Impact of Nickel Toxicity on Growth, Fruit Quality and Antioxidant Response in Zucchini Squash (Cucurbita pepo L.)
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Culture and Treatments
2.2. Parameters of Growth
- S/R = DW shoots/DW roots
- TI (%) = DW-treated plants/DW control plants × 100
- RGR = (ln (W2) − ln (W1)/(t2 − t1)
- W is the fresh matter at the beginning of treatment (W1) and at the end (W2).
- (t2 − t1) is the duration of this period.
2.3. Assay of Nickel and Minerals Elements
2.4. Assay of Total Phenolics and Flavonoids
2.5. Assay of Proline
2.6. Assay of Proteins
2.7. Statistical Analysis
3. Results
3.1. Mineral Nutrition
3.2. Nickel Content
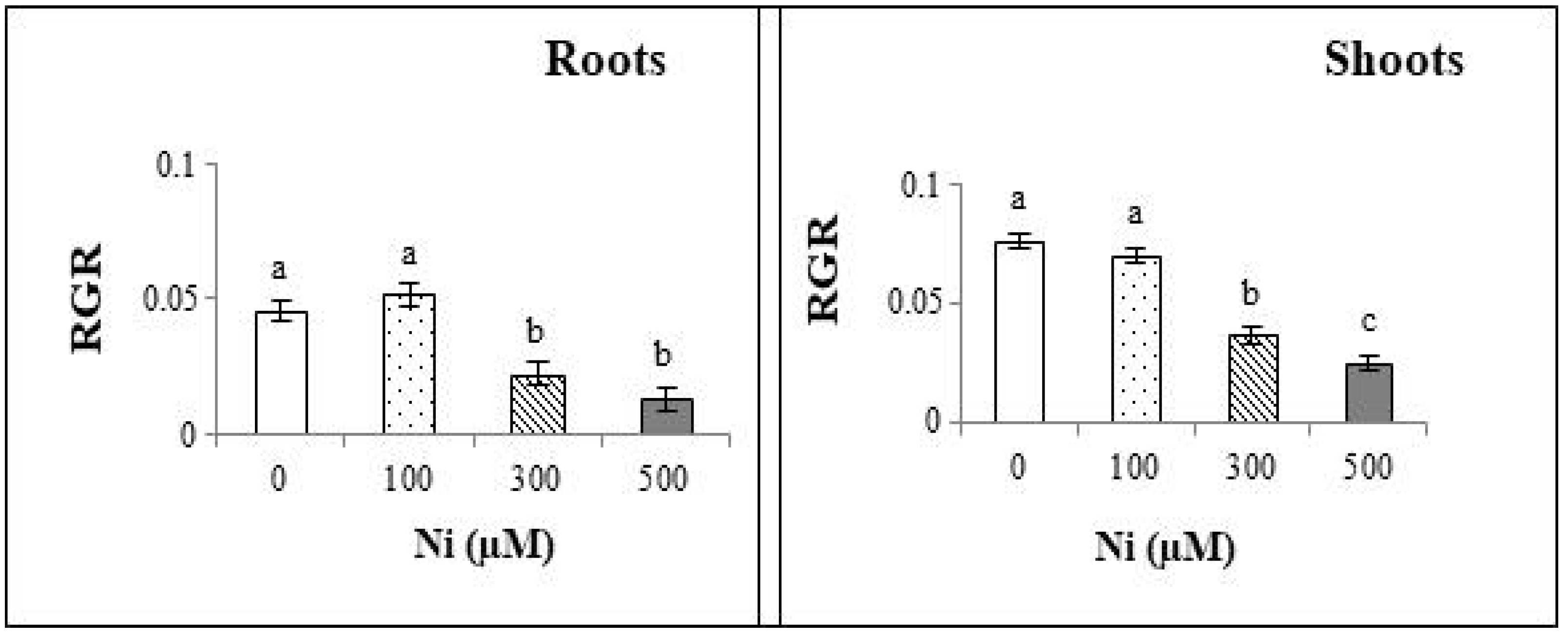
3.3. Plant Morphology and Growth
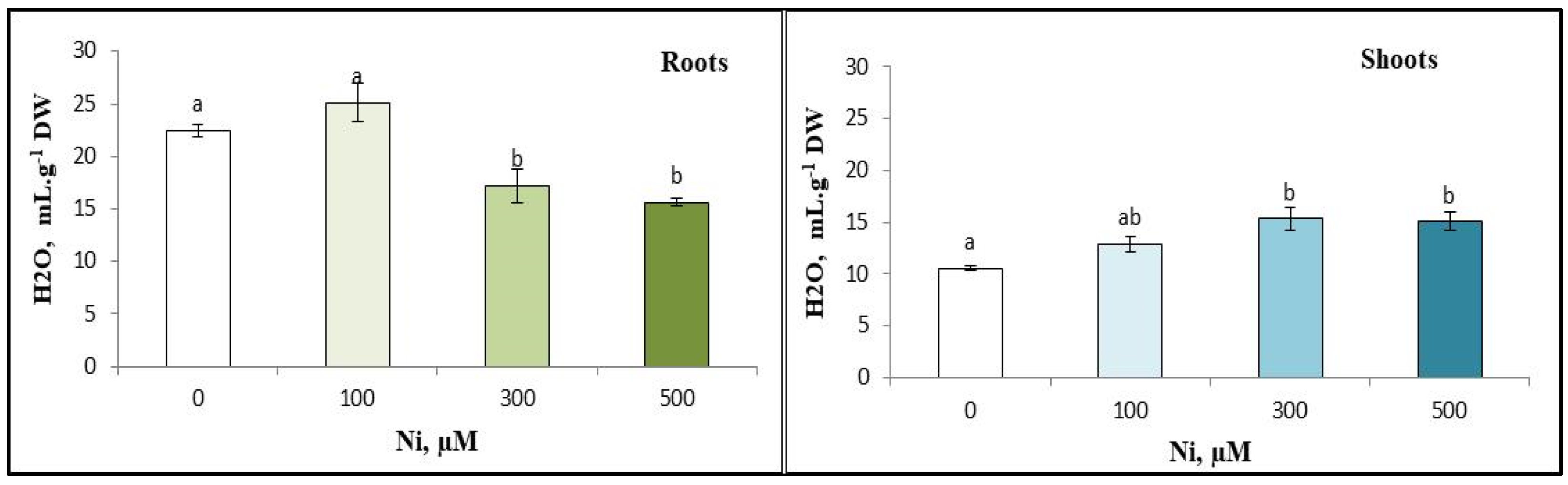
3.4. Water Content
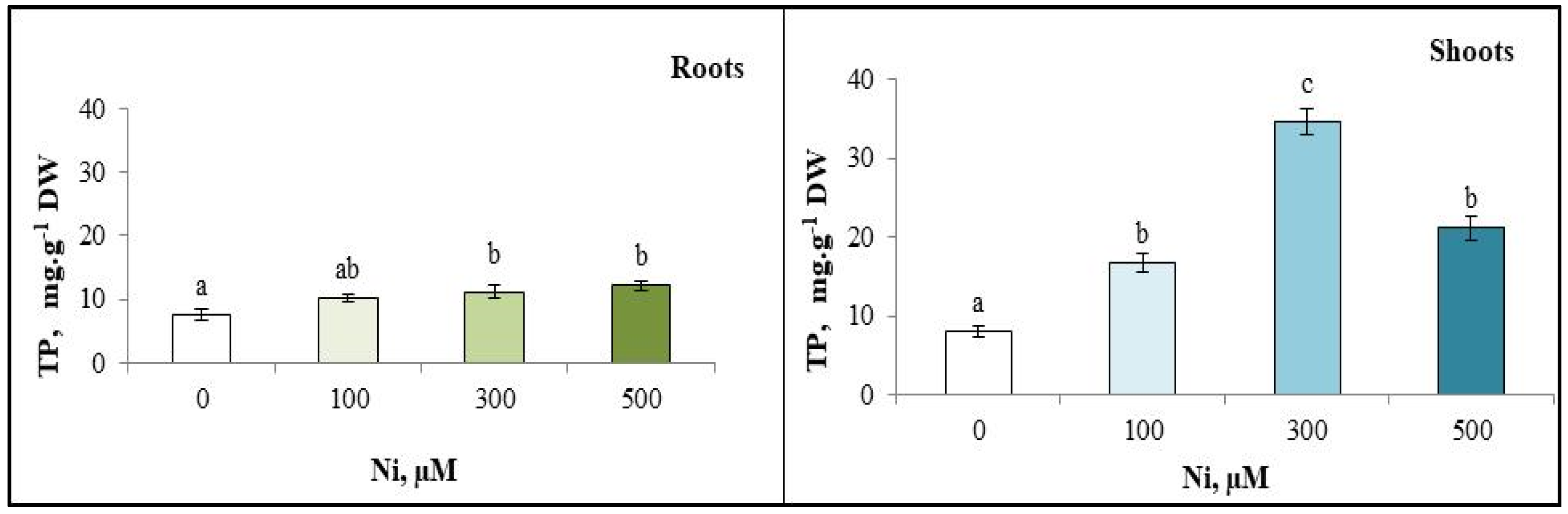
3.5. Total Polyphenol (TP) Content
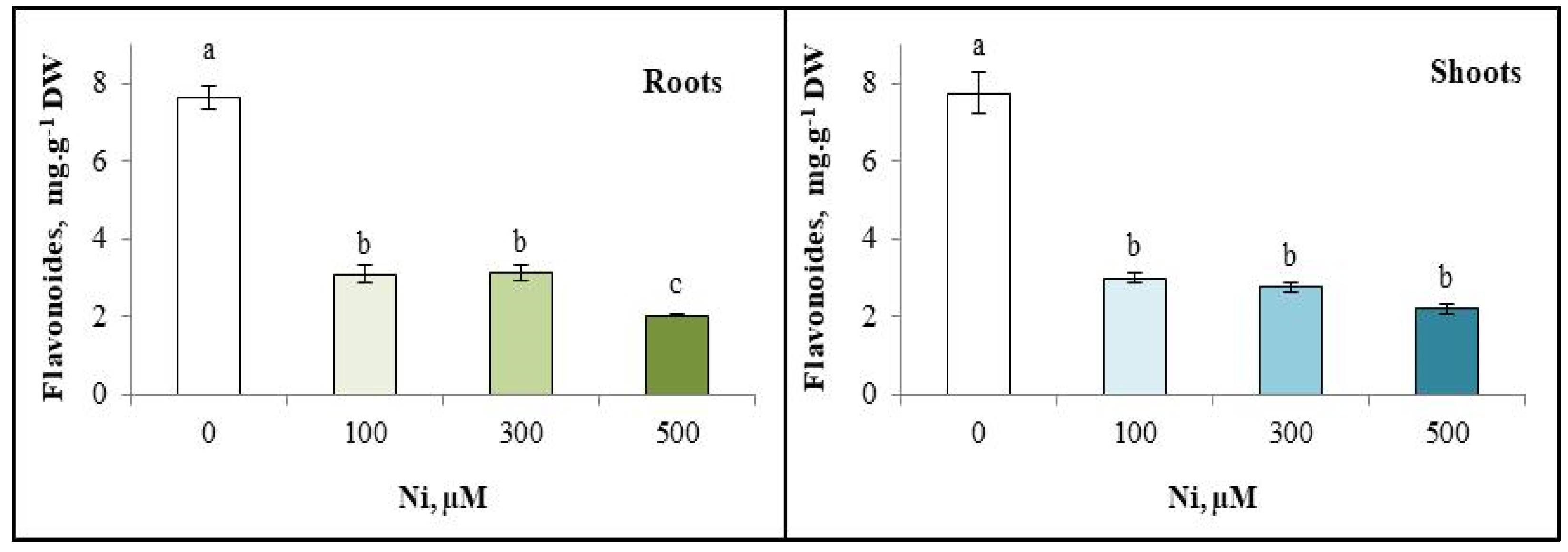
3.6. Flavonoid Content
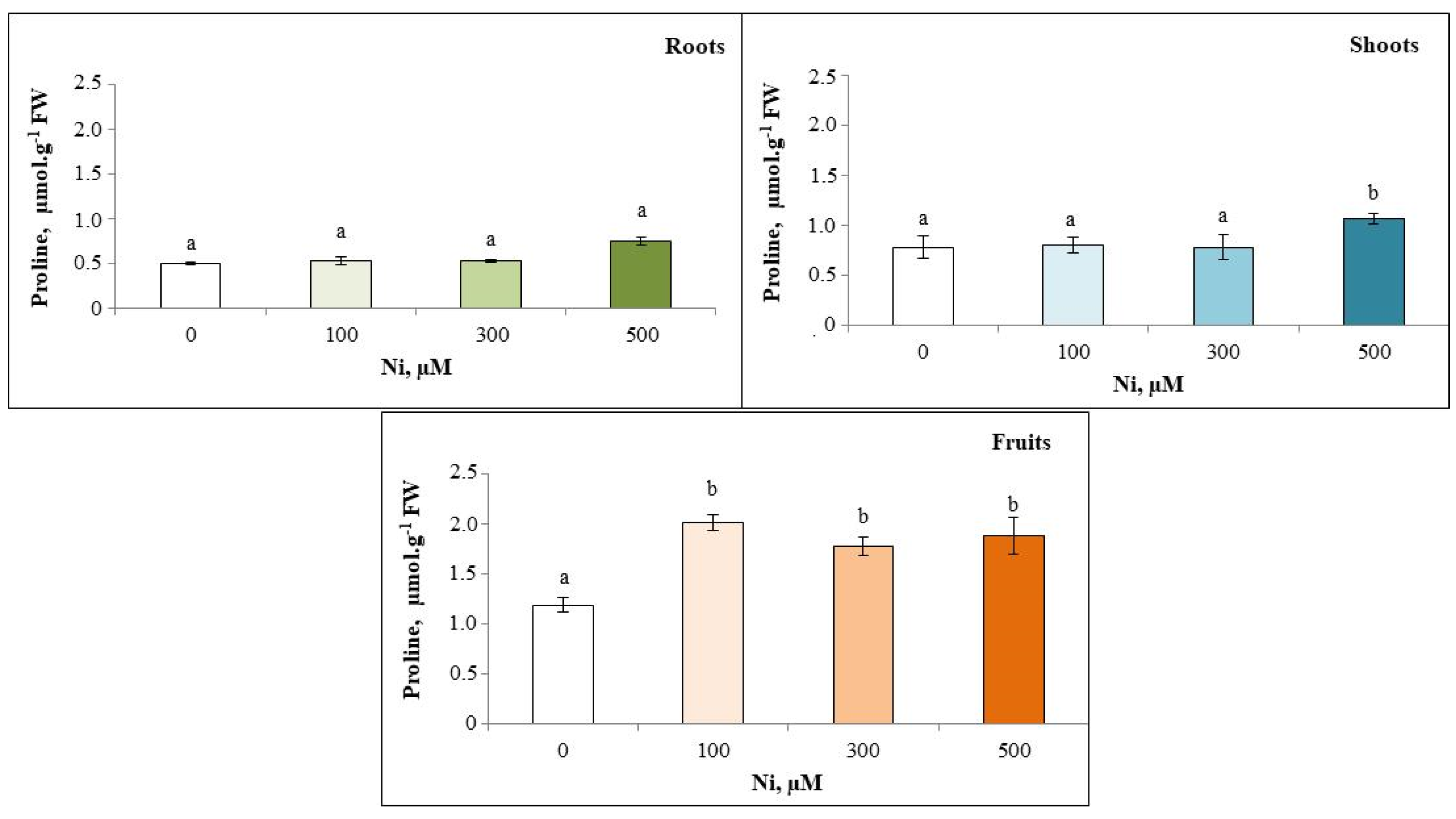
3.7. Proline Content
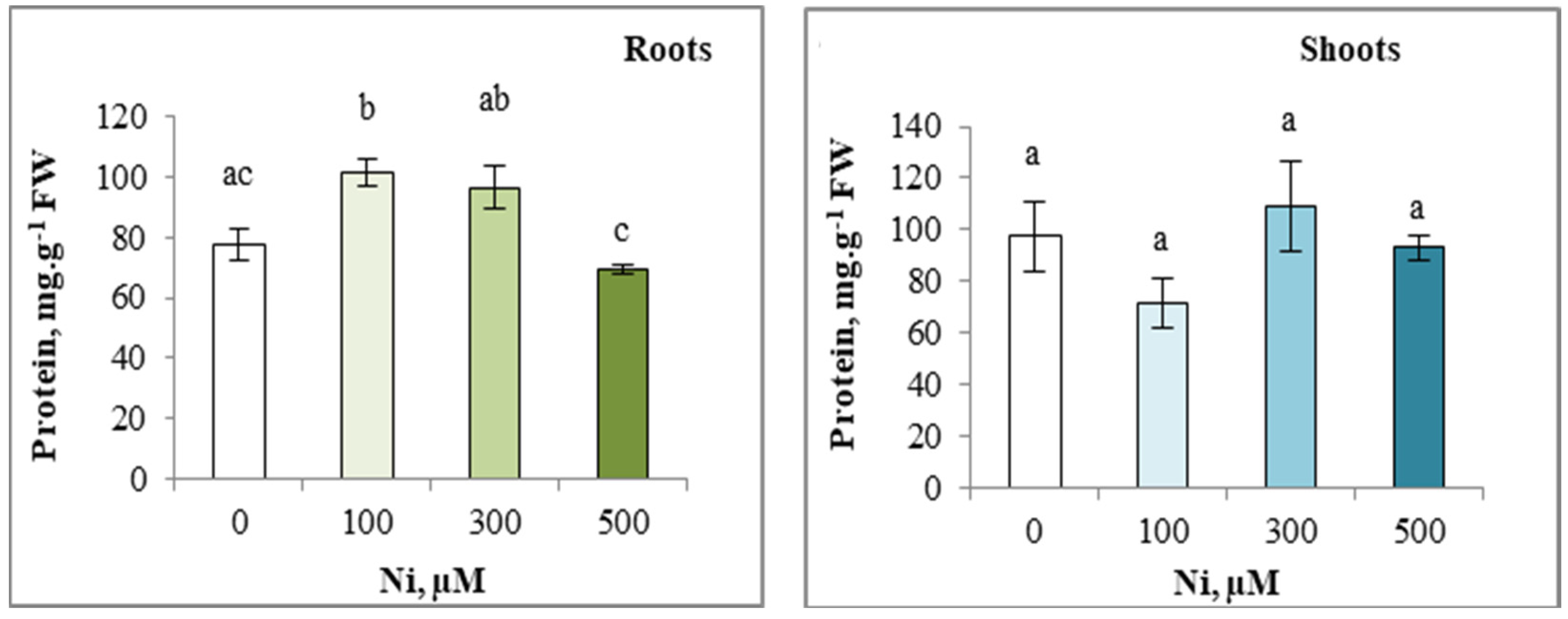
3.8. Total Protein Content
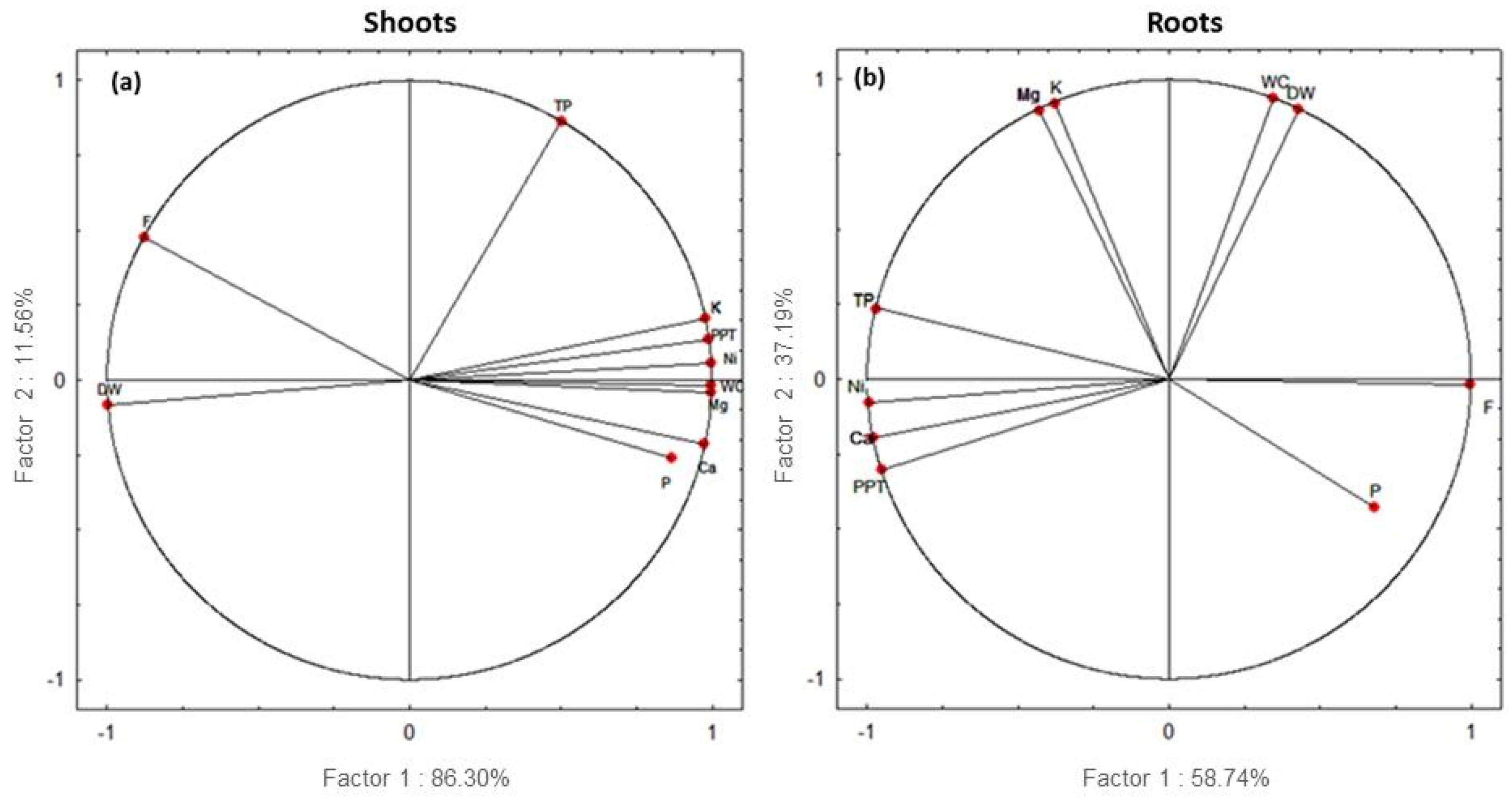
3.9. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neethu, S.; Midhun, S.J.; Radhakrishnan, E.K.; Jyothis, M. Microbially synthesized nanomaterials for remediation of contaminated soil and water environment. In Microbe Mediated Remediation of Environmental Contaminants, Series in Food Science, Technology and Nutrition; Kumar, A., Singh, V.K., Singh, P., Mishra, V.K., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 157–176. [Google Scholar] [CrossRef]
- Carre, F.; Caudeville, J.; Bonnard, R.; Bert, V.; Boucard, P.; Martine, R. Soil contamination and human health: A major challenge for global soil security. In Global Soil Security Symposium; Springer: College Station, TX, USA, 2015; pp. 275–295. [Google Scholar]
- Sleimi, N.; Bankaji, I.; Dallai, M.; Kefi, O. Accumulation des éléments traces et tolérance au stress métallique chez les halophytes colonisant les bordures de la lagune de Bizerte. Rev. Ecol. (Terre Vie) 2014, 69, 49–59. [Google Scholar] [CrossRef]
- Karimi, R.; Solhi, S.; Salehi, M.; Solhi, M.; Mollahosaini, H. Effects of Cd, Pb and Ni on growth and macronutrient contents of Vicia faba L and Brassica arvensis L. Int. J. Agron. Plant Prod. 2013, 4, 739–744. [Google Scholar]
- Cachada, A.; Rocha-Santos, T.; Duarte, A.C. Soil and pollution: An introduction to the main issues. In Soil Pollution; Duarte, A.C., Cachada, A., Rocha-Santos, T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–28. [Google Scholar] [CrossRef]
- Hseu, Z.Y.; Lai, Y.J. Nickel accumulation in paddy rice on serpentine soils containing high geogenic nickel contents in Taiwan. Environ. Geochem. Health 2017, 39, 1325–1334. [Google Scholar] [CrossRef]
- Masciandaro, G.; Macci, C.; Peruzzi, E.; Ceccanti, B.; Doni, S. Organic matter–microorganism–plant in soil bioremediation: A synergic approach. Rev. Environ. Sci. Biotechnol. 2013, 12, 399–419. [Google Scholar] [CrossRef]
