Rapid Reduction of Phytotoxicity in Green Waste for Use as Peat Substitute: Optimization of Ammonium Incubation Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.2.1. The Effects of Detoxifiers
2.2.2. The Effects of Environmental Conditions
2.2.3. Cultivation Experiment of Green Waste as a Peat Substitute
2.3. Analytical Techniques
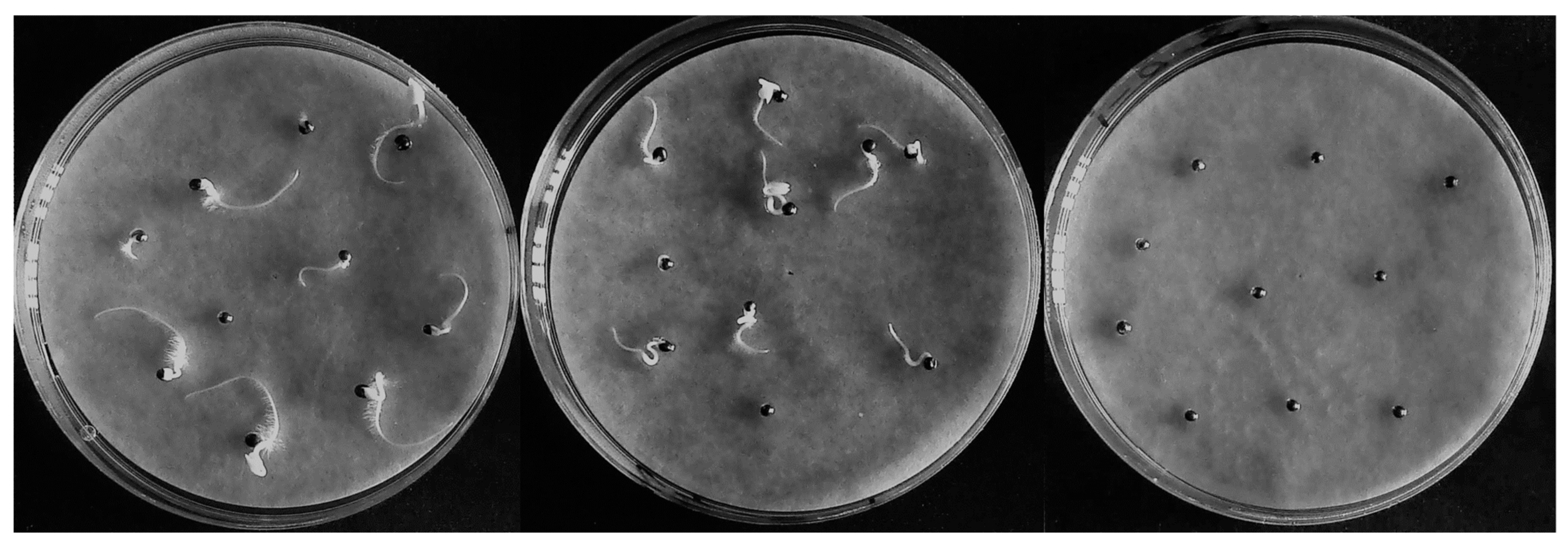
2.3.1. Phytotoxicity Assessment
2.3.2. pH, EC
2.3.3. Determination of Total Phenol
2.3.4. Lettuce Biomass Measurement
2.3.5. OJIP Parameters Measurement
2.4. Statistical Analyses
3. Results and Discussion
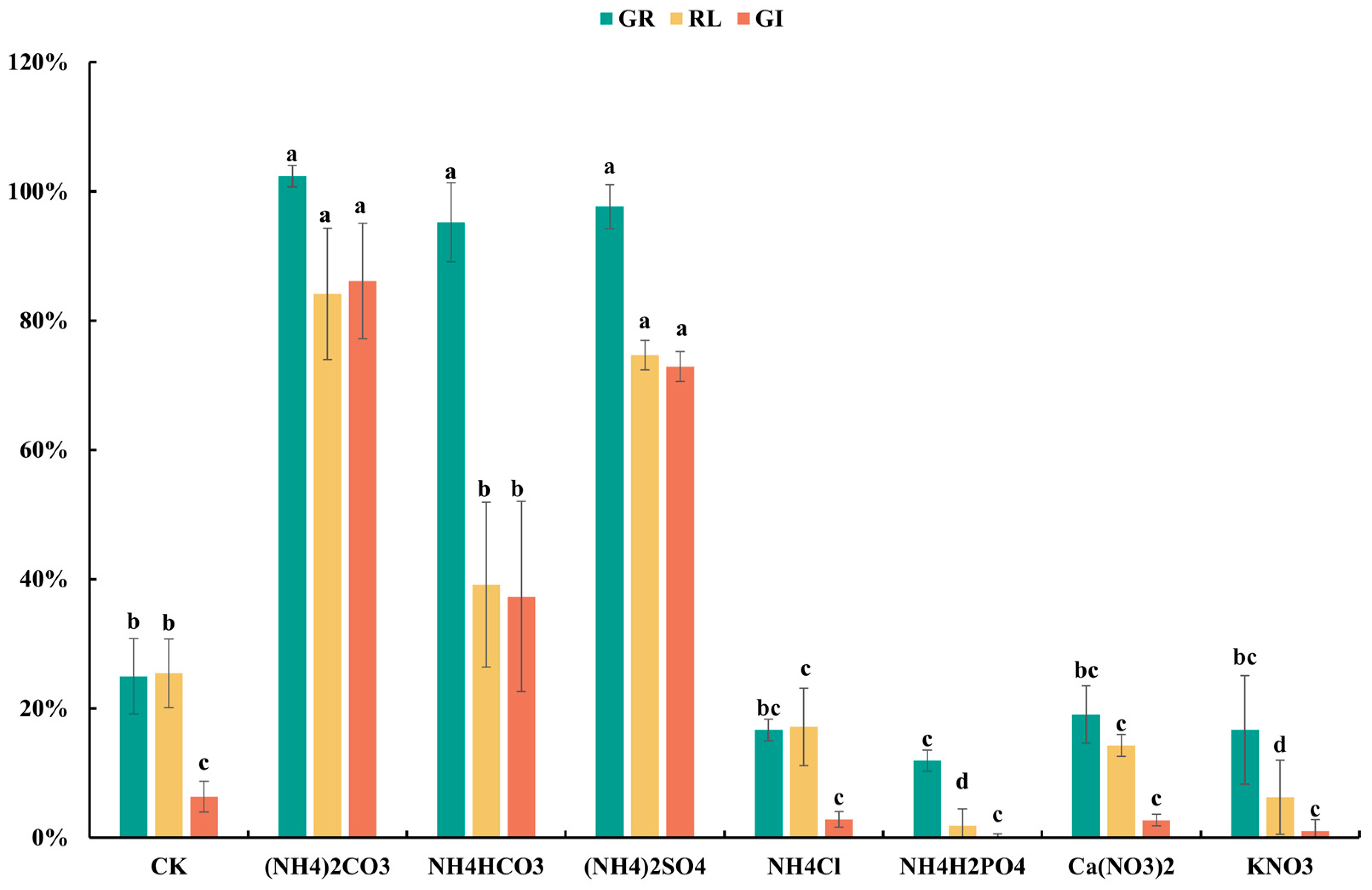
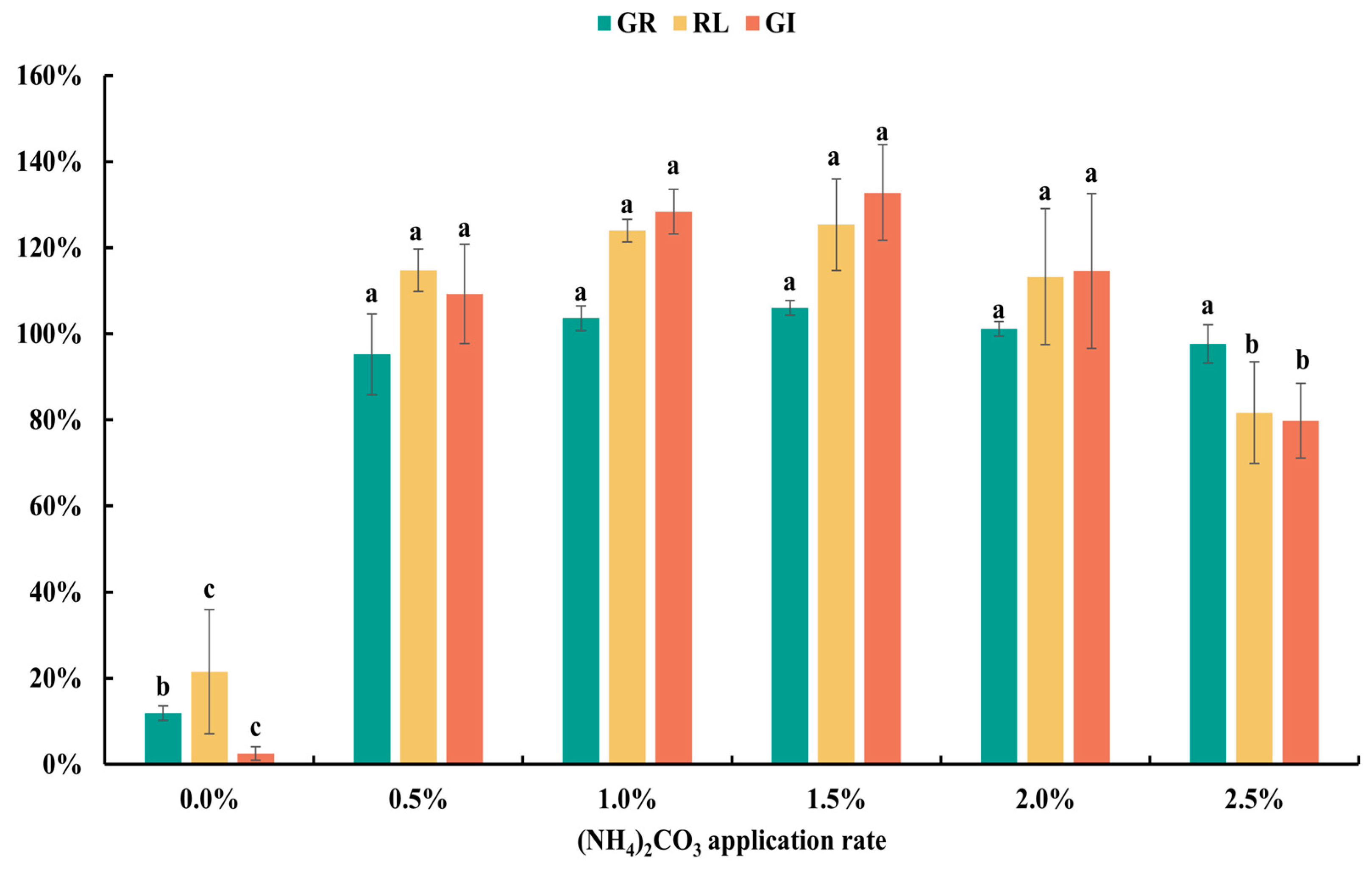
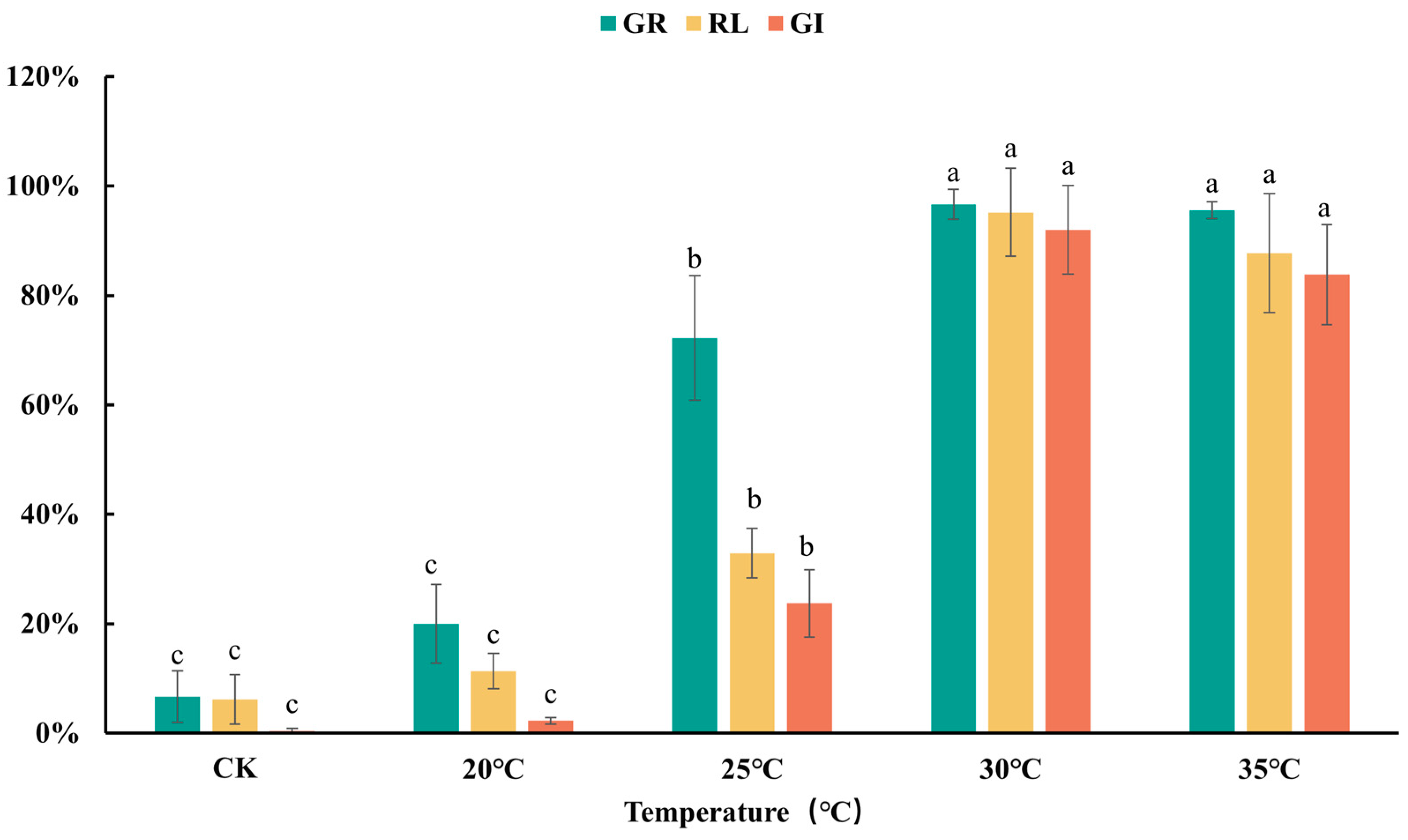
3.1. Effects of Detoxifying Agent Types and Dosages on Detoxification Efficacy
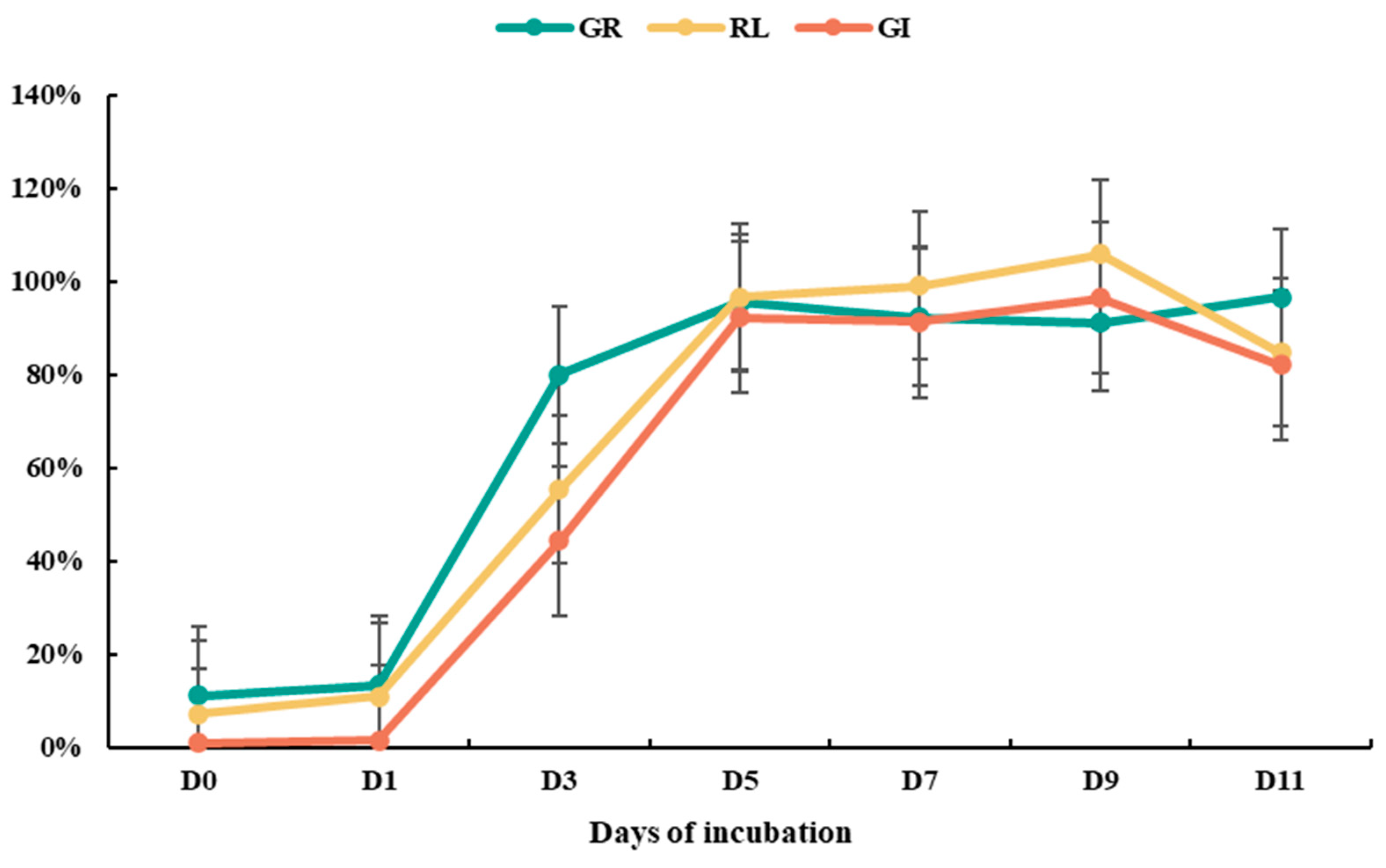
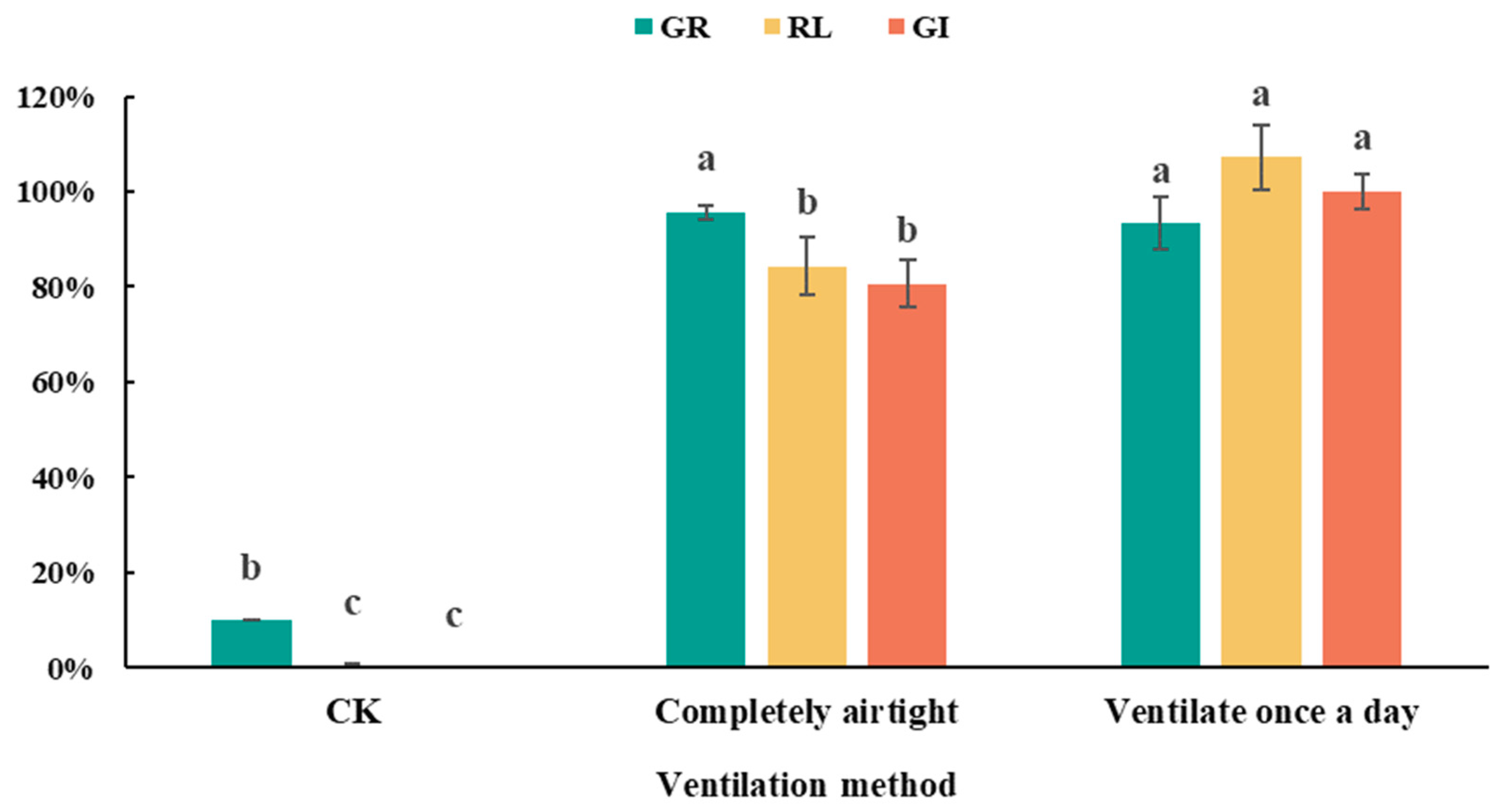
3.2. Effects of Environmental Conditions on Detoxification Efficacy
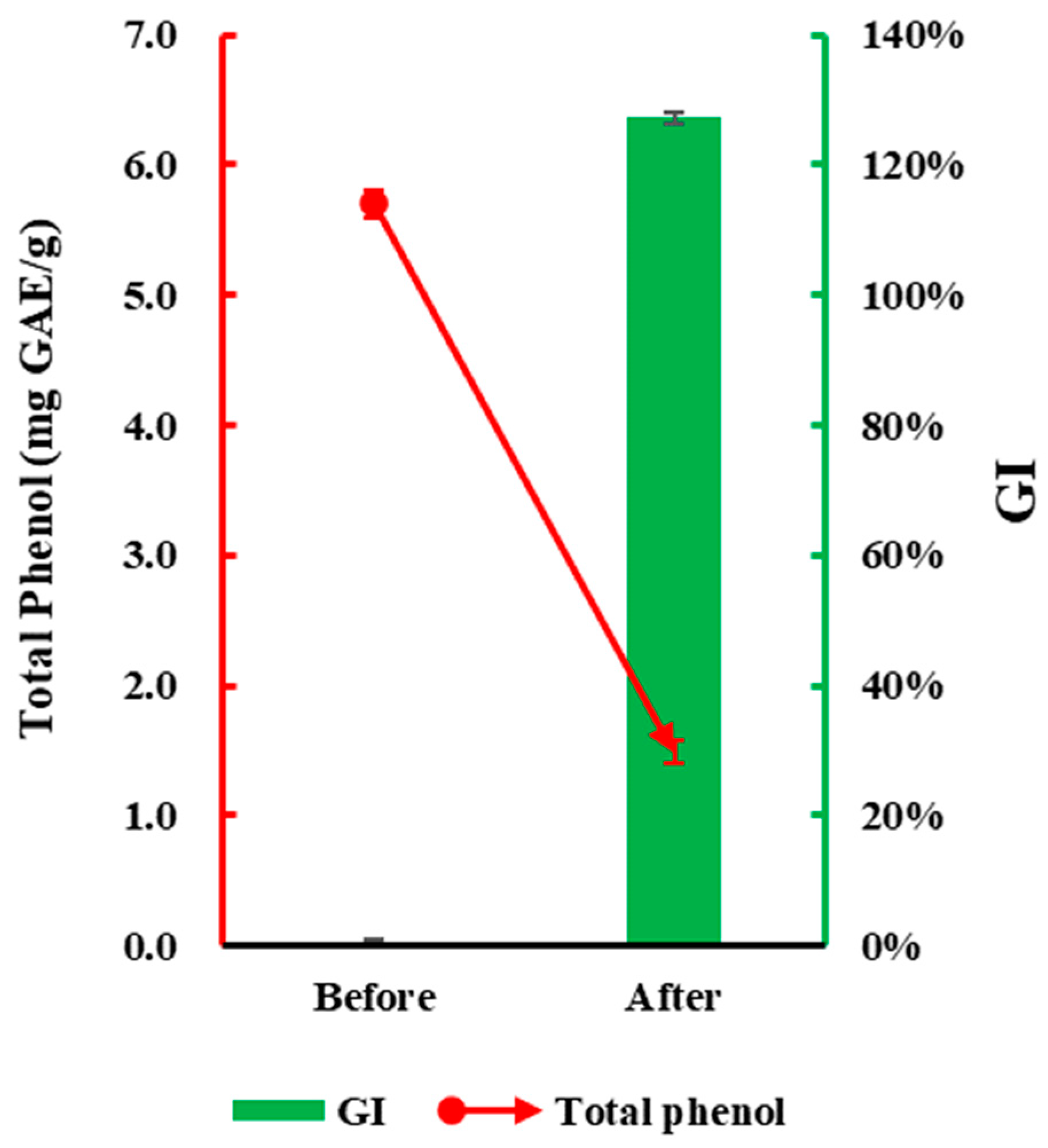
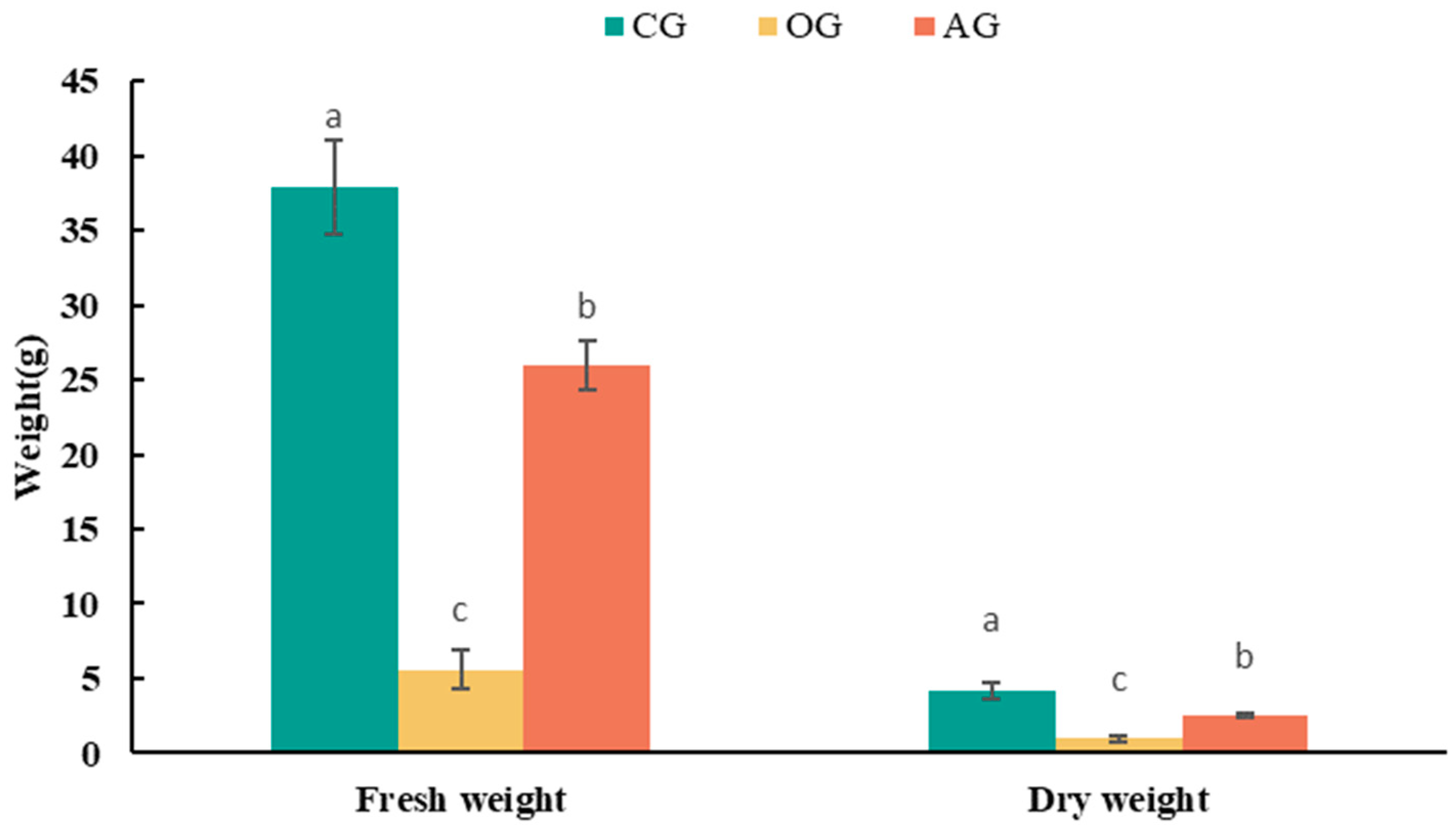
3.3. Verification of Detoxification Effects of Ammonium Incubation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Calculation Formulas | Parameters Interpretation |
|---|---|
| F0 (Minimum Fluorescence): In the state of dark adaptation, when all reaction centers are open, i.e., the primary electron acceptor QA in the reaction center is in an oxidized state, the fluorescence value is observed. This reflects the baseline fluorescence of the reaction center of Photosystem II (PSII), mainly originating from antenna pigments. | |
| FM (Maximum Fluorescence): The fluorescence value when all reaction centers are closed in the state of dark adaptation. This reflects the fluorescence of antenna pigments after maximum photochemical quenching. | |
| Energy flux absorbed by the active reaction center of PSII per unit. | |
| The approximate initial slope (per millisecond) of transient fluorescence V = f(t). This parameter reflects the closing rate of the active reaction centers (RCs) of PSII. | |
