Nano-Biochar Suspension Mediated Alterations in Growth, Physio-Biochemical Activities and Nutrient Content in Wheat (Triticum aestivum L.) at the Vegetative Stage
Abstract
:1. Introduction
2. Results
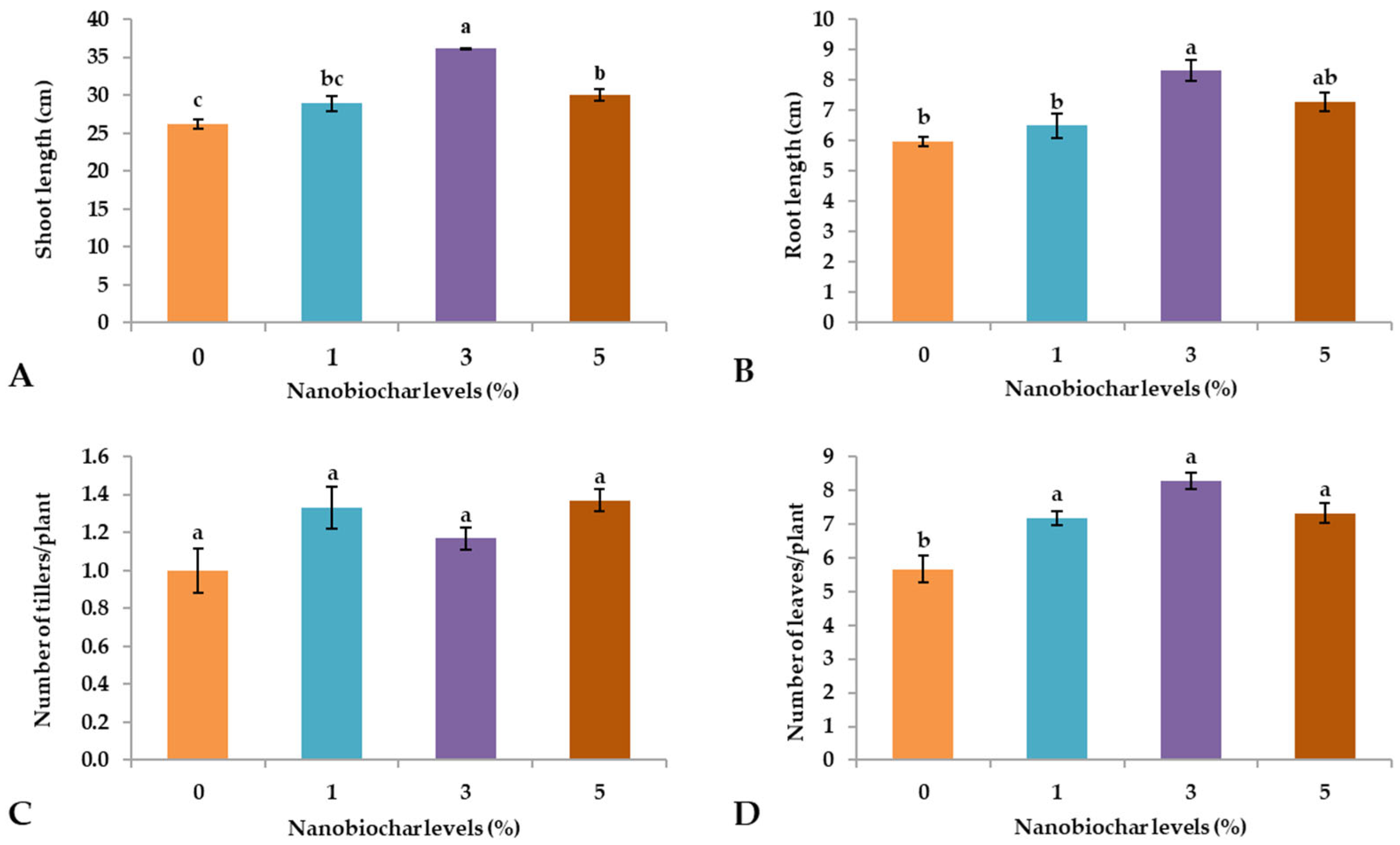
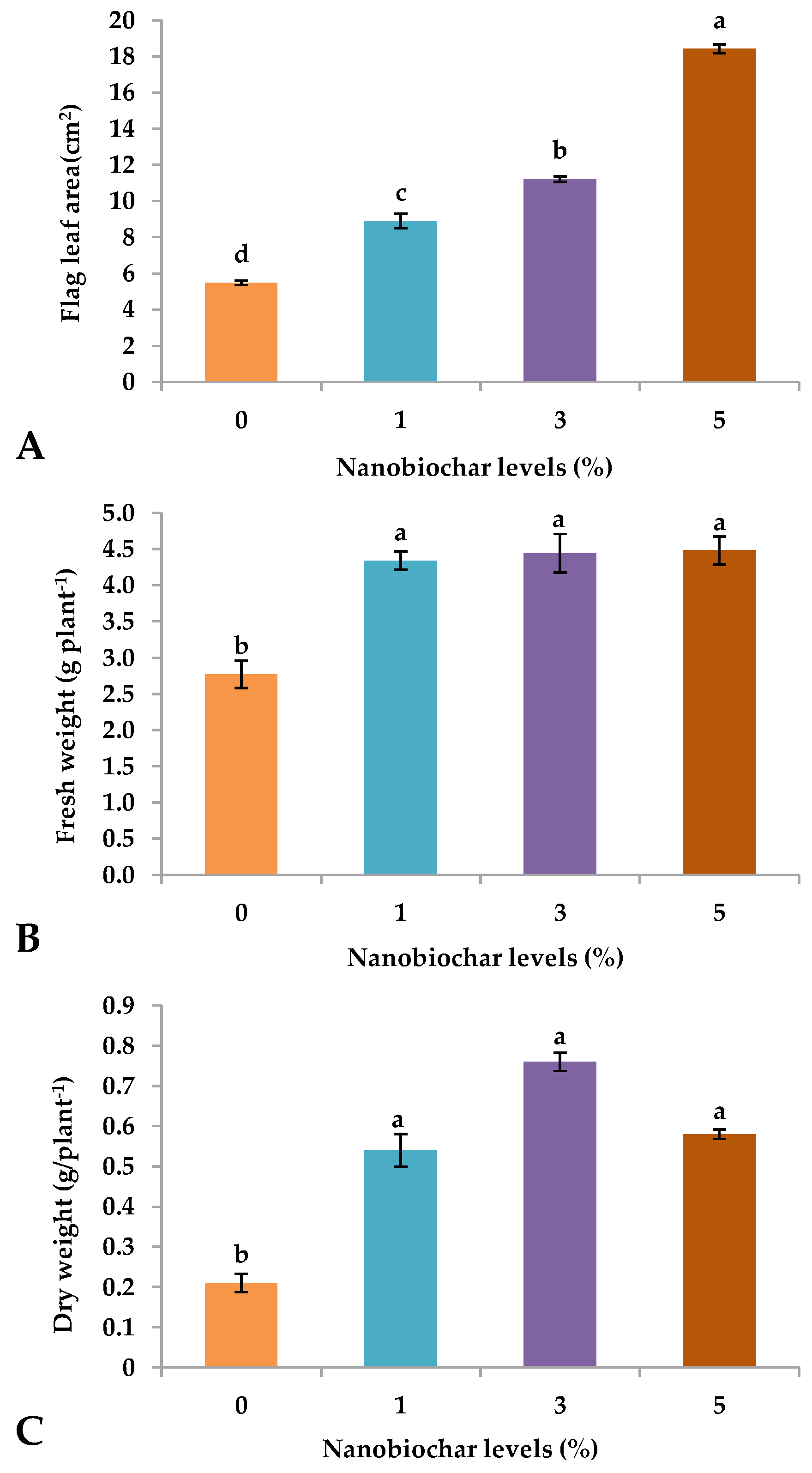
2.1. Growth Parameters
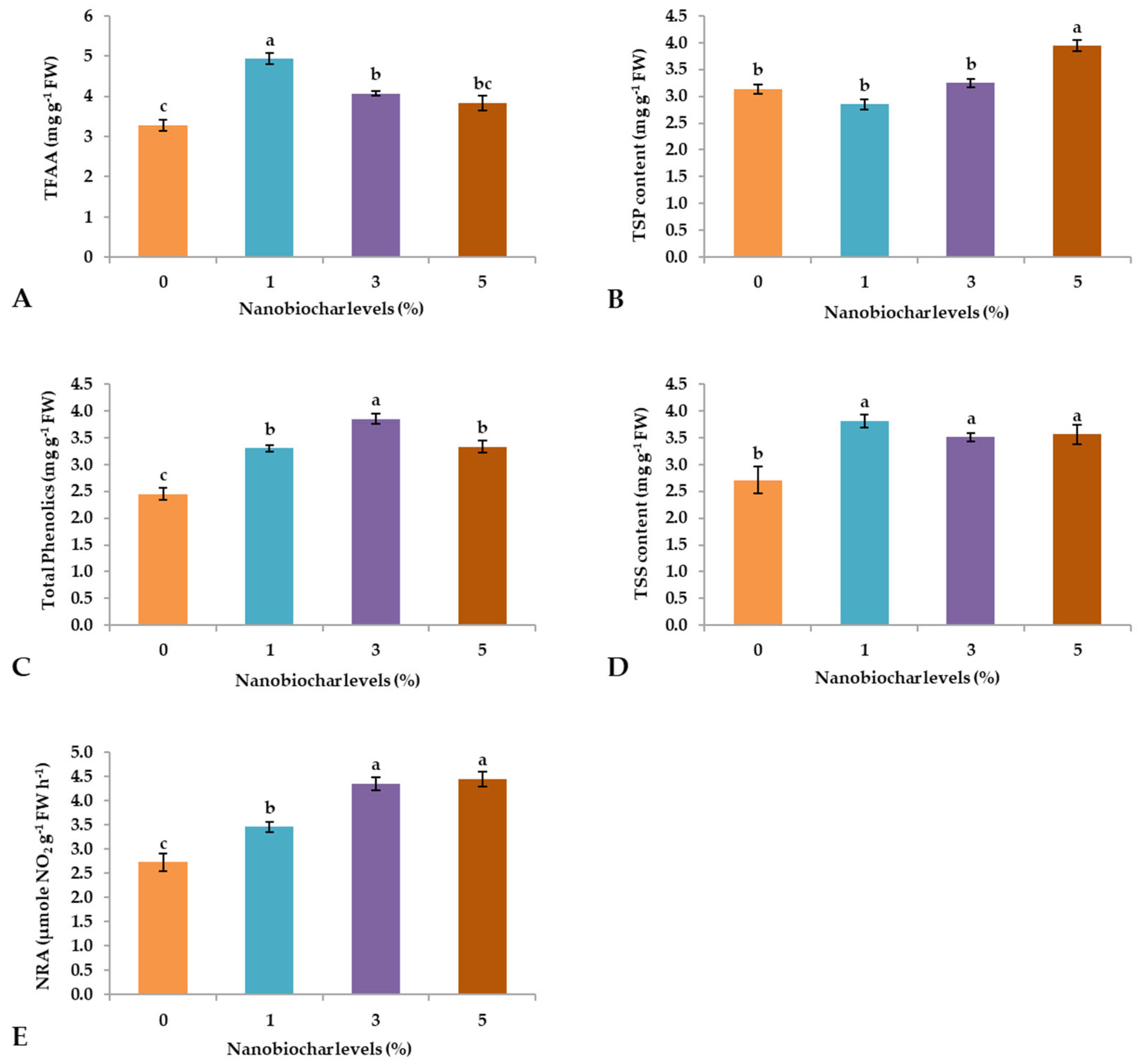
2.2. Biochemical Alterations
2.3. Physio-Biochemical Attributes
2.4. Nutrient Contents
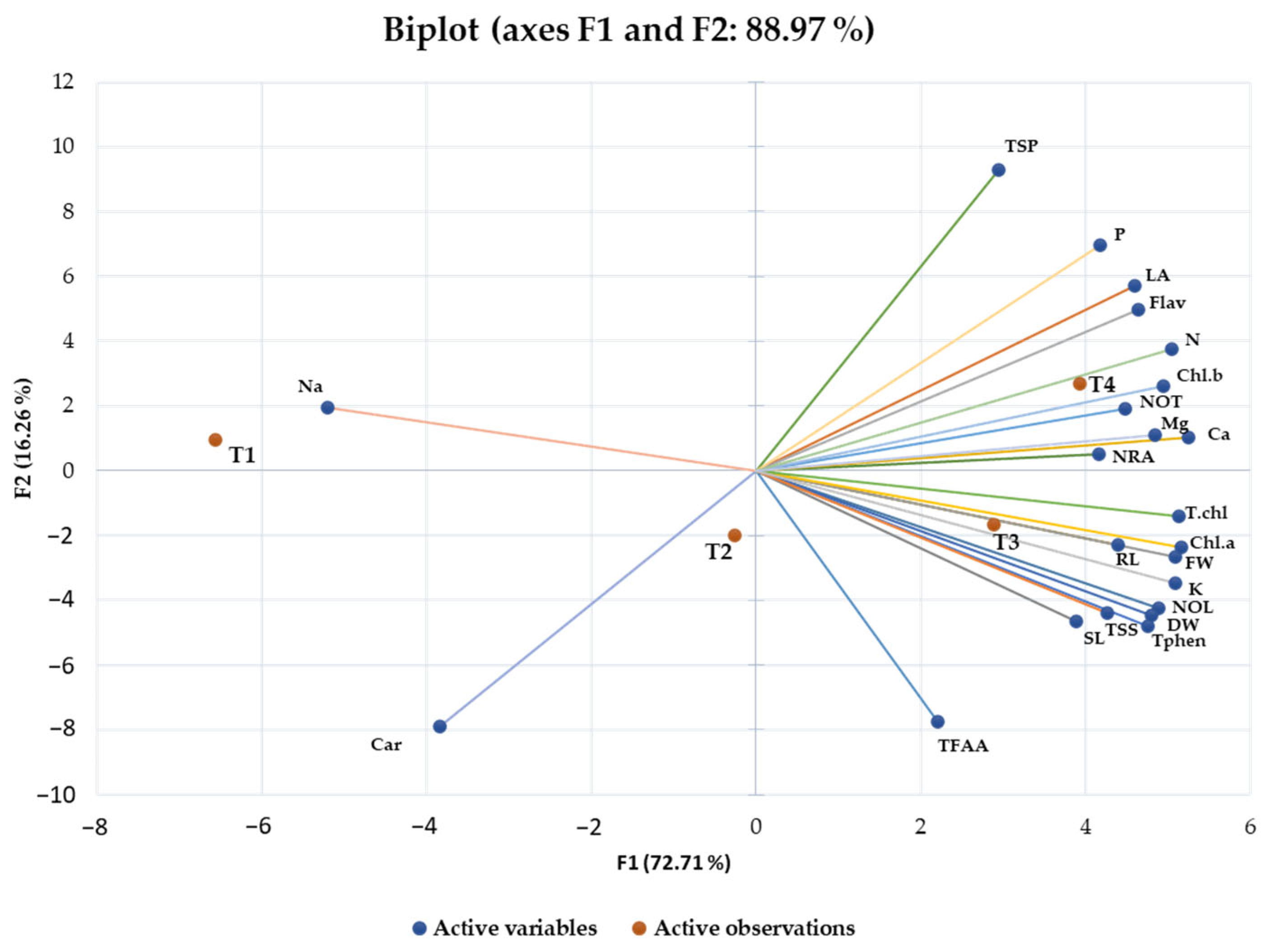
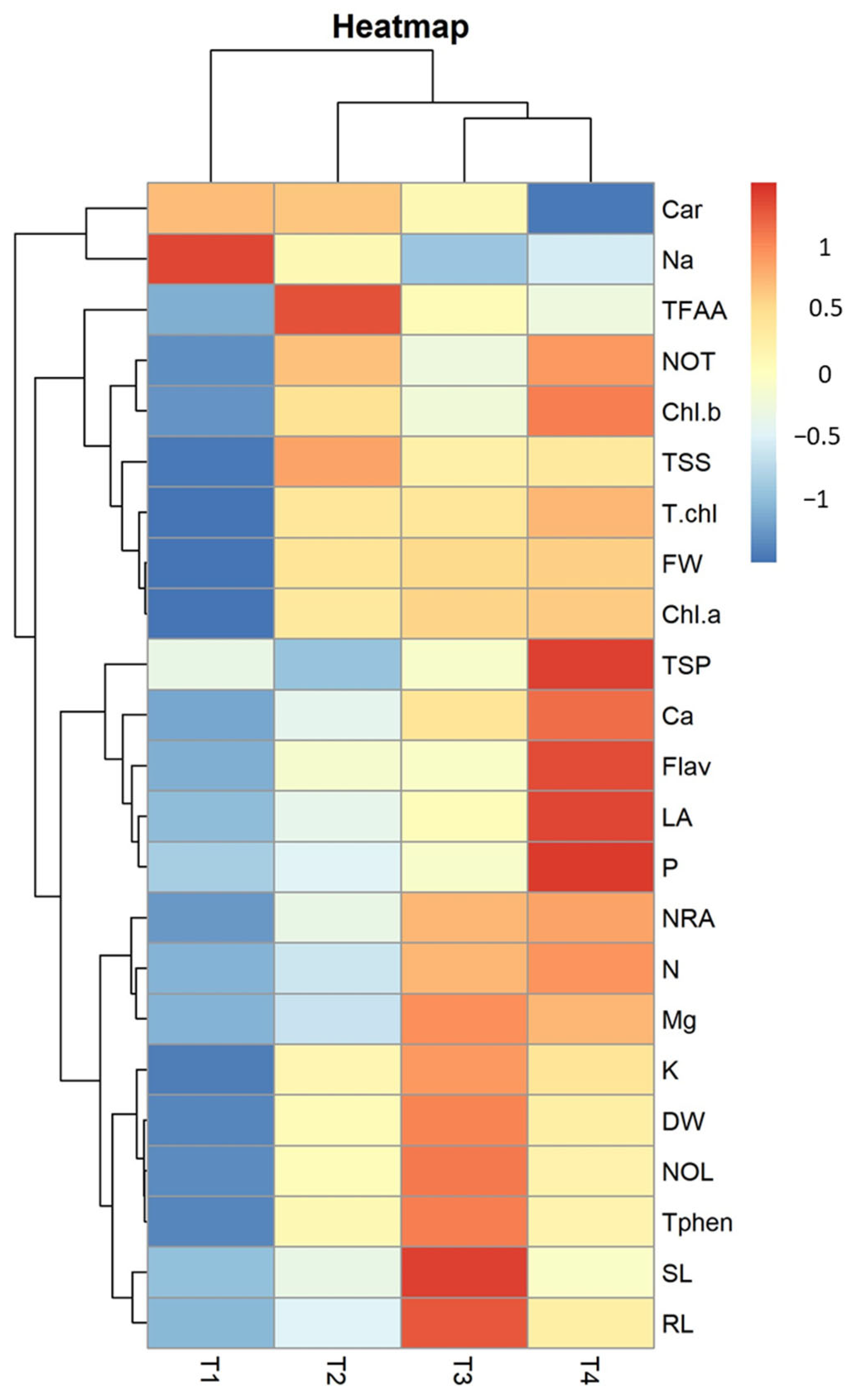
2.5. Principal Component and Heatmap Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Soil Analyses
4.2. Characterization of Nanobiochar Suspension
4.3. Estimation of Physio-Biochemical and Nutrient Contents at the Seedling Stages
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anjum, F.; Zafar, M.I.; Asghar, K.; Riaz, A. Gender Dimensions of Agriculture: Status, Trends, and Gap. In Developing Sustainable Agriculture in Pakistan; CRC Press: Boca Raton, FL, USA, 2018; pp. 613–634. [Google Scholar]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Alam, M.A.; Syed, M.A.; Islam, T. Consequences and mitigation strategies of abiotic stresses in wheat (Triticum aestivum L.) under the changing climate. Agronomy 2021, 11, 241. [Google Scholar] [CrossRef]
- Katileviciute, A.; Plakys, G.; Budreviciute, A.; Onder, K.; Damiati, S.; Kodzius, R. A sight to wheat bran: High value-added products. Biomolecules 2019, 9, 887. [Google Scholar] [CrossRef]
- Ladha, J.K.; Peoples, M.B.; Reddy, P.M.; Biswas, J.C.; Bennett, A.; Jat, M.L.; Krupnik, T.J. Biological nitrogen fixation and prospects for ecological intensification in cereal-based cropping systems. Field Crops Res. 2022, 283, 108541. [Google Scholar] [CrossRef]
