Sugarcane/Soybean Intercropping with Reduced Nitrogen Application Synergistically Increases Plant Carbon Fixation and Soil Organic Carbon Sequestration
Abstract
1. Introduction
2. Results
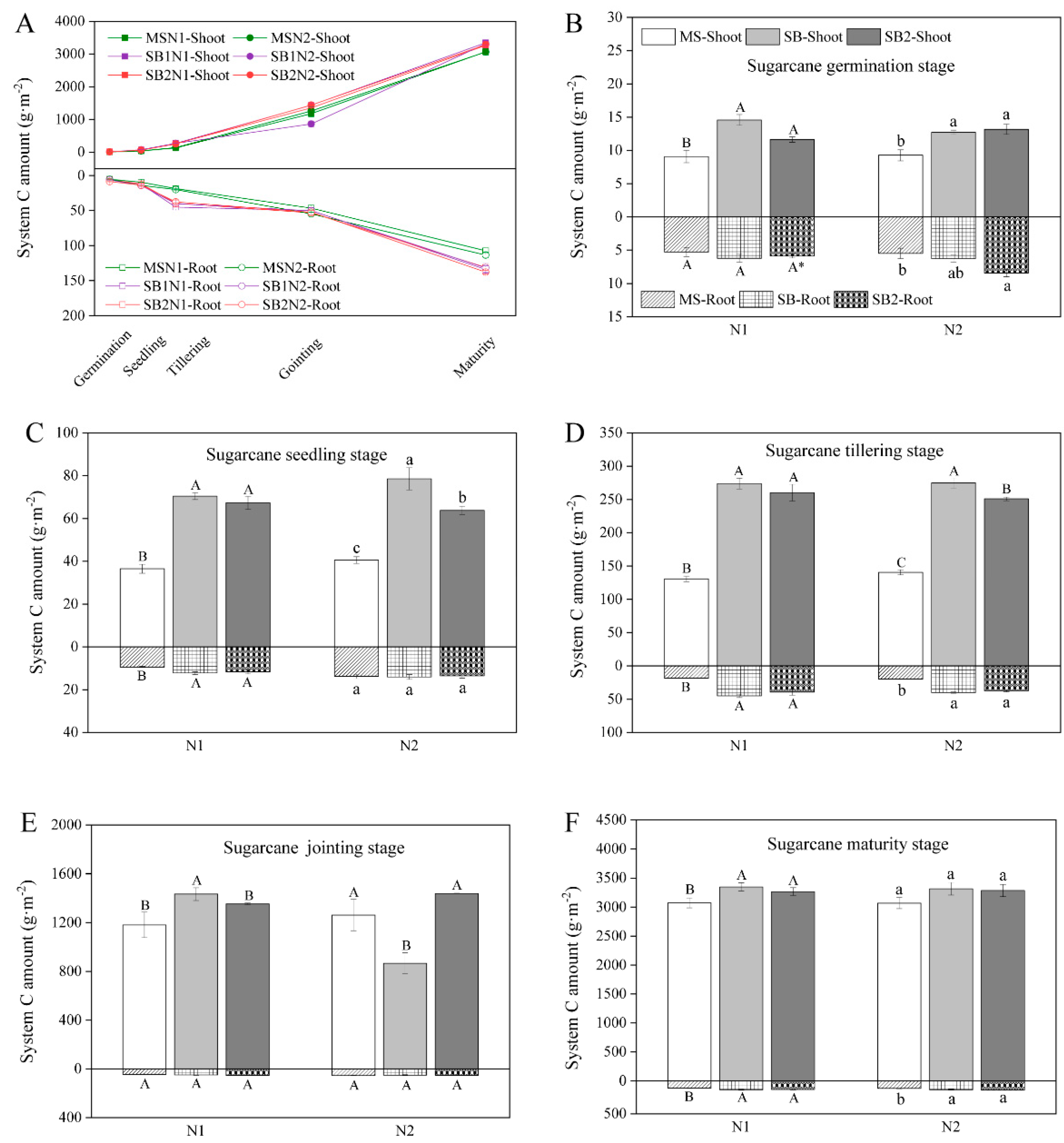
2.1. Dynamic Changes of C Sequestration in the Shoots and Roots of Sugarcane
2.2. Dynamic Changes of C Sequestration in the Shoots and Roots of Soybean
2.3. Dynamic Changes of C Sequestration in the Shoots and Roots of the System
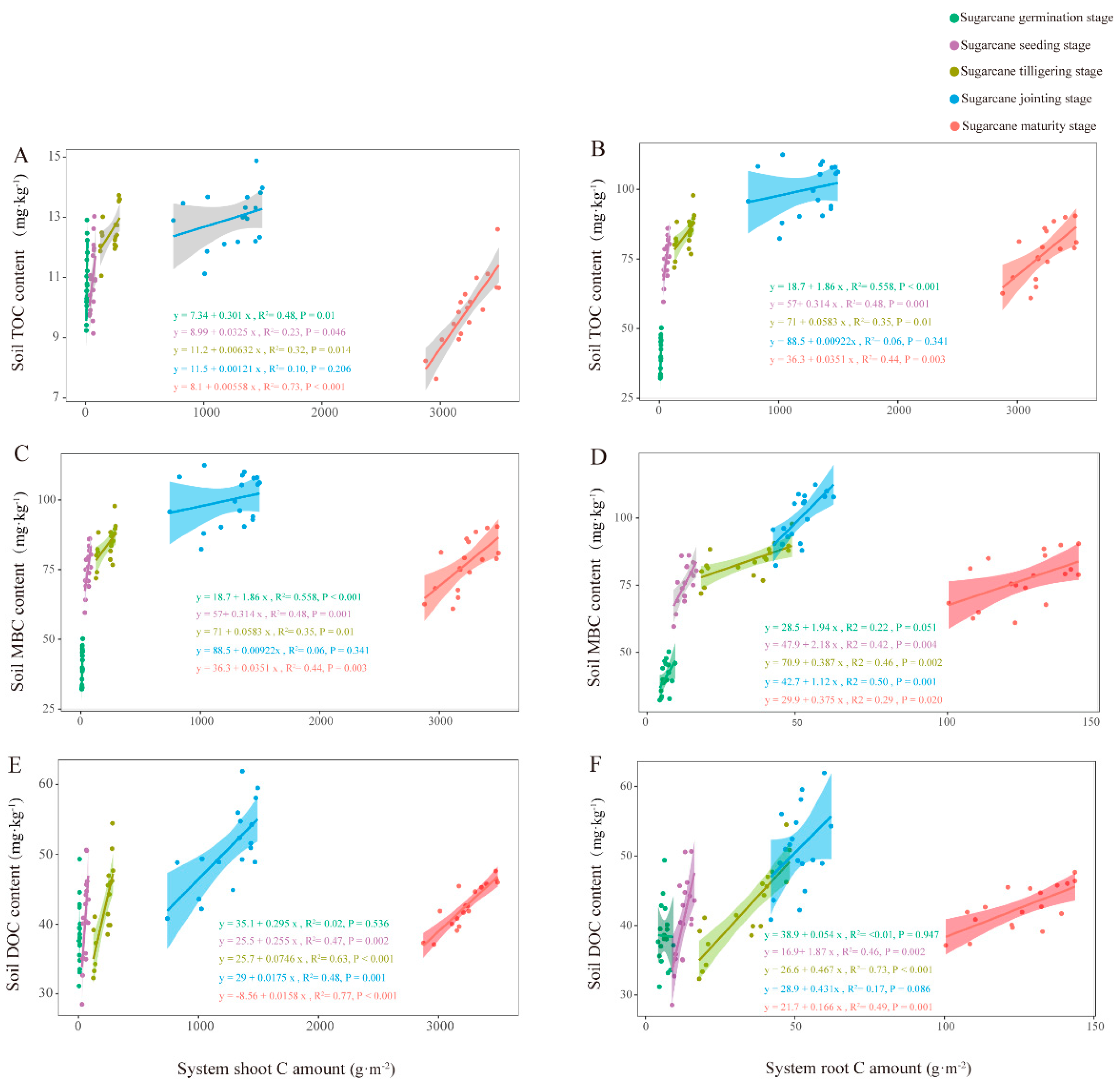
2.4. Dynamic Changes of Soil TOC Content
2.5. Dynamics Changes of Soil MBC Content
2.6. Dynamic Changes of Soil DOC Content
2.7. Regression Analysis of Plant C Sequestration and Soil Organic C Components
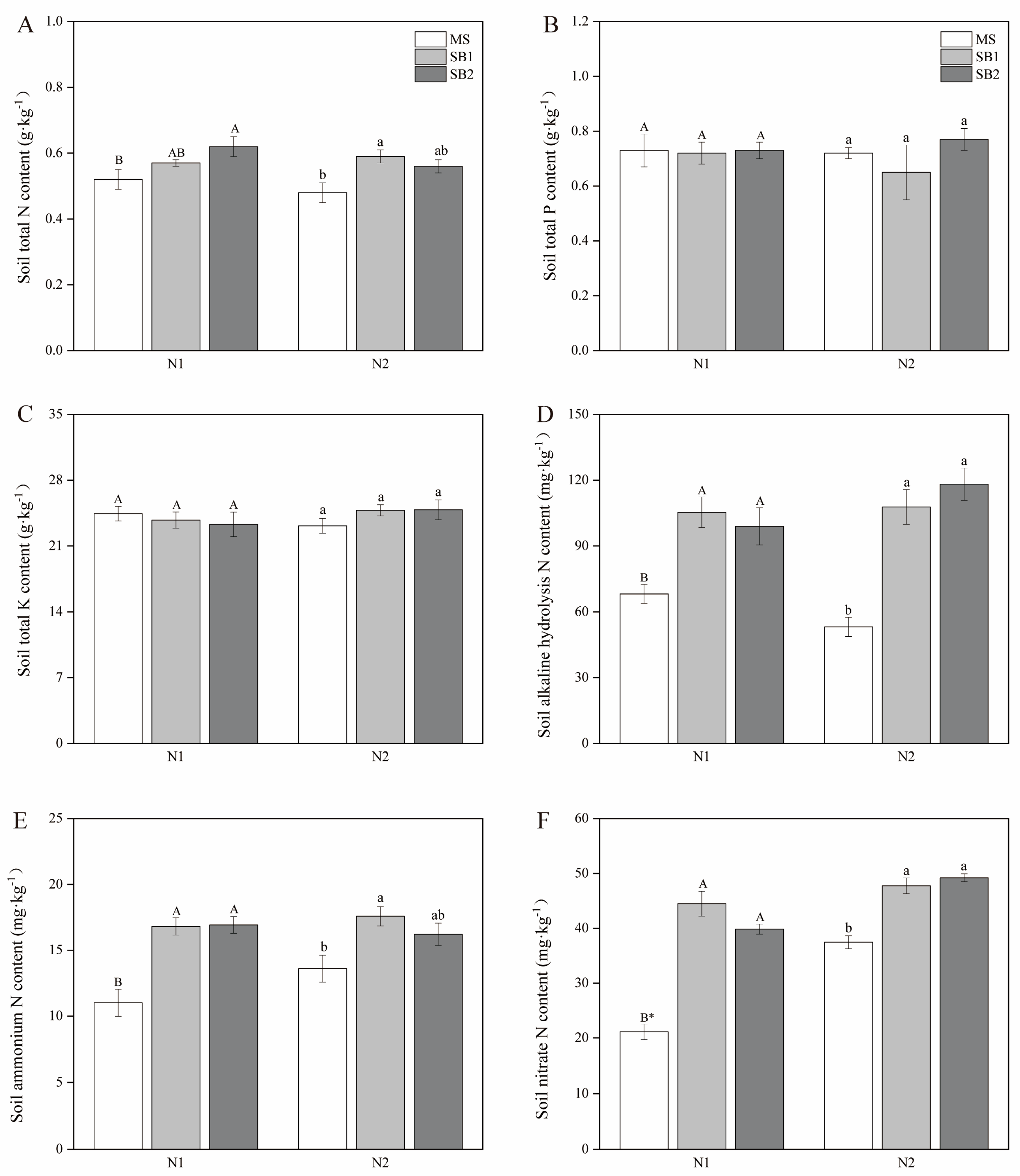
2.8. Effects of Planting Pattern and N Application on Soil Nutrients and Sugarcane Yield
3. Discussion
3.1. Plant C Sequestration in the Sugarcane/Soybean Intercropping System
3.2. Soil TOC Fixation in the Sugarcane/Soybean Intercropping System
3.3. Fixation of Soil Labile Organic C in the Sugarcane/Soybean Intercropping System
4. Materials and Methods
4.1. Field Sites
4.2. Experimental Materials
4.3. Experiment Design
4.4. Soil and Plant Sampling Collection
4.5. Contents of TOC, MBC, and DOC in Soil
4.6. Calculation Method
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse gases emissions and global climate change: Examining the influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peoples, M.B.; Boddey, R.M.; Gresshoff, P.M.; Hauggaard-Nielsen, H.; Alves, B.J.R.; Morrison, M.J. Legumes for mitigation of climate change and the provision of feedstock for biofuels and biorefineries. A review. Agron. Sustain. Dev. 2012, 32, 329–364. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Rosinger, C.; Bodner, G.; Bernardini, L.G.; Huber, S.; Mentler, A.; Sae-Tun, O.; Scharf, B.; Steiner, P.; Tintner-Olifiers, J.; Keiblinger, K. Benchmarking carbon sequestration potentials in arable soils by on-farm research on innovative pioneer farms. Plant Soil 2023, 488, 137–156. [Google Scholar] [CrossRef]