| Energy flux captured by the active reaction center of PSII per unit at t = 0 | |
| Energy flux transmitted by the active reaction center of PSII per unit at t = 0 | |
| At t = 0, the ratio of electron flux reducing the terminal electron acceptor of PSⅠ per active reaction center of PS.Ⅱ | |
| At t = 0, the energy flux dissipated as heat per active reaction center of PSII. | |
| At t = 0, the maximum quantum yield of the primary photochemical reaction, which refers to the quantum yield of photon capture by the PSII reaction center, also represents the maximum photochemical efficiency of the PSII reaction center in the state of dark adaptation. | |
| At t = 0, the ratio of electrons captured by the active reaction center of PSII that are transferred. | |
| At t = 0, the quantum efficiency of electrons transferred from QA- to the electron transport chain. | |
| At t = 0, the ratio of electrons captured by the active reaction center of PSII that are transferred to PSI. | |
| The ratio of electrons reducing the terminal electron acceptor of PSI to the electrons in the electron transport chain. | |
| Quantum yield of reducing the terminal electron acceptor on the acceptor side of PSI. | |
| At t = 0, the quantum yield of heat dissipation reflects the proportion of absorbed light energy in Photosystem II (PSII) that is dissipated by the non-photochemical quenching process. | |
| Performance index based on absorption: It describes the performance index from the absorption of photons to the reduction of the electron transport chain. |
References
- Caputo, S.; Rumble, H.; Schaefer, M. “I like to Get My Hands Stuck in the Soil”: A Pilot Study in the Acceptance of Soil-Less Methods of Cultivation in Community Gardens. J. Clean. Prod. 2020, 258, 120585. [Google Scholar] [CrossRef]
- Amesbury, M.J.; Gallego-Sala, A.; Loisel, J. Peatlands as Prolific Carbon Sinks. Nat. Geosci. 2019, 12, 880–881. [Google Scholar] [CrossRef]
- Gruda, N. Sustainable Peat Alternative Growing Media. Acta Hortic. 2012, 927, 973–979. [Google Scholar] [CrossRef]
