Endophytic Colletotrichum fructicola KL19 and Its Derived SeNPs Mitigate Cd-Stress-Associated Damages in Spinacia oleracea L.
Abstract
:1. Introduction
2. Results
2.1. Screening and Characterization of the Endophytic Fungus
2.1.1. Screening of Effective Strains for Synthesizing SeNPs
2.1.2. Morphological and Molecular Identification
2.2. Fitting Models of SeNPs Yields and Analysis of the BBD
2.2.1. Analysis of 2D and 3D Response Surface Plots
2.2.2. Optimization and Validation
2.3. Biogenic SeNPs Characterization
2.4. Soil-Pot Experimentation
2.4.1. Strain KL19 and SeNPs Improved the Growth of Small-Leaf Spinach under
Cd Stress
2.4.2. Effect of Strain KL19 and SeNPs on Spinach Leaf Physiology
2.4.3. Cd Uptake and Translocation and Se Influx in Small-Leaf Spinach
3. Discussion
4. Materials and Methods
4.1. Experimental Reagents
4.2. Screening of Endophytic Fungi
Taxonomic Identification and Phylogenetic Analysis of Strain KL19
4.3. Optimization and Acquisition of Extracellular SeNPs
4.4. Characterization of SeNPs
4.5. Pot Experiment
4.5.1. Soil Treatment and Planting of Small-Leaf Spinach
4.5.2. Measurement of Growth Parameters and Chlorophyll Content
4.5.3. Antioxidant Capacity Detection
4.5.4. Cd and Se Contents Detection
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.J.; Li, G.J.; Xia, M.L.; Chen, Y.M.; Chen, Y.; Kumar, S.; Sun, Z.L.; Li, X.Z.; Zhao, X.Y.; Hou, H.W. Combined effects of temperature and nutrients on the toxicity of cadmium in duckweed (Lemna aequinoctialis). J. Hazard. Mater. 2022, 432, 128646. [Google Scholar] [CrossRef]
- Vainio, H.; Heseltine, E.; Partensky, C.; Wilbourn, J. Meeting of the Iarc Working Group on Beryllium, Cadmium, Mercury and Exposures in the Glass Manufacturing-Industry. Scand. J. Work. Environ. Health 1993, 19, 360–363. [Google Scholar]
- Qin, X.M.; Nie, Z.J.; Liu, H.E.; Zhao, P.; Qin, S.Y.; Shi, Z.W. Influence of selenium on root morphology and photosynthetic characteristics of winter wheat under cadmium stress. Environ. Exp. Bot. 2018, 150, 232–239. [Google Scholar] [CrossRef]
- Sardar, R.; Ahmed, S.; Shah, A.A.; Yasin, N.A. Selenium nanoparticles reduced cadmium uptake, regulated nutritional homeostasis and antioxidative system in Coriandrum sativum grown in cadmium toxic conditions. Chemosphere 2022, 287, 132332. [Google Scholar] [CrossRef]
- Bashir, S.; Ali, U.; Shaaban, M.; Gulshan, A.B.; Iqbal, J.; Khan, S.; Husain, A.; Ahmed, N.; Mehmood, S.; Kamran, M.; et al. Role of sepiolite for cadmium (Cd) polluted soil restoration and spinach growth in wastewater irrigated agricultural soil. J. Environ. Manag. 2020, 258, 110020. [Google Scholar] [CrossRef] [PubMed]
