How Do Plant Growth-Promoting Bacteria Use Plant Hormones to Regulate Stress Reactions?
Abstract
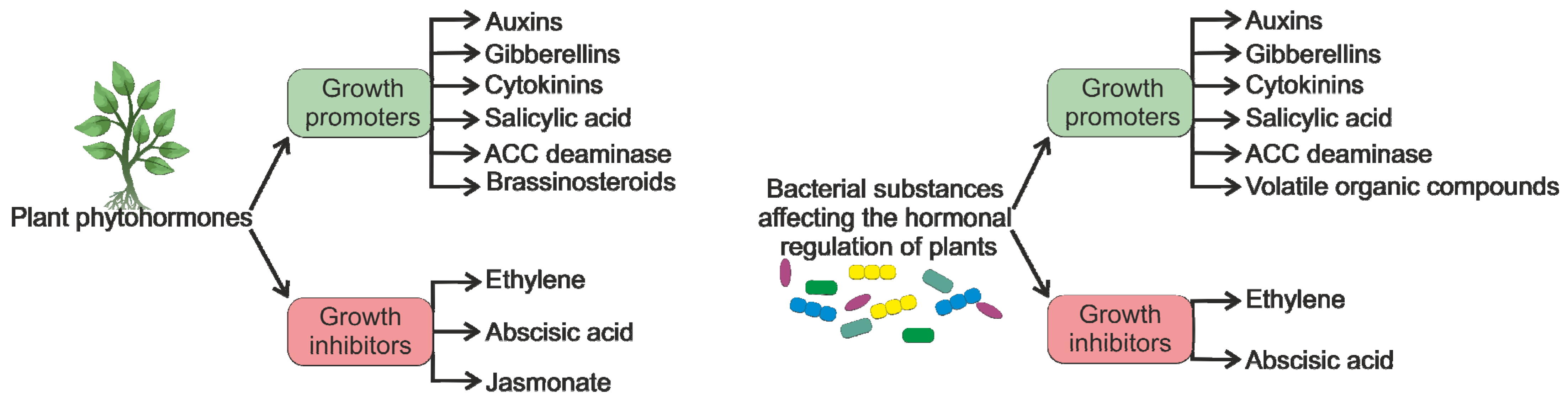
:1. Introduction
2. PGPB and Phytohormones in the Rhizosphere
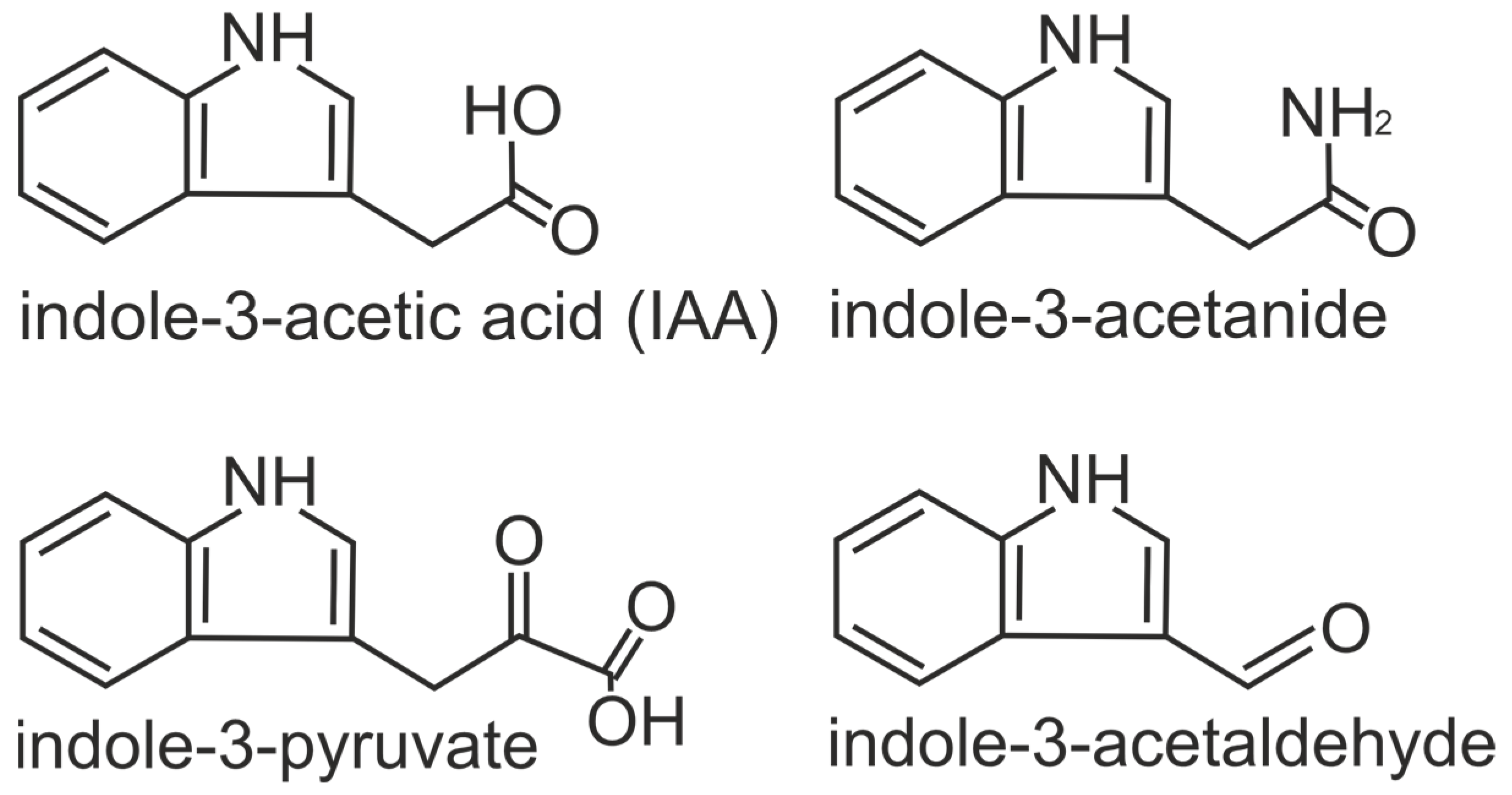
2.1. Auxins
2.2. Cytokinins
2.3. Gibberellins
2.4. Salicylic Acid
2.5. Abscisic Acid
2.6. Volatile Organic Compounds
2.7. Ethylene and ACC Deaminase
3. Synergistic Effects of PGPB on Plant Growth through the Interaction of Multiple Pathways
3.1. Effect of IAA on ACC Deaminase and Ethylene Synthesis
3.2. Interactions among Phytohormones
4. Strategies for Assessing the Ability of PGPB to Synthesize Phytohormones
4.1. Determination of the Potential for IAA Synthesis
4.2. Detection of ACC Deaminase Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cui, M.; Guo, Y.; Chen, J. Influence of Transfer Plot Area and Location on Chemical Input Reduction in Agricultural Production: Evidence from China. Agriculture 2023, 13, 1794. [Google Scholar] [CrossRef]
- Devi, P.I.; Manjula, M.; Bhavani, R.V. Agrochemicals, Environment, and Human Health. Annu. Rev. Environ. Resour. 2022, 47, 399–421. [Google Scholar] [CrossRef]
- Akinnawo, S.O. Eutrophication: Causes, Consequences, Physical, Chemical and Biological Techniques for Mitigation Strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Chataut, G.; Bhatta, B.; Joshi, D.; Subedi, K.; Kafle, K. Greenhouse Gases Emission from Agricultural Soil: A Review. J. Agric. Food Res. 2023, 11, 100533. [Google Scholar] [CrossRef]
- Ortiz-Monasterio, J.I.; Raun, W. Paper Presented At International Workshop On Increasing Wheat Yield Potential, Cimmyt, Obregon, Mexico, 20–24 MARCH 2006 Reduced Nitrogen and Improved Farm Income for Irrigated Spring Wheat in the Yaqui Valley, Mexico, Using Sensor Based Nitrogen Manageme. J. Agric. Sci. 2007, 145, 215–222. [Google Scholar] [CrossRef]
- Majeed, A.; Abbasi, M.K.; Hameed, S.; Imran, A.; Rahim, N. Isolation and Characterization of Plant Growth-Promoting Rhizobacteria from Wheat Rhizosphere and Their Effect on Plant Growth Promotion. Front. Microbiol. 2015, 6, 198. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant Growth-Promoting Soil Bacteria: Nitrogen Fixation, Phosphate Solubilization, Siderophore Production, and Other Biological Activities. Plants 2023, 12, 4074. [Google Scholar] [CrossRef]
- Montoya-Martínez, A.C.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Beneficial Microorganisms in Sustainable Agriculture: Harnessing Microbes’ Potential to Help Feed the World. Plants 2022, 11, 372. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Bacterial Siderophores: Classification, Biosynthesis, Perspectives of Use in Agriculture. Plants 2022, 11, 3065. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef]
- Gurska, J.; Glick, B.R.; Greenberg, B.M. Gene Expression of Secale Cereale (Fall Rye) Grown in Petroleum Hydrocarbon (PHC) Impacted Soil With and Without Plant Growth-Promoting Rhizobacteria (PGPR), Pseudomonas putida. Water Air Soil Pollut. 2015, 226, 308. [Google Scholar] [CrossRef]
- Chet, I.; Inbar, J. Biological Control of Fungal Pathogens. Appl. Biochem. Biotechnol. 1994, 48, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Penrose, D.M.; Glick, B.R. Methods for Isolating and Characterizing ACC Deaminase-containing Plant Growth-promoting Rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Asif, R.; Yasmin, R.; Mustafa, M.; Ambreen, A.; Mazhar, M.; Rehman, A.; Umbreen, S.; Ahmad, M. Phytohormones as Plant Growth Regulators and Safe Protectors against Biotic and Abiotic Stress. In Plant Hormones—Recent Advances, New Perspectives and Applications; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and Beneficial Microbes: Essential Components for Plants to Balance Stress and Fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.V.; Zygadlo, J.; Giordano, W.; Banchio, E. Volatile Organic Compounds from Rhizobacteria Increase Biosynthesis of Essential Oils and Growth Parameters in Peppermint (Mentha piperita). Plant Physiol. Biochem. 2011, 49, 1177–1182. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Glick, B.R.; Rossi, M.J. Multiple Plant Hormone Catabolism Activities: An Adaptation to a Plant-associated Lifestyle by Achromobacter Spp. Environ. Microbiol. Rep. 2021, 13, 533–539. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.I.; Oves, M. Bacteria and Fungi Can Contribute to Nutrients Bioavailability and Aggregate Formation in Degraded Soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef]
- Novo, L.A.B.; Mahler, C.F.; González, L. Plants to Harvest Rhenium: Scientific and Economic Viability. Environ. Chem. Lett. 2015, 13, 439–445. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying Mechanics of Plant Growth Promoting Rhizobacteria (PGPR): A Review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Evseeva, N.V.; Tkachenko, O.V.; Burygin, G.L.; Vysotskaya, L.B.; Akhtyamova, Z.A.; Kudoyarova, G.R. Rhizobacteria Inoculation Effects on Phytohormone Status of Potato Microclones Cultivated In Vitro under Osmotic Stress. Biomolecules 2020, 10, 1231. [Google Scholar] [CrossRef]