- Maher, M.; Prasad, M.; Raviv, M. Organic Soilless Media Components. In Soilless Culture; Elsevier: Amsterdam, The Netherlands, 2008; pp. 459–504. ISBN 978-0-444-52975-6. [Google Scholar]
- Roehrdanz, M.; Greve, T.; De Jager, M.; Buchwald, R.; Wark, M. Co-Composted Hydrochar Substrates as Growing Media for Horticultural Crops. Sci. Hortic. 2019, 252, 96–103. [Google Scholar] [CrossRef]
- Chemetova, C.; Quilhó, T.; Braga, S.; Fabião, A.; Gominho, J.; Ribeiro, H. Aged Acacia Melanoxylon Bark as an Organic Peat Replacement in Container Media. J. Clean. Prod. 2019, 232, 1103–1111. [Google Scholar] [CrossRef]
- Chemetova, C.; Mota, D.; Fabião, A.; Gominho, J.; Ribeiro, H. Low-Temperature Hydrothermally Treated Eucalyptus Globulus Bark: From by-Product to Horticultural Fiber-Based Growing Media Viability. J. Clean. Prod. 2021, 319, 128805. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, Q.; Yang, R.; Lin, W.; Wang, H.; Zhou, W.; Qi, Z.; Ouyang, L. Slight Carbonization as a New Approach to Obtain Peat Alternative. Ind. Crops Prod. 2023, 202, 117041. [Google Scholar] [CrossRef]
- Altland, J.E.; Owen, J.S.; Jackson, B.E.; Fields, J.S. Physical and Hydraulic Properties of Commercial Pine-Bark Substrate Products Used in Production of Containerized Crops. HortScience 2018, 53, 1883–1890. [Google Scholar] [CrossRef]
- Witcher, A.L.; Blythe, E.K.; Fain, G.B.; Curry, K.J.; Pounders, C.T. Assessing Phytotoxicity in Fresh and Aged Whole Pine Tree Substrates©. Comb. Proc. Int. Plant Propagators’ Soc. 2011, 61, 477–482. [Google Scholar]
- Li, Z. Resource Utilization of Agricultural Solid Waste. J. Integr. Agric. 2021, 20, 1119–1120. [Google Scholar] [CrossRef]
