Climate Change Impacts on and Response Strategies for Kiwifruit Production: A Comprehensive Review
Abstract
:1. Introduction
2. Climate Change and Its Manifestations in Subtropical Regions
2.1. Global Temperature Rise and Its Impact on Kiwifruit Production
2.2. Frosting and Its Impact on Kiwifruit-Producing Regions
2.3. Changes in Extreme Weather Events: Droughts, Floods, and Typhoons
3. Effects of Climate Change on Kiwifruit Trees
3.1. Phenological Changes: Flowering, Fruiting, and Ripening
3.2. Shift in Distribution Ranges and Suitable Growing Regions
3.3. Impact on Plant Physiology: Photosynthesis, Respiration, and Water Stress
3.4. Increased Susceptibility to Pests and Diseases
4. Economic and Social Implications for Kiwifruit Production
4.1. Losses in Kiwifruit Production and Yield
4.2. Impacts on Livelihoods of Kiwifruit Farmers and Agricultural Communities
4.3. Market Fluctuations and Global Trade Dynamics of Kiwifruit
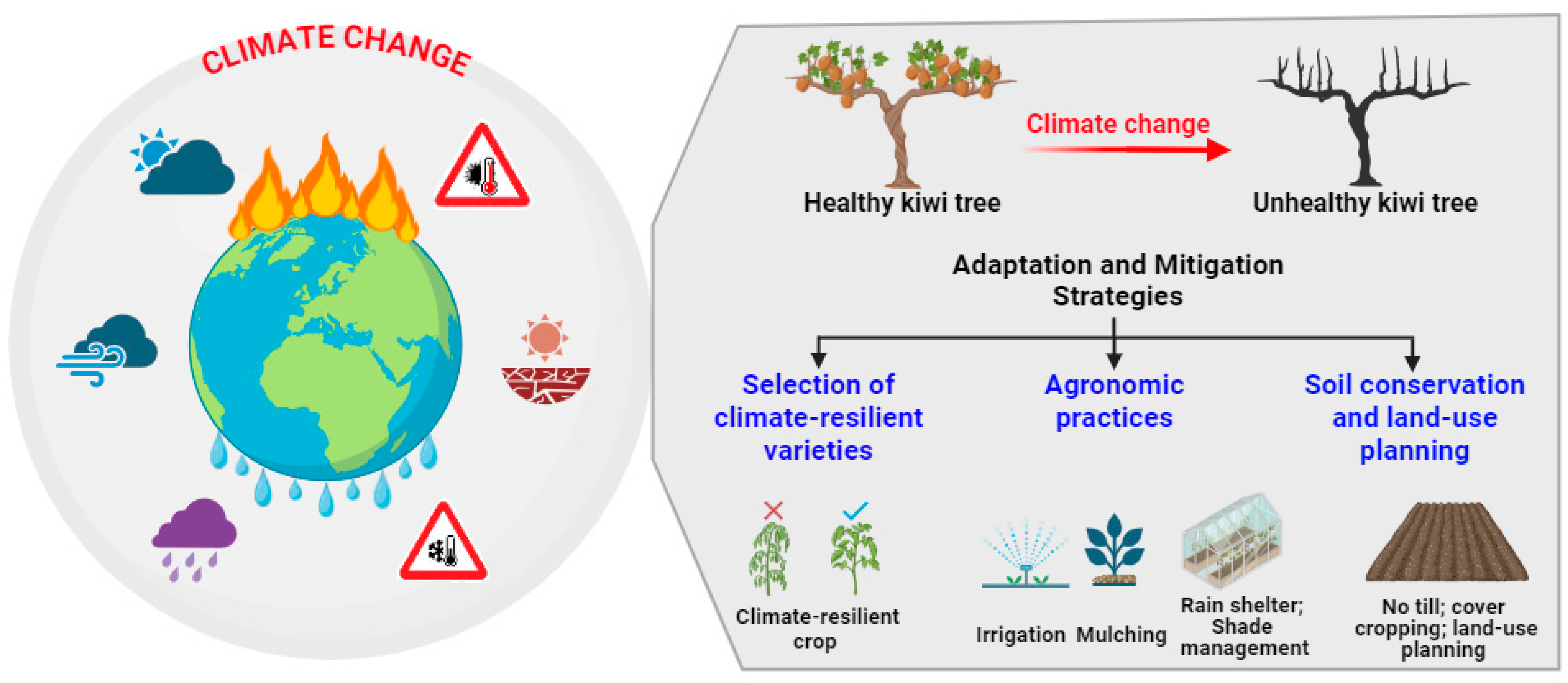
5. Adaptation and Mitigation Strategies for Kiwifruit Cultivation
5.1. Breeding and Selection of Climate-Resilient Kiwifruit Cultivars
5.2. Agronomic Practices: Irrigation, Mulching, Rain-Shelter Cultivation System, and Shade Management for Kiwifruit Orchards
5.3. Soil Conservation and Land-Use Planning Specific to Kiwifruit Farming
6. Case Studies and Success Stories of Kiwifruit Farming
6.1. Examples of Climate Change Adaptation in Kiwifruit Cultivation
6.1.1. Phenology Management
6.1.2. Water Resource Management
6.1.3. Pest and Disease Control
7. Methods
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kaviani, B.; Kulus, D. Cryopreservation of endangered ornamental plants and fruit crops from tropical and subtropical regions. Biology 2022, 11, 847. [Google Scholar] [CrossRef]
- Galán Saúco, V.; Farré Massip, J.M. Tropical and subtropical fruits in spain. Acta Hortic. 2005, 694, 259–264. [Google Scholar] [CrossRef]
- Jat, R.K.; Kumar, M.; Lal Jat, M.; Shivran, J.S. A Review on Use of Micronutrients in Tropical and Subtropical Fruit Crops. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2744–2753. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Mohd Adzahan, N.; Abdul Rahman, R.; Zainal Abedin, N.H.; Hussain, N.; Sulaiman, R.; Chong, G.H. Current trends of tropical fruit waste utilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 335–361. [Google Scholar] [CrossRef] [PubMed]
- Sayago-Ayerdi, S.; García-Martínez, D.L.; Ramírez-Castillo, A.C.; Ramírez-Concepción, H.R.; Viuda-Martos, M. Tropical Fruits and Their Co-Products as Bioactive Compounds and Their Health Effects: A Review. Foods 2021, 10, 1952. [Google Scholar] [CrossRef]
- Suriati, L.; Utama, I.M.S.; Harjosuwono, B.A.; Gunam, I.B.W. Physicochemical Characteristics of Fresh-cut Tropical Fruit during Storage. Int. J. Adv. Sci. Eng. Inf. Technol. 2020, 10, 1731–1736. [Google Scholar] [CrossRef]
- Lasekan, O.; Abbas, K.A. Distinctive Exotic Flavor and Aroma Compounds of some Exotic Tropical Fruits and Berries: A Review. Crit. Rev. Food Sci. Nutr. 2012, 52, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Maia, G.A.; Da Silva, L.M.R.; Do Prado, G.M.; Fonseca, A.V.V.; De Sousa, P.H.M.; De Figueiredo, R.W. Development of Mixed Beverages Based on Tropical Fruits. In Non-Alcoholic Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 129–162. [Google Scholar]
- He, Z.; Lu, X.; Cui, N.; Jiang, S.; Zheng, S.; Chen, F.; Qiu, R.; Liu, C.; Fan, J.; Wang, Y.; et al. Effect of soil water content threshold on kiwifruit quality at different growth stages with drip irrigation in the humid area of Southern China. Sci. Hortic. 2023, 307, 111477. [Google Scholar] [CrossRef]
- Mammadzade, E.A.; Aliyeva, A.Y.; Malikzade, J.T. Development perspectives of agriculture of the southern region. Ecoenergetics 2022, 18, 3–8. [Google Scholar]
- Ahmad, I.; Chwee, C.P. An overview of the world production and marketing of tropical and subtropical fruits. Acta Hortic. 2008, 787, 47–58. [Google Scholar] [CrossRef]
- Mohamed, Z.; AbdLatif, I.; Mahir Abdullah, A. Economic importance of tropical and subtropical fruits. In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–20. [Google Scholar]
- Sarkar, T.; Salauddin, M.; Roy, A.; Sharma, N.; Sharma, A.; Yadav, S.; Jha, V.; Rebezov, M.; Khayrullin, M.; Thiruvengadam, M.; et al. Minor tropical fruits as a potential source of bioactive and functional foods. Crit. Rev. Food Sci. Nutr. 2023, 63, 6491–6535. [Google Scholar] [CrossRef]
- Yahia, E.M.; De Jesus Ornelas-Paz, J.; Gonzalez-Aguilar, G.A. Nutritional and health-promoting properties of tropical and subtropical fruits. In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Elsevier: Amsterdam, The Netherlands, 2011; pp. 21–78. [Google Scholar]
