Ectopic Expression of AetPGL from Aegilops tauschii Enhances Cadmium Tolerance and Accumulation Capacity in Arabidopsis thaliana
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Culture and Treatments
2.2. Phylogenetic and Conserved Motif Analysis of AetPGL Proteins
2.3. RNA Isolation and qRT-PCR
2.4. Construction of the AetPGL Expression Vector and Genetic Transformation of Arabidopsis thaliana
2.5. Phenotypic Analysis and Physiological Index Determination
2.6. Determination of Cadmium Content
2.7. RNA-Seq Analysis
2.8. Statistical Analysis
2.9. Primers
3. Results
3.1. AetPGL Conserved Motif and Phylogenetic Analysis
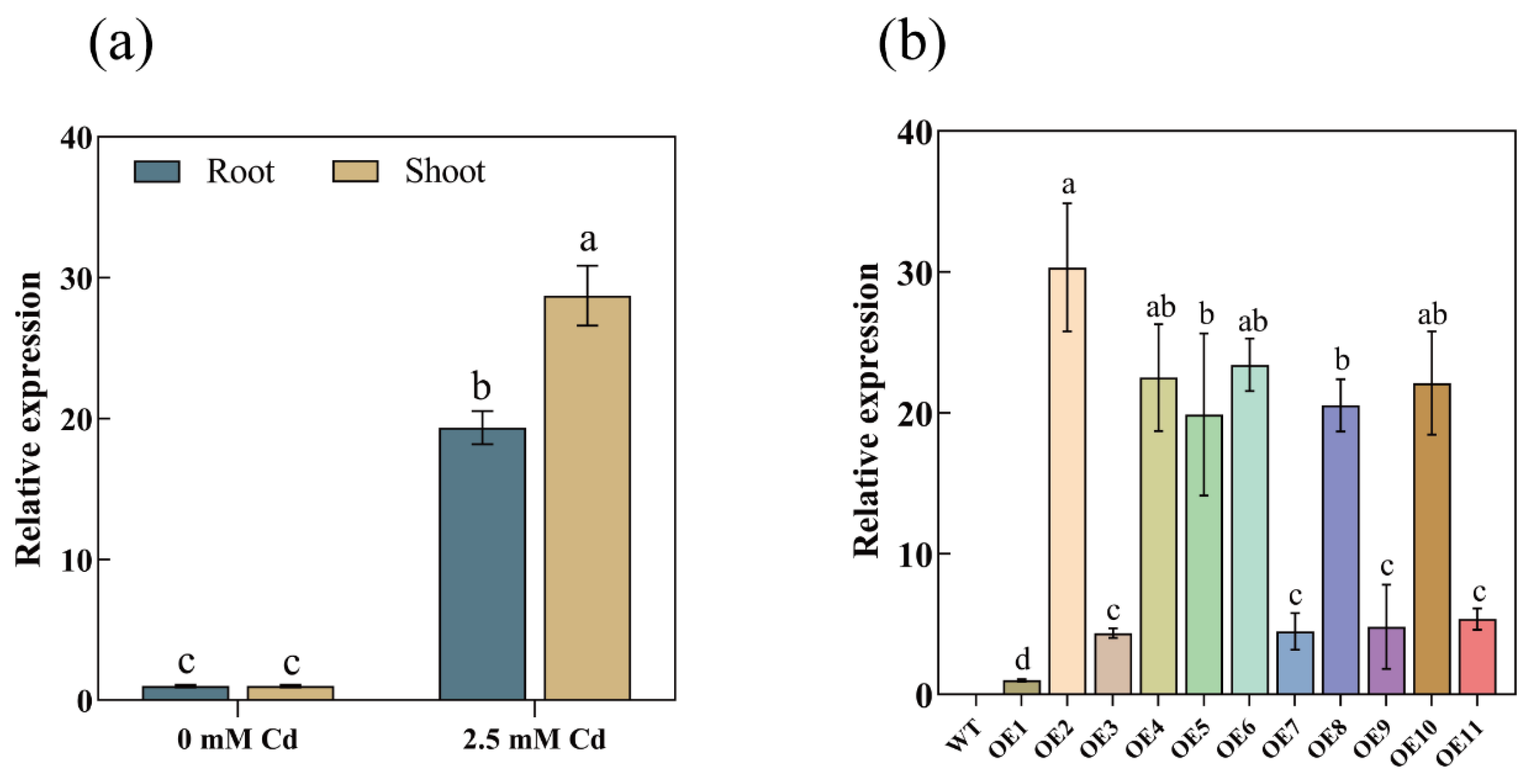
3.2. Cd-Induced AetPGL Expression
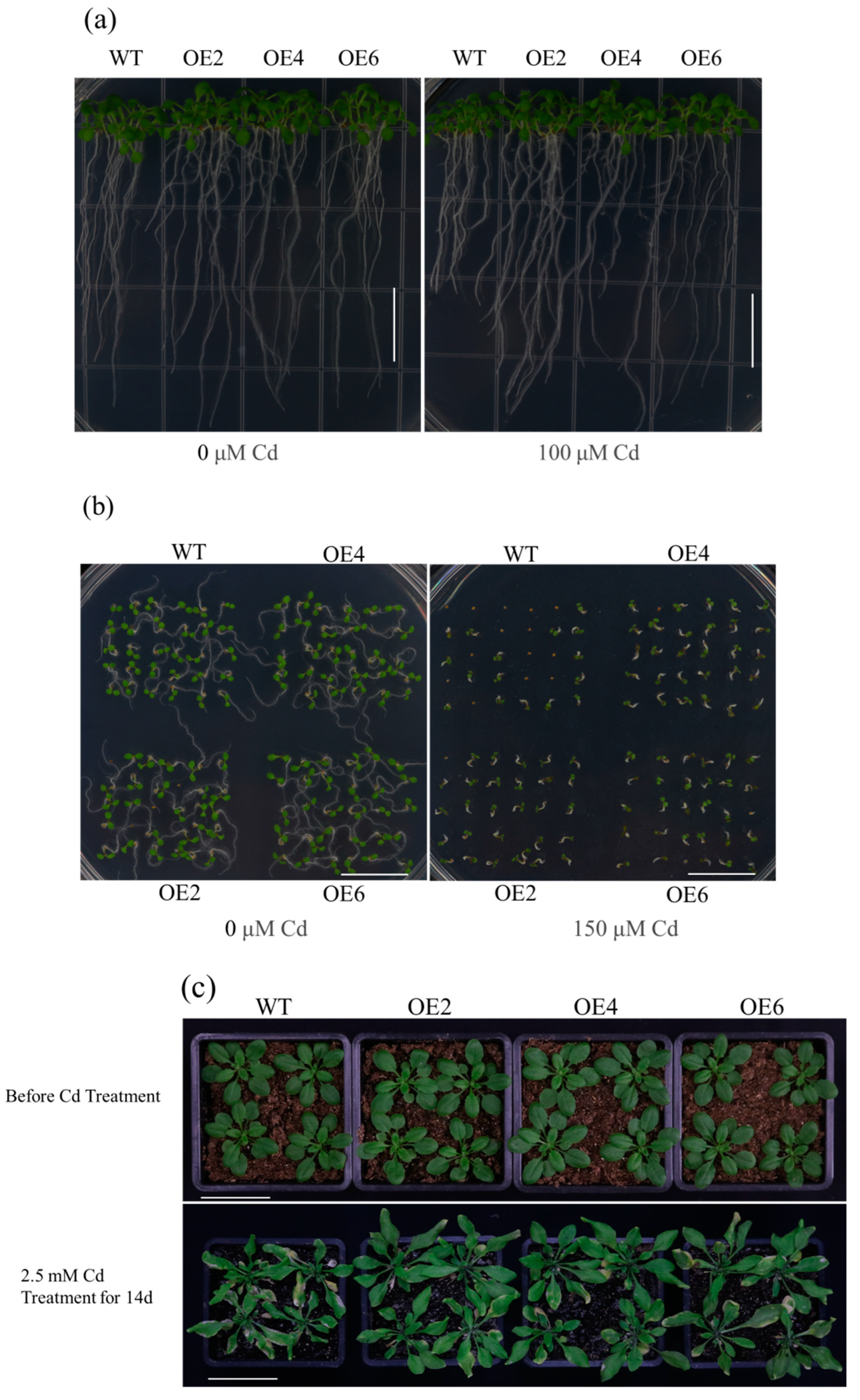
3.3. Overexpression of AetPGL Enhanced the Tolerance of Arabidopsis to Cd
3.4. Overexpression of AetPGL Enhanced the Antioxidant Capacity of Transgenic Arabidopsis
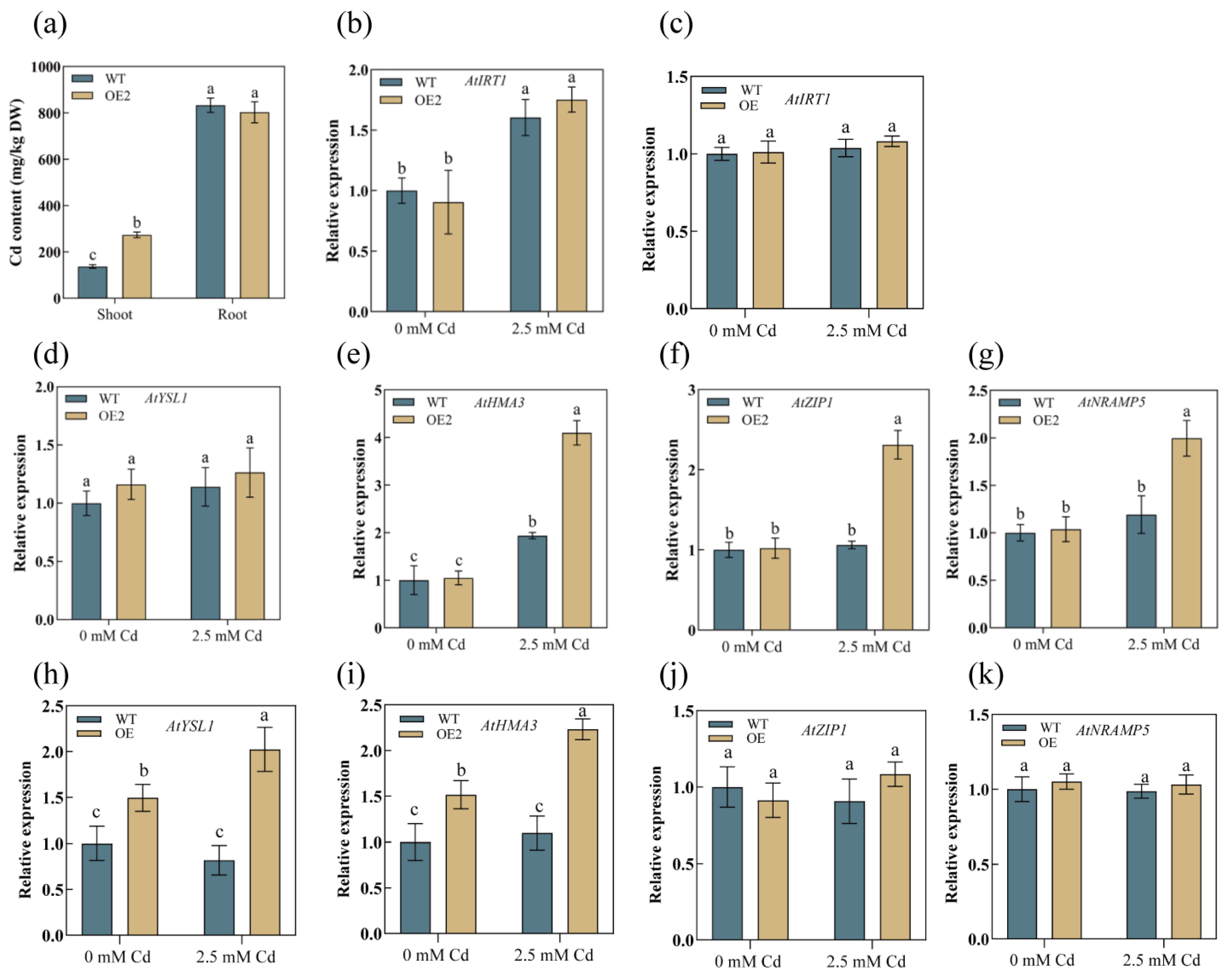
3.5. AetPGL Increased Cadmium Uptake by Regulating the Expression of Heavy Metal Transporters in Roots
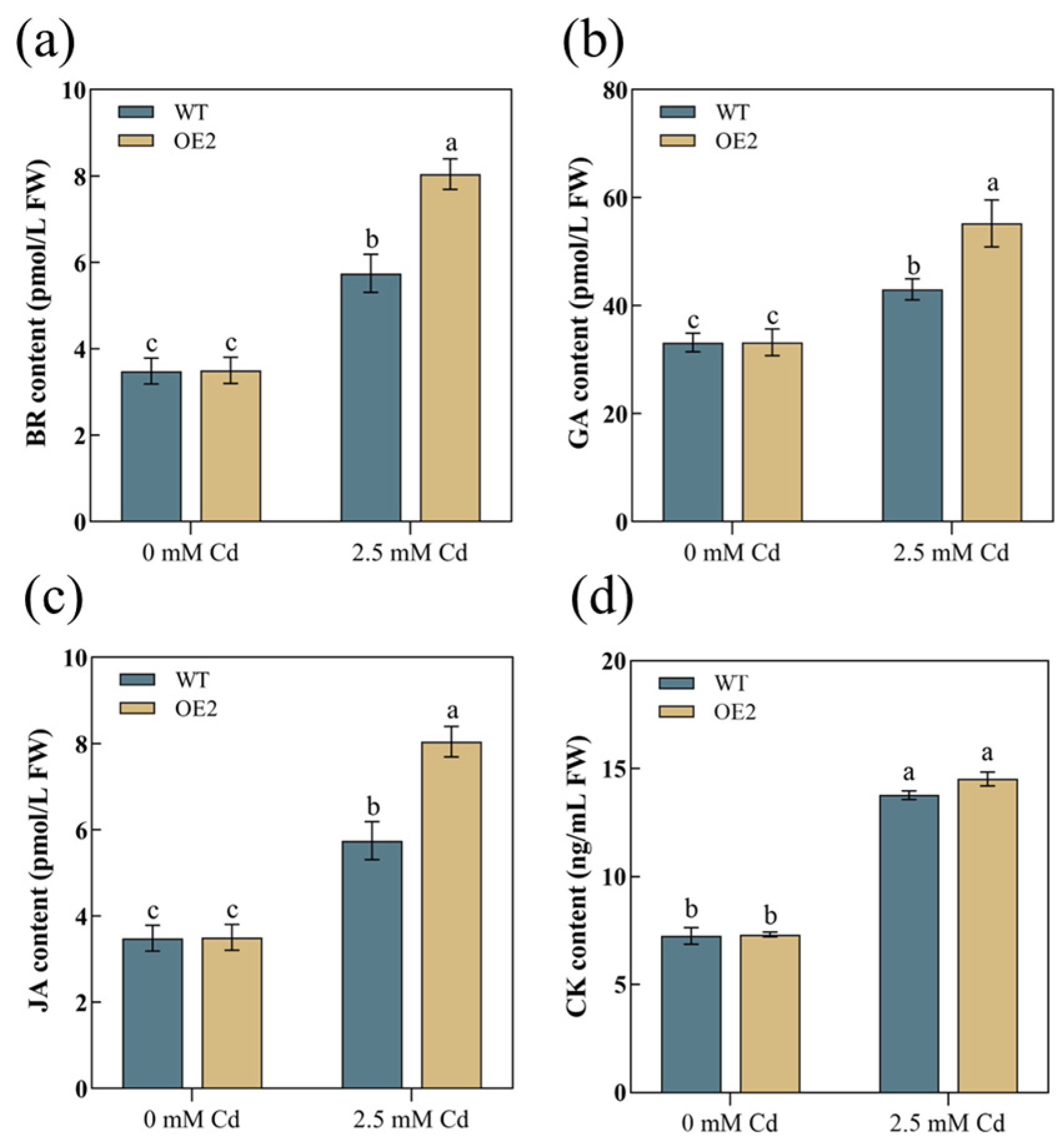
3.6. AetPGL Promotes Phytohormone Synthesis in Aboveground Parts
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| pBI121-AetPGL | CGCGGATCCGCGATGGAGAGGGAAATGGCTG | CCCAAGCTTGGGCTAGTACTCACGATGCTGCTGCT |
| AtACTIN | CTTAACCCAAAGGCCAACAGA | GCAAGGTCAAGACGGAGGAT |
| qAetPGL | TGGATTGGTCTAAATGGT | ACGAATTTGTCATCAGGCAG |
| qAtHMA3 | TGCTGCTCATAAGGCAAGCA | CTAGTCCAGTATCCGCGATCACT |
| qAtNRAMP5 | ACAGCCACGGTTCGAATTG | CCGTGGGCATCCGACCA |
| qAtYSL1 | TGCGAAAGGATGTGGCAGCCGTTCCCGA | CAACGCATAAGAACCTCTTTGA |
| qAtIRT1 | TATCGCCAAATGGGCTTAAC | AAACCGATAGAGAATCGAGACG |
| qAtZIP1 | ATGTGTTGTGCCTCGAGTGAT | ATCAACGGTAGACTCACGCC |
| qAtAHP4 | GCTCCAAGATGATGCAAACCC | TCGTGCTGCTTCCCTTAAACT |
| qAtRGL1 | AAGACCGGGTAGAGAGGCAT | CGTTTGCCATCCAAGCAACA |
| qAtBES1 | CCGTTTTATGCGGTGTCTGC | CGAGGTTGGCACCATAGAGG |
| qAtTCH4 | ATCACTTGGGGTGATGGTCG | TCGTCCCATGTTGTTCCAGG |
| qAtTIFY7 | ATCATGTTATGCGCCGGGAA | GGGTGTGTCCCTACACCTTG |
| qAtTIFY10B | AAAAACCGCAGCACAAGAGC | CTGAGCCAAGCTGGGTTAGT |
| qAtJAZ3 | AGTAGCACAAACGGACTCGG | TCGTGACCCTTTCTTTGCGT |
| KEGG | GeneID | GeneName | CdOE_1 | CdOE_2 | CdOE_3 | CdWT_1 | CdWT_2 | CdWT_3 | log2FC |
|---|---|---|---|---|---|---|---|---|---|
| Cytokinine | AT3G16360 | AHP4 | 10.0256 | 10.0559 | 16.1601 | 6.4350 | 11.3637 | 5.4429 | 0.6409 |
| AT1G67710 | ARR11 | 1.4158 | 0.7991 | 1.3316 | 0.5446 | 0.3158 | 0.6660 | 1.2162 | |
| Gibbenellin | AT1G66350 | RGL1 | 7.7941 | 9.9622 | 14.5231 | 7.6902 | 11.2410 | 4.7497 | 0.4469 |