- Li, R.; Wang, Q.; Qu, G.; Zhang, Z.; Wang, H. Green Utilization of Organic Waste Resource. Environ. Sci. Pollut. Res. 2023, 30, 8899–8901. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X. A Triumph of Reducing Carbon Emission by Banning Open Straw Burning. Sci. Bull. 2023, 68, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Dhiman, S.K.; Kumar, V.; Babu, R.; Shree, K.; Priyadarshani, A.; Singh, A.; Shakya, L.; Nautiyal, A.; Saluja, S. Crop Residue Burning and Its Relationship between Health, Agriculture Value Addition, and Regional Finance. Atmosphere 2022, 13, 1405. [Google Scholar] [CrossRef]
- Hu, W.-N.; Wu, S.-D.; Cai, Z.; Lin, Y.-C.; Sun, X.-D. Study on the Recycling Technology of Street Tree Waste Recycling and New Lead Carbon Alloy Processing Technology. IOP Conf. Ser. Mater. Sci. Eng. 2020, 774, 012136. [Google Scholar] [CrossRef]
- Bhuvaneshwari, S.; Hettiarachchi, H.; Meegoda, J. Crop Residue Burning in India: Policy Challenges and Potential Solutions. Int. J. Environ. Res. Public Health 2019, 16, 832. [Google Scholar] [CrossRef]
- Trushna, T.; Diwan, V.; Nandi, S.S.; Aher, S.B.; Tiwari, R.R.; Sabde, Y.D. A Mixed-Methods Community-Based Participatory Research to Explore Stakeholder’s Perspectives and to Quantify the Effect of Crop Residue Burning on Air and Human Health in Central India: Study Protocol. BMC Public Health 2020, 20, 1824. [Google Scholar] [CrossRef]
- Cui, W.; Bai, Q.; Liu, J.; Chen, J.; Qi, Z.; Zhou, W. Phytotoxicity Removal Technologies for Agricultural Waste as a Growing Media Component: A Review. Agronomy 2023, 14, 40. [Google Scholar] [CrossRef]
- Zhou, W.; Liao, J.; Zhou, B.; Yang, R.; Lin, W.; Zhang, D.; Wang, H.; Qi, Z. Rapidly Reducing Phytotoxicity of Green Waste for Growing Media by Incubation with Ammonium. Environ. Technol. Innov. 2023, 31, 103136. [Google Scholar] [CrossRef]
- Arshad, M.; Khan, A.H.A.; Hussain, I.; Badar-uz-Zaman; Anees, M.; Iqbal, M.; Soja, G.; Linde, C.; Yousaf, S. The Reduction of Chromium (VI) Phytotoxicity and Phytoavailability to Wheat (Triticum aestivum L.) Using Biochar and Bacteria. Appl. Soil Ecol. 2017, 114, 90–98. [Google Scholar] [CrossRef]