- Sári, D.; Ferroudj, A.; Abdalla, N.; El-Ramady, H.; Dobránszki, J.; Prokisch, J. Nano-Management Approaches for Salt Tolerance in Plants under Field and In Vitro Conditions. Agronomy 2023, 13, 2695. [Google Scholar] [CrossRef]
- Jat, M.L.; Stirling, C.M.; Jat, H.S.; Tetarwal, J.P.; Jat, R.K.; Singh, R.; Shirsath, P.B. Soil processes and wheat cropping under emerging climate change scenarios in South Asia. Adv. Agron. 2018, 148, 111–171. [Google Scholar]
- Abhiram, G. Contributions of Nano-Nitrogen Fertilizers to Sustainable Development Goals: A Comprehensive Review. Nitrogen 2023, 4, 397–415. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.L.; Li, Y.R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef]
- Shaukat, M.; Sun, M.; Ali, M.; Mahmood, T.; Naseer, S.; Maqbool, S.; Rehman, S.; Mahmood, Z.; Hao, Y.; Xia, X.; et al. Genetic gain for grain micronutrients and their association with phenology in historical wheat cultivars released between 1911 and 2016 in Pakistan. Agronomy 2021, 11, 1247. [Google Scholar] [CrossRef]
- Lowe, N.M.; Zaman, M.; Khan, M.J.; Brazier, A.K.; Shahzad, B.; Ullah, U.; Khobana, G.; Ohly, H.; Broadley, M.R.; Zia, M.H.; et al. Biofortified wheat increases dietary zinc intake: A randomised controlled efficacy study of Zincol-2016 in rural Pakistan. Front. Nutr. 2022, 8, 809783. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.; Wu, W. Soil and crop management strategies to ensure higher crop productivity within sustainable environments. Sustainability 2019, 11, 1485. [Google Scholar] [CrossRef]