- Mitra, S.K.; Devi, H.L.; Debnath, S. Tropical and subtropical fruits and human health. Acta Hortic. 2014, 1024, 39–47. [Google Scholar] [CrossRef]
- Huang, Z.G.; Tan, C.Y. Chinese Gooseberry, Kiwifruit (Actinidia spp.). In Crops, I.I.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 166–180. [Google Scholar]
- Gorinstein, S.; Haruenkit, R.; Poovarodom, S.; Park, Y.S.; Vearasilp, S.; Vearasilp, S.; Suhaj, M.; Ham, K.-S.; Heo, B.-G.; Cho, J.-Y.; et al. The comparative characteristics of snake and kiwi fruits. Food Chem. Toxicol. 2009, 47, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Mishra, A.K.; Priya, S. Potential Health Benefits of Kiwifruits: The King of Fruits. J. Sci. Technol. 2020, 5, 126–131. [Google Scholar]
- Medda, S.; Fadda, A.; Mulas, M. Influence of Climate Change on Metabolism and Biological Characteristics in Perennial Woody Fruit Crops in the Mediterranean Environment. Horticulturae 2022, 8, 273. [Google Scholar] [CrossRef]
- Bardi, L.; Nari, L.; Morone, C.; Faga, M.G.; Malusà, E. Possible Role of High Temperature and Soil Biological Fertility on Kiwifruit Early Decline Syndrome. Front. Agron. 2020, 2, 580659. [Google Scholar] [CrossRef]
- Burdon, J.; Lallu, N. Kiwifruit (Actinidia spp.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Elsevier: Amsterdam, The Netherlands, 2011; pp. 326–362e. [Google Scholar]
- Ward, C.; Courtney, D. Kiwifruit: Taking its place in the global fruit bowl. Adv. Food Nutr. Res. 2013, 68, 1–14. [Google Scholar]
- Guroo, I.; Wani, S.A.; Wani, S.M.; Ahmad, M.; Mir, S.A.; Masoodi, F.A. A review of production and processing of kiwifruit. J. Food Process. Technol. 2017, 8, 699. [Google Scholar]
- Rymbai, H.; Talang, H.D.; Dayal, V.; Deshmukh, N.A.; Assumi, S.R.; Devi, M.B.; Jha, A.K. Kiwifruit—A High Value Crop For Hilly Terrain. In Book-Natural Resource Management in Horticultural Crops; Today & Tomorrow’s Printers and Publishers: New Delhi, India, 2022; pp. 49–91. [Google Scholar]
- Nayik, G.A.; Gull, A. Antioxidants in Fruits: Properties and Health Benefits; Springer: Berlin/Heidelberg, Germany, 2020; pp. 201–225. [Google Scholar]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559. [Google Scholar] [CrossRef]
- Gornall, J.; Betts, R.; Burke, E.; Clark, R.; Camp, J.; Willett, K.; Wiltshire, A. Implications of climate change for agricultural productivity in the early twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2973–2989. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Spasojevic, M.J.; Li, D. Climate and plant community diversity in space and time. Proc. Natl. Acad. Sci. USA 2020, 117, 4464–4470. [Google Scholar] [CrossRef]
- Habib-ur-Rahman, M.; Ahmad, A.; Raza, A.; Hasnain, M.U.; Alharby, H.F.; Alzahrani, Y.M.; Bamagoos, A.A.; Hakeem, K.R.; Ahmad, S.; Nasim, W.; et al. Impact of climate change on agricultural production; Issues, challenges, and opportunities in Asia. Front. Plant Sci. 2022, 13, 925548. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The Impact of Climate Change on Agricultural Insect Pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Orlowsky, B.; Seneviratne, S.I. Global changes in extreme events: Regional and seasonal dimension. Clim. Chang. 2012, 110, 669–696. [Google Scholar] [CrossRef]
- Mishra, V.; Ganguly, A.R.; Nijssen, B.; Lettenmaier, D.P. Changes in observed climate extremes in global urban areas. Environ. Res. Lett. 2015, 10, 024005. [Google Scholar] [CrossRef]
- Ye, J.S.; Gong, Y.H.; Zhang, F.; Ren, J.; Bai, X.K.; Zheng, Y. Which Temperature and Precipitation Extremes Best Explain the Variation of Warm versus Cold Years and Wet versus Dry Years? J. Clim. 2018, 31, 45–59. [Google Scholar] [CrossRef]
- Spinoni, J.; Barbosa, P.; De Jager, A.; McCormick, N.; Naumann, G.; Vogt, J.V.; Magni, D.; Masante, D.; Mazzeschi, M. A new global database of meteorological drought events from 1951 to 2016. J. Hydrol. Reg. Stud. 2019, 22, 100593. [Google Scholar] [CrossRef] [PubMed]
- Cradock-Henry, N.A. New Zealand kiwifruit growers’ vulnerability to climate and other stressors. Reg. Environ. Chang. 2017, 17, 245–259. [Google Scholar] [CrossRef]
- Bardi, L. Early kiwifruit decline: A soil-borne disease syndrome or a climate change effect on plant–soil relations? Front. Agron. 2020, 2, 3. [Google Scholar] [CrossRef]
- Tait, A.; Paul, V.; Sood, A.; Mowat, A. Potential impact of climate change on Hayward kiwifruit production viability in New Zealand. N. Z. J. Crop Hortic. Sci. 2018, 46, 175–197. [Google Scholar] [CrossRef]
- Gurbuz, I.B.; Ozkan, G.; Er, S. Exploring Kiwi Fruit Producers’ Climate Change Perceptions. Appl. Fruit Sci. 2024, 66, 475–483. [Google Scholar] [CrossRef]