- Bohacz, J. Composts and Water Extracts of Lignocellulosic Composts in the Aspect of Fertilization, Humus-Forming, Sanitary, Phytosanitary and Phytotoxicity Value Assessment. Waste Biomass Valor 2019, 10, 2837–2850. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed Germination Test for Toxicity Evaluation of Compost: Its Roles, Problems and Prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef]
- Yakefu, Z.; Huannixi, W.; Ye, C.; Zheng, T.; Chen, S.; Peng, X.; Tian, Z.; Wang, J.; Yang, Y.; Ma, Z.; et al. Inhibitory Effects of Extracts from Cinnamomum Camphora Fallen Leaves on Algae. Water Sci. Technol. 2018, 77, 2545–2554. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, L.; Wu, H.; Jing, T.; Li, T.; Li, J.; Kong, L.; Zhu, F. Application of Chlorophyll Fluorescence Analysis Technique in Studying the Response of Lettuce (Lactuca sativa L.) to Cadmium Stress. Sensors 2024, 24, 1501. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to Salt Stress in Lettuce: Changes in Chlorophyll Fluorescence Parameters, Phytochemical Contents, and Antioxidant Activities. Agronomy 2020, 10, 1627. [Google Scholar] [CrossRef]
- Buss, W.; Mašek, O. Mobile Organic Compounds in Biochar—A Potential Source of Contamination—Phytotoxic Effects on Cress Seed (Lepidium sativum) Germination. J. Environ. Manag. 2014, 137, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Paschalidis, K.; Tsaniklidis, G.; Wang, B.-Q.; Delis, C.; Trantas, E.; Loulakakis, K.; Makky, M.; Sarris, P.F.; Ververidis, F.; Liu, J.-H. The Interplay among Polyamines and Nitrogen in Plant Stress Responses. Plants 2019, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Jiang, Z.; Zhang, L.; Hussain, I.; Yang, X. Insights on Phytohormonal Crosstalk in Plant Response to Nitrogen Stress: A Focus on Plant Root Growth and Development. Int. J. Mol. Sci. 2023, 24, 3631. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Xie, Y.; Wang, Z.; Luo, L.; Zhang, C.; Pélissier, P.; Parizot, B.; Qi, W.; Zhang, J.; Hu, Z.; et al. Rice Plants Respond to Ammonium Stress by Adopting a Helical Root Growth Pattern. Plant J. 2020, 104, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within Composting: A Review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef]