- Pankievicz, V.C.S.; do Amaral, F.P.; Ané, J.-M.; Stacey, G. Diazotrophic Bacteria and Their Mechanisms to Interact and Benefit Cereals. Mol. Plant-Microbe Interact. 2021, 34, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, C.; Oliveira, R.S.; Freitas, H.; Luo, Y. Bioaugmentation with Endophytic Bacterium E6S Homologous to Achromobacter piechaudii Enhances Metal Rhizoaccumulation in Host Sedum plumbizincicola. Front. Plant Sci. 2016, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and Molecular Mechanisms of Plant-Microbe-Metal Interactions: Relevance for Phytoremediation. Front. Plant Sci. 2016, 7, 918. [Google Scholar] [CrossRef]
- Spence, C.; Bais, H. Role of Plant Growth Regulators as Chemical Signals in Plant–Microbe Interactions: A Double Edged Sword. Curr. Opin. Plant Biol. 2015, 27, 52–58. [Google Scholar] [CrossRef]
- Dodd, I.C.; Zinovkina, N.Y.; Safronova, V.I.; Belimov, A.A. Rhizobacterial Mediation of Plant Hormone Status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Taylor, J.L.; Zaharia, L.I.; Chen, H.; Anderson, E.; Abrams, S.R. Biotransformation of Adenine and Cytokinins by the Rhizobacterium Serratia proteamaculans. Phytochemistry 2006, 67, 1887–1894. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Khan, N. Climate Change and Salinity Effects on Crops and Chemical Communication Between Plants and Plant Growth-Promoting Microorganisms Under Stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Yen, K.-M.; Serdar, C.M.; Gunsalus, I.C. Genetics of Naphthalene Catabolism in Pseudomonads. CRC Crit. Rev. Microbiol. 1988, 15, 247–268. [Google Scholar] [CrossRef]
- Sazonova, O.I.; Izmalkova, T.Y.; Kosheleva, I.A.; Boronin, A.M. Salicylate Degradation by Pseudomonas putida Strains Not Involving the “Classical” Nah2 Operon. Microbiology 2008, 77, 710–716. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Glick, B.R.; Rossi, M.J. Isolation and Characterization of Novel Soil- and Plant-Associated Bacteria with Multiple Phytohormone-Degrading Activities Using a Targeted Methodology. Access Microbiol. 2019, 1, e000053. [Google Scholar] [CrossRef] [PubMed]
- Fukami, J.; Nogueira, M.A.; Araujo, R.S.; Hungria, M. Accessing Inoculation Methods of Maize and Wheat with Azospirillum brasilense. AMB Express 2016, 6, 3. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Ansari, M.W.; Pradhan, M.; Dangar, T.K.; Mohanty, S.; Tuteja, N. Phenotypic and Molecular Characterization of Native Azospirillum Strains from Rice Fields to Improve Crop Productivity. Protoplasma 2014, 251, 943–953. [Google Scholar] [CrossRef]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits That Go Far beyond Biological Nitrogen Fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.C.; Bottini, R.; Piccoli, P.N. Azospirillum brasilense Sp 245 Produces ABA in Chemically-Defined Culture Medium and Increases ABA Content in Arabidopsis Plants. Plant Growth Regul. 2008, 54, 97–103. [Google Scholar] [CrossRef]
- Weijers, D.; Wagner, D. Transcriptional Responses to the Auxin Hormone. Annu. Rev. Plant Biol. 2016, 67, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-Acetic Acid in Microbial and Microorganism-Plant Signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Korasick, D.A.; Enders, T.A.; Strader, L.C. Auxin Biosynthesis and Storage Forms. J. Exp. Bot. 2013, 64, 2541–2555. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Bacterial Biosynthesis of Indole-3-Acetic Acid. Can. J. Microbiol. 1996, 42, 207–220. [Google Scholar] [CrossRef]
- Khalid, A.; Tahir, S.; Arshad, M.; Zahir, Z.A. Relative Efficiency of Rhizobacteria for Auxin Biosynthesis in Rhizosphere and Non-Rhizosphere Soils. Soil Res. 2004, 42, 921. [Google Scholar] [CrossRef]
- Cassán, F.; Coniglio, A.; López, G.; Molina, R.; Nievas, S.; de Carlan, C.L.N.; Donadio, F.; Torres, D.; Rosas, S.; Pedrosa, F.O.; et al. Everything You Must Know about Azospirillum and Its Impact on Agriculture and Beyond. Biol. Fertil. Soils 2020, 56, 461–479. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin and Plant-Microbe Interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef]
- Zúñiga, A.; Poupin, M.J.; Donoso, R.; Ledger, T.; Guiliani, N.; Gutiérrez, R.A.; González, B. Quorum Sensing and Indole-3-Acetic Acid Degradation Play a Role in Colonization and Plant Growth Promotion of Arabidopsis thaliana by Burkholderia Phytofirmans PsJN. Mol. Plant-Microbe Interact. 2013, 26, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Leveau, J.H.J.; Gerards, S. Discovery of a Bacterial Gene Cluster for Catabolism of the Plant Hormone Indole 3-Acetic Acid. FEMS Microbiol. Ecol. 2008, 65, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Calatrava, V.; Hom, E.F.Y.; Guan, Q.; Llamas, A.; Fernández, E.; Galván, A. Genetic Evidence for Algal Auxin Production in Chlamydomonas and Its Role in Algal-Bacterial Mutualism. iScience 2024, 27, 108762. [Google Scholar] [CrossRef] [PubMed]