| Brassinosteroid | AT1G19350 | BES1 | 38.3013 | 22.0161 | 25.5946 | 15.7426 | 12.6653 | 19.8737 | 0.8314 |
| AT5G57560 | TCH4 | 7.8951 | 50.0741 | 179.4677 | 11.7427 | 6.5290 | 29.7952 | 2.3044 | |
| jasmonicacid | AT1G70700 | TIFY7 | 287.7421 | 346.7738 | 292.9672 | 302.0224 | 307.5035 | 253.3108 | 0.1042 |
| AT1G72450 | JAZ6 | 62.5468 | 145.3204 | 116.0356 | 113.8443 | 153.5590 | 75.0006 | −0.0801 | |
| AT1G74950 | TIFY10B | 71.2557 | 181.2355 | 115.7101 | 110.3789 | 127.3035 | 70.0456 | 0.2588 | |
| AT3G17860 | JAZ3 | 13.8426 | 23.7418 | 23.2512 | 17.6589 | 19.2862 | 12.5151 | 0.2987 |
References
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- DalCorso, G.; Manara, A.; Furini, A. An Overview of Heavy Metal Challenge in Plants: From Roots to Shoots. Metallomics 2013, 5, 1117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, J.; Yue, S.; Duan, K.; Yang, H. MhMAPK4 from Malus hupehensis Rehd. decreases cell death in tobacco roots by controlling Cd2+ uptake. Ecotoxicol. Environ. Saf. 2019, 168, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Tao, Q.; Zhou, Y.; Gill, R.A.; Ali, S.; Rafiq, M.T.; Xu, L.; Zhou, W. 5-Aminolevolinic acid mitigates the cadmium-induced changes in Brassica napus as revealed by the biochemical and ultra-structural evaluation of roots. Ecotoxicol. Environ. Saf. 2013, 92, 271–280. [Google Scholar] [CrossRef]
- Tahir, I.; Alkheraije, K.A. A review of important heavy metals toxicity with special emphasis on nephrotoxicity and its management in cattle. Front. Vet. Sci. 2023, 10, 1149720. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Rizwan, M.; Ali, S.; Shahid, M.S.; Li, B. Recent progress on the heavy metals ameliorating potential of engineered nanomaterials in rice paddy: A comprehensive outlook on global food safety with nanotoxicitiy issues. Crit. Rev. Food Sci. Nutr. 2023, 63, 2672–2686. [Google Scholar] [CrossRef]
- Cang, L.; Xing, J.; Liu, C.; Wang, Y.; Zhou, D. Effects of different water management strategies on the stability of cadmium and copper immobilization by biochar in rice-wheat rotation system. Ecotoxicol. Environ. Saf. 2020, 202, 110887. [Google Scholar] [CrossRef] [PubMed]
- Lejay, L.; Wirth, J.; Pervent, M.; Cross, J.M.; Tillard, P.; Gojon, A. Oxidative pentose phosphate pathway-dependent sugar sensing as a mechanism for regulation of root ion transporters by photosynthesis. Plant Physiol. 2008, 146, 2036–2053. [Google Scholar] [CrossRef]