- Li, D.; Xie, X.; Liu, X.; Cheng, C.; Guo, W.; Zhong, C.; Atak, A. Effects of Short-Term High Temperature on Gas Exchange in Kiwifruits (Actinidia spp.). Biology 2022, 11, 1686. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.C.; Marsh, K.B.; Boldingh, H.L.; Pickering, A.H.; Bulley, S.M.; Frearson, N.J.; Ferguson, A.R.; Thornber, S.E.; Bolitho, K.M.; Macrae, E.A. High growing temperatures reduce fruit carbohydrate and vitamin C in kiwifruit. Plant Cell Environ. 2004, 27, 423–435. [Google Scholar] [CrossRef]
- Jeong, Y.; Chung, U.; Kim, K. Predicting future frost damage risk of kiwifruit in Korea under climate change using an integrated modelling approach. Int. J. Climatol. 2018, 38, 5354–5367. [Google Scholar] [CrossRef]
- Patra, N.K.; Rilung, T.; Das, L.; Kumar, P. Assessing climate change and its impact on kiwi (Actinidia deliciosa Chev.) production in the Eastern Himalayan Region of India through a combined approach of people perception and meteorological data. Theor. Appl. Climatol. 2024, 155, 2347–2364. [Google Scholar] [CrossRef]
- Mills, T.M.; Li, J.; Behboudian, M.H. Physiological Responses of Gold Kiwifruit (Actinidia chinensis) to Reduced Irrigation. J. Am. Soc. Hortic. Sci. 2009, 134, 677–683. [Google Scholar] [CrossRef]
- Wurms, K.V.; Reglinski, T.; Buissink, P.; Ah Chee, A.; Fehlmann, C.; McDonald, S.; Cooney, J.; Jensen, D.; Hedderley, D.; McKenzie, C.; et al. Effects of Drought and Flooding on Phytohormones and Abscisic Acid Gene Expression in Kiwifruit. Int. J. Mol. Sci. 2023, 24, 7580. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Rennenberg, H. Molecular and physiological responses of trees to waterlogging stress. Plant Cell Environ. 2014, 37, 2245–2259. [Google Scholar] [CrossRef]
- Kim, K.-H.; Jeong, Y.M.; Cho, Y.-S.; Chung, U. Preliminary Result of Uncertainty on Variation of Flowering Date of Kiwifruit: Case Study of Kiwifruit Growing Area of Jeonlanam-do. Korean J. Agric. For. Meteorol. 2016, 18, 42–54. [Google Scholar] [CrossRef]
- Kim, C.G.; Lee, S.M.; Jeong, H.K.; Jang, J.K.; Kim, Y.H.; Lee, C.K. Impacts of climate change on Korean agriculture and its counterstrategies. In Korea Rural Economic Institute Basic Research Report; 2010; pp. 1–306. [Google Scholar]
- Calderón-Orellana, A.; Silva, D.I.; Bastías, R.M.; Bambach, N.; Aburto, F. Late-season plastic covering delays the occurrence of severe water stress and improves intrinsic water use efficiency and fruit quality in kiwifruit vines. Agric. Water Manag. 2021, 249, 106795. [Google Scholar] [CrossRef]
- Wang, W.X.; Vinocur, B.; Shoseyov, O.; Altman, A. Biotechnology of plant osmotic stress tolerance physiological and molecular considerations. Acta Hortic. 2001, 560, 285–292. [Google Scholar] [CrossRef]
- Froud, K.J.; Beresford, R.M.; Cogger, N. Risk factors for kiwifruit bacterial canker disease development in ‘Hayward’ kiwifruit blocks. Australas. Plant Pathol. 2017, 46, 421–431. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, K.H.; Son, K.I.; Choi, E.D.; Lee, Y.S.; Jung, J.S.; Koh, Y.J. Outbreak and Spread of Bacterial Canker of Kiwifruit Caused by Pseudomonas syringae pv. actinidiae Biovar 3 in Korea. Plant Pathol. J. 2016, 32, 545–551. [Google Scholar] [CrossRef]
- Vanneste, J.L. The Scientific, Economic, and Social Impacts of the New Zealand Outbreak of Bacterial Canker of Kiwifruit (Pseudomonas syringae pv. actinidiae). Annu. Rev. Phytopathol. 2017, 55, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, J.L.; Yu, J.; Cornish, D.A.; Tanner, D.J.; Windner, R.; Chapman, J.R.; Taylor, R.K.; Mackay, J.F.; Dowlut, S. Identification, Virulence, and Distribution of Two Biovars of Pseudomonas syringae pv. actinidiae in New Zealand. Plant Dis. 2013, 97, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Wilstermann, A.; Schrader, G.; Kehlenbeck, H.; Robinet, C. Potential spread of kiwifruit bacterial canker (Pseudomonas syringae pv. actinidiae) in Europe. EPPO Bull. 2017, 47, 255–262. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, J.E.; Jiang, Y.P.; Wang, R.L.; Wu, R.S. Predicting the potential distribution of Pseudomonas syringae pv. actinidiae in China using ensemble models. Plant Pathol. 2020, 69, 120–131. [Google Scholar] [CrossRef]
- Narouei-Khandan, H.A.; Worner, S.P.; Viljanen, S.L.H.; Van Bruggen, A.H.C.; Balestra, G.M.; Jones, E. The Potential Global Climate Suitability of Kiwifruit Bacterial Canker Disease (Pseudomonas syringae pv. actinidiae (Psa)) Using Three Modelling Approaches: CLIMEX, Maxent and Multimodel Framework. Climate 2022, 10, 14. [Google Scholar] [CrossRef]
