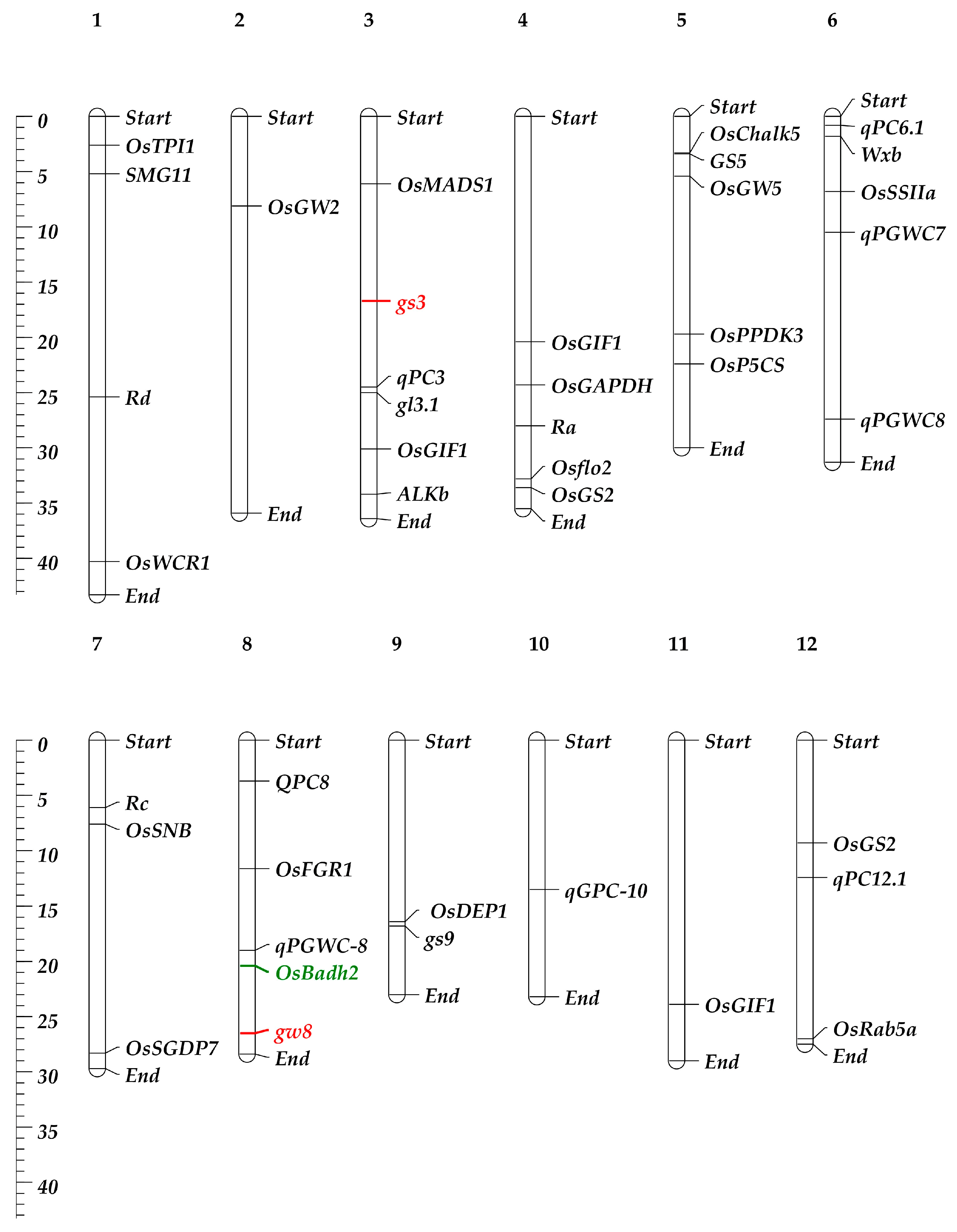
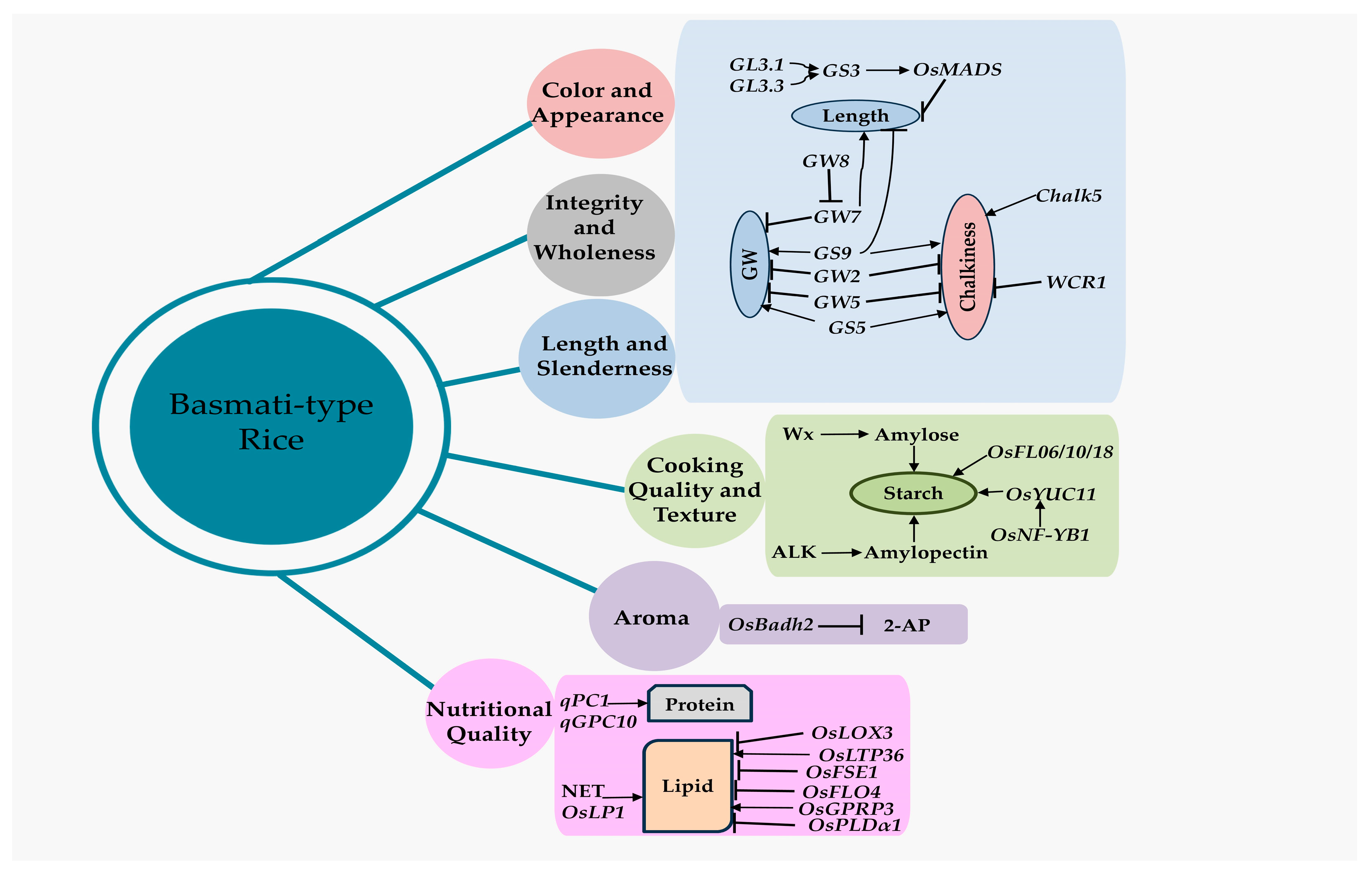


| Traits | Alleles (Genes/QTLs) | Functions | References |
|---|---|---|---|
| Color and Appearance | GS2 | Influence growth-regulating factor 4 | [47,48] |
| chalk5 | Reduce chalkiness | [49] | |
| WCR1A | Reduce chalkiness | [50] | |
| qPGWC-8 | Affect white chalkiness | [51] | |
| OsPPDK3, GIF1, ms-h, FLO2, OsRab5a, PFP1, qPGWC7, qPGWC-8 | Regulate grain chalkiness | [10,52] | |
| Grain Integrity and Wholeness | GLW, SGDP7, smg11, OsMADS1, OsSNB, qGRL1.1 | Regulate grain shape | [10,52] |
| FGR1 | Impact on thickness, transparency, and starch content | [53] | |
| Grain Length and Slenderness | gs9 | Increase grain length | [54] |
| GS5 | Affect grain width, grain weight, and grain filling | [55,56] | |
| GSE5ZJB | Produce long and bold grains with low chalkiness | [57] | |
| gw8 | Increase grain length having no yield loss | [46] | |
| OsMADSlgy3 | Increase grain length and yield | [58] | |
| dep1 | Increase grain length | [59] | |
| gl3.1 | Increase grain length and yield | [60] | |
| gs3 | Increase grain length with slender grains | [58,61,62] | |
| GW2 | Major QTL of grain width and weight | [63,64] | |
| GW5 | Increase seed width | [65,66] | |
| GW7TFA | Produce longer grains and increase grain yield | [61] | |
| Cooking Characteristics and Texture | Wxmq | Decrease amylose content to 10~15% | [67] |
| Wxb | Decrease amylose content to 10~15% | [68,69] | |
| Wxmw | Decrease amylose content to 14% and improve endosperm transparency | [70] | |
| ALKb | Decrease the amylose content and gelatinization temperature | [71] | |
| SSIIa | Control gelatinization temperature | [72,73] | |
| Aroma | OsBadh2 | Produce rice variety with aroma | [71] |
| P5CS | Proline biosynthesis and aroma | [74] | |