- Abbas, S.; Javed, M.T.; Ali, Q.; Azeem, M.; Ali, S. Nutrient deficiency stress and relation with plant growth and development. In Engineering Tolerance in Crop Plants Against Abiotic Stress; CRC Press: Boca Raton, FL, USA, 2021; pp. 239–262. [Google Scholar]
- Haider, F.U.; Coulter, J.A.; Cai, L.; Hussain, S.; Cheema, S.A.; Wu, J.; Zhang, R. An overview on biochar production, its implications, and mechanisms of biochar-induced amelioration of soil and plant characteristics. Pedosphere 2022, 32, 107–130. [Google Scholar] [CrossRef]
- Shahbaz, A.K.; Ramzani, P.M.A.; Saeed, R.; Turan, V.; Iqbal, M.; Lewińska, K.; Rahman, M.U. Effects of biochar and zeolite soil amendments with foliar proline spray on nickel immobilization, nutritional quality and nickel concentrations in wheat. Ecotoxicol. Environ. Saf. 2019, 173, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Shani, M.Y.; Ahmed, S.R.; Ashraf, M.Y.; Khan, Z.; Cocozza, C.; De Mastro, F.; Brunetti, G. Nano-Biochar Suspension Mediated Alterations in Yield and Juice Quality of Kinnow (Citrus reticulata L.). Horticulturae 2023, 9, 521. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Kumar, S.; Jasrotia, P.; Singh, D.P.; Singh, G.P. Nanotechnology in wheat production and protection. In Environmental Nanotechnology; Springer: Cham, Switzerland, 2020; Volume 4, pp. 165–194. [Google Scholar]
- Yadav, A.; Yadav, K.; Abd-Elsalam, K.A. Nanofertilizers: Types, Delivery and Advantages in Agricultural Sustainability. Agrochemicals 2023, 2, 296–336. [Google Scholar] [CrossRef]
- Shafiq, H.; Shani, M.Y.; Ashraf, M.Y.; De Mastro, F.; Cocozza, C.; Abbas, S.; Brunetti, G. Copper Oxide Nanoparticles Induced Growth and Physio-Biochemical Changes in Maize (Zea mays L.) in Saline Soil. Plants 2024, 13, 1080. [Google Scholar] [CrossRef]