- Bussell, J.D.; Keech, O.; Fenske, R.; Smith, S.M. Requirement for the plastidial oxidative pentose phosphate pathway for nitrate assimilation in Arabidopsis. Plant J. 2013, 75, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Leustek, T.; Martin, M.N.; Bick, J.A.; Davies, J.P. Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 141–165. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Hu, Y.; Hu, W.; Bi, Y. Glucose-6-phosphate dehydrogenase plays a pivotal role in tolerance to drought stress in soybean roots. Plant Cell Rep. 2013, 32, 415–429. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Li, Y.; Hou, J.; Huang, J.; Liang, W. Involvement of ABA- and H2O2-dependent cytosolic glucose-6-phosphate dehydrogenase in maintaining redox homeostasis in soybean roots under drought stress. Plant Physiol. Biochem. 2016, 107, 126–136. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, S.; Guo, H.; Zhang, Z.; Chen, X. Functional analysis of PsG6PDH, a cytosolic glucose-6-phosphate dehydrogenase gene from Populus suaveolens, and its contribution to cold tolerance improvement in tobacco plants. Biotechnol. Lett. 2013, 35, 1509–1518. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, R.; Wan, Q.; Xie, G.; Bi, Y. Glucose-6-phosphate dehydrogenase plays a pivotal role in nitric oxide-involved defense against oxidative stress under salt stress in red kidney bean roots. Plant Cell Physiol. 2007, 48, 511–522. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Wang, X.; Zhang, Y.; Jia, H.; Bi, Y. Glucose-6-phosphate dehydrogenase-dependent hydrogen peroxide production is involved in the regulation of plasma membrane H+-ATPase and Na+/H+ antiporter protein in salt-stressed callus from Carex moorcroftii. Physiol. Plant 2011, 141, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Peng, K.; Bao, Y.; Zhang, D.; Meng, J.; Wang, D.; Wang, X.; Cang, J. Glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase genes of winter wheat enhance the cold tolerance of transgenic Arabidopsis. Plant Physiol. Biochem. 2021, 161, 86–97. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H.; Wang, J.; Yang, J. Molecular cloning and characterization of rice 6-phosphogluconate dehydrogenase gene that is up-regulated by salt stress. Mol. Biol. Rep. 2003, 30, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Huang, J.; Yu, S.; Zhang, H. The 6-phosphogluconate dehydrogenase genes are responsive to abiotic stresses in rice. J. Integr. Plant Biol. 2007, 49, 655–663. [Google Scholar] [CrossRef]