- Sillen, W.; Dieleman, W.J.B. Effects of elevated CO2 and N fertilization on plant and soil carbon pools of managed grasslands: A meta-analysis. Biogeosciences 2012, 9, 2247–2258. [Google Scholar] [CrossRef]
- Felcmanova, K.; Lukes, M.; Kotabova, E.; Lawrenz, E.; Halsey, K.H.; Prasil, O. Carbon use efficiencies and allocation strategies in Prochlorococcus marinus strain PCC 9511 during nitrogen-limited growth. Photosynth. Res. 2017, 134, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gong, J.; Zihe, Z.; Yang, B.; Liu, M.; Zhu, C.; Shi, J.; Zhang, W.; Yue, K. Nitrogen addition alters photosynthetic carbon fixation, allocation of photoassimilates, and carbon partitioning of Leymus chinensis in a temperate grassland of Inner Mongolia. Agric. For. Meteorol. 2019, 279, 107743. [Google Scholar] [CrossRef]
- Padilla, F.M.; de Souza, R.; Teresa Pena-Fleitas, M.; Gallardo, M.; Gimenez, C.; Thompson, R.B. Different Responses of Various Chlorophyll Meters to Increasing Nitrogen Supply in Sweet Pepper. Front. Plant Sci. 2018, 9, 1752. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ge, T.; Gunina, A.; Li, Y.; Zhu, Z.; Peng, P.; Wu, J.; Kuzyakov, Y. Carbon and nitrogen availability in paddy soil affects rice photosynthate allocation, microbial community composition, and priming: Combining continuous 13C labeling with PLFA analysis. Plant Soil 2019, 445, 137–152. [Google Scholar] [CrossRef]
- Ge, T.; Li, B.; Zhu, Z.; Hu, Y.; Yuan, H.; Dorodnikov, M.; Jones, D.L.; Wu, J.; Kuzyakov, Y. Rice rhizodeposition and its utilization by microbial groups depends on N fertilization. Biol. Fertil. Soils 2017, 53, 37–48. [Google Scholar] [CrossRef]
- Pausch, J.; Kuzyakov, Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Chang. Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef]
- Makela, A.; Valentine, H.T.; Helmisaari, H.-S. Optimal co-allocation of carbon and nitrogen in a forest stand at steady state. New Phytol. 2008, 180, 114–123. [Google Scholar] [CrossRef]