- Lupolt, S.N.; Agnew, J.; Burke, T.A.; Kennedy, R.D.; Nachman, K.E. Key Considerations for assessing soil ingestion exposures among agricultural workers. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Fariduddin, Q.; Hayat, S.; Ahmad, A. Nickel: An overview of uptake, essentiality and toxicity in plants. Bull. Environ. Contam. Toxicol. 2011, 86, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Mandal, C.; Ghosh, N.; Adak, M.K.; Dey, N. Interaction of polyamine on oxidative stress induced by exogenously applied hydrogen peroxide in Salvinia natans Linn. Theor. Exp. Plant Physiol. 2013, 25, 223–230. [Google Scholar] [CrossRef]
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 11–50. [Google Scholar]
- Selvam, A.; Wong, J.W.C. Phytochelatin synthesis and cadmium uptake of Brassica napus. Environ. Technol. 2008, 29, 765–773. [Google Scholar] [CrossRef]
- Perriguey, J.; Sterckeman, T.; Morel, J.L. Effect of rhizosphere and plant-related factors on the cadmium uptake by maize (Zea mays L.). Environ. Exp. Bot. 2008, 63, 333–341. [Google Scholar] [CrossRef]
- Prasad, M.N.V. Cadmium Toxicity and Tolerance in Vascular Plants. Environ. Exp. Bot. 1995, 35, 525–545. [Google Scholar] [CrossRef]
- Carrier, P.; Baryla, A.; Havaux, M. Cadmium distribution and microlocalization in oilseed rape (Brassica napus) after long-term growth on cadmium-contaminated soil. Planta 2003, 216, 939–950. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Cabot, C.; Martos, S.; Gallego, B.; Barceló, J. Do Toxic Ions Induce Hormesis in Plants? Plant Sci. 2013, 212, 15–25. [Google Scholar] [CrossRef]
- Baize, D.; Paquereau, H. Teneurs Totales en Éléments Traces dans les Sols Agricoles de Seine-et-Marne. Etude Gest. Sols 1997, 4, 77–94. [Google Scholar]
- WHO. Nickel in Drinking-Water. In Background Document for Development of WHO: Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Mellis, V.E.; da Cruz, M.C.P.; Casagrande, C.J. Nickel adsorption by soils in relation to pH, organic matter, and iron oxides. Sci. Agric. 2004, 61, 190–195. [Google Scholar] [CrossRef]
- Brown, P.H.; Welch, R.M.; Cary, E.E. Nickel: A Micronutrient Essential for Higher Plants. Plant Physiol. 1987, 85, 801–803. [Google Scholar] [CrossRef]
- Reeves, R.D. Tropical hyperaccumulators of metals and their potential for phytoextraction. Plant Soil 2003, 249, 57–65. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Distribution of cadmium, lead, nickel, and strontium in imbibing maize caryopses. Russ. J. Plant Physiol. 2005, 52, 565–569. [Google Scholar] [CrossRef]
- Molas, J.; Baran, S. Relationship between the chemical form of nickel applied to the soil and its uptake and toxicity to barley plants (Hordeum vulgare L.). Geoderma 2004, 122, 247–255. [Google Scholar] [CrossRef]