- Calatrava, V.; Hom, E.F.Y.; Llamas, Á.; Fernández, E.; Galván, A. OK, Thanks! A New Mutualism between Chlamydomonas and Methylobacteria Facilitates Growth on Amino Acids and Peptides. FEMS Microbiol. Lett. 2018, 365, fny021. [Google Scholar] [CrossRef]
- Olesen, M.R.; Jochimsen, B.U. Identification of Enzymes Involved in Indole-3-Acetic Acid Degradation. Plant Soil 1996, 186, 143–149. [Google Scholar] [CrossRef]
- Mole, B.M.; Baltrus, D.A.; Dangl, J.L.; Grant, S.R. Global Virulence Regulation Networks in Phytopathogenic Bacteria. Trends Microbiol. 2007, 15, 363–371. [Google Scholar] [CrossRef]
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-Acetic Acid in Plant–Microbe Interactions. Antonie Van Leeuwenhoek 2014, 106, 85–125. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone Mediation of Interactions Between Plants and Non-Symbiotic Growth Promoting Bacteria Under Edaphic Stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Keswani, C.; Singh, S.P.; García-Estrada, C.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Rajput, V.D.; Minkina, T.M.; Ortiz, A.; Sansinenea, E. Biosynthesis and Beneficial Effects of Microbial Gibberellins on Crops for Sustainable Agriculture. J. Appl. Microbiol. 2022, 132, 1597–1615. [Google Scholar] [CrossRef] [PubMed]
- Morales-Cedeño, L.R.; Orozco-Mosqueda, M.d.C.; Loeza-Lara, P.D.; Parra-Cota, F.I.; de los Santos-Villalobos, S.; Santoyo, G. Plant Growth-Promoting Bacterial Endophytes as Biocontrol Agents of Pre- and Post-Harvest Diseases: Fundamentals, Methods of Application and Future Perspectives. Microbiol. Res. 2021, 242, 126612. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.; Defez, R. Medicago truncatula Improves Salt Tolerance When Nodulated by an Indole-3-Acetic Acid-Overproducing Sinorhizobium meliloti Strain. J. Exp. Bot. 2009, 60, 3097–3107. [Google Scholar] [CrossRef]
- Pii, Y.; Crimi, M.; Cremonese, G.; Spena, A.; Pandolfini, T. Auxin and Nitric Oxide Control Indeterminate Nodule Formation. BMC Plant Biol. 2007, 7, 21. [Google Scholar] [CrossRef]
- Camerini, S.; Senatore, B.; Lonardo, E.; Imperlini, E.; Bianco, C.; Moschetti, G.; Rotino, G.L.; Campion, B.; Defez, R. Introduction of a Novel Pathway for IAA Biosynthesis to Rhizobia Alters Vetch Root Nodule Development. Arch. Microbiol. 2008, 190, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, E.; Lerner, A.; Mirza, M.S.; El Zemrany, H.; Prigent-Combaret, C.; Jurkevich, E.; Spaepen, S.; Vanderleyden, J.; Nazaret, S.; Okon, Y.; et al. Effects of Azospirillum brasilense with Genetically Modified Auxin Biosynthesis Gene IpdC upon the Diversity of the Indigenous Microbiota of the Wheat Rhizosphere. Res. Microbiol. 2010, 161, 219–226. [Google Scholar] [CrossRef]
- Salomé, P.A.; Merchant, S.S. A Series of Fortunate Events: Introducing Chlamydomonas as a Reference Organism. Plant Cell 2019, 31, 1682–1707. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, M.-Y.; Khan, N.; Tan, L.-L.; Yang, S. Potentials, Utilization, and Bioengineering of Plant Growth-Promoting Methylobacterium for Sustainable Agriculture. Sustainability 2021, 13, 3941. [Google Scholar] [CrossRef]
- Calatrava, V.; Hom, E.F.Y.; Llamas, Á.; Fernández, E.; Galván, A. Nitrogen Scavenging from Amino Acids and Peptides in the Model Alga Chlamydomonas reinhardtii. The Role of Extracellular l-Amino Oxidase. Algal Res. 2019, 38, 101395. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin Biosynthesis: A Simple Two-Step Pathway Converts Tryptophan to Indole-3-Acetic Acid in Plants. Mol. Plant 2012, 5, 334–338. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, L.; Liao, Y.; Zhou, Y.; Xu, X.; Dong, F.; Yang, Z. An Alternative Pathway for the Formation of Aromatic Aroma Compounds Derived from L-Phenylalanine via Phenylpyruvic Acid in Tea (Camellia sinensis (L.) O. Kuntze) Leaves. Food Chem. 2019, 270, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Osugi, A.; Sakakibara, H. Q&A: How Do Plants Respond to Cytokinins and What Is Their Importance? BMC Biol. 2015, 13, 102. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin Signaling in Plant Development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef] [PubMed]
- Kieber, J.J.; Schaller, G.E. The Perception of Cytokinin: A Story 50 Years in the Making. Plant Physiol. 2010, 154, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda, M.d.C.; Santoyo, G.; Glick, B.R. Recent Advances in the Bacterial Phytohormone Modulation of Plant Growth. Plants 2023, 12, 606. [Google Scholar] [CrossRef]