- Rasheed, A.; Anwar, S.; Shafiq, F.; Khan, S.; Ashraf, M. Physiological and biochemical effects of biochar nanoparticles on spinach exposed to salinity and drought stresses. Environ. Sci. Pollut. Res. 2024, 31, 14103–14122. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, C.; Rawat, S.; Cota-Ruiz, K.; Medina-Velo, I.; Gardea-Torresdey, J.L. Evaluation of the effects of nanomaterials on rice (Oryza sativa L.) responses: Underlining the benefits of nanotechnology for agricultural applications. ACS Agric. Sci. Technol. 2021, 1, 44–54. [Google Scholar] [CrossRef]
- Joshi, K.D.; Conroy, C.; Witcombe, J.R. Agriculture, seed, and innovation in Nepal: Industry and policy issues for the future. Gates Open Res. 2019, 3, 232. [Google Scholar]
- Hong, J.; Wang, C.; Wagner, D.C.; Gardea-Torresdey, J.L.; He, F.; Rico, C.M. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano 2021, 8, 1196–1210. [Google Scholar] [CrossRef]
- Ran, C.; Gulaqa, A.; Zhu, J.; Wang, W.W.; Zhang, S.Q.; Geng, Y.Q.; Guo, L.Y.; Jin, F.; Shao, X.W. Benefits of biochar for improving ion contents, cell membrane permeability, leaf water status and yield of rice under saline-sodic paddy field condition. J. Plant Growth Regul. 2019, 39, 370–377. [Google Scholar] [CrossRef]
- Pandey, A.; Khan, M.K.; Hakki, E.E.; Gezgin, S.; Hamurcu, M. Combined Boron Toxicity and Salinity Stress—An Insight into Its Interaction in Plants. Plants 2019, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J. Potassium Control of Plant Functions: Ecological and Agricultural Implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of