- Markiv, B.; Ruiz-Azcona, L.; Expósito, A.; Santibáñez, M.; Fernández-Olmo, I. Short- and long-term exposure to trace metal(loid)s from the production of ferromanganese alloys by personal sampling and biomarkers. Environ. Geochem. Health 2022, 44, 4595–4618. [Google Scholar] [CrossRef]
- Gerendás, J.; Sattelmacher, B. Influence of Ni supply on growth and nitrogen metabolism of Brassica napus L. grown with NH4NO3 or urea as N source. Ann. Bot. 1999, 83, 65–71. [Google Scholar] [CrossRef]
- Tipu, M.I.; Ashraf, M.Y.; Sarwar, N.; Akhtar, M.; Shaheen, M.R.; Ali, S.; Damalas, C.A. Growth and physiology of maize (Zea mays L.) in a nickel-contaminated soil and phytoremediation efficiency using EDTA. J. Plant Growth Regul. 2021, 40, 774–786. [Google Scholar] [CrossRef]
- Obrero, Á.; Die, J.V.; Román, B.; Gómez, P.; Nadal, S.; González, V.; Clara, I. Selection of reference genes for gene expression studies in zucchini (Cucurbita pepo) using qPCR. J. Agric. Food Chem. 2011, 59, 5402–5411. [Google Scholar] [CrossRef]
- Labidi, O.; Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M.; Sleimi, N. Assessing of growth, antioxidant enzymes and phytohormone regulation in Cucurbita pepo under cadmium stress. Food Sci. Nutr. 2021, 9, 2021–2031. [Google Scholar] [CrossRef]
- Hewitt, E.J. Sand and water culture methods used in the study of plant nutrition. J. Assoc. Off. Anal. Chem. 1966, 49, 888–889. [Google Scholar]
- Sleimi, N.; Abdely, C. Salt-tolerance strategy of two halophytes species: Spartina alterniflora and Suaeda fruticosa. In Tasks for Vegetation Science; Lieth, H., Mochtchenko, M., Eds.; Cash Crop Halophytes: Recent Studies; Kluwer Academic Publishers: Amsterdam, The Netherlands, 2003; Volume 38, pp. 79–85. [Google Scholar] [CrossRef]
- Sleimi, N.; Abdelly, C. Growth and mineral nutrition of some halophytes under seawater irrigation. In Prospects for Saline Agriculture; Ahmad, R., Malik, K.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 403–410. [Google Scholar] [CrossRef]
- Sleimi, N.; Bankaji, I.; Kouki, R.; Dridi, N.; Duarte, B.; Caçador, I. Assessment of extraction methods of trace metallic elements in plants: Approval of a common method. Sustainability 2022, 14, 1428. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.; Paraggio, M.; Viggiano, M. A reproducible, rapid and inexpensive Folin-Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoids contents in mulberry and their scavenging effects on superoxide radicals. J. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Maheshwari, R.; Dubey, R.S. Inhibition of ribonuclease and protease activities in germinating rice seeds exposed to nickel. Acta Physiol. Plant 2008, 30, 863–872. [Google Scholar] [CrossRef]
- Gajewska, E.; Sklodowska, M. Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. Biometals 2007, 20, 27–36. [Google Scholar] [CrossRef]
- Palacios, G.; Gómez, I.; Carbonell-Barrachina, A.; Pedreño, J.N.; Mataix, J. Effect of nickel concentration on tomato plant nutrition and dry matter yield. J. Plant Nutr. 1998, 21, 2179–2191. [Google Scholar] [CrossRef]