- Nafees, M.; Sehrish, A.K.; Alomrani, S.O.; Qiu, L.L.; Saeed, A.; Ahmad, S.; Ali, S.; Guo, H.Y. Mechanism and synergistic effect of sulfadiazine (SDZ) and cadmium toxicity in spinach (Spinacia oleracea L.) and its alleviation through zinc fortification. J. Hazard. Mater. 2024, 464, 132903. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Wang, Q.Q.; Dai, F.J.; Li, H.F. Reduction of selenite to selenium nanospheres by Se(IV)-resistant Lactobacillus paralimentarius JZ07. Food Chem. 2022, 393, 133385. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Iqbal, J.; Ali, A.; Ayaz, M.; Abbas, M.; Ahmad, I.; Devkota, H.P. Microbes-mediated synthesis strategies of metal nanoparticles and their potential role in cancer therapeutics. Semin. Cancer Biol. 2022, 86, 693–705. [Google Scholar] [CrossRef]
- Farooq, M.A.; Islam, F.; Ayyaz, A.; Chen, W.; Noor, Y.; Hu, W.; Hannan, F.; Zhou, W. Mitigation effects of exogenous melatonin-selenium nanoparticles on arsenic-induced stress in Brassica napus. Environ. Pollut. 2022, 292, 118473. [Google Scholar] [CrossRef]
- Hussain, A.; Lakhan, M.N.; Hanan, A.; Soomro, I.A.; Ahmed, M.; Bibi, F.; Zehra, I. Recent progress on green synthesis of selenium nanoparticles—A review. Mater. Today Sustain. 2023, 23, 100420. [Google Scholar] [CrossRef]
- Mosallam, F.M.; El-Sayyad, G.S.; Fathy, R.M.; El-Batal, A.I. Biomolecules-mediated synthesis of selenium nanoparticles using Aspergillus oryzae fermented Lupin extract and gamma radiation for hindering the growth of some multidrug-resistant bacteria and pathogenic fungi. Microb. Pathog. 2018, 122, 108–116. [Google Scholar] [CrossRef]
- Faramarzi, S.; Anzabi, Y.; Jafarizadeh-Malmiri, H. Nanobiotechnology approach in intracellular selenium nanoparticle synthesis using Saccharomyces cerevisiae—Fabrication and characterization. Arch. Microbiol. 2020, 202, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.B.; Namvar, F.; Moniri, M.; Tahir, P.M.; Azizi, S.; Mohamad, R. Nanoparticles Biosynthesized by Fungi and Yeast: A Review of Their Preparation, Properties, and Medical Applications. Molecules 2015, 20, 16540–16565. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Mu, C.Y.; Li, Y.L.; Wang, Y.X.; Ma, W.Y.; Ge, C.H.; Cheng, C.; Shi, G.L.; Li, H.B.; Zhou, D.M. Foliar application of selenium nanoparticles alleviates cadmium toxicity in maize (Zea mays L.) seedlings: Evidence on antioxidant, gene expression, and metabolomics analysis. Sci. Total Environ. 2023, 899, 165521. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Dong, Y.W.; Zhu, N.; Jin, H.M. Foliar application of biosynthetic nano-selenium alleviates the toxicity of Cd, Pb, and Hg in Brassica chinensis by inhibiting heavy metal adsorption and improving antioxidant system in plant. Ecotoxicol. Environ. Saf. 2022, 240, 113681. [Google Scholar] [CrossRef]