exogenous proline to abiotic stresses tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Janati, W.; Benmrid, B.; Elhaissoufi, W.; Zeroual, Y.; Nasielski, J.; Bargaz, A. Will phosphate bio-solubilization stimulate biological nitrogen fixation in grain legumes? Front. Agron. 2021, 3, 637196. [Google Scholar] [CrossRef]
- Gerenfes, D.; Negasa, G. Review on phosphorus and zinc fertilizer application for enhanced performance of crops. J. Biol. Agric. Healthc. 2021, 11, 32–44. [Google Scholar]
- Saleem, S.; Mushtaq, N.U.; Rasool, A.; Shah, W.H.; Tahir, I.; Rehman, R.U. Plant nutrition and soil fertility: Physiological and molecular avenues for crop improvement. In Sustainable Plant Nutrition; Academic Press: Cambridge, MA, USA, 2023; pp. 23–49. [Google Scholar]
- Rohilla, P.; Yadav, J.P. Nitrate reductase structure, role and factors affecting its regulation: A review. Plant Arch. 2020, 20, 5787–5793. [Google Scholar]
- Junaid, M.D.; Öztürk, Z.N.; Gökçe, A.F. Drought stress effects on morphophysiological and quality characteristics of commercial carrot cultivars. Turk. J. Bot. 2023, 47, 111–126. [Google Scholar] [CrossRef]
- Khaliq, H.; Anwar, S.; Shafiq, F.; Ashraf, M.; Zhang, L.; Haider, I.; Khan, S. Interactive effects of soil and foliar-applied nanobiochar on growth, metabolites, and nutrient composition in Daucus carota. J. Plant Growth Regul. 2023, 42, 3715–3729. [Google Scholar] [CrossRef]
- Mubashir, A.; Nisa, Z.U.; Shah, A.A.; Kiran, M.; Hussain, I.; Ali, N.; AbdElgawad, H. Effect of foliar application of nano-nutrients solution on growth and biochemical attributes of tomato (Solanum lycopersicum) under drought stress. Front. Plant Sci. 2023, 13, 1066790. [Google Scholar] [CrossRef]