- Bouslimi, H.; Ferreira, R.; Dridi, N.; Brito, P.; Martins-Dias, S.; Caçador, I.; Sleimi, N. Effects of barium stress in Brassica juncea and Cakile maritima: The indicator role of some antioxidant enzymes and secondary metabolites. Phyton Int. J. Exp. Bot. 2021, 90, 145–158. [Google Scholar] [CrossRef]
- Suwa, R.K.; Jayachandran, N.T.; Nguyen, A.; Boulenouar, K.; Fujita, K.; Saneoka, H. Barium toxicity effects in soybean plants. Arch. Environ. Contam. Toxicol. 2008, 55, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.J.; Li, G.; Jin, C.W.; Liu, W.J.; Zhang, S.S.; Zhang, Y.S.; Lin, X.Y. Aluminum-induced changes in reactive oxygen species accumulation, lipid peroxidation and antioxidant capacity in wheat root tips. Biol. Plant 2012, 56, 89–96. [Google Scholar] [CrossRef]
- Bhalerao, S.A.; Sharma, A.S.; Poojari, A.C. Toxicity of Nickel in Plants. Int. J. Pure Appl. Biosci. 2015, 3, 345–355. [Google Scholar]
- Poschenrieder, C.; Gunse, B.; Barcelo, J. Influence of cadmium on water relations, stomatal-resistance, and abscisic-acid content in expanding bean leaves. Plant Physiol. 1989, 90, 1365–1371. [Google Scholar] [CrossRef]
- Kouki, R.; Ayachi, R.; Ferreira, R.; Sleimi, N. Behavior of Cucumis sativus L. in presence of aluminum stress: Germination, plant growth, and antioxidant enzymes. Food Sci. Nutr. 2021, 9, 3280–3288. [Google Scholar] [CrossRef]
- Barcelo, J.; Poschenrieder, C.H. Plant water relations as affected by heavy metal stress. J. Plant Nutr. 1990, 13, 1–37. [Google Scholar] [CrossRef]
- Molas, J. Changes in morphological and anatomical structure of cabbage (Brassica oleracea L.) outer leaves and in ultrastructure of their chloroplast caused by an in vitro excess of nickel. Photosynthetica 1997, 34, 513–522. [Google Scholar] [CrossRef]
- Brunet, J.; Varrault, G.; Zuily-Fodil, Y.; Repellin, A. Accumulation of lead in the roots of grass pea (Lathyrus sativus L.) plants triggers systemic variation in gene expression in the shoots. Chemosphere 2009, 77, 1113–1120. [Google Scholar] [CrossRef]
- Amjad, M.; Raza, H.; Murtaza, B.; Abbas, G.; Imran, M.; Shahid, M.; Naeem, M.A.; Zakir, A.; Iqbal, M.M. Nickel toxicity induced changes in nutrient dynamics and antioxidant profiling in two maize (Zea mays L.) hybrids. Plants 2020, 9, 5. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Rengel, Z.; Bose, J.; Chen, Q.; Tripathi, B.N. Magnesium alleviates plant toxicity of aluminium and heavy metals. Crop Pasture Sci. 2015, 66, 1298–1307. [Google Scholar] [CrossRef]
- Rizwan, M.; Mostofa, M.G.; Ahmad, M.Z.; Zhou, Y.; Adeel, M.; Mehmood, S.; Ahmad, M.A.; Javed, R.; Imtiaz, M.; Aziz, O.; et al. Hydrogen sulfide enhances rice tolerance to nickel through the prevention of chloroplast damage and the improvement of nitrogen metabolism under excessive nickel. Plant Physiol. Biochem. 2019, 138, 100–111. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Hernandez-Viezcas, J.A.; Zhao, L.J.; Corral-Diaz, B.; Ge, Y.; Priester, J.H.; Holde, P.A.; Gardea-Torresdey, J.L. Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiol. Biochem. 2014, 80, 128–135. [Google Scholar] [CrossRef]