- Li, D.; Zhou, C.R.; Ma, J.L.; Wu, Y.L.; Kang, L.; An, Q.S.; Zhang, J.B.; Deng, K.L.; Li, J.Q.; Pan, C.P. Nanoselenium transformation and inhibition of cadmium accumulation by regulating the lignin biosynthetic pathway and plant hormone signal transduction in pepper plants. J. Nanobiotechnol. 2021, 19, 316. [Google Scholar] [CrossRef]
- Ran, M.; Wu, J.; Jiao, Y.; Li, J. Biosynthetic selenium nanoparticles (Bio-SeNPs) mitigate the toxicity of antimony (Sb) in rice (Oryza sativa L.) by limiting Sb uptake, improving antioxidant defense system and regulating stress-related gene expression. J. Hazard. Mater. 2024, 470, 134263. [Google Scholar] [CrossRef]
- Zeeshan, M.; Hu, Y.X.; Guo, X.H.; Sun, C.Y.; Salam, A.; Ahmad, S.; Muhammad, I.; Nasar, J.; Jahan, M.S.; Fahad, S.; et al. Physiological and transcriptomic study reveal SeNPs-mediated AsIII stress detoxification mechanisms involved modulation of antioxidants, metal transporters, and transcription factors in Glycine max L. (Merr.) roots. Environ. Pollut. 2023, 317, 120637. [Google Scholar] [CrossRef]
- Chen, F.; Ren, C.-G.; Zhou, T.; Wei, Y.-J.; Dai, C.-C. A novel exopolysaccharide elicitor from endophytic fungus Gilmaniella sp. AL12 on volatile oils accumulation in Atractylodes lancea. Sci. Rep. 2016, 6, 34735. [Google Scholar] [CrossRef]
- Zheng, J.D.; Xie, X.G.; Li, C.Y.; Wang, H.X.; Yu, Y.R.; Huang, B.K. Regulation mechanism of plant response to heavy metal stress mediated by endophytic fungi. Int. J. Phytoremediat. 2023, 25, 1596–1613. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Kiran, K.; Banerjee, S.; Pande, V.; Dandapat, A. Myco-nanotechnological approach to synthesize silver oxide nanocuboids using endophytic fungus isolated from Citrus pseudolimon plant. Colloids Surf. B Biointerfaces 2021, 206, 111948. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Kalaivani, S.; Sabarathinam, S.; Vasuki, M.; Soundari, A.; Das, M.A.; Elfasakhany, A.; Pugazhendhi, A. Copper oxide nanoparticles synthesized from an endophytic fungus Aspergillus terreus: Bioactivity and anti-cancer evaluations. Environ. Res. 2021, 201, 111502. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.P.; Wang, M.Y.; Liu, J.F.; Wang, Q.Z. Green synthesis of silver nanoparticles using a novel endophytic fungus Letendraea sp. WZ07: Characterization and evaluation of antioxidant, antibacterial and catalytic activities (3-in-1 system). Inorg. Chem. Commun. 2022, 138, 109301. [Google Scholar] [CrossRef]
- Diko, C.S.; Zhang, H.; Lian, S.; Fan, S.; Li, Z.; Qu, Y. Optimal synthesis conditions and characterization of selenium nanoparticles in Trichoderma sp. WL-Go culture broth. Mater. Chem. Phys. 2020, 246, 122583. [Google Scholar] [CrossRef]