- Callaway, R.M.; Pennings, S.C.; Richards, C.L. Phenotypic plasticity and interactions among plants. Ecology 2003, 84, 1115–1128. [Google Scholar] [CrossRef]
- Grime, J.P.; Crick, J.C.; Rincon, J.E. The ecological significance of plasticity. Symp. Soc. Exp. Biol. 1986, 40, 5–29. [Google Scholar]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L. Carbon allocation in a mixed-species plantation of Eucalyptus globulusand Acacia mearnsii. For. Ecol. Manag. 2006, 233, 275–284. [Google Scholar] [CrossRef]
- Craine, J.M.; Tilman, D.; Wedin, D.; Reich, P.; Tjoelker, M.; Knops, J. Functional traits, productivity and effects on nitrogen cycling of 33 grassland species. Funct. Ecol. 2002, 16, 563–574. [Google Scholar] [CrossRef]
- Yang, F.; Huang, S.; Gao, R.; Liu, W.; Yong, T.; Wang, X.; Wu, X.; Yang, W. Growth of soybean seedlings in relay strip intercropping systems in relation to light quantity and red:far-red ratio. Field Crops Res. 2014, 155, 245–253. [Google Scholar] [CrossRef]
- Zhang, L.; van der Werf, W.; Bastiaans, L.; Zhang, S.; Li, B.; Spiertz, J.H.J. Light interception and utilization in relay intercrops of wheat and cotton. Field Crops Res. 2008, 107, 29–42. [Google Scholar] [CrossRef]
- Duchene, O.; Vian, J.-F.; Celette, F. Intercropping with legume for agroecological cropping systems: Complementarity and facilitation processes and the importance of soil microorganisms. A review. Agric. Ecosyst. Environ. 2017, 240, 148–161. [Google Scholar] [CrossRef]
- Hu, L.; Huang, R.; Deng, H.; Li, K.; Peng, J.; Zhou, L.; Ou, H. Effects of Different Intercropping Methods on Soil Organic Carbon and Aggregate Stability in Sugarcane Field. Pol. J. Environ. Stud. 2022, 31, 3587–3596. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Yu, L.; Shu, Y.; Tan, F.; Gou, Y.; Luo, S.; Yang, W.; Li, Z.; Wang, J. Sugarcane/soybean intercropping with reduced nitrogen input improves crop productivity and reduces carbon footprint in China. Sci. Total Environ. 2020, 719, 137517. [Google Scholar] [CrossRef]
