Genome-Wide Identification and Expression Analyses of the Thaumatin-Like Protein Gene Family in Tetragonia tetragonoides (Pall.) Kuntze Reveal Their Functions in Abiotic Stress Responses
Abstract
:1. Introduction
2. Results
2.1. Identification of the T. tetragonoides TLP Family
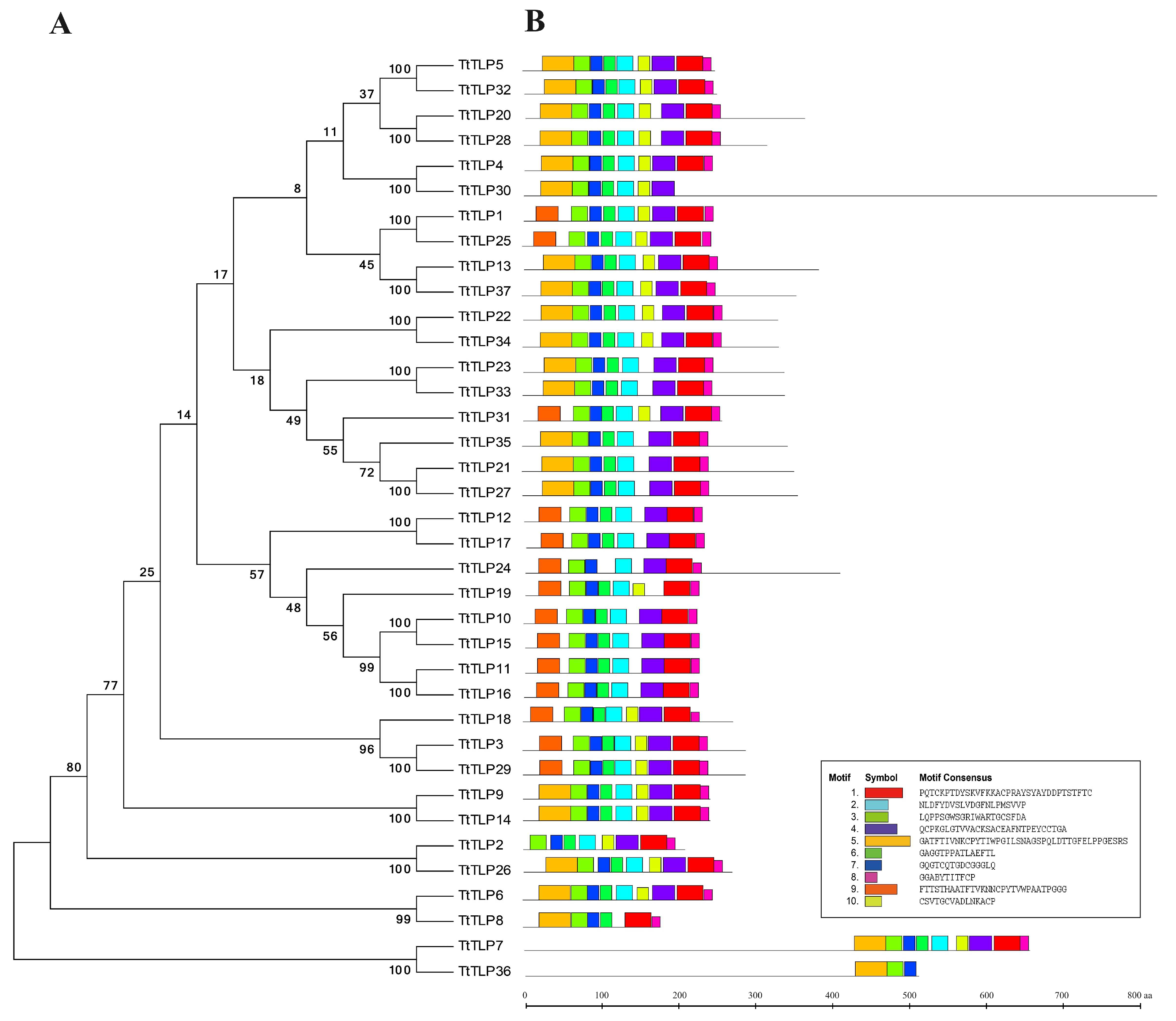
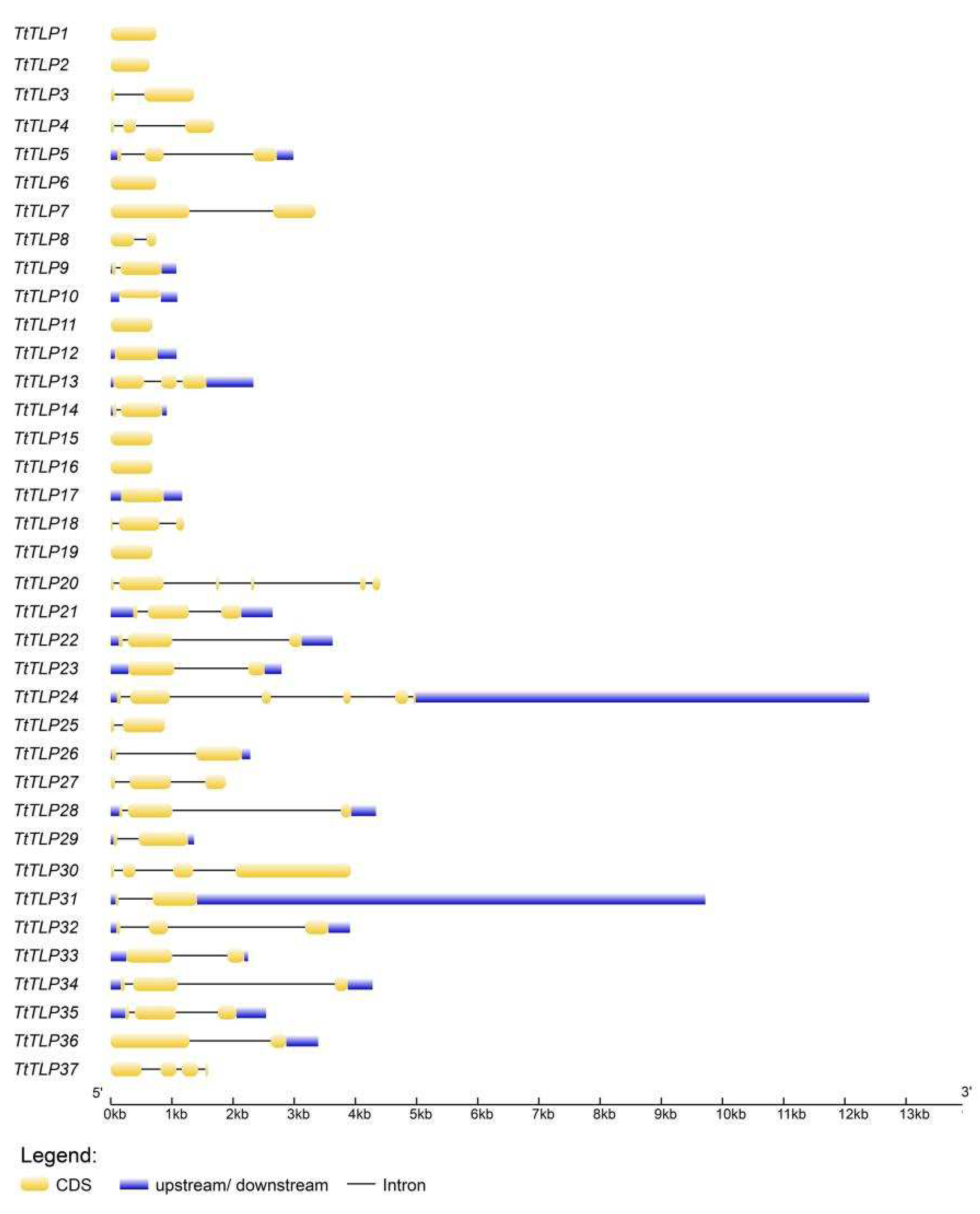
2.2. Evolutionary Characterization of TLP Proteins and TtTLP Gene Structures
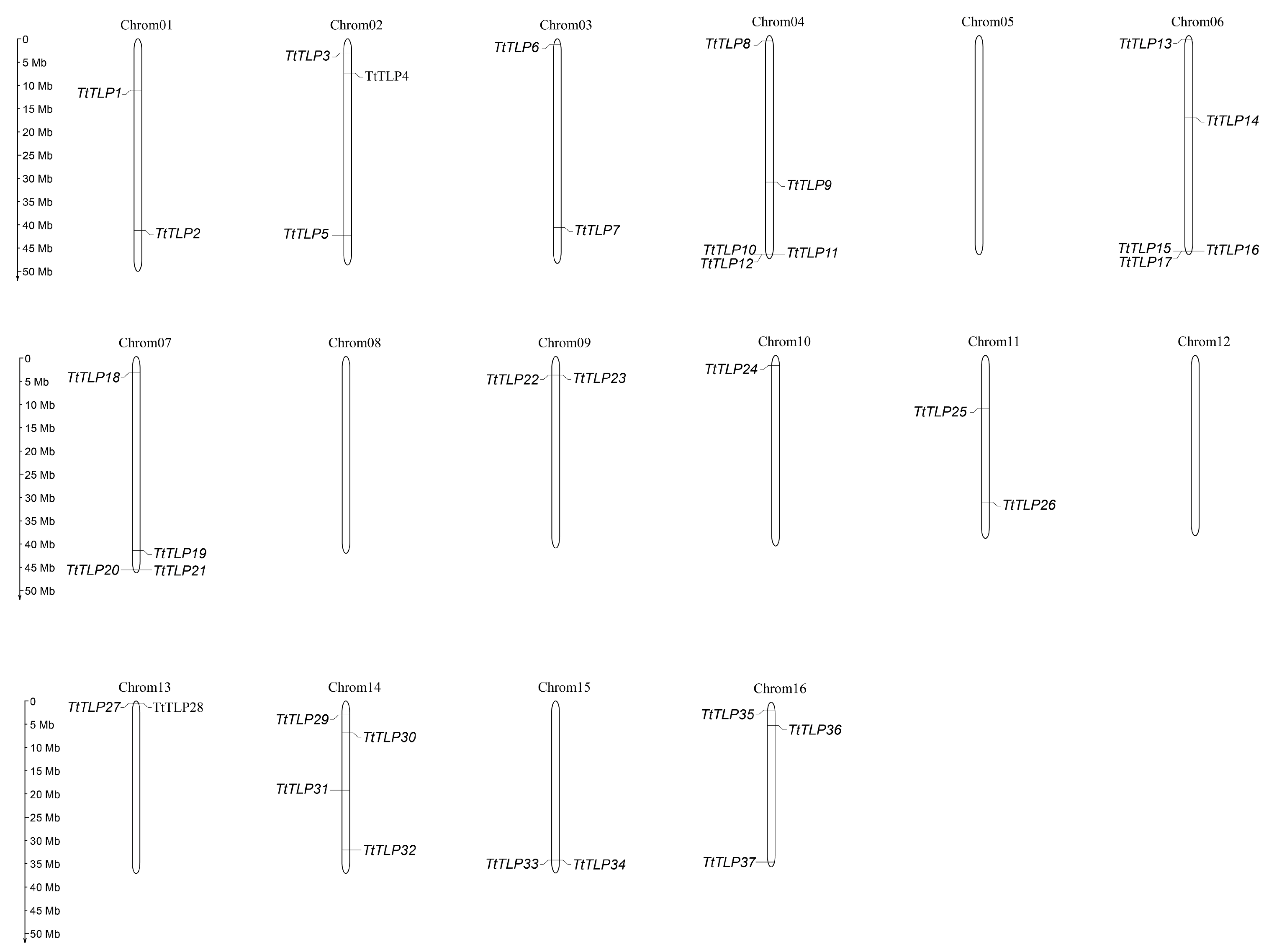
2.3. Duplication Event Investigation
2.4. Features of TtTLP Proteins
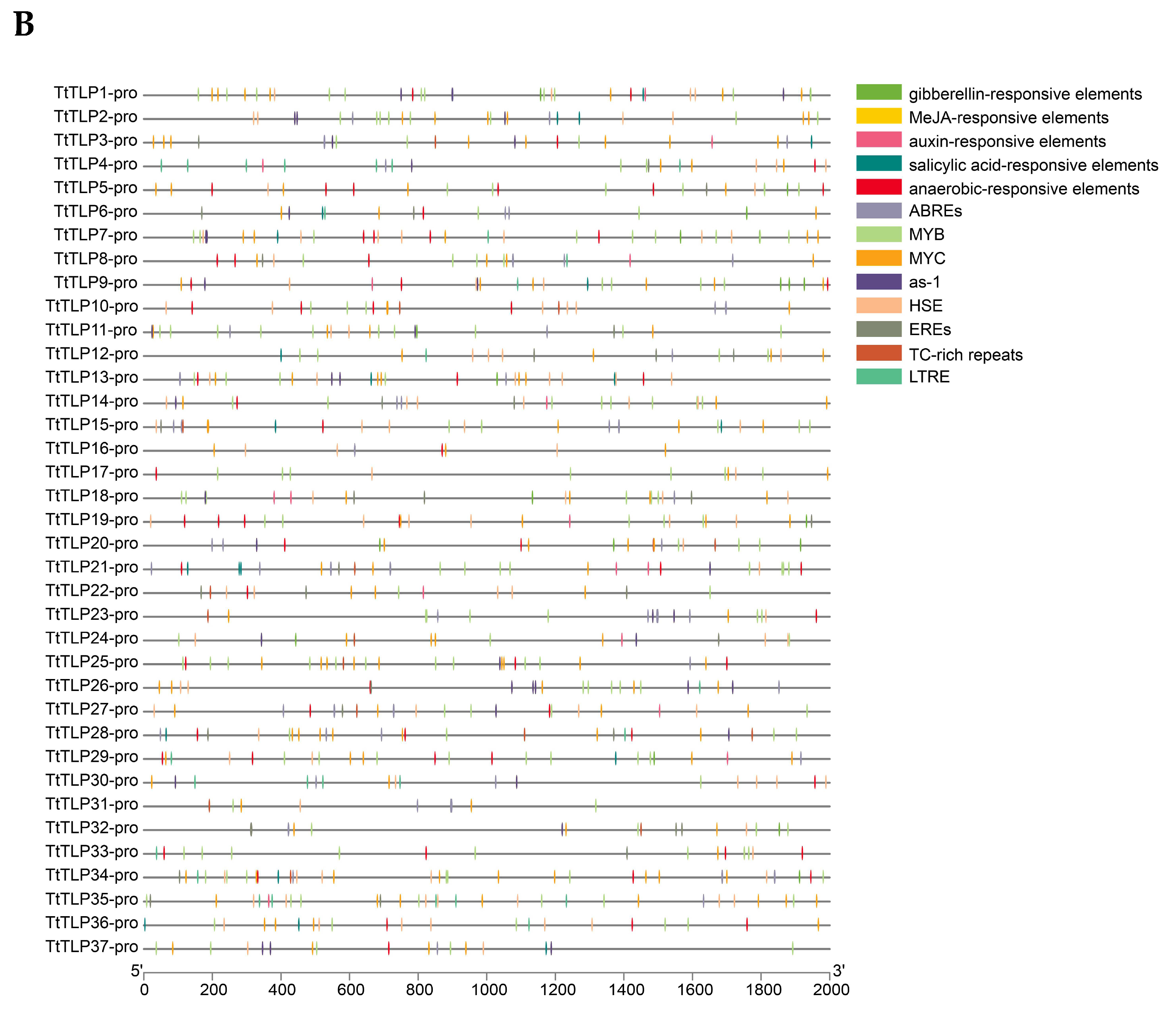
2.5. Cis-Acting Elements (CEs) of the TtTLP Promoter Sequences
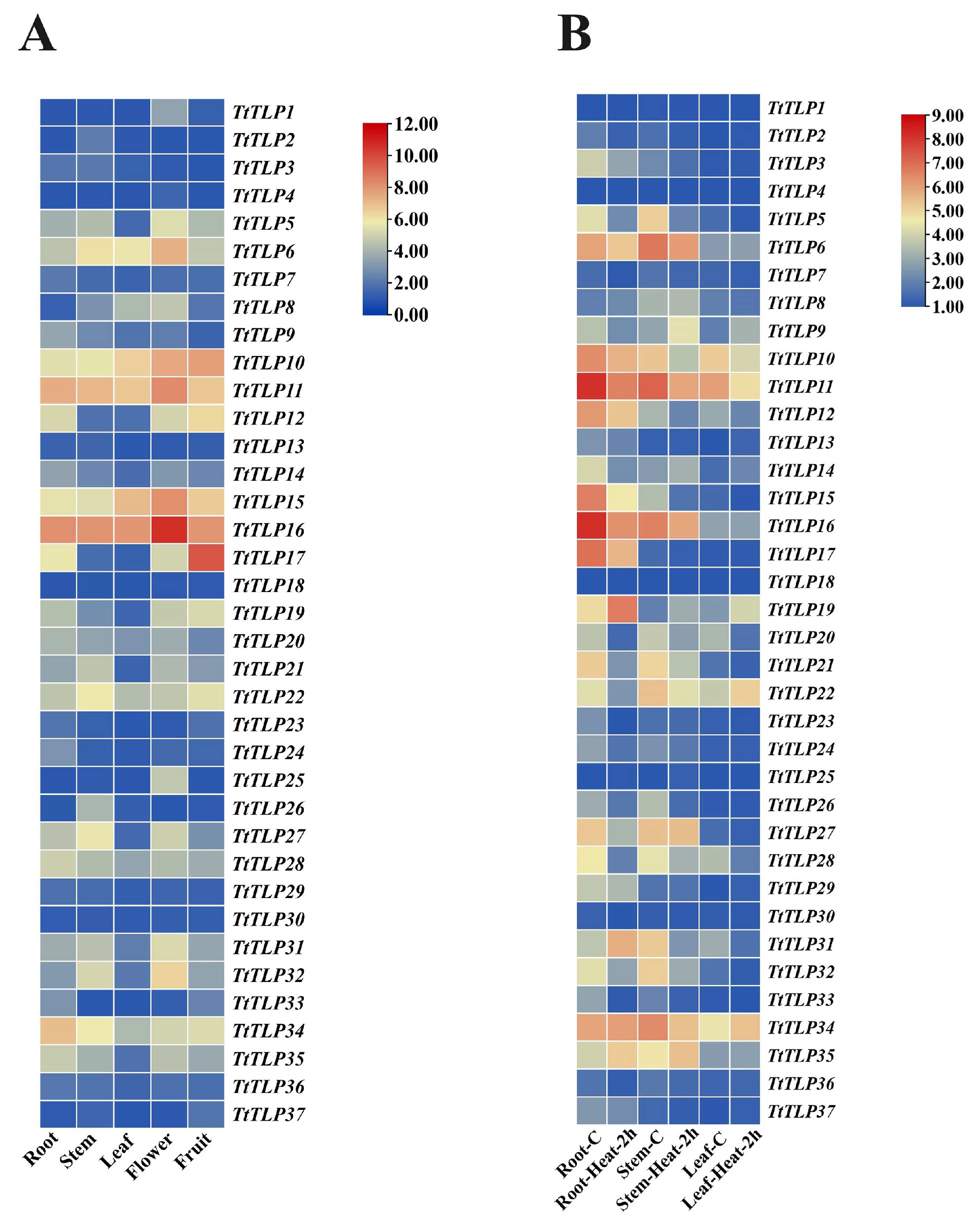
2.6. Expression Profiles of TtTLPs in Different Tissues and Plants in Response to Stress
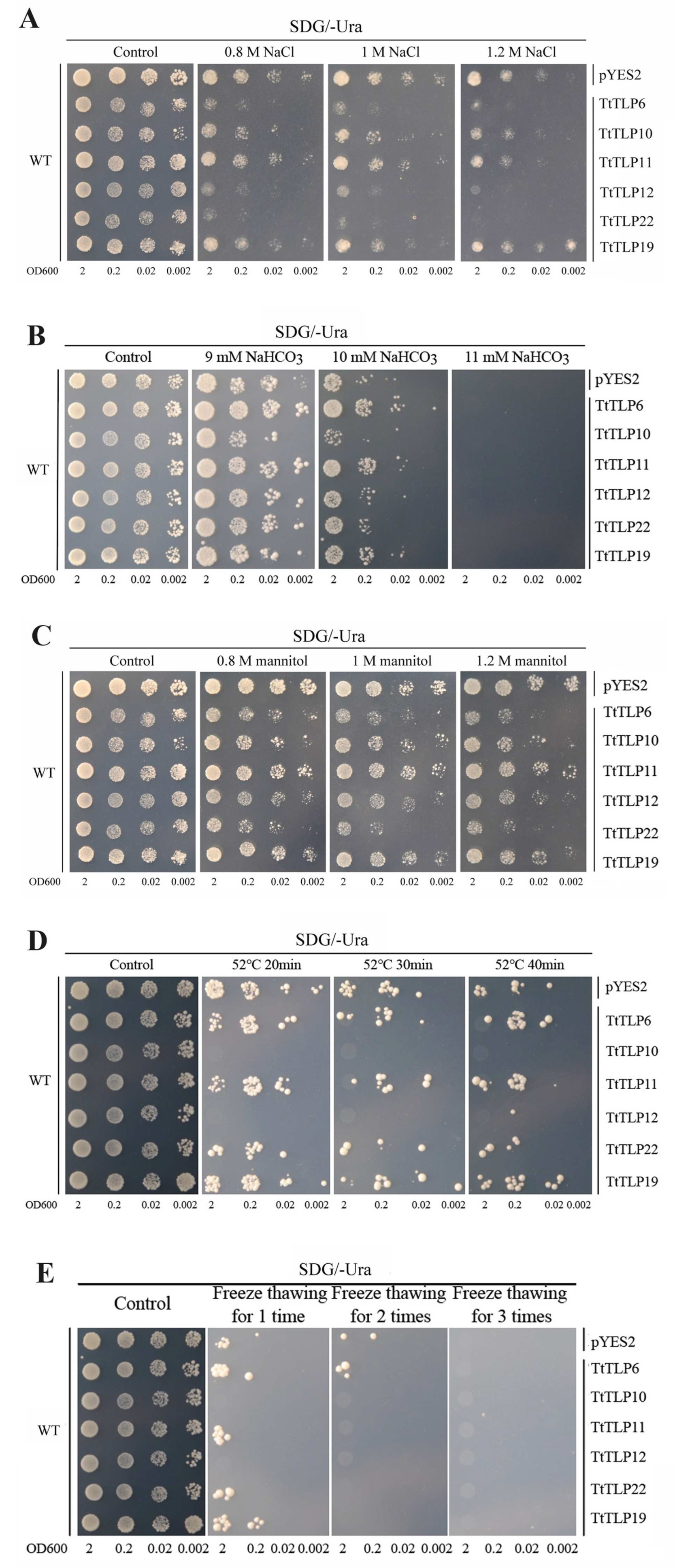
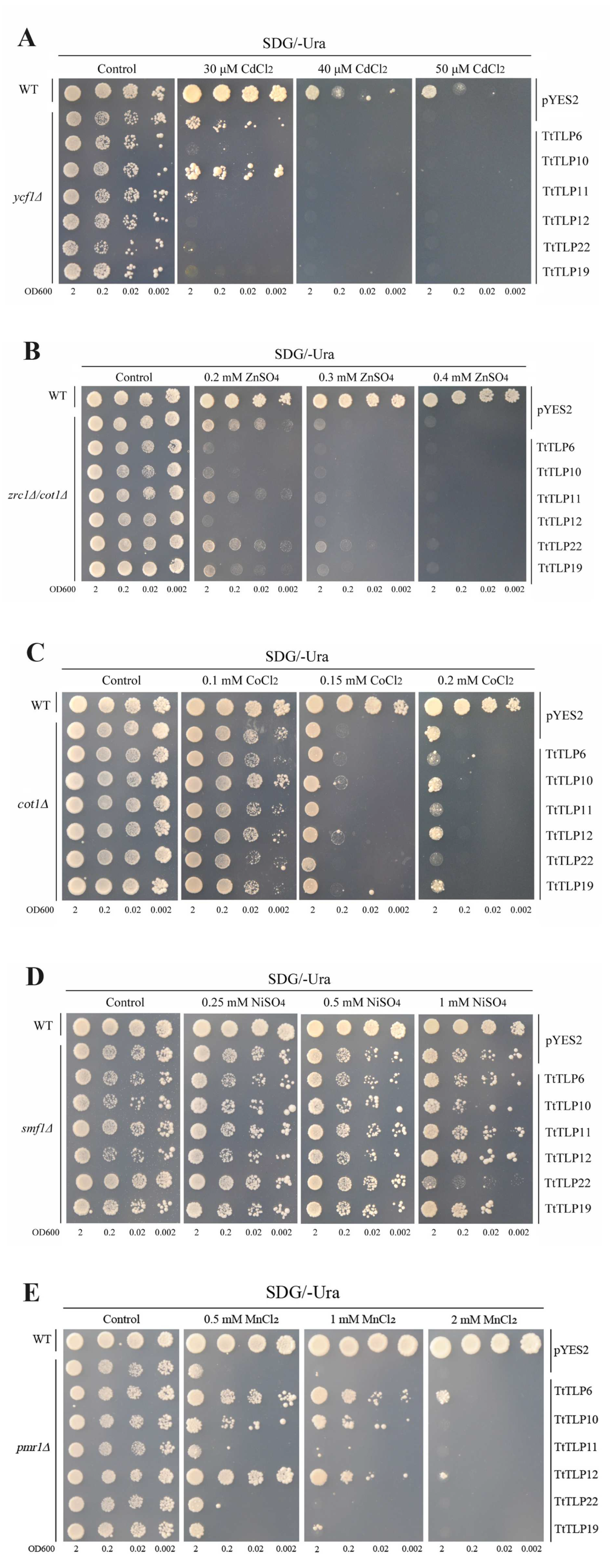
2.7. Abiotic Stress Tolerance of Yeast-Heterologous-Expressing TtTLPs
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Stress Treatments
4.2. Identification and Characterization of TtTLPs in T. tetragonoides
4.3. Multiple Sequence Alignment and Phylogenetic Analysis of TLP Proteins
4.4. Bioinformatic Analysis of T. tetragonoides TLP Genes
4.5. Promoter Sequence Profiling of TtTLPs
4.6. Expression Profiling of TtTLPs in Different Organs or Under Specific Challenges
4.7. In Vivo Stress Tolerance Assay for TtTLP Overexpression in Yeast
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TLP | Thaumatin-like protein |
| TF | Transcription factors |
| PR-5 | Pathogenesis-related protein family 5 |
| kDa | KiloDalton |
| Cys | Cysteine |
| UBQ | Ubiquitin |
| MeJA | Methyl jasmonate |
| ROS | Reactive oxygen species |
| WGD | Whole-genome duplication |
| ABA | Abscisic acid |
| TD | Tandem duplication |
| PD | Proximal duplication |
| TRD | Transposed duplication |
| DD | Dispersed duplication |
| aa | Amino acid |
| MW | Molecular weight |
| pI | Isoelectric point |
| II | Instability index |
| AI | Aliphatic index |
| GRAVY | Grand average of hydropathicity |
| REDDD | Arginine, glutamic acid, and three aspartic acid residues |
| CE | Cis-acting element |
| 5′-UTR | 5′-untranslated region |
| ABRE | CE responsive to ABA |
| WT | Wild-type |
| HM | Heavy metal |
| CRP | Cysteine-rich protein |
| LTP | Lipid transfer protein |
| ABC | ATP-binding cassette |
References
- Lee, K.Y.; Kim, S.H.; Yang, W.K.; Lee, G.J. Effect of Tetragonia tetragonoides (Pall.) Kuntze extract on andropause symptoms. Nutrients 2022, 14, 4572. [Google Scholar] [CrossRef]
- Lee, M.A.; Choi, H.J.; Kang, J.S.; Choi, Y.H.; Joo, W.H. Antioxidant activities of the solvent extracts from Tetragonia tetragonioides. J. Life Sci. 2008, 18, 220–227. [Google Scholar] [CrossRef]