- Habibi, F.; Liu, T.; Shahid, M.A.; Schaffer, B.; Sarkhosh, A. Physiological, biochemical, and molecular responses of fruit trees to root zone hypoxia. Environ. Exp. Bot. 2023, 206, 105179. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Tam, C.Y.; Lau, N.C.; Lau, D.S.D.; Mok, H.Y. Impacts of climate change on tropical cyclones and induced storm surges in the Pearl River Delta region using pseudo-global-warming method. Sci. Rep. 2020, 10, 1965. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, G.; Motavalli, P.P.; Nelson, K.A.; Orlowski, J.M.; Golden, B.R. Impacts and management strategies for crop production in waterlogged or flooded soils: A review. Agron. J. 2020, 112, 1475–1501. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, Y.; Chen, P.; Zhang, F.; Li, J.; Yan, F.; Dong, Y.; Feng, B. How Does the Waterlogging Regime Affect Crop Yield? A Global Meta-Analysis. Front. Plant Sci. 2021, 12, 634898. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Huang, S.N.; Chen, Y.H.; Wang, G.; Guo, Z.R. Identification and characterization of two waterlogging responsive alcohol dehydrogenase genes (AdADH1 and AdADH2) in Actinidia deliciosa. Mol. Breed. 2017, 37, 52. [Google Scholar] [CrossRef]
- Snelgar, W.P.; Hall, A.J.; McPherson, H.G. Modelling flower production of kiwifruit (Actinidia deliciosa) from winter chilling. N. Z. J. Crop Hortic. Sci. 2008, 36, 273–284. [Google Scholar] [CrossRef]
- McPherson, H.G.; Jill Stanley, C.; Warrington, I.J. The response of bud break and flowering to cool winter temperatures in kiwifruit (Actinidia deliciosa). J. Hortic. Sci. 1995, 70, 737–747. [Google Scholar]
- Martin, R.A. The production and marketing of new zealand kiwifruit. Acta Hortic. 2003, 25–28. [Google Scholar] [CrossRef]
- Salinger, M.J.; Kenny, G.J. Climate and kiwifruit cv. ‘Hayward’ 2. Regions in New Zealand suited for production. N. Z. J. Crop Hortic. Sci. 1995, 23, 173–184. [Google Scholar] [CrossRef]
- Richardson, A.C.; Walton, E.F.; Meekings, J.S.; Boldingh, H.L. Carbohydrate changes in kiwifruit buds during the onset and release from dormancy. Sci. Hortic. 2010, 124, 463–468. [Google Scholar] [CrossRef]
- Snelgar, W.P.; Hall, A.J.; Ferguson, A.R.; Blattmann, P. Temperature influences growth and maturation of fruit on “Hayward” kiwifruit vines. Funct. Plant Biol. 2005, 32, 631. [Google Scholar] [CrossRef]
- Kopeć, P. Climate Change—The Rise of Climate-Resilient Crops. Plants 2024, 13, 490. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Kim, S.-o.; Seo, H.; Moon, K.H.; Yun, J.I. Geographical Shift in Blooming Date of Kiwifruits in Jeju Island by Global Warming. Korean J. Agric. For. Meteorol. 2012, 14, 179–188. [Google Scholar] [CrossRef]
- Montanaro, G.; Dichio, B.; Xiloyannis, C. Response of photosynthetic machinery of field-grown kiwifruit under Mediterranean conditions during drought and re-watering. Photosynthetica 2007, 45, 533–540. [Google Scholar] [CrossRef]
- Sofo, A.; Mininni, A.N.; Dichio, B.; Mastroleo, M.; Xylogiannis, E. Physical structure and chemical quality of waterlogged soils in an Italian kiwifruit orchard. Acta Hortic. 2021, 1332, 195–202. [Google Scholar] [CrossRef]
- Kenny, G.J.; Porteous, A.S. Adapting to Climate Change in the Kiwifruit Industry; Ministry of Agriculture and Forestry: Wellington, New Zealand, 2008.
- Sambaraju, K.R.; Carroll, A.L.; Zhu, J.; Stahl, K.; Moore, R.D.; Aukema, B.H. Climate change could alter the distribution of mountain pine beetle outbreaks in western Canada. Ecography 2012, 35, 211–223. [Google Scholar] [CrossRef]
- Biber-Freudenberger, L.; Ziemacki, J.; Tonnang, H.E.Z.; Borgemeister, C. Future Risks of Pest Species under Changing Climatic Conditions. PLoS ONE 2016, 11, 0153237. [Google Scholar] [CrossRef]
- Krishnan, S.; Chander, S. Simulation of climatic change impact on crop-pest interactions: A case study of rice pink stem borer Sesamia inferens (Walker). Clim. Chang. 2015, 131, 259–272. [Google Scholar] [CrossRef]
- Schatz, A.M.; Kramer, A.M.; Drake, J.M. Accuracy of climate-based forecasts of pathogen spread. R. Soc. Open Sci. 2017, 4, 160975. [Google Scholar] [CrossRef]
- Ghini, R.; Hamada, E.; Angelotti, F.; Costa, L.B.; Bettiol, W. Research approaches, adaptation strategies, and knowledge gaps concerning the impacts of climate change on plant diseases. Trop. Plant Pathol. 2012, 37, 5–24. [Google Scholar]
- Elad, Y.; Pertot, I. Climate Change Impacts on Plant Pathogens and Plant Diseases. J. Crop Improv. 2014, 28, 99–139. [Google Scholar] [CrossRef]
- Zhao, Z.B.; Gao, X.N.; Huang, Q.L.; Huang, L.L.; Qin, H.Q.; Kang, Z.S. Identification and characterization of the causal agent of bacterial canker of kiwifruit in the Shaanxi province of China. J. Plant Pathol. 2013, 95, 155–162. [Google Scholar]
- Serizawa, S.; Ichikawa, T.; Takikawa, Y.; Tsuyumu, S.; Goto, M. Occurrence of bacterial canker of kiwifruit in Japan description of symptoms, isolation of the pathogen and screening of bactericides. Jpn. J. Phytopathol. 1989, 55, 427–436. [Google Scholar] [CrossRef]
- EPPO A1 and A2 Lists of Pests Recommended for Regulation as Quarantine Pests; European and Mediterranean Plant Protection Organization: Paris, France, 2013; pp. 1–19.