- Auer, C.A. Cytokinin Inhibition of Arabidopsis Root Growth: An Examination of Genotype, Cytokinin Activity, and N6-Benzyladenine Metabolism. J. Plant Growth Regul. 1996, 15, 201–206. [Google Scholar] [CrossRef]
- Strnad, M. The Aromatic Cytokinins. Physiol. Plant. 1997, 101, 674–688. [Google Scholar] [CrossRef]
- Tarkowská, D.; Doležal, K.; Tarkowski, P.; Åstot, C.; Holub, J.; Fuksová, K.; Schmülling, T.; Sandberg, G.; Strnad, M. Identification of New Aromatic Cytokinins in Arabidopsis thaliana and Populus × Canadensis Leaves by LC-(+)ESI-MS and Capillary Liquid Chromatography/Frit–Fast Atom Bombardment Mass Spectrometry. Physiol. Plant. 2003, 117, 579–590. [Google Scholar] [CrossRef]
- Spíchal, L.; Rakova, N.Y.; Riefler, M.; Mizuno, T.; Romanov, G.A.; Strnad, M.; Schmülling, T. Two Cytokinin Receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, Differ in Their Ligand Specificity in a Bacterial Assay. Plant Cell Physiol. 2004, 45, 1299–1305. [Google Scholar] [CrossRef]
- Hönig, M.; Plíhalová, L.; Husičková, A.; Nisler, J.; Doležal, K. Role of Cytokinins in Senescence, Antioxidant Defence and Photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef]
- Mok, D.W.; Mok, M.C. CYTOKININ METABOLISM AND ACTION. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Lomin, S.N.; Krivosheev, D.M.; Steklov, M.Y.; Arkhipov, D.V.; Osolodkin, D.I.; Schmülling, T.; Romanov, G.A. Plant Membrane Assays with Cytokinin Receptors Underpin the Unique Role of Free Cytokinin Bases as Biologically Active Ligands. J. Exp. Bot. 2015, 66, 1851–1863. [Google Scholar] [CrossRef]
- Li, Y.; Baldauf, S.; Lim, E.-K.; Bowles, D.J. Phylogenetic Analysis of the UDP-Glycosyltransferase Multigene Family of Arabidopsis thaliana. J. Biol. Chem. 2001, 276, 4338–4343. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Piotrowska, A. Conjugates of Auxin and Cytokinin. Phytochemistry 2009, 70, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Hluska, T.; Hlusková, L.; Emery, R.J.N. The Hulks and the Deadpools of the Cytokinin Universe: A Dual Strategy for Cytokinin Production, Translocation, and Signal Transduction. Biomolecules 2021, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Frebort, I.; Kowalska, M.; Hluska, T.; Frebortova, J.; Galuszka, P. Evolution of Cytokinin Biosynthesis and Degradation. J. Exp. Bot. 2011, 62, 2431–2452. [Google Scholar] [CrossRef]
- Wei, X.; Moreno-Hagelsieb, G.; Glick, B.R.; Doxey, A.C. Comparative Analysis of Adenylate Isopentenyl Transferase Genes in Plant Growth-Promoting Bacteria and Plant Pathogenic Bacteria. Heliyon 2023, 9, e13955. [Google Scholar] [CrossRef]
- Nester, E.W.; Gordon, M.P.; Amasino, R.M.; Yanofsky, M.F. Crown Gall: A Molecular and Physiological Analysis. Annu. Rev. Plant Physiol. 1984, 35, 387–413. [Google Scholar] [CrossRef]
- Zboralski, A.; Filion, M. Pseudomonas Spp. Can Help Plants Face Climate Change. Front. Microbiol. 2023, 14, 1198131. [Google Scholar] [CrossRef]
- Frébortová, J.; Greplová, M.; Seidl, M.F.; Heyl, A.; Frébort, I. Biochemical Characterization of Putative Adenylate Dimethylallyltransferase and Cytokinin Dehydrogenase from Nostoc Sp. PCC 7120. PLoS ONE 2015, 10, e0138468. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin Producing Bacteria Enhance Plant Growth in Drying Soil. Plant Soil 2007, 292, 305–315. [Google Scholar] [CrossRef]
- Großkinsky, D.K.; Tafner, R.; Moreno, M.V.; Stenglein, S.A.; García de Salamone, I.E.; Nelson, L.M.; Novák, O.; Strnad, M.; van der Graaff, E.; Roitsch, T. Cytokinin Production by Pseudomonas fluorescens G20-18 Determines Biocontrol Activity against Pseudomonas syringae in Arabidopsis. Sci. Rep. 2016, 6, 23310. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Mekureyaw, M.F.; Pandey, C.; Roitsch, T. Role of Cytokinins for Interactions of Plants With Microbial Pathogens and Pest Insects. Front. Plant Sci. 2020, 10, 1777. [Google Scholar] [CrossRef]
- De Rybel, B.; Mähönen, A.P.; Helariutta, Y.; Weijers, D. Plant Vascular Development: From Early Specification to Differentiation. Nat. Rev. Mol. Cell Biol. 2016, 17, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Brenner, W.G.; Ramireddy, E.; Heyl, A.; Schmülling, T. Gene Regulation by Cytokinin in Arabidopsis. Front. Plant Sci. 2012, 3, 8. [Google Scholar] [CrossRef]
- Aremu, A.O.; Fawole, O.A.; Makunga, N.P.; Masondo, N.A.; Moyo, M.; Buthelezi, N.M.D.; Amoo, S.O.; Spíchal, L.; Doležal, K. Applications of Cytokinins in Horticultural Fruit Crops: Trends and Future Prospects. Biomolecules 2020, 10, 1222. [Google Scholar] [CrossRef]
- AlAli, H.A.; Khalifa, A.; Almalki, M. Plant Growth-Promoting Rhizobacteria from Ocimum basilicum Improve Growth of Phaseolus vulgaris and Abelmoschus esculentus. South African J. Bot. 2021, 139, 200–209. [Google Scholar] [CrossRef]