- Atzori, G.; Nissim, W.; Macchiavelli, T.; Vita, F.; Azzarello, E.; Pandolfi, C.; Masi, E.; Mancuso, S. Tetragonia tetragonioides (Pallas) Kuntz. as promising salt-tolerant crop in a saline agricultural context. Agric. Water Manag. 2020, 240, 106261. [Google Scholar] [CrossRef]
- Rao, Y.; Peng, T.; Xue, S. Mechanisms of plant saline-alkaline tolerance. J. Plant Physiol. 2023, 281, 153916. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Billah, M.; Li, F.; Yang, Z. Regulatory network of cotton genes in response to salt, drought and wilt diseases (Verticillium and Fusarium): Progress and perspective. Front. Plant Sci. 2021, 12, 759245. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.C.; Sandeep; Kamthan, M.; Kumar, S.; Ghosh, S. A thaumatin-like protein of Ocimum basilicum confers tolerance to fungal pathogen and abiotic stress in transgenic Arabidopsis. Sci. Rep. 2016, 6, 25340. [Google Scholar] [CrossRef]
- de Jesús-Pires, C.; Ferreira-Neto, J.R.C.; Pacifico Bezerra-Neto, J.; Kido, E.A.; de Oliveira Silva, R.L.; Pandolfi, V.; Wanderley-Nogueira, A.C.; Binneck, E.; da Costa, A.F.; Pio-Ribeiro, G.; et al. Plant thaumatin-like proteins: Function, evolution and biotechnological applications. Curr. Protein Pept. Sci. 2020, 21, 36–51. [Google Scholar] [CrossRef]
- Bashir, M.A.; Silvestri, C.; Ahmad, T.; Hafiz, I.A.; Abbasi, N.A.; Manzoor, A.; Cristofori, V.; Rugini, E. Osmotin: A cationic protein leads to improve biotic and abiotic stress tolerance in plants. Plants 2020, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Hongbo, S.; Zhaolong, X.; Jia, L.; Dayong, Z.; Yihong, H. Salinity tolerance mechanism of osmotin and osmotin-like proteins: A promising candidate for enhancing plant salt tolerance. Curr. Genom. 2017, 18, 553–556. [Google Scholar] [CrossRef]
- Hakim; Ullah, A.; Hussain, A.; Shaban, M.; Khan, A.H.; Alariqi, M.; Gul, S.; Jun, Z.; Lin, S.; Li, J.; et al. Osmotin: A plant defense tool against biotic and abiotic stresses. Plant Physiol. Biochem. 2018, 123, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.L.; Wiebke-Strohm, B.; Bredemeier, C.; Margis-Pinheiro, M.; de Brito, G.G.; Rechenmacher, C.; Bertagnolli, P.F.; de Sá, M.E.; Campos Mde, A.; de Amorim, R.M.; et al. Expression of an osmotin-like protein from Solanum nigrum confers drought tolerance in transgenic soybean. BMC Plant Biol. 2014, 14, 343. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Basu, A.; Kundu, S. Overexpression of a new osmotin-like protein gene (SindOLP) confers tolerance against biotic and abiotic stresses in sesame. Front. Plant Sci. 2017, 8, 410. [Google Scholar] [CrossRef]