- Hu, H.; Wang, L.; Li, Y.; Sun, J.; Zhou, Q.; Huang, X. Insight into the mechanism of lanthanum (III) induced damage to plant photosynthesis. Ecotox. Environ. Saf. 2016, 127, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Dridi, N.; Romdhane, L.; Ferreira, R.; Sleimi, N. Fertilizer effect of composted sewage sludge and cattle manure on Pelargonium growth. J. Water Sanit. Hygi. Dev. 2020, 10, 1019–1025. [Google Scholar] [CrossRef]
- Chaudhry, A.H.; Nayab, S.; Hussain, S.B.; Ali, M.; Pan, Z. Current understandings on magnesium deficiency and future outlooks for sustainable agriculture. Int. J. Mol. Sci. 2021, 22, 1819. [Google Scholar] [CrossRef]
- Sghaier, D.B.; Bankaji, I.; Pedro, S.; Caçador, I.; Sleimi, N. Photosynthetic behaviour and mineral nutrition of Tamarix gallica cultivated under aluminum and NaCl combined stress. Phyton-Int. J. Exp. Bot. 2019, 88, 239–252. [Google Scholar]
- Wang, T.; Chen, X.; Ju, C.; Wang, C. Calcium signaling in plant mineral nutrition: From uptake to transport. Plant Commun. 2023, 4, 100678. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Sleimi, N.; Kouki, R.; Haj Ammar, M.; Ferreira, R.; Pérez-Clemente, R.M. Barium effect on germination, plant growth, and antioxidant enzymes in Cucumis sativus L. plants. Food Sci. Nutr. 2021, 9, 2086–2094. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.D.; Baker, A.J.M. Metal accumulating plant. In Phytoremediation of Toxic Metals: Using Plants to Clean up the Environment; Raskin, I., Ensley, B.D., Eds.; John Wiley and Sons: New York, NY, USA, 2000; pp. 193–230. [Google Scholar]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–168. [Google Scholar]
- Macheix, J.J.; Fleuriet, A.; Jay-Allemand, C. Les Composés Phénoliques des Végétaux: Un Exemple de Métabolites Secondaires d’Importance économique; Presses Polytechniques et Universitaires Romandes: Lausanne, Switzerland, 2005; pp. 4–5. [Google Scholar]
- Jonnala, R.S.; Irmak, S.; MacRitchie, F.; Bean, S.R. Phenolics in the bran of waxy wheat and triticale. J. Cereal Sci. 2010, 52, 509–515. [Google Scholar] [CrossRef]
- Chaib, G.; Bouchelaleg, H.A.; Talbi, R. Etude phytochimique de quelques variétés de blé tendre (Triticum aestivum) et d’orge (Hordeum vulgare) et leurs activités biologiques. Eur. Sci. J. 2015, 11, 1857–7881. [Google Scholar]
- Rastgoo, L.; Alemzadeh, A. Biochemical responses of Gouan (Aeluropus littoralis) to heavy metals stress. Aust. J. Crop Sci. 2011, 5, 375–383. [Google Scholar]