- Al-Tammar, F.K.; Khalifa, A.Y.Z. Plant Growth Promoting Bacteria Drive Food Security. Brazilian J. Biol. 2022, 82, e267257. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of Plant Response to Salt and Drought Stress and Their Alteration by Rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Veselov, S.U.; Melentiev, A.I.; Martynenko, E.V.; Kudoyarova, G.R. Ability of Bacterium Bacillus subtilis to Produce Cytokinins and to Influence the Growth and Endogenous Hormone Content of Lettuce Plants. Plant Soil 2005, 272, 201–209. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.-L.; Luo, L. Effects of Engineered Sinorhizobium meliloti on Cytokinin Synthesis and Tolerance of Alfalfa to Extreme Drought Stress. Appl. Environ. Microbiol. 2012, 78, 8056–8061. [Google Scholar] [CrossRef]
- Palberg, D.; Kisiała, A.; Jorge, G.L.; Emery, R.J.N. A Survey of Methylobacterium Species and Strains Reveals Widespread Production and Varying Profiles of Cytokinin Phytohormones. BMC Microbiol. 2022, 22, 49. [Google Scholar] [CrossRef] [PubMed]
- Mekureyaw, M.F.; Pandey, C.; Hennessy, R.C.; Nicolaisen, M.H.; Liu, F.; Nybroe, O.; Roitsch, T. The Cytokinin-Producing Plant Beneficial Bacterium Pseudomonas fluorescens G20-18 Primes Tomato (Solanum lycopersicum) for Enhanced Drought Stress Responses. J. Plant Physiol. 2022, 270, 153629. [Google Scholar] [CrossRef] [PubMed]
- Van De Velde, K.; Ruelens, P.; Geuten, K.; Rohde, A.; Van Der Straeten, D. Exploiting DELLA Signaling in Cereals. Trends Plant Sci. 2017, 22, 880–893. [Google Scholar] [CrossRef]
- Zaidi, A.; Ahmad, E.; Khan, M.S.; Saif, S.; Rizvi, A. Role of Plant Growth Promoting Rhizobacteria in Sustainable Production of Vegetables: Current Perspective. Sci. Hortic. (Amsterdam). 2015, 193, 231–239. [Google Scholar] [CrossRef]
- Khan, A.L.; Waqas, M.; Hussain, J.; Al-Harrasi, A.; Hamayun, M.; Lee, I.-J. Phytohormones Enabled Endophytic Fungal Symbiosis Improve Aluminum Phytoextraction in Tolerant Solanum lycopersicum: An Examples of Penicillium janthinellum LK5 and Comparison with Exogenous GA3. J. Hazard. Mater. 2015, 295, 70–78. [Google Scholar] [CrossRef]
- You, Y.-H. Fungal Diversity and Plant Growth Promotion of Endophytic Fungi from Six Halophytes in Suncheon Bay. J. Microbiol. Biotechnol. 2012, 22, 1549–1556. [Google Scholar] [CrossRef]
- Kozaki, A.; Aoyanagi, T. Molecular Aspects of Seed Development Controlled by Gibberellins and Abscisic Acids. Int. J. Mol. Sci. 2022, 23, 1876. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P.; Thomas, S.G. Gibberellin Biosynthesis and Its Regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Nelson, S.K.; Steber, C.M. Gibberellin Hormone Signal Perception: Down-regulating DELLA Repressors of Plant Growth and Development. In Annual Plant Reviews; Wiley: Hoboken, NJ, USA, 2016; Volume 49, pp. 153–188. [Google Scholar]
- Yamaguchi, S. Gibberellin Metabolism and Its Regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, G.; Liu, C.; Li, H.; Chen, X.; Fu, Q.; Lin, Y.; Guo, B. Salicylic Acid, a Multifaceted Hormone, Combats Abiotic Stresses in Plants. Life 2022, 12, 886. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic Acid as a Safe Plant Protector and Growth Regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Rafique, M.; Aftab, M.; Qureshi, M.A.; Javed, H.; Mujeeb, F.; Akhtar, S. Mitigation of Salinity in Chickpea by Plant Growth Promoting Rhizobacteria and Salicylic Acid. EURASIAN J. SOIL Sci. 2019, 8, 221–228. [Google Scholar] [CrossRef]
- Mehrasa, H.; Farnia, A.; Kenarsari, M.J.; Nakhjavan, S. Endophytic Bacteria and SA Application Improve Growth, Biochemical Properties, and Nutrient Uptake in White Beans Under Drought Stress. J. Soil Sci. Plant Nutr. 2022, 22, 3268–3279. [Google Scholar] [CrossRef]
- de Andrade, W.L.; de Melo, A.S.; Melo, Y.L.; da Silva Sá, F.V.; Rocha, M.M.; da Silva Oliveira, A.P.; Fernandes Júnior, P.I. Bradyrhizobium Inoculation Plus Foliar Application of Salicylic Acid Mitigates Water Deficit Effects on Cowpea. J. Plant Growth Regul. 2021, 40, 656–667. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Torrens-Spence, M.P.; Bobokalonova, A.; Carballo, V.; Glinkerman, C.M.; Pluskal, T.; Shen, A.; Weng, J.-K. PBS3 and EPS1 Complete Salicylic Acid Biosynthesis from Isochorismate in Arabidopsis. Mol. Plant 2019, 12, 1577–1586. [Google Scholar] [CrossRef]
- Rekhter, D.; Lüdke, D.; Ding, Y.; Feussner, K.; Zienkiewicz, K.; Lipka, V.; Wiermer, M.; Zhang, Y.; Feussner, I. Isochorismate-Derived Biosynthesis of the Plant Stress Hormone Salicylic Acid. Science (80-.). 2019, 365, 498–502. [Google Scholar] [CrossRef]
- Grobelak, A.; Hiller, J. Bacterial Siderophores Promote Plant Growth: Screening of Catechol and Hydroxamate Siderophores. Int. J. Phytoremediation 2017, 19, 825–833. [Google Scholar] [CrossRef]