- Darch, T.; Giles, C.D.; Blackwell, M.S.A.; George, T.S.; Brown, L.K.; Menezes-Blackburn, D.; Shand, C.A.; Stutter, M.I.; Lumsdon, D.G.; Mezeli, M.M.; et al. Inter- and intra-species intercropping of barley cultivars and legume species, as affected by soil phosphorus availability. Plant Soil 2018, 427, 125–138. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Z.; Dong, S. Interspecific competitiveness affects the total biomass yield in an alfalfa and corn intercropping system. Field Crops Res. 2011, 124, 66–73. [Google Scholar] [CrossRef]
- Fernandez-Martinez, M.; Vicca, S.; Janssens, I.A.; Sardans, J.; Luyssaert, S.; Campioli, M.; Chapin, F.S., III; Ciais, P.; Malhi, Y.; Obersteiner, M.; et al. Nutrient availability as the key regulator of global forest carbon balance. Nat. Clim. Chang. 2014, 4, 471–476. [Google Scholar] [CrossRef]
- Chowdhury, M.; Rosario, E.J.T. Comparison of nitrogen, phosphorus and potassium utilization efficiency in maize/mungbean intercropping. J. Agric. Sci. 1994, 122, 193–199. [Google Scholar] [CrossRef]
- Zhang, F.S.; Li, L. Using competitive and facilitative interactions in intercropping systems enhances crop productivity and nutrient-use efficiency. Plant Soil 2003, 248, 305–312. [Google Scholar] [CrossRef]
- Neilson, E.H.; Goodger, J.Q.D.; Woodrow, I.E.; Moller, B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2013, 18, 250–258. [Google Scholar] [CrossRef]
- Chen, X.; Sun, N.; Gu, Y.; Liu, Y.; Li, J.; Wu, C.; Wang, Z. Maize-Soybean Strip Intercropping Improved Lodging Resistance and Productivity of Maize. Int. J. Agric. Biol. 2020, 24, 1383–1392. [Google Scholar] [CrossRef]
- Yang, W.; Li, Z.; Wang, J.; Wu, P.; Zhang, Y. Crop yield, nitrogen acquisition and sugarcane quality as affected by interspecific competition and nitrogen application. Field Crops Res. 2013, 146, 44–50. [Google Scholar] [CrossRef]
- Feng, C.; Sun, Z.; Zhang, L.; Feng, L.; Zheng, J.; Bai, W.; Gu, C.; Wang, Q.; Xu, Z.; van der Werf, W. Maize/peanut intercropping increases land productivity: A meta-analysis. Field Crops Res. 2021, 270, 108208. [Google Scholar] [CrossRef]