- Le, T.T.T.; Williams, B.; Mundree, S.G. An osmotin from the resurrection plant Tripogon loliiformis (TlOsm) confers tolerance to multiple abiotic stresses in transgenic rice. Physiol. Plant 2018, 162, 13–34. [Google Scholar] [CrossRef]
- Yan, J.; Ninkuu, V.; Fu, Z.; Yang, T.; Ren, J.; Li, G.; Yang, X.; Zeng, H. OsOLP1 contributes to drought tolerance in rice by regulating ABA biosynthesis and lignin accumulation. Front. Plant Sci. 2023, 14, 1163939. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Cui, Y.; Qiao, K.; Zhu, L.; Fan, S.; Ma, Q. Comprehensive genome-wide analysis of thaumatin-like gene family in four cotton species and functional identification of GhTLP19 involved in regulating tolerance to Verticillium dahlia and drought. Front. Plant Sci. 2020, 11, 575015. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, H.; Rajput, R.; Pandey, A.; Upadhyay, S.K. Molecular characterization revealed the role of thaumatin-like proteins of bread wheat in stress response. Front. Plant Sci. 2022, 12, 807448. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sui, X.; Wang, Y.; Zhu, M.; Zhou, Y.; Gao, F. Genome-wide analyses of thaumatin-like protein family genes reveal the involvement in the response to low-temperature stress in Ammopiptanthus nanus. Int. J. Mol. Sci. 2023, 24, 2209. [Google Scholar] [CrossRef]
- Tong, Z.; Sun, Y.; Wang, D.; Wang, L.; Li, L.; Meng, X.; Feng, W.; Wurtele, E.S.; Wang, X. Identification and functional characterization of HbOsmotin from Hevea brasiliensis. Plant Physiol. Biochem. 2016, 109, 171–180. [Google Scholar] [CrossRef]
- Petre, B.; Major, I.; Rouhier, N.; Duplessis, S. Genome-wide analysis of eukaryote thaumatin-like proteins (TLPs) with an emphasis on poplar. BMC Plant Biol. 2011, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Qiao, H.; Zhang, X.; Guo, C.; Wang, M.; Wang, Y.; Wang, X. Analysis of the grape (Vitis vinifera L.) thaumatin-like protein (TLP) gene family and demonstration that TLP29 contributes to disease resistance. Sci. Rep. 2017, 7, 4269. [Google Scholar] [CrossRef]
- Iqbal, I.; Tripathi, R.K.; Wilkins, O.; Singh, J. Thaumatin-like protein (TLP) gene family in barley: Genome-wide exploration and expression analysis during germination. Genes 2020, 11, 1080. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, J.; Zhou, X.; Luan, Y.; Luan, F. Genome-wide identification, characterization and expression analysis of the TLP gene family in melon (Cucumis melo L.). Genomics 2020, 112, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, W.; Sang, X.; Wang, T.; Gong, H.; Zhao, Y.; Zhao, P.; Wang, H. Genome-wide identification of the thaumatin-like protein family genes in Gossypium barbadense and analysis of their responses to Verticillium dahliae infection. Plants 2021, 10, 2647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Miao, L.; Yang, X.; Jiang, G. Genome-wide characterization and expression of the TLP gene family associated with Colletotrichum gloeosporioides inoculation in Fragaria × ananassa. PeerJ 2022, 10, e12979. [Google Scholar] [CrossRef]