- Kjærbølling, I.; Mortensen, U.H.; Vesth, T.; Andersen, M.R. Strategies to Establish the Link between Biosynthetic Gene Clusters and Secondary Metabolites. Fungal Genet. Biol. 2019, 130, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Baek, K.-H. Salicylic Acid Biosynthesis and Metabolism: A Divergent Pathway for Plants and Bacteria. Biomolecules 2021, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Kim, H.U. The Secondary Metabolite Bioinformatics Portal: Computational Tools to Facilitate Synthetic Biology of Secondary Metabolite Production. Synth. Syst. Biotechnol. 2016, 1, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Zeevaart, J.A.D.; Creelman, R.A. Metabolism and Physiology of Abscisic Acid. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 439–473. [Google Scholar] [CrossRef]
- Melcher, K.; Xu, Y.; Ng, L.-M.; Zhou, X.E.; Soon, F.-F.; Chinnusamy, V.; Suino-Powell, K.M.; Kovach, A.; Tham, F.S.; Cutler, S.R.; et al. Identification and Mechanism of ABA Receptor Antagonism. Nat. Struct. Mol. Biol. 2010, 17, 1102–1108. [Google Scholar] [CrossRef]
- Liu, S.; Lv, Z.; Liu, Y.; Li, L.; Zhang, L. Network Analysis of ABA-Dependent and ABA-Independent Drought Responsive Genes in Arabidopsis thaliana. Genet. Mol. Biol. 2018, 41, 624–637. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. ABSCISIC ACID BIOSYNTHESIS AND CATABOLISM. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.; Bressan, R.A.; Song, C.; Zhu, J.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Hartung, W.; Sauter, A.; Turner, N.C.; Fillery, I.; Heilmeier, H. Abscisic Acid in Soils: What Is Its Function and Which Factors and Mechanisms Influence Its Concentration? Plant Soil 1996, 184, 105–110. [Google Scholar] [CrossRef]
- Forchetti, G.; Masciarelli, O.; Alemano, S.; Alvarez, D.; Abdala, G. Endophytic Bacteria in Sunflower (Helianthus annuus L.): Isolation, Characterization, and Production of Jasmonates and Abscisic Acid in Culture Medium. Appl. Microbiol. Biotechnol. 2007, 76, 1145–1152. [Google Scholar] [CrossRef]
- Cohen, A.C.; Travaglia, C.N.; Bottini, R.; Piccoli, P.N. Participation of Abscisic Acid and Gibberellins Produced by Endophytic Azospirillum in the Alleviation of Drought Effects in Maize. Botany 2009, 87, 455–462. [Google Scholar] [CrossRef]
- Sgroy, V.; Cassán, F.; Masciarelli, O.; Del Papa, M.F.; Lagares, A.; Luna, V. Isolation and Characterization of Endophytic Plant Growth-Promoting (PGPB) or Stress Homeostasis-Regulating (PSHB) Bacteria Associated to the Halophyte Prosopis Strombulifera. Appl. Microbiol. Biotechnol. 2009, 85, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Hartung, W. The Evolution of Abscisic Acid (ABA) and ABA Function in Lower Plants, Fungi and Lichen. Funct. Plant Biol. 2010, 37, 806. [Google Scholar] [CrossRef]
- Crocoll, C.; Kettner, J.; Dörffling, K. Abscisic Acid in Saprophytic and Parasitic Species of Fungi. Phytochemistry 1991, 30, 1059–1060. [Google Scholar] [CrossRef]
- Belimov, A.A.; Dodd, I.C.; Safronova, V.I.; Dumova, V.A.; Shaposhnikov, A.I.; Ladatko, A.G.; Davies, W.J. Abscisic Acid Metabolizing Rhizobacteria Decrease ABA Concentrations in Planta and Alter Plant Growth. Plant Physiol. Biochem. 2014, 74, 84–91. [Google Scholar] [CrossRef]
- Zhang, J.; Schurr, U.; Davies, W.J. Control of Stomatal Behaviour by Abscisic Acid Which Apparently Originates in the Roots. J. Exp. Bot. 1987, 38, 1174–1181. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant Growth-Promoting Rhizobacteria and Root System Functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hu, W.; Deng, X.; Ma, Z.; Chen, L.; Huang, C.; Wang, C.; Wang, J.; He, Y.; Yang, G.; et al. Overexpression of the Wheat Aquaporin Gene, TaAQP7, Enhances Drought Tolerance in Transgenic Tobacco. PLoS ONE 2012, 7, e52439. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Poling, S.M.; Maier, V.P.; Bennett, R.D. Metabolism of Abscisic Acid: Bacterial Conversion to Dehydrovomifoliol and Vomifoliol Dehydrogenase Activity. Phytochemistry 1984, 23, 2769–2771. [Google Scholar] [CrossRef]
- Montes-Osuna, N.; Cernava, T.; Gómez-Lama Cabanás, C.; Berg, G.; Mercado-Blanco, J. Identification of Volatile Organic Compounds Emitted by Two Beneficial Endophytic Pseudomonas Strains from Olive Roots. Plants 2022, 11, 318. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.-M.; Zhang, S. Induced Systemic Resistance and Promotion of Plant Growth by Bacillus Spp. Phytopathology® 2004, 94, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Effmert, U.; Piechulla, B. Bacterial-Plant-Interactions: Approaches to Unravel the Biological Function of Bacterial Volatiles in the Rhizosphere. Front. Microbiol. 2016, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Choub, V.; Won, S.-J.; Ajuna, H.B.; Moon, J.-H.; Choi, S.-I.; Lim, H.-I.; Ahn, Y.S. Antifungal Activity of Volatile Organic Compounds from Bacillus velezensis CE 100 against Colletotrichum Gloeosporioides. Horticulturae 2022, 8, 557. [Google Scholar] [CrossRef]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Raza, W.; Hanif, A.; Wu, L.; Colman, M.V.; Gao, X. Plant Growth Promotion by Volatile Organic Compounds Produced by Bacillus subtilis SYST2. Front. Microbiol. 2017, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Venneman, J.; Vandermeersch, L.; Walgraeve, C.; Audenaert, K.; Ameye, M.; Verwaeren, J.; Steppe, K.; Van Langenhove, H.; Haesaert, G.; Vereecke, D. Respiratory CO2 Combined With a Blend of Volatiles Emitted by Endophytic Serendipita Strains Strongly Stimulate Growth of Arabidopsis Implicating Auxin and Cytokinin Signaling. Front. Plant Sci. 2020, 11, 544435. [Google Scholar] [CrossRef]