- Li, L.; Tilman, D.; Lambers, H.; Zhang, F.-S. Plant diversity and overyielding: Insights from belowground facilitation of intercropping in agriculture. New Phytol. 2014, 203, 63–69. [Google Scholar] [CrossRef]
- Li, L.; Sun, J.H.; Zhang, F.S.; Guo, T.W.; Bao, X.G.; Smith, F.A.; Smith, S.E. Root distribution and interactions between intercropped species. Oecologia 2006, 147, 280–290. [Google Scholar] [CrossRef]
- Yu, Y.; Stomph, T.-J.; Makowski, D.; van der Werf, W. Temporal niche differentiation increases the land equivalent ratio of annual intercrops: A meta-analysis. Field Crops Res. 2015, 184, 133–144. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, L.; Yang, H.; Gao, Y.; Liu, W.; Yang, W. Ameliorated light conditions increase the P uptake capability of soybean in a relay-strip intercropping system by altering root morphology and physiology in the areas with low solar radiation. Sci. Total Environ. 2019, 688, 1069–1080. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, L.; Shao, Y.; Wang, J. Root and hyphal interactions influence N transfer by arbuscular mycorrhizal fungi in soybean/maize intercropping systems. Fungal Ecol. 2023, 64, 101240. [Google Scholar] [CrossRef]
- Ortez, O.A.; Salvagiotti, F.; Enrico, J.M.; Prasad, P.V.V.; Armstrong, P.; Ciampitti, I.A. Exploring Nitrogen Limitation for Historical and Modern Soybean Genotypes. Agron. J. 2018, 110, 2080–2090. [Google Scholar] [CrossRef]
- Roohi, M.; Arif, M.S.; Guillaume, T.; Yasmeen, T.; Riaz, M.; Shakoor, A.; Farooq, T.H.; Shahzad, S.M.; Bragazza, L. Role of fertilization regime on soil carbon sequestration and crop yield in a maize-cowpea intercropping system on low fertility soils. Geoderma 2022, 428, 116152. [Google Scholar] [CrossRef]
- Tian, J.; Pausch, J.; Fan, M.; Li, X.; Tang, Q.; Kuzyakov, Y. Allocation and dynamics of assimilated carbon in rice-soil system depending on water management. Plant Soil 2013, 363, 273–285. [Google Scholar] [CrossRef]