- Anisimova, O.K.; Kochieva, E.Z.; Shchennikova, A.V.; Filyushin, M.A. Thaumatin-like protein (TLP) genes in garlic (Allium sativum L.): Genome-wide identification, characterization, and expression in response to Fusarium proliferatum infection. Plants 2022, 11, 748. [Google Scholar] [CrossRef] [PubMed]
- Faillace, G.R.; Turchetto-Zolet, A.C.; Guzman, F.L.; de Oliveira-Busatto, L.A.; Bodanese-Zanettini, M.H. Genome-wide analysis and evolution of plant thaumatin-like proteins: A focus on the origin and diversification of osmotins. Mol. Genet. Genom. 2019, 294, 1137–1157. [Google Scholar] [CrossRef]
- Hrmova, M.; Hussain, S.S. Plant transcription factors involved in drought and associated stresses. Int. J. Mol. Sci. 2021, 22, 5662. [Google Scholar] [CrossRef]
- Li, S.; Hu, Y.; Yang, H.; Tian, S.; Wei, D.; Tang, Q.; Yang, Y.; Wang, Z. The regulatory roles of MYC TFs in plant stamen development. Plant Sci. 2023, 333, 111734. [Google Scholar] [CrossRef]
- Abdin, M.Z.; Kiran, U.; Alam, A. Analysis of osmotin, a PR protein as metabolic modulator in plants. Bioinformation 2011, 5, 336–340. [Google Scholar] [CrossRef]
- Viktorova, J.; Krasny, L.; Kamlar, M.; Novakova, M.; Mackova, M.; Macek, T. Osmotin, a pathogenesis-related protein. Curr. Protein Pept. Sci. 2012, 13, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Harada, E.; Kim, J.A.; Meyer, A.J.; Hell, R.; Clemens, S.; Choi, Y.E. Expression profiling of tobacco leaf trichomes identifies genes for biotic and abiotic stresses. Plant Cell Physiol. 2010, 51, 1627–1637. [Google Scholar] [CrossRef]
- Walliwalagedara, C.; Atkinson, I.; van Keulen, H.; Cutright, T.; Wei, R. Differential expression of proteins induced by lead in the Dwarf Sunflower Helianthus annuus. Phytochemistry 2010, 1, 1460–1465. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Lesch, S.M.; Grieve, C.M. Growth stage modulates salinity tolerance of New Zealand spinach (Tetragonia tetragonioides Pall.) and red orach (Atriplex hortensis L.). Ann. Bot. 2000, 85, 501–509. [Google Scholar] [CrossRef]
- Wang, L.; Wu, C.B.; Xu, W.F.; Guo, J.C.; Fu, S.P.; Liu, J.; Li, R.M.; Duan, R.J. Effect of seawater stress on growth, osmotic adjustment substances and cell membrane permeability of Tetragonia tetragonoides (In Chinese). Jiangsu Agric. Sci. 2016, 44, 248–251. [Google Scholar] [CrossRef]