- Liu, C.; Tian, J.; Chen, L.; He, Q.; Liu, X.; Bian, R.; Zheng, J.; Cheng, K.; Xia, S.; Zhang, X.; et al. Biochar boosted high oleic peanut production with enhanced root development and biological N fixation by diazotrophs in a sand-loamy Primisol. Sci. Total Environ. 2024, 932, 173061. [Google Scholar] [CrossRef]
- Ahmad, S.; Zhai, X.; Wang, M.; Shi, Y.; Chen, Y.; Liang, Q.; He, B.; Wen, R. Biochar amendments improve soil functionalities, microbial community and reduce Pokkah boeng disease of sugarcane. Chem. Biol. Technol. Agric. 2024, 11, 28. [Google Scholar]
- Dewis, J.; Freitas, P. Physical and Chemical Methods of Soil and Water Analysis; FAO (Food and Agricultural Organization of the United Nations): Rome, Italy, 1970; Bulletin No. 10; p. 275. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall: Hoboken, NJ, USA, 1962; p. 498. [Google Scholar]
- Shani, M.Y.; Ashraf, M.Y.; Butt, A.K.; Abbas, S.; Nasif, M.; Khan, Z.; Amin, F. Potassium Nutrition Induced Salinity Mitigation in Mungbean [Vigna radiata (L.) Wilczek] by Altering Biomass and Physio-Biochemical Processes. Horticulturae 2024, 10, 549. [Google Scholar] [CrossRef]
- Miller, T.D. Growth Stages of Wheat: Identification and Understandings Improve Crop Management; Potash and Phosphate Institute (PPI): Norcorss, GA, USA, 1992; SCS 1999-16. [Google Scholar]
- Sym, G.J. Optimisation of the in-vivo Assay conditions for Nitrate Reductase in Barley (Hordeum vulgare). J. Sci. Food Agric. 1984, 35, 725–730. [Google Scholar] [CrossRef]