| TPI | Glycolysis and aroma | [75] | |
| GAPDH | Energy metabolism and aroma | [74] | |
| Nutritional quality | qPC3, QPC8, qPC6.1, qPC12.1 | Grain protein content | [76] |
| qCPC5, qGPC-1, qGPC-10, Ra, Rc, Rd | Protein, fat, and phenolic content | [10,77] |
| Name of Compounds | Aroma and Flavor | References |
|---|---|---|
| (E)-2-nonenal | Metallic | [133] |
| (E)-2-octenal | Nutty, cooked flour | |
| (E)-hept-2-enal | Green | |
| (E,E)-2,4-decadienal | Musty, cooked starch aromas | |
| (E,E)-2,4-nonadienal | Fatty, metallic | |
| 1-octen-3-ol | Straw, mushroom | |
| 2-heptanone | Fruit, spicy | |
| Decanal | Fatty, fruity | |
| Guaiacol | Smoky, sweet, vanilla-like | |
| Heptanal | Grass, fresh | |
| Hexanal | Green | |
| 2-Acetyl-1-pyrroline (2-AP) | Popcorn-like | [124,130,131] |
| 2-ethyl-1-hexanol | Citrus | [134] |
| 2-ethylhexyl acetate | Fruity, pleasant | |
| 2-ethylyhexyl acetate | Fruity, pleasant | |
| 2-methyl-1-propenylbenzene | Green | |
| Bisthiophene | Nutty hazelnut | |
| 2-pentylfuran | Floral, fruit, nutty | [129,131] |
| 2-phenylethanol | Pleasant rosy | [131] |
| Alk-2-enals | Metallic | |
| Alka(E)-2,4-dienals | Sweet aromatic | |
| Alkanals | Green | |
| 6,10,14-trimethyl-pentadecan-2-one | Sweet, floral | [130] |
| Benzaldehyde | Nutty, sweet | |
| Hexadecanol | Odorless or faint | |
| Pentanol | Moderately strong | |
| 6-methyl-5-hepten-2-one | Herby, green | [134,135] |
| Acetaldehyde | Strong, fruity | [136,137] |
| Propionaldehyde | Strong fruity | |
| Hexanol | Green | [138] |
| Methyl oleate | Pleasant fatty ester | [133,134] |
| Nonanal | Grassy, citrus, floral | |
| Octanal | Citrusy | [133,134,139] |
| Pentadecan-2-one | Fatty and spicy, floral nuance | [129,130] |
| Month | Weather | Panjab, Pakistan | Western, India | California, USA | Texas, USA |