- Li, C.; Li, K.; Zheng, M.; Liu, X.; Ding, X.; Gai, J.; Yang, S. Gm6PGDH1, a cytosolic 6-phosphogluconate dehydrogenase, enhanced tolerance to phosphate starvation by improving root system development and modifying the antioxidant system in soybean. Front. Plant Sci. 2021, 12, 704983. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Xiong, Y.; DeFraia, C.; Williams, D.; Zhang, X.; Mou, Z. Characterization of Arabidopsis 6-phosphogluconolactonase T-DNA insertion mutants reveals an essential role for the oxidative section of the plastidic pentose phosphate pathway in plant growth and development. Plant Cell Physiol. 2009, 50, 1277–1291. [Google Scholar] [CrossRef]
- Lansing, H.; Doering, L.; Fischer, K.; Baune, M.C.; Schaewen, A.V. Analysis of potential redundancy among Arabidopsis 6-phosphogluconolactonase isoforms in peroxisomes. J. Exp. Bot. 2020, 71, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Peres, A.; Soares, J.S.; Tavares, R.G.; Righetto, G.; Zullo, M.; Mandava, N.B.; Menossi, M. Brassinosteroids, the sixth class of phytohormones: A molecular view from the discovery to hormonal interactions in plant development and stress adaptation. Int. J. Mol. Sci. 2019, 20, 331. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Kaur, N.; Pati, P.K. Phytohormones: Key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol. Environ. Saf. 2021, 223, 112578. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tan, H.; Huang, L.; Cai, C.; Ding, Y.; Bao, H.; Chen, Z.; Zhu, C. Application of exogenous salicylic acid reduces Cd toxicity and Cd accumulation in rice. Ecotoxicol. Environ. Saf. 2021, 207, 111198. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, A.K.; Suprasanna, P.; D’Souza, S.F. Identification and profiling of arsenic stress-induced microRNAs in Brassica juncea. J. Exp. Bot. 2013, 64, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Nazir, F.; Maheshwari, C.; Kaur, H.; Gupta, R.; Siddique, K.; Khan, M. Plant hormones and secondary metabolites under environmental stresses: Enlightening defense molecules. Plant Physiol. Biochem. 2024, 206, 108238. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Ji, J.; Li, X.; Jin, C.; Wang, G. LcMKK, a MAPK kinase from Lycium chinense, confers cadmium tolerance in transgenic tobacco by transcriptional upregulation of ethylene responsive transcription factor gene. J. Genet. 2016, 95, 875–885. [Google Scholar] [CrossRef]