- Kim, Y.U.; Webber, H.; Adiku, S.G.K.; Nóia Júnior, R.D.S.; Deswarte, J.-C.; Asseng, S.; Ewert, F. Mechanisms and modelling approaches for excessive rainfall stress on cereals: Waterlogging, submergence, lodging, pests and diseases. Agric. For. Meteorol. 2024, 344, 109819. [Google Scholar] [CrossRef]
- Bano, S.; Scrimgeour, F. The Export Growth and Revealed Comparative Advantage of the New Zealand Kiwifruit Industry. Int. Bus. Res. 2012, 5, 73. [Google Scholar] [CrossRef]
- Scrimgeour, F.; Hughes, W.; Kumar, V. The Economic Contribution of Kiwifruit Industry Expansion to the Bay of Plenty, Northland and New Zealand Economies; Institute for Business Research: Hamilton, New Zealand, 2017; pp. 1–36. [Google Scholar]
- Ferguson, A.R. Kiwifruit: Evolution of a crop. Acta Hortic. 2011, 913, 31–42. [Google Scholar] [CrossRef]
- Laiopoulou, N. A study of the factors influencing the kiwifruit production cost. New Medit 2002, 424, 400. [Google Scholar]
- Ausseil, A.G.E.; Daigneault, A.J.; Frame, B.; Teixeira, E.I. Towards an integrated assessment of climate and socio-economic change impacts and implications in New Zealand. Environ. Model. Softw. 2019, 119, 1–20. [Google Scholar] [CrossRef]
- Lawrence, J.; Blackett, P.; Cradock-Henry, N.; Flood, S.; Greenaway, A.; Dunningham, A. Climate Change Impacts and Implications for New Zealand to 2100. In Synthesis Report RA4: Enhancing Capacity and Increasing Coordination to Support Decision Making; MBIE Contract C01X1225: Wellington, New Zealand, 2016. [Google Scholar]
- Kenny, G. Adaptation in agriculture: Lessons for resilience from eastern regions of New Zealand. Clim. Chang. 2011, 106, 441–462. [Google Scholar] [CrossRef]
- Malla, S.; Bista, L.; Sapkota, R. Prospects of Kiwi Production and Marketing in the Advancement of Household Economy in Dolakha District. Turk. J. Agric. Food Sci. Technol. 2022, 10, 2039–2044. [Google Scholar] [CrossRef]
- Wang, H.; Sarkar, A.; Qian, L. Evaluations of the Roles of Organizational Support, Organizational Norms and Organizational Learning for Adopting Environmentally Friendly Technologies: A Case of Kiwifruit Farmers’ Cooperatives of Meixian, China. Land 2021, 10, 284. [Google Scholar] [CrossRef]
- Sarkar, A.; Wang, H.; Rahman, A.; Qian, L.; Memon, W.H. Evaluating the roles of the farmer’s cooperative for fostering environmentally friendly production technologies-a case of kiwi-fruit farmers in Meixian, China. J. Environ. Manag. 2022, 301, 113858. [Google Scholar] [CrossRef]
- Saeed, M. Labour Shortage–The Role of Technology led Innovation in the Kiwifruit Industry; KELLOGG Rural Leadership Programme: 2021. Available online: https://ruralleaders.co.nz/wp-content/uploads/2021/11/Munazza-Saeed-The-role-ot-technology-led-innovation-in-the-Kiwifruit-Industry-K44-2021.pdf (accessed on 20 August 2024).
- Ferguson, A.R. Kiwifruit in the world-2014. VIII Int. Symp. Kiwifruit 2014, 1096, 33–46. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Cheng, Y.J.; Cai, L.H.; Zeng, Y.L.; Qi, C.J. Impact of the COVID-19 on global trade of kiwifruit. J. Fruit Sci. 2021, 1790–1801. [Google Scholar] [CrossRef]
- Qin, Y.; Shi, X.; Li, X.; Yan, J. Geographical indication agricultural products, livelihood capital, and resilience to meteorological disasters: Evidence from kiwifruit farmers in China. Environ. Sci. Pollut. Res. 2021, 28, 65832–65847. [Google Scholar] [CrossRef] [PubMed]
- Folke, C. Resilience: The emergence of a perspective for social–ecological systems analyses. Glob. Environ. Chang. 2006, 16, 253–267. [Google Scholar] [CrossRef]
- Nelson, D.R.; Adger, W.N.; Brown, K. Adaptation to Environmental Change: Contributions of a Resilience Framework. Annu. Rev. Environ. Resour. 2007, 32, 395–419. [Google Scholar] [CrossRef]
- Rajan, R.; Feza, M.; Pandey, K.; Aman, A.; Kumar, V. Climate change and resilience in fruit crops. In Climate Change and Its Effects on Agriculture; BIOTEC: Tokyo, Japan, 2020; pp. 337–354. [Google Scholar]
- Jo, Y.-S.; Cho, H.-S.; Park, M.-Y.; Bang, G.-P.; Kim, W.-S. Drought stress tolerance of actinidia arguta and actinidia eriantha. Acta Hortic. 2008, 773, 283–287. [Google Scholar]
- Naqvi, R.Z.; Siddiqui, H.A.; Mahmood, M.A.; Najeebullah, S.; Ehsan, A.; Azhar, M.; Farooq, M.; Amin, I.; Asad, S.; Mukhtar, Z.; et al. Smart breeding approaches in post-genomics era for developing climate-resilient food crops. Front. Plant Sci. 2022, 13, 972164. [Google Scholar] [CrossRef]
- Aswini, M.S.; Kiran; Lenka, B.; Shaniware, Y.A.; Pandey, P.; Dash, A.P.; Haokip, S.W. The Role of Genetics and Plant Breeding for Crop Improvement: Current Progress and Future Prospects. Int. J. Plant Soil Sci 2023, 35, 190–202. [Google Scholar]
- Singh, R.K.; Prasad, A.; Muthamilarasan, M.; Parida, S.K.; Prasad, M. Breeding and biotechnological interventions for trait improvement: Status and prospects. Planta 2020, 252, 54. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Abid, M.; Luo, J.; Zhang, Y.; Yang, E.; Cai, X.; Gao, P.; Huang, H.; Wang, Z. Genome-wide identification of the heat shock transcription factor gene family in two kiwifruit species. Front. Plant Sci. 2023, 14, 1075013. [Google Scholar] [CrossRef]
- Zha, Y.; Chen, F.; Wang, Z.; Jiang, S.; Cui, N. Effects of water and fertilizer deficit regulation with drip irrigation at different growth stages on fruit quality improvement of kiwifruit in seasonal arid areas of Southwest China. J. Integr. Agric. 2023, 22, 3042–3058. [Google Scholar] [CrossRef]
- Di Biase, R.; Calabritto, M.; Sofo, A.; Reyes, F.; Mininni, A.N.; Mastroleo, M.; Xylogiannis, E.; Dichio, B. Assessment of kiwifruit physiological decline: Irrigation and soil management strategy to recover from waterlogging. Acta Hortic. 2023, 1373, 11–18. [Google Scholar] [CrossRef]
- Yang, S.Y.; Cao, H.X.; Sun, T.; Nan, X.P.; Zhao, K.; Li, Z.J. Effects of dual mulching on soil moisture, growth and yield of kiwifruit in Guanzhong Plain. In Agricultural Research in the Arid Areas; Northwest A&F University: Yangling, China, 2023; pp. 187–197. [Google Scholar]
- Liu, L.H.; Wang, H.; Tan, S.; Hu, C.W.; Lu, J.Y.; Tong, C.H. Effects of different mulching patterns on soil moisture, soil temperature and yield of kiwifruit. J. Drain. Irrig. Mach. Eng. 2022, 188–195. [Google Scholar] [CrossRef]