- Wang, J.; Chadwick, D.R.; Cheng, Y.; Yan, X. Global Analysis of Agricultural Soil Denitrification in Response to Fertilizer Nitrogen. Sci. Total Environ. 2018, 616–617, 908–917. [Google Scholar] [CrossRef]
- Ma, J.; Lu, C.; Bai, L.; Zhang, J.; Shen, Y. Phytotoxic Phenols from the Needles of Cedrus Deodara. Phytochemistry 2024, 219, 113977. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Seda, M.; Sarraga, C.; Montero, J.I.; Castellari, M.; Muñoz, P. Municipal Solid Waste Composting: Application as a Tomato Fertilizer and Its Effect on Crop Yield, Fruit Quality and Phenolic Content. Renew. Agric. Food Syst. 2017, 32, 358–365. [Google Scholar] [CrossRef]
- Wang, G.; Yang, Y.; Kong, Y.; Ma, R.; Yuan, J.; Li, G. Key Factors Affecting Seed Germination in Phytotoxicity Tests during Sheep Manure Composting with Carbon Additives. J. Hazard. Mater. 2022, 421, 126809. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Kong, Y.; Yang, Y.; Ma, R.; Shen, Y.; Li, G.; Yuan, J. Superphosphate, Biochar, and a Microbial Inoculum Regulate Phytotoxicity and Humification during Chicken Manure Composting. Sci. Total Environ. 2022, 824, 153958. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Dai, S.; Zhao, J.; Huang, X.; Sun, Y.; Chen, J.; Cai, Z.; Zhang, J. The Effect of C:N Ratio on Heterotrophic Nitrification in Acidic Soils. Soil Biol. Biochem. 2019, 137, 107562. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Effects of Bean Dregs and Crab Shell Powder Additives on the Composting of Green Waste. Bioresour. Technol. 2018, 260, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.; Wang, X.; Wang, S.; Ma, L.; Ma, W. Transformation of Nitrogen and Carbon during Composting of Manure Litter with Different Methods. Bioresour. Technol. 2019, 293, 122046. [Google Scholar] [CrossRef]