- Zou, C.; Chen, A.; Xiao, L.; Muller, H.M.; Ache, P.; Haberer, G.; Zhang, M.; Jia, W.; Deng, P.; Huang, R.; et al. A high-quality genome assembly of quinoa provides insights into the molecular basis of salt bladder-based salinity tolerance and the exceptional nutritional value. Cell Res. 2017, 27, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Bose, J.; Hedrich, R. Salt bladders: Do they matter? Trends Plant Sci. 2014, 19, 687–691. [Google Scholar] [CrossRef]
- Sels, J.; Mathys, J.; De Coninck, B.M.; Cammue, B.P.; De Bolle, M.F. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef]
- Liu, J.J.; Sturrock, R.; Ekramoddoullah, A.K. The superfamily of thaumatin-like proteins: Its origin, evolution, and expression towards biological function. Plant Cell Rep. 2010, 29, 419–436. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Zhang, J.; Huang, L.; Shi, Z.; Tian, Z.; Sha, A.; Lu, G. Thaumatin-like protein family genes VfTLP4-3 and VfTLP5 are critical for faba bean’s response to drought stress at the seedling stage. Plant Physiol. Biochem. 2024, 206, 108243. [Google Scholar] [CrossRef]
- Sharma, A.; Shumayla; Tyagi, S.; Alok, A.; Singh, K.; Upadhyay, S.K. Thaumatin-like protein kinases: Molecular characterization and transcriptional profiling in five cereal crops. Plant Sci. 2020, 290, 110317. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Z.; Yin, W.; Xu, K.; Wang, S.; Shang, Q.; Sa, W.; Liang, J.; Wang, L. Genome-wide analysis of the thaumatin-like gene family in Qingke (Hordeum vulgare L. var. nudum) uncovers candidates involved in plant defense against biotic and abiotic stresses. Front. Plant Sci. 2022, 13, 912296. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, H.; He, S.; Zhang, P.; Ma, X. Genome-wide identification and characterization of the TLP gene family in Phyllostachys edulis and association with Witches’ Broom disease resistance in bamboo. Int. J. Mol. Sci. 2023, 24, 10257. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, N.K.; Tomasiak, T.M. Structural basis for autoinhibition by the dephosphorylated regulatory domain of Ycf1. Nat. Commun. 2024, 15, 2389. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Chi, S.D.; He, Z.M. The development of Aspergillus flavus and biosynthesis of aflatoxin B1 are regulated by the Golgi-localized Mn2+ transporter pmr1. J. Agric. Food Chem. 2024, 72, 1276–1291. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Zheng, S.; Dai, H.; Meng, Q.; Huang, R.; Tong, H.; Yuan, L. Identification and expression analysis of the ZRT, IRT-like protein (ZIP) gene family in Camellia sinensis (L.) O. Kuntze. Plant Physiol. Biochem. 2022, 172, 87–100. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Q.; Cao, L.; Huang, Z.; Wang, Z.; Zhang, M.; Jian, S. Identification of the abscisic acid-, stress-, and ripening-induced (ASR) family involved in the adaptation of Tetragonia tetragonoides (Pall.) Kuntze to saline-alkaline and drought habitats. Int. J. Mol. Sci. 2023, 24, 15815. [Google Scholar] [CrossRef]