- Neggaz, N.E.; Reguieg Yssaad, H.A. Effect of lead stress on polyphenols, flavonoids, and proline contents in radish (Raphanus sativus L.). Int. J. Biosci. 2018, 12, 135–144. [Google Scholar]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. J. Exp. Bot. 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Kattab, H. Role of glutathione and polyadenylic acid on the oxidative defense systems of two different cultivars of canola seedlings grown under saline condition. Aust. J. Basic. Appl. Sci. 2007, 1, 323–334. [Google Scholar]
- Dridi, N.; Kouki, R.; Bouslimi, H.; Ferreira, R.; Hidouri, S.; Caçador, I.; Sleimi, N. Assessment of photochemical performance of photosystem II in Limbarda crithmoides and Helianthus annuus under Pb stress using chlorophyll a fluorescence kinetics. World. J. Biol. Biotech. 2023, 8, 15–19. [Google Scholar] [CrossRef]
- Ma, C.; Liu, H.; Guo, H.; Musante, C.; Coskun, S.H.; Nelson, B.C.; White, J.C.; Xing, B.; Dhankher, O.P. Defense mechanisms and nutrient displacement in Arabidopsis thaliana upon exposure to CeO2 and In2O3 nanoparticles. Environ. Sci. Nano 2016, 3, 1369–1379. [Google Scholar] [CrossRef]
- Şen, A. Oxidative stress studies in plant tissue culture, antioxidant enzyme. Techopen 2012, 3, 59–88. [Google Scholar]
- Finger-Teixeira, A.; Ferrarese, M.dL.L.; Soares, A.R.; da Silva, D.; Ferrarese-Filho, O. Cadmium-induced lignification restricts soybean root growth. Ecotoxicol. Environ. Saf. 2010, 73, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Q.; Lu, H.; Li, J.; Yang, D.; Liu, J.; Yan, C. Phenolic metabolism and related heavy metal tolerance mechanism in Kandelia Obovata under Cd and Zn stress. Ecotoxicol. Environ. Saf. 2019, 169, 134–143. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Bouslimi, H.; Dridi, N.; Ferreira, R.; Brito, P.; Caçador, I.; Hidouri, S.; Sleimi, N. Appraisal of the physiological response of Cakile maritima and Brassica juncea for tolerating lanthanum stress. J. Mar. Sci. Eng. 2024, 12, 65. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Barros, L.; Soares, M.E.; Bastos, M.L.; Pereira, J.A. Antioxidant activity and phenolic contents of Olea europaea L. leaves sprayed with different copper formulations. J. Food Chem. 2007, 103, 188–195. [Google Scholar] [CrossRef]
- Khatun, S.; Ali, M.B.; Hahn, E.J.; Paek, K.Y. Copper toxicity in Withania somnifera: Growth and antioxidant enzymes responses of in vitro grown plants. Environ. Exp. Bot. 2008, 64, 279–285. [Google Scholar] [CrossRef]
- Murch, S.J.; Haq, K.; Rupasinghe, H.P.V.; Saxena, P.K. Nickel contamination affects growth and secondary metabolite composition of St. John’s wort (Hypericum perforatum L.). Environ. Exp. Bot. 2003, 49, 251–257. [Google Scholar] [CrossRef]
- Ikram, K.; Abdelhakim, R.Y.H.; Topcuoglu, B.; Badiaa, O.; Houria, T. Accumulation of polyphenols and flavonoids in Atriplex canescens (Pursh) Nutt stressed by heavy metals (zinc, lead and cadmium). Malays. J. Fundam. Appl. Sci. 2020, 16, 334–337. [Google Scholar] [CrossRef]