- Tao, Q.; Jupa, R.; Dong, Q.; Yang, X.; Liu, Y.; Li, B.; Yuan, S.; Yin, J.; Xu, Q.; Li, T.; et al. Abscisic acid-mediated modifications in water transport continuum are involved in cadmium hyperaccumulation in Sedum alfredii. Chemosphere 2021, 268, 129339. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.C.; Gu, Y.Q.; Puiu, D.; Wang, H.; Twardziok, S.O.; Deal, K.R.; Huo, N.; Zhu, T.; Wang, L.; Wang, Y.; et al. Genome sequence of the progenitor of the wheat D genome Aegilops tauschii. Nature 2017, 551, 498–502. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Aghaei, M.J.; Ahmadi, K.; Naghibi, A. Climate-change-driven shifts in Aegilops tauschii species distribution: Implications for food security and ecological conservation. Diversity 2024, 16, 241. [Google Scholar] [CrossRef]
- De Vries, S.; Hoge, H.; Bisseling, T. Isolation of Total and Polysomal RNA from Plant Tissues. In Plant Molecular Biology Manual; Gelvin, S.B., Schilperoort, R.A., Verma, D.P.S., Eds.; Springer: Dordrecht, The Netherlands, 1989; pp. 323–335. ISBN 978-94-010-6918-2. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Renu, K.; Chakraborty, R.; Myakala, H.; Koti, R.; Famurewa, A.C.; Madhyastha, H.; Vellingiri, B.; George, A.; Valsala, G.A. Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium)—Induced hepatotoxicity—A review. Chemosphere 2021, 271, 129735. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, K.E.; Gerwing, P.D.; Greenberg, B.M. Opinion: Taking phytoremediation from proven technology to accepted practice. Plant Sci. 2017, 256, 170–185. [Google Scholar] [CrossRef]
- Kamar, V.; Dagalp, R.; Tastekin, M. Determination of heavy metals in almonds and mistletoe as a parasite growing on the almond tree using ICP-OES or ICP-MS. Biol. Trace Elem. Res. 2018, 185, 226–235. [Google Scholar] [CrossRef]
- Komal, T.; Mustafa, M.; Ali, Z.; Kazi, A.G. Heavy Metal Uptake and Transport in Plants. In Heavy Metal Contamination of Soils; Sherameti, I., Varma, A., Eds.; Soil Biology; Springer International Publishing: Cham, Switzerland, 2015; Volume 44, pp. 181–194. ISBN 978-3-319-14525-9. [Google Scholar]
- Hurbain, J.; Thommen, Q.; Anquez, F.; Pfeuty, B. Quantitative modeling of pentose phosphate pathway response to oxidative stress reveals a cooperative regulatory strategy. Iscience 2022, 25, 104681. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Kandhol, N.; Sharma, S.; Singh, V.P.; Chauhan, D.K.; Ludwig-Muller, J.; Corpas, F.J.; Tripathi, D.K. Auxin crosstalk with reactive oxygen and nitrogen species in plant development and abiotic stress. Plant Cell Physiol. 2023, 63, 1814–1825. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Siddique, K.; Ahmad, P. Understanding drought tolerance in plants. Physiol. Plant 2021, 172, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, M.; Tahmasebi-Sarvestani, Z.; Emam, Y.; Mokhtassi-Bidgoli, A. Physiological and antioxidant responses of winter wheat cultivars to strigolactone and salicylic acid in drought. Plant Physiol. Biochem. 2017, 119, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; Rehman, S.U.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef]