- Yu, L.; Lan, G.; Yang, Y.; Tang, Y.; Li, Z.; She, X.; He, Z. First report of anthracnose caused by Colletotrichum fructicola on Brassica parachinensis in China. Crop Prot. 2022, 154, 105842. [Google Scholar] [CrossRef]
- Aeindartehran, L.; Talesh, S.S.A. Enhanced photocatalytic degradation of Acid Blue 1 using Ni-Decorated ZnO NPs synthesized by sol-gel method: RSM optimization approach. Ceram. Int. 2021, 47, 27294–27304. [Google Scholar] [CrossRef]
- Laxmi, V.; Kaushik, G.; Raza, K. Potential of novel Dunaliella salina from sambhar salt lake, India, for bioremediation of hexavalent chromium from aqueous effluents: An optimized green approach. Ecotoxicol. Environ. Saf. 2020, 180, 430–438. [Google Scholar]
- Borah, S.N.; Goswami, L.; Sen, S.; Sachan, D.; Sarma, H.; Montes, M.; Peralta-Videa, J.R.; Pakshirajan, K.; Narayan, M. Selenite bioreduction and biosynthesis of selenium nanoparticles by Bacillus paramycoides SP3 isolated from coal mine overburden leachate. Environ. Pollut. 2021, 285, 117519. [Google Scholar] [CrossRef]
- Yu, Y.; Yuan, S.; Zhuang, J.; Wan, Y.; Wang, Q.; Zhang, J.; Li, H. Effect of selenium on the uptake kinetics and accumulation of and oxidative stress induced by cadmium in Brassica chinensis. Ecotoxicol. Environ. Saf. 2018, 162, 571–580. [Google Scholar] [CrossRef]
- Karthik, K.K.; Cheriyan, B.V.; Rajeshkumar, S.; Gopalakrishnan, M. A review on selenium nanoparticles and their biomedical applications. Biomed. Technol. 2024, 6, 61–74. [Google Scholar] [CrossRef]
- Suryavanshi, P.; Pandit, R.; Gade, A.; Derita, M.; Zachino, S.; Rai, M. Colletotrichum sp.-mediated synthesis of sulphur and aluminium oxide nanoparticles and its in vitro activity against selected food-borne pathogens. LWT Food Sci. Technol. 2017, 81, 188–194. [Google Scholar] [CrossRef]
- Mukherjee, D.; Pramanik, K.; Mandal, S.; Mandal, N.C. Augmented growth of Cd-stressed rice seedlings with the application of phytostimulating, root-colonizing, Cd-tolerant, leaf-endophytic fungi Colletotrichum spp. isolated from Eupatorium triplinerve. J. Hazard. Mater. 2022, 438, 129508. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Dai, H.P.; Wei, S.H.; Skuza, L.; Chen, Y.Q. Effects of Cd-resistant fungi on uptake and translocation of Cd by soybean seedlings. Chemosphere 2022, 291, 132908. [Google Scholar] [CrossRef]
- Ge, M.; Zhou, S.; Li, D.; Song, D.; Yang, S.; Xu, M. Reduction of selenite to selenium nanoparticles by highly selenite-tolerant bacteria isolated from seleniferous soil. J. Hazard. Mater. 2024, 472, 134491. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sevonkaev, I.; Goia, D.V. Synthesis of selenium particles with various morphologies. J. Colloid. Interface Sci. 2014, 416, 119–123. [Google Scholar] [CrossRef]