| Name | Locus | Length (aa) and MW (kDa) | Major Amino Acid (%) | PI | II | AI | GRAVY | Disordered aa (%) | TMHs and Topologies | WoLF_PSORT | Plant-PLoc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TtTLP1 | 01G0009210 | 247–26.38 | T (11.7%), A (8.1%), P (8.1%) | 7.39 | 40.68 | 72.27 | 0.005 | 70.45 | None/outside | chlo: 14 | Extracellular |
| TtTLP2 | 01G0018050 | 211–22.82 | S (10.0%), L (9.0%), V (7.6%) | 5.29 | 49.66 | 72.99 | −0.226 | 84.83 | None/outside | nucl: 7, chlo: 6 | Nucleus |
| TtTLP3 | 02G0003200 | 291–31.30 | G (10.3%), S (9.3%), L (7.9%) | 8.24 | 37.81 | 71.37 | 0.099 | 80.07 | 1/out to in | golg: 3.5, golg_plas: 3.5, vacu: 3, plas: 2.5, chlo: 2, extr: 2 | Extracellular |
| TtTLP4 | 02G0007540 | 245–25.82 | S (13.9%), G (10.2%), T (10.2%) | 6.65 | 48.32 | 57.39 | −0.129 | 87.76 | None/outside | extr: 9, chlo: 4 | Extracellular |
| TtTLP5 | 02G0018220 | 251–26.39 | A (11.6%), G (11.2%), S (8.8%) | 8.81 | 44.10 | 67.37 | 0.133 | 84.86 | 1/in to out | chlo: 9, extr: 2, vacu: 2 | Extracellular |
| TtTLP6 | 03G0001120 | 247–26.44 | G (10.9%), L (9.3%), P (8.9%) | 7.88 | 50.49 | 66.76 | −0.065 | 87.85 | None/outside | extr: 6, chlo: 3, vacu: 2, nucl: 1, mito: 1 | Cell wall |
| TtTLP7 | 03G0019340 | 659–73.67 | L (10.3%), G (8.3%), V (7.1%) | 8.47 | 33.15 | 84.43 | −0.192 | 59.79 | None/outside | chlo: 5, nucl: 4, cyto: 4 | Chloroplast |
| TtTLP8 | 04G0001170 | 179–19.33 | G (10.1%), L (8.9%), T (8.9%) | 8.13 | 53.30 | 66.03 | −0.040 | 78.77 | None/outside | extr: 7, chlo: 2, vacu: 2, nucl: 1, mito: 1 | Cell wall |
| TtTLP9 | 04G0015010 | 244–26.17 | G (10.7%), L (7.8%), V (7.8%) | 8.09 | 41.77 | 73.48 | −0.015 | 83.20 | 1/in to out | chlo: 12, extr: 2 | Extracellular |
| TtTLP10 | 04G0025610 | 226–23.58 | G (11.9%), T (11.1%), S (8.4%) | 4.68 | 29.98 | 50.53 | −0.133 | 92.04 | 1/in to out | chlo: 5, extr: 4, vacu: 4 | Extracellular |
| TtTLP11 | 04G0025620 | 227–24.02 | G (11.9%), T (10.1%), P (8.4%) | 8.40 | 19.66 | 54.58 | −0.202 | 88.55 | None/outside | chlo: 8, extr: 4, vacu: 2 | Vacuole |
| TtTLP12 | 04G0025640 | 232–24.86 | G (9.9%), T (9.5%), P (8.2%) | 6.08 | 42.10 | 60.60 | −0.314 | 88.36 | None/outside | extr: 8, chlo: 4, vacu: 1 | Extracellular |
| TtTLP13 | 06G0000860 | 384–39.82 | G (13.5%), S (13.0%), P (11.5%) | 4.80 | 56.96 | 57.92 | −0.128 | 85.42 | 2/in to in | vacu: 4, golg_plas: 4, plas: 3.5, golg: 3.5, extr: 3 | Extracellular |