- Jin, J.; Wang, G.H.; Liu, J.D.; Liu, X.B.; Liu, J.J.; Yu, Z.H.; Herbert, S.J. Seasonal allocation of photosynthetically fixed carbon to the soybean-grown Mollisols in Northeast China. Crop Pasture Sci. 2011, 62, 563–570. [Google Scholar] [CrossRef]
- Lian, T.; Mu, Y.; Jin, J.; Ma, Q.; Cheng, Y.; Cai, Z.; Nian, H. Impact of intercropping on the coupling between soil microbial community structure, activity, and nutrient-use efficiencies. Peerj 2019, 7, e6412. [Google Scholar] [CrossRef]
- Altieri, M.A. The ecological role of biodiversity in agroecosystems. In Invertebrate Biodiversity as Bioindicators of Sustainable Landscapes; Elsevier: Amsterdam, The Netherlands, 1999; pp. 19–31. [Google Scholar] [CrossRef]
- Muscolo, A.; Settineri, G.; Attina, E. Early warning indicators of changes in soil ecosystem functioning. Ecol. Indic. 2015, 48, 542–549. [Google Scholar] [CrossRef]
- Lemaire, G.; Franzluebbers, A.; de Faccio Carvalho, P.C.; Dedieu, B. Integrated crop-livestock systems: Strategies to achieve synergy between agricultural production and environmental quality. Agric. Ecosyst. Environ. 2014, 190, 4–8. [Google Scholar] [CrossRef]
- Silva, L.S.; dos Santos Laroca, J.V.; Coelho, A.P.; Gonçalves, E.C.; Gomes, R.P.; Pacheco, L.P.; de Faccio Carvalho, P.C.; Pires, G.C.; Oliveira, R.L.; de Souza, J.M.A. Does grass-legume intercropping change soil quality and grain yield in integrated crop-livestock systems? Appl. Soil Ecol. 2022, 170, 104257. [Google Scholar] [CrossRef]
- Tang, X.; Bernard, L.; Brauman, A.; Daufresne, T.; Deleporte, P.; Desclaux, D.; Souche, G.; Placella, S.A.; Hinsinger, P. Increase in microbial biomass and phosphorus availability in the rhizosphere of intercropped cereal and legumes under field conditions. Soil Biol. Biochem. 2014, 75, 86–93. [Google Scholar] [CrossRef]
- Chavez, L.F.; Escobar, L.F.; Anghinoni, I.; de Faccio Carvalho, P.C.; Meurer, E.J. Metabolic diversity and microbial activity in the soil in an integrated crop-livestock system under grazing intensities. Pesqui. Agropecu. Bras. 2011, 46, 1254–1261. [Google Scholar] [CrossRef]