|---|---|---|---|---|---|
| January | Daily maximum temperatures (°C) | 18.4 | 28.8 | 15.2 | 15.7 |
| Night-time low temperatures (°C) | 5.2 | 14.5 | 4.6 | 2.5 | |
| Humidity (%) | 64 | 56 | 71 | 66 | |
| Sunshine hours per day | 5.2 | 6.0 | 8.2 | 6.0 | |
| February | Daily maximum temperatures (°C) | 22.4 | 31.2 | 16.6 | 17.6 |
| Night-time low temperatures (°C) | 8.6 | 16.5 | 5.3 | 4.3 | |
| Humidity (%) | 60 | 52 | 69 | 63 | |
| Sunshine hours per day | 6.9 | 6.7 | 9.1 | 7.3 | |
| March | Daily maximum temperatures (°C) | 27.9 | 34.3 | 18.6 | 22.5 |
| Night-time low temperatures (°C) | 14 | 20.1 | 7.2 | 9.1 | |
| Humidity (%) | 57 | 48 | 67 | 58 | |
| Sunshine hours per day | 7.3 | 7.1 | 8.5 | 8.7 | |
| April | Daily maximum temperatures (°C) | 34.4 | 36.9 | 21.3 | 26.2 |
| Night-time low temperatures (°C) | 19.1 | 23.6 | 9.1 | 12.8 | |
| Humidity (%) | 46 | 49 | 62 | 57 | |
| Sunshine hours per day | 8.0 | 8.2 | 9.0 | 10.2 | |
| May | Daily maximum temperatures (°C) | 38.9 | 37.9 | 23.9 | 30 |
| Night-time low temperatures (°C) | 23.8 | 26.1 | 11.8 | 17.6 | |
| Humidity (%) | 37 | 53 | 61 | 61 | |
| Sunshine hours per day | 8.4 | 8.2 | 9.2 | 10.7 | |
| June | Daily maximum temperatures (°C) | 39.7 | 34.5 | 27.8 | 34.2 |
| Night-time low temperatures (°C) | 26.4 | 25.5 | 14.8 | 22 | |
| Humidity (%) | 42 | 69 | 60 | 60 | |
| Sunshine hours per day | 8.6 | 9.6 | 5.6 | 11.2 | |
| July | Daily maximum temperatures (°C) | 36.7 | 30.5 | 30.2 | 35.4 |
| Night-time low temperatures (°C) | 26.9 | 24.3 | 16.9 | 23.3 | |
| Humidity (%) | 63 | 80 | 61 | 61 | |
| Sunshine hours per day | 6.6 | 9.2 | 2.9 | 11.5 | |
| August | Daily maximum temperatures (°C) | 35.6 | 29.8 | 30 | 35.6 |
| Night-time low temperatures (°C) | 26.3 | 23.7 | 16.7 | 23.1 | |
| Humidity (%) | 69 | 82 | 63 | 62 | |