- Sharifi, R.; Ryu, C.-M. Revisiting Bacterial Volatile-Mediated Plant Growth Promotion: Lessons from the Past and Objectives for the Future. Ann. Bot. 2018, 122, 349–358. [Google Scholar] [CrossRef]
- del Carmen Orozco-Mosqueda, M.; Macías-Rodríguez, L.I.; Santoyo, G.; Farías-Rodríguez, R.; Valencia-Cantero, E. Medicago truncatula Increases Its Iron-Uptake Mechanisms in Response to Volatile Organic Compounds Produced by Sinorhizobium meliloti. Folia Microbiol. (Praha) 2013, 58, 579–585. [Google Scholar] [CrossRef]
- Lemfack, M.C.; Nickel, J.; Dunkel, M.; Preissner, R.; Piechulla, B. MVOC: A Database of Microbial Volatiles. Nucleic Acids Res. 2014, 42, D744–D748. [Google Scholar] [CrossRef]
- Yasmin, H.; Rashid, U.; Hassan, M.N.; Nosheen, A.; Naz, R.; Ilyas, N.; Sajjad, M.; Azmat, A.; Alyemeni, M.N. Volatile Organic Compounds Produced by Pseudomonas pseudoalcaligenes Alleviated Drought Stress by Modulating Defense System in Maize (Zea mays L.). Physiol. Plant. 2021, 172, 896–911. [Google Scholar] [CrossRef]
- Sudha, A.; Durgadevi, D.; Archana, S.; Muthukumar, A.; Suthin Raj, T.; Nakkeeran, S.; Poczai, P.; Nasif, O.; Ansari, M.J.; Sayyed, R.Z. Unraveling the Tripartite Interaction of Volatile Compounds of Streptomyces rochei with Grain Mold Pathogens Infecting Sorghum. Front. Microbiol. 2022, 13, 923360. [Google Scholar] [CrossRef]
- Poveda, J. Beneficial Effects of Microbial Volatile Organic Compounds (MVOCs) in Plants. Appl. Soil Ecol. 2021, 168, 104118. [Google Scholar] [CrossRef]
- Lemfack, M.C.; Gohlke, B.-O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. MVOC 2.0: A Database of Microbial Volatiles. Nucleic Acids Res. 2018, 46, D1261–D1265. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, A.; De Stradis, A.; Lo Cantore, P.; Iacobellis, N.S. Biocide Effects of Volatile Organic Compounds Produced by Potential Biocontrol Rhizobacteria on Sclerotinia sclerotiorum. Front. Microbiol. 2015, 6, 1056. [Google Scholar] [CrossRef] [PubMed]
- Rybakova, D.; Rack-Wetzlinger, U.; Cernava, T.; Schaefer, A.; Schmuck, M.; Berg, G. Aerial Warfare: A Volatile Dialogue between the Plant Pathogen Verticillium longisporum and Its Antagonist Paenibacillus polymyxa. Front. Plant Sci. 2017, 8, 1294. [Google Scholar] [CrossRef]
- Kai, M.; Effmert, U.; Berg, G.; Piechulla, B. Volatiles of Bacterial Antagonists Inhibit Mycelial Growth of the Plant Pathogen Rhizoctonia solani. Arch. Microbiol. 2007, 187, 351–360. [Google Scholar] [CrossRef]
- Rojas-Solís, D.; Zetter-Salmón, E.; Contreras-Pérez, M.; Rocha-Granados, M.d.C.; Macías-Rodríguez, L.; Santoyo, G. Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR71 Endophytes Produce Antifungal Volatile Organic Compounds and Exhibit Additive Plant Growth-Promoting Effects. Biocatal. Agric. Biotechnol. 2018, 13, 46–52. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Verma, R.; Singh, K.; Nisha, N.; Keisham, M.; Bhati, K.K.; Kim, S.T.; Gupta, R. Ethylene: A Master Regulator of Salinity Stress Tolerance in Plants. Biomolecules 2020, 10, 959. [Google Scholar] [CrossRef]
- Burg, S.P.; Burg, E.A. Molecular Requirements for the Biological Activity of Ethylene. Plant Physiol. 1967, 42, 144–152. [Google Scholar] [CrossRef]
- Guzmán, P.; Ecker, J.R. Exploiting the Triple Response of Arabidopsis to Identify Ethylene-Related Mutants. Plant Cell 1990, 2, 513–523. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.C.; Glick, B.R. Amelioration of High Salinity Stress Damage by Plant Growth-Promoting Bacterial Endophytes That Contain ACC Deaminase. Plant Physiol. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef]
- Gómez-Cadenas, A.; Tadeo, F.R.; Primo-Millo, E.; Talon, M. Involvement of Abscisic Acid and Ethylene in the Responses of Citrus Seedlings to Salt Shock. Physiol. Plant. 1998, 103, 475–484. [Google Scholar] [CrossRef]