- Budamagunta, V.; Shameem, N.; Irusappan, S.; Parray, J.A.; Thomas, M.; Marimuthu, S.; Kirubakaran, R.; Arul Jothi, K.N.; Sayyed, R.Z.; Show, P.L. Nanovesicle and extracellular polymeric substance synthesis from the remediation of heavy metal ions from soil. Environ. Res. 2023, 219, 114997. [Google Scholar] [CrossRef]
- Wang, D.; Rensing, C.; Zheng, S. Microbial reduction and resistance to selenium: Mechanisms, applications and prospects. J. Hazard. Mater. 2022, 421, 126684. [Google Scholar] [CrossRef]
- Wyantuti, S.; Fadhilatunnisa, B.; Fauzia, R.P.; Jia, Q.; Rahmani, A.A.; Irkham; Bahti, H.H. Response surface methodology box-behnken design to optimise the hydrothermal synthesis of gadolinium nanoparticles. Chin. J. Anal. Chem. 2023, 51, 100316. [Google Scholar] [CrossRef]
- Sharma, K.; Guleria, S.; Salaria, K.H.; Majeed, A.; Sharma, N.; Pawar, K.D.; Thakur, V.K.; Gupta, V.K. Photocatalytic and biological properties of silver nanoparticles synthesized using Callistemon lanceolatus leaf extract. Ind. Crop. Prod. 2023, 202, 116951. [Google Scholar] [CrossRef]
- Jiang, J.; Zhai, H.; Li, H.; Wang, Z.; Chen, Y.; Hong, N.; Wang, G.; Chofong, G.N.; Xu, W. Identification and characterization of Colletotrichum fructicola causing black spots on young fruits related to bitter rot of pear (Pyrus bretschneideri Rehd.) in China. Crop Prot. 2014, 58, 41–48. [Google Scholar] [CrossRef]
- Lin, Z.H.; Wang, C.R.C. Evidence on the size-dependent absorption spectral evolution of selenium nanoparticles. Mater. Chem. Phys. 2005, 92, 591–594. [Google Scholar] [CrossRef]
- Gaur, D.K.; Agrahari, K.; Singh, B.P.; Alam, M.B.; Parmar, A.S.; Manohar, R.; Singh, S. Optical properties and zeta potential of polyvinyl pyrrolidone capped gold nanoparticles dispersed nematic liquid crystal mixture E7. Opt. Mater. 2023, 145, 114317. [Google Scholar] [CrossRef]
- Wang, N.; Hsu, C.; Zhu, L.H.; Tseng, S.J.; Hsu, J.P. Influence of metal oxide nanoparticles concentration on their zeta potential. J. Colloid Interface Sci. 2013, 407, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Celik, H.; Sanele, S.S. Assessment of Heavy Metal Amounts of Spinach Plants (Spinach oleracea L.) Grown on Cd and Chicken Manure Applied Soil Conditions. Pol. J. Environ. Stud. 2021, 2 Pt 1, 30. [Google Scholar]
- Qi, W.-Y.; Li, Q.; Chen, H.; Liu, J.; Xing, S.-F.; Xu, M.; Yan, Z.; Song, C.; Wang, S.-G. Selenium nanoparticles ameliorate Brassica napus L. cadmium toxicity by inhibiting the respiratory burst and scavenging reactive oxygen species. J. Hazard. Mater. 2021, 417, 125900. [Google Scholar] [CrossRef]
- Muhammad, L.; Khan, A.; Zhou, Y.; He, M.; Alrefaei, A.F.; Khan, M.; Ali, S. Physiological and Ultrastructural Changes in Dendranthema morifolium Cultivars Exposed to Different Cadmium Stress Conditions. Agriculture 2023, 13, 317. [Google Scholar] [CrossRef]