| TtTLP14 | 06G0011700 | 244–26.12 | G (10.7%), L (8.2%), C (7.4%), S (7.4%) | 7.80 | 42.85 | 71.52 | −0.029 | 81.97 | None/outside | extr: 7, chlo: 3, nucl: 1, cyto: 1, vacu: 1 | Extracellular |
| TtTLP15 | 06G0023190 | 227–23.67 | G (12.3%), T (11.0%), S (8.4%) | 4.68 | 28.01 | 51.19 | −0.138 | 91.63 | 1/in to out | chlo: 6, vacu: 4, extr: 3 | Extracellular |
| TtTLP16 | 06G0023200 | 227–24.00 | G (11.9%), T (10.1%), P (8.8%) | 8.40 | 19.01 | 54.58 | −0.203 | 88.55 | None/outside | chlo: 7, extr: 4, vacu: 2 | Extracellular |
| TtTLP17 | 06G0023220 | 232–24.87 | G (9.9%), T (9.9%), P (9.1%) | 5.08 | 38.91 | 61.03 | −0.284 | 89.66 | None/outside | extr: 8, chlo: 4, vacu: 1 | Vacuole |
| TtTLP18 | 07G0002190 | 274–29.60 | S (11.7%), T (9.1%), G (8.0%) | 5.14 | 46.68 | 68.36 | −0.094 | 84.67 | None/outside | nucl: 6, mito: 4, chlo: 3 | Extracellular |
| TtTLP19 | 07G0015670 | 226–24.49 | T (9.3%), G (8.8%), N (8.4%) | 7.84 | 30.83 | 60.04 | −0.188 | 85.84 | None/outside | chlo: 14 | Vacuole |
| TtTLP20 | 07G0019970 | 364–39.62 | L (9.3%), A (8.8%), S (8.8%) | 8.64 | 42.92 | 79.09 | 0.001 | 71.43 | None/outside | chlo: 4, mito: 3, vacu: 3, extr: 2, nucl: 1 | Cell wall |
| TtTLP21 | 07G0019980 | 355–36.81 | G (13.0%), T (13.0%), S (11.5%) | 4.31 | 31.77 | 57.21 | −0.032 | 90.14 | 1/out to in | chlo: 4, extr: 4, vacu: 3, nucl: 2 | Chloroplast |
| TtTLP22 | 09G0001980 | 332–34.63 | S (12.0%), G (10.8%), T (10.5%) | 4.70 | 38.64 | 64.70 | −0.121 | 78.31 | None/outside | chlo: 11, extr: 2 | Extracellular |
| TtTLP23 | 09G0001990 | 340–35.38 | S (14.7%), G (12.1%), T (10.0%) | 4.73 | 47.62 | 55.18 | 0.010 | 83.82 | 2/out to out | extr: 5, golg: 3.5, golg_plas: 2.5, chlo: 2, vacu: 2 | Extracellular |
| TtTLP24 | 10G0001950 | 410–45.74 | L (10.5%), T (9.8%), S (8.8%) | 9.28 | 39.04 | 83.17 | −0.092 | 66.59 | 1/in to out | plas: 8.5, golg_plas: 5.5, golg: 1.5, chlo: 1, cyto: 1, vacu: 1 | Chloroplast |
| TtTLP25 | 11G0010740 | 246–26.20 | T (13.0%), P (8.5%), G (7.7%), L (7.7%) | 6.66 | 37.24 | 70.16 | 0.080 | 81.71 | None/outside | chlo: 14 | Extracellular |
| TtTLP26 | 11G0017940 | 272–29.88 | L (8.5%), P (7.4%), S (7.4%) | 6.77 | 49.27 | 81.73 | −0.024 | 75.37 | 1/in to out | chlo: 8, extr: 2, vacu: 2, nucl: 1 | Chloroplast |
| TtTLP27 | 13G0000550 | 359–37.26 | T (12.8%), G (12.5%), S (11.7%) | 4.27 | 31.77 | 58.25 | −0.025 | 86.91 | 1/out to in | chlo: 4, extr: 3, nucl: 2, vacu: 2, E.R.: 1.5, E.R._plas: 1.5 | Extracellular |
| TtTLP28 | 13G0000560 | 317–33.62 | L (10.7%), A (10.4%), G (9.5%) | 8.27 | 37.09 | 83.47 | 0.268 | 72.56 | 1/out to in | chlo: 6, extr: 4, plas: 3 | Cell wall |
| TtTLP29 | 14G0003240 | 290–31.12 | G (10.3%), S (9.0%), L (7.6%), T (7.6%), V (7.6%) | 7.35 | 35.08 | 70.59 | 0.108 | 77.59 | 2/in to in | golg: 3.5, golg_plas: 3.5, extr: 3, vacu: 3, plas: 2.5, chlo: 2 | Extracellular |