| Sunshine hours per day | 7.0 | 9.5 | 3.4 | 10.8 | |
| September | Daily maximum temperatures (°C) | 35.1 | 31 | 28.4 | 32.2 |
| Night-time low temperatures (°C) | 24.3 | 23.4 | 14.8 | 19.9 | |
| Humidity (%) | 62 | 77 | 62 | 65 | |
| Sunshine hours per day | 7.4 | 7.8 | 5.0 | 9.8 | |
| October | Daily maximum temperatures (°C) | 32.3 | 32.8 | 24.3 | 27 |
| Night-time low temperatures (°C) | 18.3 | 21.6 | 11.1 | 13.7 | |
| Humidity (%) | 57 | 66 | 64 | 63 | |
| Sunshine hours per day | 7.5 | 7.4 | 7.4 | 8.4 | |
| November | Daily maximum temperatures (°C) | 26.6 | 31.7 | 18.9 | 20.9 |
| Night-time low temperatures (°C) | 11.5 | 18.5 | 6.8 | 7.8 | |
| Humidity (%) | 62 | 58 | 68 | 64 | |
| Sunshine hours per day | 6.4 | 6.4 | 8.0 | 6.6 | |
| December | Daily maximum temperatures (°C) | 21.3 | 29.6 | 14.7 | 16.6 |
| Night-time low temperatures (°C) | 6.3 | 15.6 | 4.3 | 3.9 | |
| Humidity (%) | 66 | 56 | 71 | 67 | |
| Sunshine hours per day | 4.8 | 5.7 | 7.8 | 5.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prodhan, Z.H.; Samonte, S.O.P.B.; Sanchez, D.L.; Talukder, S.K. Profiling and Improvement of Grain Quality Traits for Consumer Preferable Basmati Rice in the United States. Plants 2024, 13, 2326. https://doi.org/10.3390/plants13162326
Prodhan ZH, Samonte SOPB, Sanchez DL, Talukder SK. Profiling and Improvement of Grain Quality Traits for Consumer Preferable Basmati Rice in the United States. Plants. 2024; 13(16):2326. https://doi.org/10.3390/plants13162326
Chicago/Turabian StyleProdhan, Zakaria Hossain, Stanley Omar P. B. Samonte, Darlene Lonjas Sanchez, and Shyamal Krishna Talukder. 2024. "Profiling and Improvement of Grain Quality Traits for Consumer Preferable Basmati Rice in the United States" Plants 13, no. 16: 2326. https://doi.org/10.3390/plants13162326