- Moulick, D.; Mukherjee, A.; Das, A.; Roy, A.; Majumdar, A.; Dhar, A.; Pattanaik, B.K.; Chowardhara, B.; Ghosh, D.; Upadhyay, M.K.; et al. Selenium—An environmentally friendly micronutrient in agroecosystem in the modern era: An overview of 50-year findings. Ecotoxicol. Environ. Saf. 2024, 270, 115832. [Google Scholar] [CrossRef]
- Gu, D.; You, J.K.; Xiao, Q.; Yu, X.Y.; Zhao, Y.T. Comprehensive understanding of the regulatory mechanism by which selenium nanoparticles boost CO2 fixation and cadmium tolerance in lipid-producing green algae under recycled medium. Water Res. 2023, 245, 120556. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Pan, Y.; Ma, L.; Fang, Y.; Pan, C.; Qiang, Y.; Cao, X.; Xu, H. Nano-selenium promotes the product quality and plant defense of Salvia miltiorrhiza by inducing tanshinones and salvianolic acids accumulation. Ind. Crop. Prod. 2023, 195, 116436. [Google Scholar] [CrossRef]
- Behbahani, S.R.; Iranbakhsh, A.; Ebadi, M.; Majd, A.; Ardebili, Z.O. Red elemental selenium nanoparticles mediated substantial variations in growth, tissue differentiation, metabolism, gene transcription, epigenetic cytosine DNA methylation, and callogenesis in bittermelon (Momordica charantia); an in vitro experiment. PLoS ONE 2020, 15, e0235556. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.M.; Zhao, X.H.; Hu, C.X. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.J.; Xu, D.C.; Chen, Y.S.; Zhang, Z. Heavy metals translocation and accumulation from the rhizosphere soils to the edible parts of the medicinal plant Fengdan (Paeonia ostii) grown on a metal mining area, China. Ecotoxicol. Environ. Saf. 2017, 143, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.H.; Wu, X.Y.; Deng, X.Y.; Lin, Z.; Liu, C.G.; Zhang, J.X.; He, T.; Yi, Y.Q.; Liu, H.; Wang, Y.F.; et al. Mechanisms of low cadmium accumulation in crops: A comprehensive overview from rhizosphere soil to edible parts. Environ. Res. 2024, 245, 118054. [Google Scholar] [CrossRef]
- Filek, M.; Keskinen, R.; Hartikainen, H.; Szarejko, I.; Janiak, A.; Miszalski, Z.; Golda, A. The protective role of selenium in rape seedlings subjected to cadmium stress. J. Plant Physiol. 2008, 165, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhou, C.R.; Wu, Y.L.; An, Q.S.; Zhang, J.B.; Fang, Y.; Li, J.Q.; Pan, C.P. Nanoselenium integrates soil-pepper plant homeostasis by recruiting rhizosphere-beneficial microbiomes and allocating signaling molecule levels under Cd stress. J. Hazard. Mater. 2022, 432, 128763. [Google Scholar] [CrossRef]