- Labidi, O.; Vives-Peris, V.; Gomez-Cadenas, A.; Perez-Clemente, R.M.; Sleimi, N. Assessing of growth, antioxidant enzymes, and phytohormone regulation in Cucurbita pepo under cadmium stress. Food Sci. Nutr. 2021, 9, 2021–2031. [Google Scholar] [CrossRef]
- Morel, M.; Crouzet, J.; Gravot, A.; Auroy, P.; Leonhardt, N.; Vavasseur, A.; Richaud, P. AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol. 2009, 149, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, P.; Wang, P.; Yang, M.; Lian, X.; Tang, Z.; Huang, C.F.; Salt, D.E.; Zhao, F.J. A loss-of-function allele of OsHMA3 associated with high cadmium accumulation in shoots and grain of Japonica rice cultivars. Plant Cell Environ. 2016, 39, 1941–1954. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E.; Mills, R.F. P1B-ATPases—An Ancient Family of Transition Metal Pumps with Diverse Functions in Plants. Trends Plant Sci. 2005, 10, 491–502. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Takahashi, R.; Bashir, K.; Shimo, H.; Senoura, T.; Sugimoto, K.; Ono, K.; Yano, M.; Ishikawa, S.; Arao, T.; et al. Characterizing the role of rice NRAMP5 in Manganese, Iron and Cadmium Transport. Sci. Rep. 2012, 2, 286. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Wang, M.; Zhang, T.; Liu, X.; Jiang, H.; Sun, Y.; Cheng, X.; Yan, Q. Enhanced cadmium accumulation and tolerance in transgenic hairy roots of Solanum nigrum L. expressing Iron-Regulated Transporter gene IRT1. Life 2020, 10, 324. [Google Scholar] [CrossRef]
- Xu, Z.R.; You, T.T.; Liu, W.Y.; Ye, K.; Zhao, F.J.; Wang, P. Mitigating cadmium accumulation in dicotyledonous vegetables by iron fertilizer through inhibiting Fe transporter IRT1-mediated Cd uptake. Chemosphere 2024, 346, 140559. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Su, N.; Yue, X.; Fang, B.; Zou, J.; Chen, Y.; Shen, Z.; Cui, J. IRT1 and ZIP2 were involved in exogenous hydrogen-rich water-reduced cadmium accumulation in Brassica chinensis and Arabidopsis thaliana. J. Hazard. Mater. 2021, 407, 124599. [Google Scholar] [CrossRef]
- Meng, Y.; Huang, J.; Jing, H.; Wu, Q.; Shen, R.; Zhu, X. Exogenous abscisic acid alleviates Cd toxicity in Arabidopsis thaliana by inhibiting Cd uptake, translocation and accumulation, and promoting Cd chelation and efflux. Plant Sci. 2022, 325, 111464. [Google Scholar] [CrossRef]