- Zheng, S.; Jiang, S.; Cui, N.; Zhao, L.; Gong, D.; Wang, Y.; Wu, Z.; Liu, Q. Deficit drip irrigation improves kiwifruit quality and water productivity under rain-shelter cultivation in the humid area of South China. Agric. Water Manag. 2023, 289, 108530. [Google Scholar] [CrossRef]
- Sui, Y.; Zhao, Q.; Wang, Z.; Liu, J.; Jiang, M.; Yue, J.; Lan, J.; Liu, J.; Liao, Q.; Wang, Q.; et al. A comparative analysis of the microbiome of kiwifruit at harvest under open-field and rain-shelter cultivation systems. Front. Microbiol. 2021, 12, 757719. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lin, L.J.; Song, H.Y.; Chen, D.; Sun, S.X.; Li, J.; Xu, Z.H.; Liu, C.Y.; Jiang, G.L.; Tu, M.Y. Effects of rain-shelter cultivation on microclimate environment and main diseases of kiwifruit orchard. Southwest China J. Agric. Sci. 2021, 2613–2620. [Google Scholar] [CrossRef]
- Gullo, G.; Dattola, A.; Vonella, V.; Zappia, R. Effects of photoselective colour nets on the vegetative, productive, and qualitative behaviour of kiwifruit, jintao cultivar. J. Berry Res. 2021, 11, 1–19. [Google Scholar] [CrossRef]
- Pinto, R.; Valin, M.I.; Brito, L.M.; Rego, R.; Rodrigues, R.; Santos, C.; Moura, L. Avaliação do efeito de diferentes redes de proteção nas necessidades hídricas e na produção de kiwi cv. Hayward [Assessment of the effect of different protection nets on water needs and production of kiwi cv. Hayward]. Actas Port. Hortic. 2020, 34, 33–40. [Google Scholar]
- Moura, L.; Pinto, R.; Rodrigues, R.; Brito, L.M.; Rego, R.; Valín, M.I.; Mariz-Ponte, N.; Santos, C.; Mourão, I.M. Effect of Photo-Selective Nets on Yield, Fruit Quality and Psa Disease Progression in a ‘Hayward’ Kiwifruit Orchard. Horticulturae 2022, 8, 1062. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Ling-ling, Z.H.; Qing-mei, L.I.; Meng-yuan, J.I.; Yan-jun, Z.H.; Jian-ning, Z.H.; Dian-lin, Y.A.; Hua-ling, W.A.; Hui, W.A. Effects of cover crops on gene abundance and community structure of soil ammonia-oxidizing microorganism in a kiwifruit orchard. J. Plant Nutr. Fertil. 2021, 27, 417–428. [Google Scholar]
- Li, Q.M.; Zhang, L.L.; Zhao, J.N.; Zhang, Y.J.; Liu, H.M.; Wang, H.L.; Wang, H.; Yang, D.L.; Zhang, F.; Weng, C.M. Effects of different cover crop treatments on soil microbial community composition in kiwifruit orchard. J. Agric. Resour. Environ. 2020, 319–325. [Google Scholar] [CrossRef]
- Li, Q.; Qi, X.-X.; Zhang, H.; Zhang, Y.; Liu, H.; Zhao, J.; Yang, D.; Wang, H. Responses of soil nematode abundance and food web to cover crops in a kiwifruit orchard. Front. Plant Sci. 2023, 14, 1173157. [Google Scholar] [CrossRef] [PubMed]
- Geneletti, D. Assessing the impact of alternative land-use zoning policies on future ecosystem services. Environ. Impact Assess. Rev. 2013, 40, 25–35. [Google Scholar] [CrossRef]
- Kärkkäinen, L.; Haakana, H.; Hirvelä, H.; Lempinen, R.; Packalen, T. Assessing the Impacts of Land-Use Zoning Decisions on the Supply of Forest Ecosystem Services. Forests 2020, 11, 931. [Google Scholar] [CrossRef]
- Banerjee, A.; Meena, R.S.; Jhariya, M.K.; Yadav, D.K. (Eds.) Agroecological Footprints Management for Sustainable Food System; Springer: Berlin/Heidelberg, Germany, 2021; p. 514. [Google Scholar]
- Wei, A.S.; Chen, Z.J.; Kang, T.T.; Zhang, X.J.; Yan, B.; Zhou, J.B. Spatial variability of soil nutrients of Yujia catchment in Zhouzhi at the northern piedmont of Qinling Mountain. J. Soil Water Conserv. 2015, 29, 128–132. [Google Scholar]
- Sun, Z.J.; Fu, C.X. Shaanxi kiwifruit area and production rank first in world. Chin. Fruit News 2009, 12, 26–50. [Google Scholar]
- Gao, J.; Wang, S.; Li, Z.; Wang, L.; Chen, Z.; Zhou, J. High Nitrate Accumulation in the Vadose Zone after Land-Use Change from Croplands to Orchards. Environ. Sci. Technol. 2021, 55, 5782–5790. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Wei, A.; Gao, J.; Lu, Y.; Zhou, J. Land-use change from arable lands to orchards reduced soil erosion and increased nutrient loss in a small catchment. Sci. Total Environ. 2019, 648, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Maurya, A.C.; Verma, S.K.; Kumar, S.; Shekhar, S.; Taria, S.; Alam, B.; Yadav, A.; Banjara, T.R. Agronomic management: Irrigation scheduling, mulching and integrated nutrient management influences growth, yields, quality, and economics of summer groundnut in a subtropical condition of India. J. Plant Nutr. 2024, 47, 1200–1213. [Google Scholar] [CrossRef]
- Jiang, S.; Tang, D.; Zhao, L.; Liang, C.; Cui, N.; Gong, D.; Wang, Y.; Feng, Y.; Hu, X.; Peng, Y. Effects of different photovoltaic shading levels on kiwifruit growth, yield and water productivity under “agrivoltaic” system in Southwest China. Agric. Water Manag. 2022, 269, 107675. [Google Scholar] [CrossRef]
- Aarushi; Sharma, C.; Jaryal, R.; Dilta, B.; Rana, V.S. Effect of mulching and pre-emergence herbicides on production of kiwifruit cuttings. Int. J. Farm. Sci. 2023, 13, 10–15. [Google Scholar] [CrossRef]
- Singh, V.P.; Jat, R.; Kumar, V.; Singh, R. Mulches and Their Impact on Floor Management and Performance of Fruit Crops: A Review. Curr. J. Appl. Sci. Technol. 2020, 39, 62–78. [Google Scholar] [CrossRef]
- Dumanski, J.; Peiretti, R. Modern concepts of soil conservation. Int. Soil Water Conserv. Res. 2013, 1, 19–23. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, X.; Li, K.; Wang, C.; Guna, A.; Zhang, J. Prediction of Potential Geographical Distribution Patterns of Actinidia arguta under Different Climate Scenarios. Sustainability 2021, 13, 3526. [Google Scholar] [CrossRef]
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z. Shade netting on subtropical fruit: Effect on environmental conditions, tree physiology and fruit quality. Sci. Hortic. 2019, 256, 108556. [Google Scholar] [CrossRef]
- Chen, Z.-w.; Li, X.-l.; Ji, X.-m.; Zhang, H.; Yue, Y.-z.; Zhai, J.-h.; Zheng, B.-x.; Li, L.-j.; Zhao, Z.-y. Evaluation of heat tolerance of kiwifruit cultivars in Wuhan. Hubei Agric. Sci. 2022, 61, 69. [Google Scholar]
- Kataoka, I.; Matsuoka, M.; Beppu, K.; Ohtani, M. High temperature tolerance of Sanuki Kiwicco® kiwifruit interspecific hybrid Actinidia rufa× A. chinensis var. chinensis. Int. Symp. Kiwifruit 2021, 23–30. [Google Scholar] [CrossRef]