- Kooch, Y.; Sanji, R.; Tabari, M. Increasing tree diversity enhances microbial and enzyme activities in temperate Iranian forests. Trees-Struct. Funct. 2018, 32, 809–822. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, J.; Lu, C.; Ou, X.; Luo, K.; Li, C.; He, M.; Zhang, H.; Yan, H. Intercropping With Turmeric or Ginger Reduce the Continuous Cropping Obstacles That Affect Pogostemon cablin (Patchouli). Front. Microbiol. 2020, 11, 579719. [Google Scholar] [CrossRef]
- Yao, M.; Rui, J.; Li, J.; Dai, Y.; Bai, Y.; Hedenec, P.; Wang, J.; Zhang, S.; Pei, K.; Liu, C.; et al. Rate-specific responses of prokaryotic diversity and structure to nitrogen deposition in the Leymus chinensis steppe. Soil Biol. Biochem. 2014, 79, 81–90. [Google Scholar] [CrossRef]
- Chu, H.; Fierer, N.; Lauber, C.L.; Caporaso, J.; Knight, R.; Grogan, P. Soil bacterial diversity in the Arctic is not fundamentally different from that found in other biomes. Environ. Microbiol. 2010, 12, 2998–3006. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Xiong, J.; Zhang, H.; Feng, Y.; Lin, X.; Li, X.; Liang, W.; Chu, H. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- Ritson, J.P.; Graham, N.J.D.; Templeton, M.R.; Clark, J.M.; Gough, R.; Freeman, C. The impact of climate change on the treatability of dissolved organic matter (DOM) in upland water supplies: A UK perspective. Sci. Total Environ. 2014, 473, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Liu, C.; Li, J.; Luo, Y.; Yang, Q.; Zhang, W.; Yang, P.; Feng, B. Responses of rhizosphere soil properties, enzyme activities and microbial diversity to intercropping patterns on the Loess Plateau of China. Soil Tillage Res. 2019, 195, 104355. [Google Scholar] [CrossRef]
- Wang, D.; Yi, W.; Zhou, Y.; He, S.; Tang, L.; Yin, X.; Zhao, P.; Long, G. Intercropping and N application enhance soil dissolved organic carbon concentration with complicated chemical composition. Soil Tillage Res. 2021, 210, 104979. [Google Scholar] [CrossRef]