- Shafiq, S.; Ali, A.; Sajjad, Y.; Zeb, Q.; Shahzad, M.; Khan, A.R.; Nazir, R.; Widemann, E. The interplay between toxic and essential metals for their uptake and translocation is likely governed by DNA methylation and histone deacetylation in maize. Int. J. Mol. Sci. 2020, 21, 6959. [Google Scholar] [CrossRef]
- Bucker-Neto, L.; Paiva, A.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, C.; Liu, S.; Xie, Z.; Chang, H.; Wu, T. Phytohormones-Mediated Strategies for Mitigation of Heavy Metals Toxicity in Plants Focused on Sustainable Production. Plant Cell Rep. 2024, 43, 99. [Google Scholar] [CrossRef]
- Bilal, S.; Saad, J.S.; Shahid, M.; Asaf, S.; Khan, A.L.; Lubna; Al-Rawahi, A.; Lee, I.J.; Al-Harrasi, A. Novel insights into exogenous phytohormones: Central regulators in the modulation of physiological, biochemical, and molecular responses in rice under metal(loid) stress. Metabolites 2023, 13, 1036. [Google Scholar] [CrossRef]
- Dai, Z.; Guan, D.; Bundschuh, J.; Ma, L.Q. Roles of phytohormones in mitigating abiotic stress in plants induced by metal(loid)s As, Cd, Cr, Hg, and Pb. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1310–1330. [Google Scholar] [CrossRef]
- Großkinsky, D.K.; Van Der Graaff, E.; Roitsch, T. Regulation of Abiotic and Biotic Stress Responses by Plant Hormones. In Plant Pathogen Resistance Biotechnology; Collinge, D.B., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 131–154. ISBN 978-1-118-86776-1. [Google Scholar]
- Gong, Q.; Li, Z.H.; Wang, L.; Zhou, J.Y.; Kang, Q.; Niu, D.D. Gibberellic acid application on biomass, oxidative stress response, and photosynthesis in spinach (Spinacia oleracea L.) seedlings under copper stress. Environ. Sci. Pollut. Res. Int. 2021, 28, 53594–53604. [Google Scholar] [CrossRef] [PubMed]
- Javed, T.; Ali, M.M.; Shabbir, R.; Anwar, R.; Afzal, I.; Mauro, R.P. Alleviation of copper-induced stress in pea (Pisum sativum L.) through foliar application of gibberellic acid. Biology 2021, 10, 120. [Google Scholar] [CrossRef]
- Gangwar, S.; Singh, V.P.; Srivastava, P.K.; Maurya, J.N. Modification of chromium (VI) phytotoxicity by exogenous gibberellic acid application in Pisum sativum (L.) seedlings. Acta Physiol. Plant 2011, 33, 1385–1397. [Google Scholar] [CrossRef]
- Uzal, O.; Yasar, F. Effects of GA3 hormone treatments on ion uptake and growth of pepper plants under cadmium stress. Appl. Ecol. Environ. Res. 2017, 15, 1347–1357. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Ahanger, M.A.; Alamri, S.A. Jasmonic acid alleviates negative impacts of cadmium stress by modifying osmolytes and antioxidants in faba bean (Vicia faba L.). Arch. Agron. Soil Sci. 2017, 63, 1889–1899. [Google Scholar] [CrossRef]