- Bretzel, F.; Benvenuti, S.; Pistelli, L. Metal contamination in urban street sediment in Pisa (Italy) can affect the production of antioxidant metabolites in Taraxacum officinale Weber. Environ. Sci. Pollut. Res. 2014, 21, 2325–2333. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Keilig, K.; Ludwig-Mueller, J. Effect of flavonoids on heavy metal tolerance in Arabidopsis thaliana seedlings. Bot. Stud. 2009, 50, 311–318. [Google Scholar]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Berni, R.; Luyckxc, M.; Xud, X.; Legayd, S.; Sergeantd, K.; Hausman, J.F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Djerroudi-Zidane, O.; Belkhodja, M.; Bissati, S. Effect du Stress Salin sur l’accumulation de proline chez deux espèces d’Atriplex Halimus L. et Atriplex canescens (Pursh) Nutt. Eur. J. Sci. Res. 2010, 41, 248–259. [Google Scholar]
- Kandziora-Ciupa, M.; Nadgórska-Socha1, A.; Barczyk, G.; Ciepał, R. Bioaccumulation of heavy metals and ecophysiological responses to heavy metal stress in selected populations of Vaccinium myrtillus L. and Vaccinium vitis-idaea L. Ecotoxicology 2017, 26, 966–980. [Google Scholar] [CrossRef]
- Kandziora-Ciupa, M.; Ciepał, R.; Nadgórska-Socha, A.; Barczyk, G. Accumulation of heavy metals and antioxidant responses in Pinus sylvestris L. needles in polluted and non-polluted sites. Ecotoxicology 2016, 25, 970–981. [Google Scholar] [CrossRef]
- Hadjadj, S.; Djerroudi, O.; Bissati, S. Etude comparative des mécanismes biochimiques de tolérance au stress salin de deux espèces d’atriplex: Atriplex halimus L. et Atriplex canescens (Purch) Nutt. Alger. J. Arid. Environ. 2011, 1, 3–10. [Google Scholar]
- Garg, G.; Neha, P. Plant transcription factors networking of pyrroline-5-carboxylate (P5C) enzyme under stress condition: A review. Plant Arch. 2019, 19, 562–569. [Google Scholar]
- Kouki, R.; Dridi, N.; Vives-Peris, V.; Gómez-Cadenas, A.; Caçador, I.; Pérez-Clemente, R.M.; Sleimi, N. Appraisal of Abelmoschus esculentus L. response to aluminum and barium stress. Plants 2023, 12, 179. [Google Scholar] [CrossRef]
- Brinis, A.; Belkhodja, M. Salinity effect on physiological and biochemical parameters of Atriplex halimus L. Sci. Technol. Synthèse 2015, 31, 42–51. [Google Scholar]
- Spormann, S.; Nadais, P.; Sousa, F.; Pinto, M.; Martins, M.; Sousa, B.; Fidalgo, F.; Soares, C. Accumulation of proline in plants under contaminated soils-Are we on the same page? Antioxidants 2023, 12, 666. [Google Scholar] [CrossRef]
- Tihana, T.; John, T.H.; Meri, E.; Nada, P.; Vera, C.; Hrvoje, L.; Ivna, Š.; Drago, B. Antioxidative responses in radish (Raphanus sativus L.) Plants stressed by copper and lead in nutrient solution and soil. Acta Biol. Cracoviensia Ser. Bot. 2008, 50, 79–86. [Google Scholar]
- John, R.; Ahmad, P.; Gadgil, K.; Sharma, S. Heavy metal toxicity: Effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Int. J. Plant Prod. 2009, 3, 65–75. [Google Scholar]
- Dridi, N.; Bouslimi, H.; Caçador, I.; Sleimi, N. Lead tolerance, accumulation and translocation in two Asteraceae plants: Limbarda crithmoides and Helianthus annuus. S. Afr. J. Bot. 2022, 150, 986–996. [Google Scholar] [CrossRef]