- Yu, Y.; Fu, P.; Huang, Q.; Zhang, J.; Li, H. Accumulation, subcellular distribution, and oxidative stress of cadmium in Brassica chinensis supplied with selenite and selenate at different growth stages. Chemosphere 2019, 216, 331–340. [Google Scholar] [CrossRef]
- Li, D.; Zhou, C.R.; Zou, N.; Wu, Y.L.; Zhang, J.B.; An, Q.S.; Li, J.Q.; Pan, C.P. Nanoselenium foliar application enhances biosynthesis of tea leaves in metabolic cycles and associated responsive pathways. Environ. Pollut. 2021, 273, 116503. [Google Scholar] [CrossRef]
- Omomowo, I.O.; Amao, J.A.; Abubakar, A.; Ogundola, A.F.; Ezediuno, L.O.; Bamigboye, C.O. A review on the trends of endophytic fungi bioactivities. Sci. Afr. 2023, 20, e01594. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Hema, P.; Murali, M.; Shilpa, N.; Nataraj, K.; Basavaraj, G.L.; Singh, S.B.; Aiyaz, M.; Udayashankar, A.C.; Amruthesh, K.N. Fungal Endophytes as Mitigators against Biotic and Abiotic Stresses in Crop Plants. J. Fungi 2024, 10, 116. [Google Scholar] [CrossRef]
- Aziz, L.; Hamayun, M.; Rauf, M.; Iqbal, A.; Husssin, A.; Khan, S.A.; Shafique, M.; Arif, M.; Ahmad, A.; Rehman, G.; et al. Aspergillus violaceofuscus alleviates cadmium and chromium stress in Okra through biochemical modulation. PLoS ONE 2022, 17, e0273908. [Google Scholar] [CrossRef]
- Qin, D.; You, C.; Lan, W.Y.; Wang, Y.M.; Yu, B.H.; Peng, Y.J.; Xu, J.R.; Dong, J.Y. Microbial assemblages of Schisandraceae plants and the correlations between endophytic species and the accumulation of secondary metabolites. Plant Soil. 2023, 483, 85–107. [Google Scholar] [CrossRef]
- Valix, M.; Loon, L.O. Adaptive tolerance behaviour of fungi in heavy metals. Miner. Eng. 2003, 16, 193–198. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Wan, S.; Yan, Z.; Qin, Z.; Gao, H. Identification and characterization of the antifungal proteins from Bacillus velezensis KL-2 to target plant pathogenic fungi. Food Biosci. 2024, 59, 104019. [Google Scholar] [CrossRef]
- Sutton, B.C. The Coelomycetes: Fungi Imperfecti with Pycnidia, Acervuli and Stromata; Commonwealth Mycological Institute: Kew, UK, 1980; 696p. [Google Scholar]
- Zhang, D.; Yang, Y.; Castlebury, L.A.; Cerniglia, C.E. A method for the large scale isolation of high transformation efficiency fungal genomic DNA. FEMS Microbiol. Lett. 1996, 145, 261–265. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Biswas, K.C.; Barton, L.L.; Tsui, W.L.; Shuman, K.; Gillespie, J.; Eze, C.S. A novel method for the measurement of elemental selenium produced by bacterial reduction of selenite. J. Microbiol. Methods 2011, 86, 140–144. [Google Scholar] [CrossRef]
- Sun, C.C.; Chen, S.; Jin, Y.J.; Song, H.; Ruan, S.L.; Fu, Z.W.; Asad, M.A.U.; Qian, H.F. Effects of the Herbicide Imazethapyr on Photosynthesis in PGR5-and NDH-Deficient at the Biochemical, Transcriptomic, and Proteomic Levels. J. Agric. Food Chem. 2016, 64, 4497–4504. [Google Scholar] [CrossRef]