- Ali, E.; Hussain, N.; Shamsi, I.H.; Jabeen, Z.; Siddiqui, M.H.; Jiang, L.X. Role of jasmonic acid in improving tolerance of rapeseed (Brassica napus L.) to Cd toxicity. J. Zhejiang Univ. Sci. B 2018, 19, 130–146. [Google Scholar] [CrossRef]
- Manzoor, H.; Mehwish; Bukhat, S.; Rasul, S.; Rehmani, M.; Noreen, S.; Athar, H.U.; Zafar, Z.U.; Skalicky, M.; Soufan, W.; et al. Methyl jasmonate alleviated the adverse effects of cadmium stress in pea (Pisum sativum L.): A nexus of photosystem II activity and dynamics of redox balance. Front. Plant Sci. 2022, 13, 860664. [Google Scholar] [CrossRef] [PubMed]
- Bruno, L.; Pacenza, M.; Forgione, I.; Lamerton, L.R.; Greco, M.; Chiappetta, A.; Bitonti, M.B. In Arabidopsis thaliana cadmium impact on the growth of primary root by altering SCR expression and auxin-cytokinin cross-talk. Front. Plant Sci. 2017, 8, 1323. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Danish, M.; Saleem, M.H.; Malik, Z.; Parveen, A.; Abbasi, G.H.; Jamil, M.; Ali, S.; Afzal, S.; Riaz, M.; et al. Application of abscisic acid and 6-benzylaminopurine modulated morpho-physiological and antioxidative defense responses of tomato (Solanum lycopersicum L.) by minimizing cobalt uptake. Chemosphere 2021, 263, 128169. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Hu, X.; Zhou, L.; Ye, L.; Zeng, T.; Du, X.; Gu, L.; Zhu, B.; Zhang, Y.; Wang, H. Ectopic Expression of AetPGL from Aegilops tauschii Enhances Cadmium Tolerance and Accumulation Capacity in Arabidopsis thaliana. Plants 2024, 13, 2370. https://doi.org/10.3390/plants13172370
Yu J, Hu X, Zhou L, Ye L, Zeng T, Du X, Gu L, Zhu B, Zhang Y, Wang H. Ectopic Expression of AetPGL from Aegilops tauschii Enhances Cadmium Tolerance and Accumulation Capacity in Arabidopsis thaliana. Plants. 2024; 13(17):2370. https://doi.org/10.3390/plants13172370
Chicago/Turabian StyleYu, Junxing, Xiaopan Hu, Lizhou Zhou, Lvlan Ye, Tuo Zeng, Xuye Du, Lei Gu, Bin Zhu, Yingying Zhang, and Hongcheng Wang. 2024. "Ectopic Expression of AetPGL from Aegilops tauschii Enhances Cadmium Tolerance and Accumulation Capacity in Arabidopsis thaliana" Plants 13, no. 17: 2370. https://doi.org/10.3390/plants13172370
APA StyleYu, J., Hu, X., Zhou, L., Ye, L., Zeng, T., Du, X., Gu, L., Zhu, B., Zhang, Y., & Wang, H. (2024). Ectopic Expression of AetPGL from Aegilops tauschii Enhances Cadmium Tolerance and Accumulation Capacity in Arabidopsis thaliana. Plants, 13(17), 2370. https://doi.org/10.3390/plants13172370