| TtTLP30 | 14G0007370 | 825–91.27 | L (9.5%), S (9.2%), G (7.5%) | 8.58 | 51.43 | 81.22 | −0.168 | 46.55 | None/outside | nucl: 6, chlo: 4, mito: 2, plas: 1 | Cytoplasm |
| TtTLP31 | 14G0013960 | 259–26.33 | G (15.1%), S (11.6%), T (9.3%) | 4.23 | 36.07 | 58.03 | −0.020 | 84.94 | 1/in to out | chlo: 13 | Extracellular |
| TtTLP32 | 14G0017910 | 251–26.39 | A (12.4%), G (11.2%), S (8.4%) | 9.01 | 42.22 | 69.32 | 0.100 | 86.45 | None/outside | chlo: 10, mito: 3 | Cell wall |
| TtTLP33 | 15G0017250 | 342–36.53 | S (14.9%), G (12.6%), T (8.5%) | 4.90 | 46.67 | 59.42 | 0.011 | 85.96 | 2/out to out | extr: 7, chlo: 3, vacu: 2, mito: 1 | Extracellular |
| TtTLP34 | 15G0017260 | 334–34.89 | S (12.3%), T (10.5%), G (10.2%) | 4.64 | 38.09 | 61.41 | −0.122 | 82.93 | None/outside | nucl: 7.5, chlo: 5, cyto_nucl: 4.5 | Extracellular |
| TtTLP35 | 16G0001800 | 346–35.10 | G (14.2%), S (13.9%), T (12.7%) | 4.51 | 43.80 | 52.57 | −0.136 | 91.91 | None/outside | extr: 5, chlo: 2, nucl: 2, plas: 2, vacu: 2 | Chloroplast |
| TtTLP36 | 16G0005420 | 513–58.38 | L (11.5%), G (7.2%), K (6.8%) | 8.40 | 30.92 | 89.84 | −0.248 | 44.64 | None/outside | cyto: 12, nucl: 2 | Chloroplast |
| TtTLP37 | 16G0019580 | 358–37.04 | G (13.7%), S (11.2%), P (10.3%) | 4.80 | 53.04 | 54.53 | −0.219 | 87.71 | 1/in to out | extr: 9, vacu: 3, golg: 2 | Extracellular |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Ding, Q.; Wang, Z.; Jian, S.; Zhang, M. Genome-Wide Identification and Expression Analyses of the Thaumatin-Like Protein Gene Family in Tetragonia tetragonoides (Pall.) Kuntze Reveal Their Functions in Abiotic Stress Responses. Plants 2024, 13, 2355. https://doi.org/10.3390/plants13172355
Huang Z, Ding Q, Wang Z, Jian S, Zhang M. Genome-Wide Identification and Expression Analyses of the Thaumatin-Like Protein Gene Family in Tetragonia tetragonoides (Pall.) Kuntze Reveal Their Functions in Abiotic Stress Responses. Plants. 2024; 13(17):2355. https://doi.org/10.3390/plants13172355
Chicago/Turabian StyleHuang, Zengwang, Qianqian Ding, Zhengfeng Wang, Shuguang Jian, and Mei Zhang. 2024. "Genome-Wide Identification and Expression Analyses of the Thaumatin-Like Protein Gene Family in Tetragonia tetragonoides (Pall.) Kuntze Reveal Their Functions in Abiotic Stress Responses" Plants 13, no. 17: 2355. https://doi.org/10.3390/plants13172355
APA StyleHuang, Z., Ding, Q., Wang, Z., Jian, S., & Zhang, M. (2024). Genome-Wide Identification and Expression Analyses of the Thaumatin-Like Protein Gene Family in Tetragonia tetragonoides (Pall.) Kuntze Reveal Their Functions in Abiotic Stress Responses. Plants, 13(17), 2355. https://doi.org/10.3390/plants13172355