- Ortiz, J.; Soto, J.; Fuentes, A.; Herrera, H.; Meneses, C.; Arriagada, C. The Endophytic Fungus Chaetomium cupreum Regulates Expression of Genes Involved in the Tolerance to Metals and Plant Growth Promotion in Eucalyptus globulus Roots. Microorganisms 2019, 7, 490. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar]
- Zang, D.; Wang, C.; Ji, X.; Wang, Y. Tamarix hispida zinc finger protein ThZFP1 participates in salt and osmotic stress tolerance by increasing proline content and SOD and POD activities. Plant Sci. 2015, 235, 111–121. [Google Scholar] [CrossRef]
- Narayanasamy, M.; Dhanasekaran, D.; Vinothini, G.; Thajuddin, N. Extraction and recovery of precious metals from electronic waste printed circuit boards by bioleaching acidophilic fungi. Int. J. Environ. Sci. Technol. 2018, 15, 119–132. [Google Scholar] [CrossRef]
- Mahajan, P.; Kaushal, J.; Pandey, V.C. Assessment of herbaceous ornamental plant species as potential remediation agents for cadmium contaminated environments. J. Geochem. Explor. 2024, 256, 107333. [Google Scholar] [CrossRef]
- Mshora, A.M.; Mshora, A.M.; Msigwa, C.; Komanya, A.; Shimo, S. Bio-concentration and translocation of chromium in soil-plant system: Health risks in Usangu agro-ecosystem. Case Stud. Chem. Environ. Eng. 2023, 8, 100398. [Google Scholar] [CrossRef]
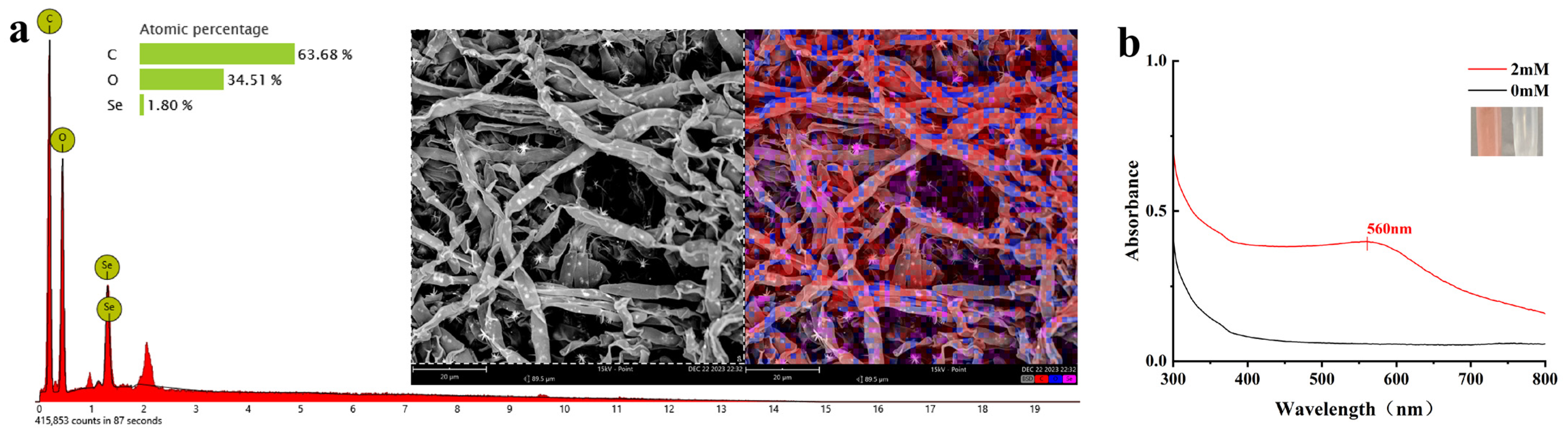



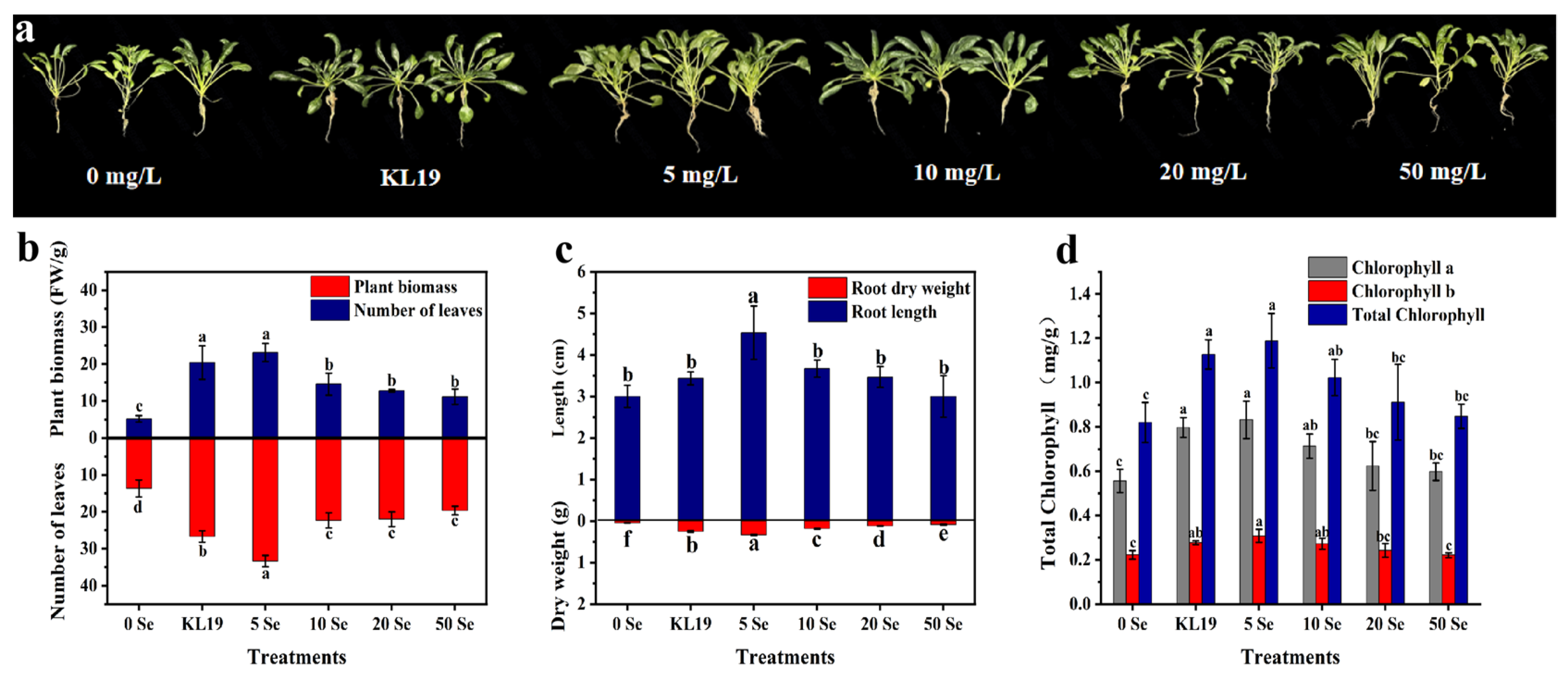
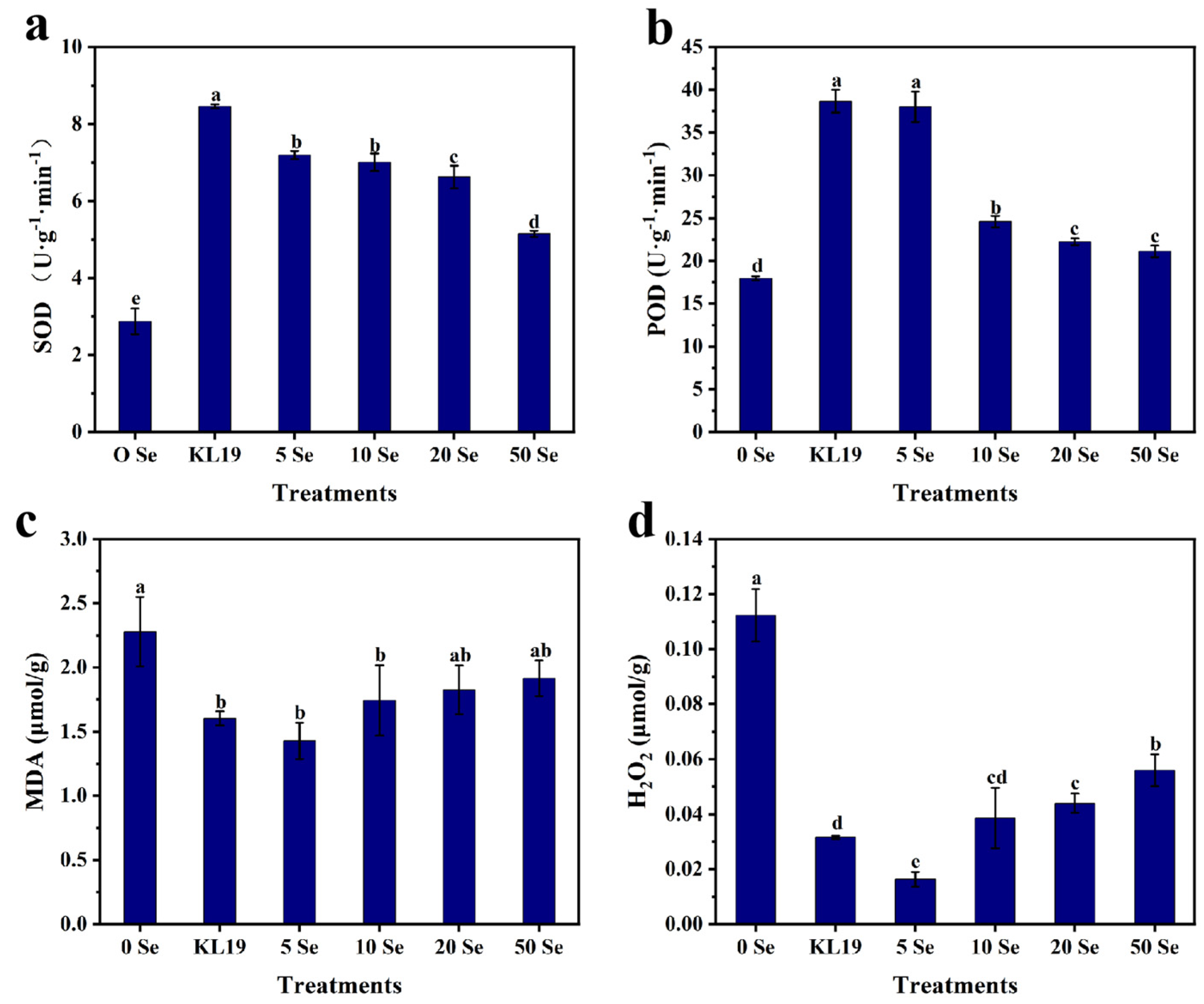
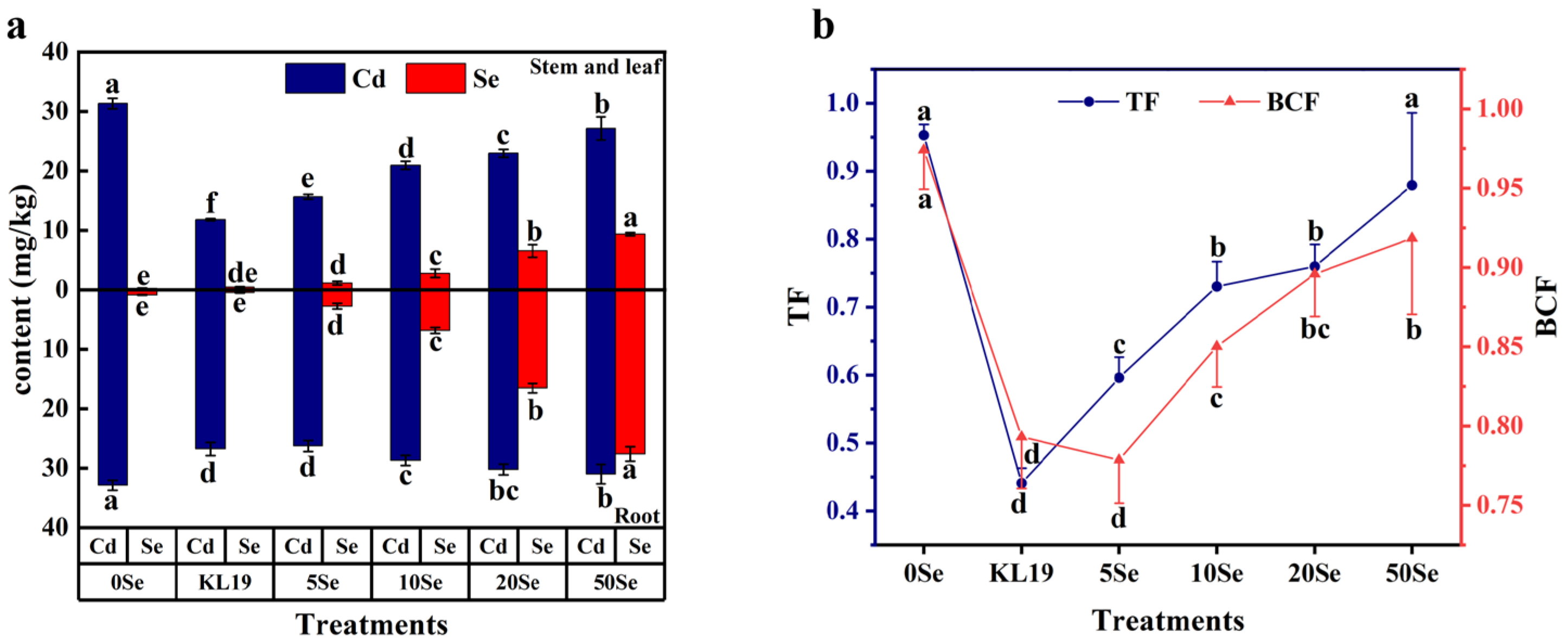
| Factor | Low Level (−1) | Medium Level (0) | High Level (+1) |
|---|---|---|---|
| Fresh weight (g) | 1 | 2 | 3 |
| Concentration of Na2SeO3 (mM) | 2 | 4.5 | 7 |
| pH | 6 | 7 | 8 |
| Fresh Weight (g) | Na2SeO3 (mM) | pH | Se0 (Pre) | Se0 (Act) |
|---|---|---|---|---|
| 2.62 | 4.56 | 6.25 | 1.02 | 1.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Huang, S.; Tian, W.; Yang, S.; Shen, W.; Dong, J. Endophytic Colletotrichum fructicola KL19 and Its Derived SeNPs Mitigate Cd-Stress-Associated Damages in Spinacia oleracea L. Plants 2024, 13, 2359. https://doi.org/10.3390/plants13172359
Wu Y, Huang S, Tian W, Yang S, Shen W, Dong J. Endophytic Colletotrichum fructicola KL19 and Its Derived SeNPs Mitigate Cd-Stress-Associated Damages in Spinacia oleracea L. Plants. 2024; 13(17):2359. https://doi.org/10.3390/plants13172359
Chicago/Turabian StyleWu, Yingxia, Shiru Huang, Wei Tian, Shengyu Yang, Wenshu Shen, and Jinyan Dong. 2024. "Endophytic Colletotrichum fructicola KL19 and Its Derived SeNPs Mitigate Cd-Stress-Associated Damages in Spinacia oleracea L." Plants 13, no. 17: 2359. https://doi.org/10.3390/plants13172359