- Gupta, D.; Huang, H.; Yang, X.; Razafindrabe, B.; Inouhe, M. The detoxification of lead in Sedum alfredii H. is not related to phytochelatins but the glutathione. J. Hazard. Mater. 2010, 177, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Wollgieh, R.; Newmann, D. Stress response of tomato cell cultures to toxic metals and heat shock: Differences and similarities. J. PlantPhysiol. 1995, 146, 736–742. [Google Scholar]


| Treatments (Ni, µM) | Ca2+, mg·g−1 DW | Mg2+, mg·g−1 DW | K+, mg·g−1 DW | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Roots | Shoots | Fruits | Roots | Shoots | Fruits | Roots | Shoots | Fruits | |
| 0 µM | 54.51 ± 1.38 a | 55.90 ± 2.19 a | 13.58 ± 1.01 a | 7.22 ± 0.20 a | 11.48 ± 0.40 a | 12.66 ± 0.70 a | 11.33 ± 0.52 a | 17.63 ± 0.66 a | 71.92 ± 3.16 a |
| 100 µM | 67.67 ± 4.48 b | 75.01 ± 2.21 b | 11.94 ± 1.11 ab | 23.55 ± 0.98 b | 17.31 ± 0.51 b | 14.01 ± 0.76 a | 73.44 ± 2.26 b | 26.74 ± 1.00 b | 73.86 ± 3.69 a |
| 300 µM | 71.15 ± 1.43 b | 84.08 ± 2.59 b | 15.20 ± 1.57 a | 9.16 ± 0.30 a | 11.92 ± 1.11 c | 12.48 ± 2.02 a | 15.47 ± 1.26 a | 53.40 ± 2.78 c | 58.13 ± 3.53 a |
| 500 µM | 71.25 ± 3.13 b | 81.26 ± 3.28 b | 7.64 ± 0.89 b | 7.18 ± 0.38 a | 9.15 ± 0.29 a | 12.04 ± 1.60 a | 10.70 ± 0.64 a | 19.35 ± 0.50 a | 73.77 ± 6.72 a |
| Treatments (Ni, µM) | DW (g) | S/R | TI (%) | |
|---|---|---|---|---|
| Roots | Shoots | |||
| 0 | 186.9 ± 9.00 a | 2699.9 ± 173.8 a | 15.1 ± 1.8 a | |
| 100 | 204.9 ± 15.2 a | 1901.9 ± 118.0 b | 9.8 ± 1.0 b | 73.9 ± 6 |
| 300 | 127.9 ± 10.5 b | 617.9 ± 49.9 c | 5.1 ± 0.5 c | 22.2 ± 1.6 |
| 500 | 103.4 ± 7.2 b | 442.5 ± 33.5 c | 4.4 ± 0.4 c | 14.6 ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labidi, O.; Kouki, R.; Hidouri, S.; Bouzahouane, H.; Caçador, I.; Pérez-Clemente, R.M.; Sleimi, N. Impact of Nickel Toxicity on Growth, Fruit Quality and Antioxidant Response in Zucchini Squash (Cucurbita pepo L.). Plants 2024, 13, 2361. https://doi.org/10.3390/plants13172361
Labidi O, Kouki R, Hidouri S, Bouzahouane H, Caçador I, Pérez-Clemente RM, Sleimi N. Impact of Nickel Toxicity on Growth, Fruit Quality and Antioxidant Response in Zucchini Squash (Cucurbita pepo L.). Plants. 2024; 13(17):2361. https://doi.org/10.3390/plants13172361
Chicago/Turabian StyleLabidi, Oumayma, Rim Kouki, Saida Hidouri, Hana Bouzahouane, Isabel Caçador, Rosa M. Pérez-Clemente, and Noomene Sleimi. 2024. "Impact of Nickel Toxicity on Growth, Fruit Quality and Antioxidant Response in Zucchini Squash (Cucurbita pepo L.)" Plants 13, no. 17: 2361. https://doi.org/10.3390/plants13172361
APA StyleLabidi, O., Kouki, R., Hidouri, S., Bouzahouane, H., Caçador, I., Pérez-Clemente, R. M., & Sleimi, N. (2024). Impact of Nickel Toxicity on Growth, Fruit Quality and Antioxidant Response in Zucchini Squash (Cucurbita pepo L.). Plants, 13(17), 2361. https://doi.org/10.3390/plants13172361