- Gibbs, B.M.; Shephard, L.R.; Third, K.A.; Cord-Ruwisch, R. The Presence of Ammonium Facilitates Nitrite Reduction under PHB Driven Simultaneous Nitrification and Denitrification. Water Sci. Technol. 2004, 50, 181–188. [Google Scholar] [CrossRef]
- Prinčič, A.; Mahne, I.; Megušar, F.; Paul, E.A.; Tiedje, J.M. Effects of pH and Oxygen and Ammonium Concentrations on the Community Structure of Nitrifying Bacteria from Wastewater. Appl. Environ. Microbiol. 1998, 64, 3584–3590. [Google Scholar] [CrossRef]
- Ji, B.; Qian, Y.; Zhang, H.; Al-Gabr, H.M.; Xu, M.; Zhang, K.; Zhang, D. Optimizing Heterotrophic Nitrification Process: The Significance of Demand-Driven Aeration and Organic Matter Concentration. Bioresour. Technol. 2023, 376, 128907. [Google Scholar] [CrossRef]
- Phan, D.C.; Vazquez-Munoz, R.; Matta, A.; Kapoor, V. Short-Term Effects of Mn2O3 Nanoparticles on Physiological Activities and Gene Expression of Nitrifying Bacteria under Low and High Dissolved Oxygen Conditions. Chemosphere 2020, 261, 127775. [Google Scholar] [CrossRef] [PubMed]
- Courtens, E.N.P.; Boon, N.; De Clippeleir, H.; Berckmoes, K.; Mosquera, M.; Seuntjens, D.; Vlaeminck, S.E. Control of Nitratation in an Oxygen-Limited Autotrophic Nitrification/Denitrification Rotating Biological Contactor through Disc Immersion Level Variation. Bioresour. Technol. 2014, 155, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.S.; Karami, A.; Haghighi, T.M.; Alizadeh, S.; Maggi, F. Phytotoxic Potential and Phenolic Profile of Extracts from Scrophularia Striata. Plants 2021, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Pinho, I.A.; Lopes, D.V.; Martins, R.C.; Quina, M.J. Phytotoxicity Assessment of Olive Mill Solid Wastes and the Influence of Phenolic Compounds. Chemosphere 2017, 185, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently Asked Questions about Chlorophyll Fluorescence, the Sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, X.; Hussein, Z.; Wang, P.; Chen, R.; Yuan, Q.; Gao, Y.; Song, N.; Gouda, S.G. Influence of the Structure and Properties of Lignocellulose on the Physicochemical Characteristics of Lignocellulose-Based Residues Used as an Environmentally Friendly Substrate. Sci. Total Environ. 