- Zhang, P.; Nie, M.; Li, B.; Wu, J. The transfer and allocation of newly fixed C by invasive Spartina alterniflora and native Phragmites australis to soil microbiota. Soil Biol. Biochem. 2017, 113, 231–239. [Google Scholar] [CrossRef]
- Wang, Z.-g.; Bao, X.-g.; Li, X.-f.; Jin, X.; Zhao, J.-h.; Sun, J.-h.; Christie, P.; Li, L. Intercropping maintains soil fertility in terms of chemical properties and enzyme activities on a timescale of one decade. Plant Soil 2015, 391, 265–282. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil—ScienceDirect. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]







| Treatment | Nitrogen Application | Cropping Patterns |
|---|---|---|
| MSN1 | 300 | Sugarcane monocropping |
| SB1N1 | 300 | Sugarcane/soybean intercropping (1:1) |
| SB2N1 | 300 | Sugarcane/soybean intercropping (1:2) |
| MSN2 | 525 | Sugarcane monocropping |
| SB1N2 | 525 | Sugarcane/soybean intercropping (1:1) |
| SB2N2 | 525 | Sugarcane/soybean intercropping (1:2) |
| MB | 0 | Soybean monocropping |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Liu, Y.; Li, L. Sugarcane/Soybean Intercropping with Reduced Nitrogen Application Synergistically Increases Plant Carbon Fixation and Soil Organic Carbon Sequestration. Plants 2024, 13, 2337. https://doi.org/10.3390/plants13162337
Zhang T, Liu Y, Li L. Sugarcane/Soybean Intercropping with Reduced Nitrogen Application Synergistically Increases Plant Carbon Fixation and Soil Organic Carbon Sequestration. Plants. 2024; 13(16):2337. https://doi.org/10.3390/plants13162337
Chicago/Turabian StyleZhang, Tantan, Yali Liu, and Lin Li. 2024. "Sugarcane/Soybean Intercropping with Reduced Nitrogen Application Synergistically Increases Plant Carbon Fixation and Soil Organic Carbon Sequestration" Plants 13, no. 16: 2337. https://doi.org/10.3390/plants13162337
APA StyleZhang, T., Liu, Y., & Li, L. (2024). Sugarcane/Soybean Intercropping with Reduced Nitrogen Application Synergistically Increases Plant Carbon Fixation and Soil Organic Carbon Sequestration. Plants, 13(16), 2337. https://doi.org/10.3390/plants13162337