- Basile, B.; Giaccone, M.; Cirillo, C.; Ritieni, A.; Graziani, G.; Shahak, Y.; Forlani, M. Photo-selective hail nets affect fruit size and quality in Hayward kiwifruit. Sci. Hortic. 2012, 141, 91–97. [Google Scholar] [CrossRef]
- Basile, B.; Giaccone, M.; Shahak, Y.; Forlani, M.; Cirillo, C. Regulation of the vegetative growth of kiwifruit vines by photo-selective anti-hail netting. Sci. Hortic. 2014, 172, 300–307. [Google Scholar] [CrossRef]
- Al-Ghobari, H.M.; Dewidar, A.Z. Integrating deficit irrigation into surface and subsurface drip irrigation as a strategy to save water in arid regions. Agric. Water Manag. 2018, 209, 55–61. [Google Scholar] [CrossRef]
- Pratima, P.; Sharma, N.; Kaushal, R. Effect of deficit irrigation and in situ moisture conservation on soil moisture content and frequency of irrigation in kiwifruit cultivar Allison. J. Appl. Nat. Sci. 2016, 8, 2093–2098. [Google Scholar] [CrossRef]
- Zheng, S.S.; Cui, N.B.; Zhao, L.; Zhang, Y.X.; Gong, D.Z.; Hu, X.T.; Feng, Y. The effect of deficit drip irrigation on photosynthetic characteristics of kiwifruit. J. Irrig. Drain. 2020, 39, 19–28. [Google Scholar]
- Li, Z.; Bai, D.; Zhong, Y.; Abid, M.; Qi, X.; Hu, C.; Fang, J. Physiological Responses of Two Contrasting Kiwifruit (Actinidia spp.) Rootstocks against Waterlogging Stress. Plants 2021, 10, 2586. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Li, Z.; Gu, S.; Li, Q.; Sun, L.; Qi, X.; Fang, J.; Zhong, Y.; Hu, C. Effects of Kiwifruit Rootstocks with Opposite Tolerance on Physiological Responses of Grafting Combinations under Waterlogging Stress. Plants 2022, 11, 2098. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Ferguson, R.; Huang, H.; Testolin, R. Main changes in the kiwifruit industry since its introduction: Present situation and future. Acta Hortic. 2018, 1218, 1–16. [Google Scholar] [CrossRef]
- Balestra, G.M.; Mazzaglia, A.; Quattrucci, A.; Renzi, M.; Rossetti, A. Current status of bacterial canker spread on kiwifruit in Italy. Australas. Plant Dis. Notes 2009, 4, 34–36. [Google Scholar] [CrossRef]
- Kisaki, G.; Tanaka, S.; Ishihara, A.; Igarashi, C.; Morimoto, T.; Hamano, K.; Endo, A.; Sugita-Konishi, S.; Tabuchi, M.; Gomi, K.; et al. Evaluation of various cultivars of Actinidia species and breeding source Actinidia rufa for resistance to Pseudomonas syringae pv. actinidiae biovar 3. J. Gen. Plant Pathol. 2018, 84, 399–406. [Google Scholar] [CrossRef]
- Qin, H.Y.; Zhao, Y.; Chen, X.L.; Zhang, B.X.; Wen, X.; Li, C.Y.; Fan, S.T.; Wang, Y.; Yang, Y.M.; Xu, P.L.; et al. Pathogens identification and resistance evaluation on bacterial canker in Actinidia arguta germplasm. J. Plant Pathol. 2023, 105, 973–985. [Google Scholar] [CrossRef]
- Yadav, S.; Korat, J.R.; Yadav, S.; Mondal, K.; Kumar, A.; Homeshvari; Kumar, S. Impacts of Climate Change on Fruit Crops: A Comprehensive Review of Physiological, Phenological, and Pest-Related Responses. Int. J. Environ. Clim. Chang. 2023, 13, 363–371. [Google Scholar] [CrossRef]
- Kalsoom, T.; Khan, M.A.; Rana, R.M.; Ahmed, M.; Ditta, A.; Soufan, W.; Werbrouck, S.; El Sabagh, A. Quantification of Phenological, Physiological, and Morphological Response of KiwifruitVarieties under Rainfed Conditions. Pol. J. Environ. Stud. 2024, 33, 3701–3719. [Google Scholar] [CrossRef]
- Bryan, E.; Ringler, C.; Okoba, B.; Roncoli, C.; Silvestri, S.; Herrero, M. Adapting agriculture to climate change in Kenya: Household strategies and determinants. J. Environ. Manag. 2013, 114, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Federal Research Centre the Subtropical Scientific Centre, The Russian Academy of Sciences; Besedina, T.D. On specific influence of the agroecological conditions of humid subtropics of russia on productive potential of Actinida deliciosa (kiwifruit). Selskokhozyaistvennaya Biol. 2021, 56, 999–1010. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, Y. Analysis of the Response of Kiwifruit to Soil Water Deficit. J. Mod. Crop Sci. 2022, 1, 59–66. [Google Scholar]
- Li, Z.; Bai, D.; Zhong, Y.; Lin, M.; Sun, L.; Qi, X.; Hu, C.; Fang, J. Full-Length Transcriptome and RNA-Seq Analyses Reveal the Mechanisms Underlying Waterlogging Tolerance in Kiwifruit (Actinidia valvata). Int. J. Mol. Sci. 2022, 23, 3237. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhong, Y.; Bai, D.; Lin, M.; Qi, X.; Fang, J. Comparative analysis of physiological traits of three Actinidia valvata Dunn genotypes during waterlogging and post-waterlogging recovery. Hortic. Environ. Biotechnol. 2020, 61, 825–836. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, Y.; Bai, D.; Abid, M.; Zhang, Y.; Lin, M.; Qi, X.; Hu, C.; Fang, J. Comparative Transcriptome Analysis of Two Contrasting Kiwifruit (Actinidia) Genotypes under Waterlogging Stress. Preprints 2020, 2020070395. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Huang, S.N.; Mo, Z.H.; Xuan, J.P.; Jia, X.D.; Wang, G.; Guo, Z.R. De novo transcriptome sequencing and comparative analysis of differentially expressed genes in kiwifruit under waterlogging stress. Mol. Breed. 2015, 35, 208. [Google Scholar] [CrossRef]
- Xing, M.; Huang, K.; Zhang, C.; Xi, D.; Luo, H.; Pei, J.; Ruan, R.; Liu, H. Transcriptome Analysis Reveals the Molecular Mechanism and Responsive Genes of Waterlogging Stress in Actinidia deliciosa Planch Kiwifruit Plants. Int. J. Mol. Sci. 2023, 24, 15887. [Google Scholar] [CrossRef]
| S. No. | Global Climate Change or Its Effect | Specific Impact on Kiwifruit Production | Location | Study Details or Observations | References |
|---|---|---|---|---|---|
| 1 | Global temperature rise | Decreased winter chilling, leading to reduced viability for ‘Hayward’ kiwifruit. | Te Puke, New Zealand | Production becoming non-viable by century’s end due to insufficient chilling. | [37] |
| 2 | Temperature fluctuations | Impact on photosynthesis and vine health; severe leaf damage at high temperatures. | Global | Photosynthetic rate impairment and irreversible leaf damage at 52 °C. | [39] |
| 3 | Frosting events | Increased risk of spring frost damage. | Boseong, Korea | Rising risks due to earlier frost dates and accelerated bud burst. | [41,42] |