- Hamilton, T. Mad like Tesla: Underdog Inventors and Their Relentless Pursuit of Clean Energy; ECW Press: Toronto, ON, Canada, 2011. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951, 191, 265–275. [Google Scholar] [CrossRef]
- Riazi, A.K.; Matruda, A.; Arslam, A. Water stress induce changes in concentration of proline and other solutes in growing regions. J. Exp. Bot. 1985, 36, 1716–1725. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reduction for flavonoid food. Anal. Methods 2014, 7, 1776–1872. [Google Scholar] [CrossRef]
- Arnon, D.T. Copper enzymes in isolated chloroplasts polyphenaloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Davies, B.H. Carotenoids. In Chemistry and Biochemistry of Plant Pigments; Academic Press: London, UK, 1976; Volume II. [Google Scholar]
- Bremner, J.M. Total nitrogen and inorganic forms of nitrogen. In Methods of Soil Analysis; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; Part 2; pp. 1149–1237. [Google Scholar]



| (a) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | DF | SL | RL | FW | DW | NOL | NOT | LA | NRA | TFAA | TSP | T.Phen | TSSs | Flav | Chl.a | Chl.b | T.chl | Car |
| Treatment | 3 | 53 *** | 3.07 *** | 2.05 ** | 0.15 * | 3.49 * | 0.08 ns | 89.94 *** | 1.97 ** | 1.36 ** | 0.64 ** | 1.007 *** | 0.68 ** | 0.52 *** | 0.137 ** | 0.022 * | 0.11 ** | 1.04 ** |
| Error | 6 | 4.10 | 0.03 | 0.12 | 0.013 | 0.25 | 0.02 | 0.14 | 0.06 | 0.06 | 0.03 | 0.001 | 0.01 | 0.002 | 0.004 | 0.003 | 0.004 | 0.06 |
| Total | 11 | |||||||||||||||||
| (b) | ||||||||||||||||||
| Source | DF | Na | K | P | N | Ca | Mg | |||||||||||
| Treatment | 3 | 1.02 *** | 20.06 *** | 0.39 ** | 25.52 *** | 0.14 ** | 1.21 ** | |||||||||||
| Error | 6 | 0.006 | 0.22 | 0.008 | 0.07 | 0.01 | 0.02 | |||||||||||