2021, 790, 148089. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Allaire, S.E.; Akram, N.A.; Méndez, A.; Younis, A.; Peerzada, A.M.; Shaukat, N.; Wright, S.R. Challenges in Organic Component Selection and Biochar as an Opportunity in Potting Substrates: A Review. J. Plant Nutr. 2019, 42, 1386–1401. [Google Scholar] [CrossRef]

| Growing Media | ϕPo | ϕEo | δRo | ϕRo | PIabs |
|---|---|---|---|---|---|
| AG | 0.83 ± 0.01 | 0.50 ± 0.02 | 0.16 ± 0.01 | 0.08 ± 0.01 | 4.80 ± 0.66 |
| OG | 0.79 ± 0.02 * | 0.44 ± 0.03 ** | 0.13 ± 0.06 * | 0.06 ± 0.03 * | 2.24 ± 0.92 ** |
| Growing Media | BD (g/cm3) | TP | WHC | AFP |
|---|---|---|---|---|
| CG | 0.172 ± 0.006 b | 65.0% ± 4.1% b | 57.0% ± 4.5% b | 8.0% ± 0.5% a |
| AG | 0.260 ± 0.013 a | 73.7% ± 3.2% a | 69.0% ± 2.4% a | 4.7% ± 1.1% b |
| OG | 0.259 ± 0.002 a | 70.2% ± 1.4% ab | 68.1% ± 1.5% a | 2.1% ± 0.1% c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, W.; Liu, J.; Bai, Q.; Wu, L.; Qi, Z.; Zhou, W. Rapid Reduction of Phytotoxicity in Green Waste for Use as Peat Substitute: Optimization of Ammonium Incubation Process. Plants 2024, 13, 2360. https://doi.org/10.3390/plants13172360
Cui W, Liu J, Bai Q, Wu L, Qi Z, Zhou W. Rapid Reduction of Phytotoxicity in Green Waste for Use as Peat Substitute: Optimization of Ammonium Incubation Process. Plants. 2024; 13(17):2360. https://doi.org/10.3390/plants13172360
Chicago/Turabian StyleCui, Wenzhong, Juncheng Liu, Qi Bai, Lingyi Wu, Zhiyong Qi, and Wanlai Zhou. 2024. "Rapid Reduction of Phytotoxicity in Green Waste for Use as Peat Substitute: Optimization of Ammonium Incubation Process" Plants 13, no. 17: 2360. https://doi.org/10.3390/plants13172360
APA StyleCui, W., Liu, J., Bai, Q., Wu, L., Qi, Z., & Zhou, W. (2024). Rapid Reduction of Phytotoxicity in Green Waste for Use as Peat Substitute: Optimization of Ammonium Incubation Process. Plants, 13(17), 2360. https://doi.org/10.3390/plants13172360