| 4 | Droughts and floods | Disruption in photosynthesis and fruit quality; increased abscisic acid levels during droughts. | New Zealand, Global | Water stress management crucial; impact of droughts and floods on yield and fruit quality. | [43,44] |
| 5 | Typhoons and strong winds | Structural damage to plants, disrupted fruit setting, and lowered pollination rates. | Global | Strong wind causes significant orchard damage and affects various growth stages. | [42,45] |
| 6 | Phenological changes | Increase in flowering days; shifts in flowering and fruiting periods. | Korea, New Zealand | Northward expansion and earlier flowering of ‘Haegeum’; less winter chilling affecting ‘Hayward’ in Te Puke. | [37,46] |
| 7 | Shifts in suitable growing regions | Expansion of kiwifruit cultivation to new areas due to warming temperatures. | Korea | Cultivation spreads to new regions, like Sacheon and Jeju Island. | [47] |
| 8 | Physiological impacts | Altered photosynthesis and respiration rates due to temperature and humidity stress. | Global | Need for water management to sustain photosynthetic activity; respiration inhibited above 44.5 °C. | [39,48,49] |
| 9 | Increased pests and diseases | Heightened prevalence of Psa and susceptibility to new pathogens. | Global | Rising temperatures foster conditions favorable for pathogens like Psa. | [50,51,52,53,54,55,56] |
| S. No. | Category | Strategy/Approach | Description | References |
|---|---|---|---|---|
| 1 | Breeding and Selection | Climate-resilient cultivars | Developing kiwifruit cultivars with heat tolerance, drought resistance, and disease resilience to withstand extreme weather events. | [39,100,101,102,103] |
| High-temperature tolerance genes | Identifying heat shock transcription factor (Hsf) genes in A. chinensis and A. eriantha to breed heat-stress-resistant cultivars. | [104] | ||
| 2 | Agronomic Practices | Irrigation management | Utilizing drip and sprinkler irrigation to minimize water loss and optimize water use efficiency based on soil moisture levels, weather conditions, and plant growth. | [105,106] |
| Mulching | Applying organic or synthetic mulch to conserve soil moisture, suppress weeds, moderate soil temperatures, and prevent erosion. | [107,108] | ||
| Rain-shelter cultivation system | Implementing rain-shelter systems to protect kiwifruit flowers from heavy rainfall, reduce disease incidence, and improve fruit quality. | [109,110,111] | ||
| Shade management | Using shade nets or strategically planted trees to reduce sunburn damage and create favorable microclimates within orchards. | [112,113,114] | ||
| 3 | Soil Conservation and Land-Use Planning | Conservation tillage (no-till farming) | Minimizing soil disturbance to preserve organic matter, enhance microbial activity, improve water retention, and reduce erosion. | [115] |
| Cover cropping | Planting cover crops to protect soil from erosion, improve soil structure, suppress weeds, and enhance nutrient cycling and beneficial soil microbial communities. | [116,117,118] | ||
| Strategic land-use planning | Implementing zoning, land capability assessments, and agro-ecological planning to optimize land use, preserve biodiversity, and minimize environmental impacts. | [119,120,121] | ||
| Managing shifts in land use | Transitioning from traditional crops to kiwifruit orchards, addressing challenges like soil erosion and nutrient loss, and promoting sustainable land management. | [122,123,124,125] |
| S. No. | Category | Strategy/Approach | Description | References |
|---|---|---|---|---|
| 1 | Phenology management | Adaptive planting | Adjust planting dates and select cultivars tolerant to heat and drought to match growth cycles with new climatic conditions. | [132] |
| Microclimate management | Use of shade netting to manage orchard temperatures and moisture, reducing heat and water stress. | [133] | ||
| 2 | Breeding | Selection of climate-resilient cultivars | Develop and use kiwifruit cultivars with improved drought and heat tolerance, like A. arguta and A. eriantha. | [100] |
| Heat-resistant cultivars | Focus on species with higher heat resistance for breeding, such as A. rufa and Jintao cultivars. | [39,134,135] | ||
| 3 | Orchard management | Photo-selective nets | Implement pearl photo-selective nets in orchards to create optimal microclimates, enhance productivity, and manage diseases like bacterial kiwifruit canker. | [114] |
| Hail netting | Use colored hail netting to optimize fruit quality and orchard productivity. | [136,137] | ||
| 4 | Water management | Advanced irrigation technologies | Use drip irrigation systems and soil moisture sensors to optimize water use efficiency. | [138] |
| Deficit drip irrigation | Apply less water than full crop requirements to improve water productivity and kiwifruit quality. | [105,109,139,140] | ||
| Rainwater harvesting | Implement systems to collect and store rainwater, supplementing irrigation needs during dry periods. | [141] | ||
| 5 | Waterlogging mitigation | Grafting onto waterlogging-tolerant rootstocks | Use KR5 rootstock for better resilience of kiwifruit plants under waterlogging conditions. | [142] |
| 6 | Pest and disease control | Integrated pest management (IPM) | Implement IPM strategies to replace conventional pesticide use, reducing health risks and environmental impact. | [143] |
| Development of disease-resistant cultivars | Breed and utilize kiwifruit cultivars resistant to diseases like Psa3, enhancing resilience to climate-related pathogens. | [52,144,145,146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajan, P.; Natraj, P.; Kim, M.; Lee, M.; Jang, Y.J.; Lee, Y.J.; Kim, S.C. Climate Change Impacts on and Response Strategies for Kiwifruit Production: A Comprehensive Review. Plants 2024, 13, 2354. https://doi.org/10.3390/plants13172354
Rajan P, Natraj P, Kim M, Lee M, Jang YJ, Lee YJ, Kim SC. Climate Change Impacts on and Response Strategies for Kiwifruit Production: A Comprehensive Review. Plants. 2024; 13(17):2354. https://doi.org/10.3390/plants13172354
Chicago/Turabian StyleRajan, Priyanka, Premkumar Natraj, Misun Kim, Mockhee Lee, Yeon Jin Jang, Young Jae Lee, and Seong Cheol Kim. 2024. "Climate Change Impacts on and Response Strategies for Kiwifruit Production: A Comprehensive Review" Plants 13, no. 17: 2354. https://doi.org/10.3390/plants13172354