| Total | 11 | |||||||||||||||||
| Soil Characteristics | Values |
|---|---|
| Physiological characters | |
| Soil texture | Clay loam |
| Chemical characters | |
| Saturation percentage (%) | 40–41 |
| ECe (dS m−1) | 1.7–1.9 |
| pH | 7.5–7.8 |
| Organic matter (%) | 0.5–0.7 |
| Ca+Mg (meq L−1) | 2.6–4.8 |
| CO3 (meq L−1) | Nil |
| HCO3 (meq L−1) | 2.5–4.8 |
| NO3-N (mg kg−1) | 3.5–4.5 |
| Total nitrogen (g kg−1) | 0.4–0.5 |
| Available K (mg kg−1) | 75–80 |
| Available P (mg kg−1) | 1.5–2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shani, M.Y.; Ahmad, S.; Ashraf, M.Y.; Nawaz, M.; Arshad, I.; Anjum, A.; De Mastro, F.; Cocozza, C.; Khan, Z.; Gul, N.; et al. Nano-Biochar Suspension Mediated Alterations in Growth, Physio-Biochemical Activities and Nutrient Content in Wheat (Triticum aestivum L.) at the Vegetative Stage. Plants 2024, 13, 2347. https://doi.org/10.3390/plants13172347
Shani MY, Ahmad S, Ashraf MY, Nawaz M, Arshad I, Anjum A, De Mastro F, Cocozza C, Khan Z, Gul N, et al. Nano-Biochar Suspension Mediated Alterations in Growth, Physio-Biochemical Activities and Nutrient Content in Wheat (Triticum aestivum L.) at the Vegetative Stage. Plants. 2024; 13(17):2347. https://doi.org/10.3390/plants13172347
Chicago/Turabian StyleShani, Muhammad Yousaf, Samia Ahmad, Muhammad Yasin Ashraf, Maria Nawaz, Iqra Arshad, Arslan Anjum, Francesco De Mastro, Claudio Cocozza, Zafran Khan, Nimra Gul, and et al. 2024. "Nano-Biochar Suspension Mediated Alterations in Growth, Physio-Biochemical Activities and Nutrient Content in Wheat (Triticum aestivum L.) at the Vegetative Stage" Plants 13, no. 17: 2347. https://doi.org/10.3390/plants13172347