- Arraes, F.B.M.; Beneventi, M.A.; Lisei de Sa, M.E.; Paixao, J.F.R.; Albuquerque, E.V.S.; Marin, S.R.R.; Purgatto, E.; Nepomuceno, A.L.; Grossi-de-Sa, M.F. Implications of Ethylene Biosynthesis and Signaling in Soybean Drought Stress Tolerance. BMC Plant Biol. 2015, 15, 213. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Min, C.W.; Kim, S.W.; Yoo, J.S.; Moon, A.-R.; Shin, A.-Y.; Kwon, S.-Y.; Kim, S.T. A TMT-Based Quantitative Proteome Analysis to Elucidate the TSWV Induced Signaling Cascade in Susceptible and Resistant Cultivars of Solanum lycopersicum. Plants 2020, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Min, C.W.; Kim, Y.-J.; Kim, S.T. Identification of Msp1-Induced Signaling Components in Rice Leaves by Integrated Proteomic and Phosphoproteomic Analysis. Int. J. Mol. Sci. 2019, 20, 4135. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Modulation of Plant Ethylene Levels by the Bacterial Enzyme ACC Deaminase. FEMS Microbiol. Lett. 2005, 251, 1–7. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Siddikee, M.A.; Chauhan, P.S.; Sa, T. Regulation of Ethylene Biosynthesis Under Salt Stress in Red Pepper (Capsicum annuum L.) by 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Deaminase-Producing Halotolerant Bacteria. J. Plant Growth Regul. 2012, 31, 265–272. [Google Scholar] [CrossRef]
- Ma, W.; Charles, T.C.; Glick, B.R. Expression of an Exogenous 1-Aminocyclopropane-1-Carboxylate Deaminase Gene in Sinorhizobium Meliloti Increases Its Ability To Nodulate Alfalfa. Appl. Environ. Microbiol. 2004, 70, 5891–5897. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Brígido, C.; Glick, B.R.; Oliveira, S. ACC Deaminase Genes Are Conserved among Mesorhizobium Species Able to Nodulate the Same Host Plant. FEMS Microbiol. Lett. 2012, 336, 26–37. [Google Scholar] [CrossRef]
- Ma, W.; Sebestianova, S.B.; Sebestian, J.; Burd, G.I.; Guinel, F.C.; Glick, B.R. Prevalence of 1-Aminocyclopropane-1-Carboxylate Deaminase in Rhizobium Spp. Antonie Van Leeuwenhoek 2003, 83, 285–291. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, Z.; Glick, B.R. The Presence of a 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase Deletion Mutation Alters the Physiology of the Endophytic Plant Growth-Promoting Bacterium Burkholderia Phytofirmans PsJN. FEMS Microbiol. Lett. 2009, 296, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Charles, T.C.; Glick, B.R. Delay of Flower Senescence by Bacterial Endophytes Expressing 1-Aminocyclopropane-1-Carboxylate Deaminase. J. Appl. Microbiol. 2012, 113, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.X.; Rossi, M.J.; Soares, C.R.F.S.; McConkey, B.J.; Glick, B.R. New Insights into 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase Phylogeny, Evolution and Ecological Significance. PLoS ONE 2014, 9, e99168. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Bacteria with ACC Deaminase Can Promote Plant Growth and Help to Feed the World. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Sheehy, R.E.; Honma, M.; Yamada, M.; Sasaki, T.; Martineau, B.; Hiatt, W.R. Isolation, Sequence, and Expression in Escherichia coli of the Pseudomonas Sp. Strain ACP Gene Encoding 1-Aminocyclopropane-1-Carboxylate Deaminase. J. Bacteriol. 1991, 173, 5260–5265. [Google Scholar] [CrossRef]
- Jacobson, C.B.; Pasternak, J.J.; Glick, B.R. Partial Purification and Characterization of 1-Aminocyclopropane-1-Carboxylate Deaminase from the Plant Growth Promoting Rhizobacterium Pseudomonas putida GR12-2. Can. J. Microbiol. 1994, 40, 1019–1025. [Google Scholar] [CrossRef]
- Hontzeas, N.; Zoidakis, J.; Glick, B.R.; Abu-Omar, M.M. Expression and Characterization of 1-Aminocyclopropane-1-Carboxylate Deaminase from the Rhizobacterium Pseudomonas putida UW4: A Key Enzyme in Bacterial Plant Growth Promotion. Biochim. Biophys. Acta Proteins Proteomics 2004, 1703, 11–19. [Google Scholar] [CrossRef]
- Khalifa, A. ACC Deaminase-Containing Rhizobacteria from Rhizosphere of Zygophyllum coccineum Alleviate Salt Stress Impact on Wheat (Triticum aestivum L.). Basic Appl. Sci. Sci. J. King Faisal Univ. 2020, 21, 89–101. [Google Scholar] [CrossRef]
- Ali, S.; Glick, B.R. Plant–Bacterial Interactions in Management of Plant Growth under Abiotic Stresses. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 21–45. [Google Scholar]
- Araya, M.A.; Valenzuela, T.; Inostroza, N.G.; Maruyama, F.; Jorquera, M.A.; Acuña, J.J. Isolation and Characterization of Cold-Tolerant Hyper-ACC-Degrading Bacteria from the Rhizosphere, Endosphere, and Phyllosphere of Antarctic Vascular Plants. Microorganisms 2020, 8, 1788. [Google Scholar] [CrossRef]
- Bal, H.B.; Adhya, T.K. Alleviation of Submergence Stress in Rice Seedlings by Plant Growth-Promoting Rhizobacteria With ACC Deaminase Activity. Front. Sustain. Food Syst. 2021, 5, 606158. [Google Scholar] [CrossRef]
- Cheng, Z.; Park, E.; Glick, B.R. 1-Aminocyclopropane-1-Carboxylate Deaminase from Pseudomonas putida UW4 Facilitates the Growth of Canola in the Presence of Salt. Can. J. Microbiol. 2007, 53, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Belimov, A.A.; Dodd, I.C.; Hontzeas, N.; Theobald, J.C.; Safronova, V.I.; Davies, W.J. Rhizosphere Bacteria Containing 1-aminocyclopropane-1-carboxylate Deaminase Increase Yield of Plants Grown in Drying Soil via Both Local and Systemic Hormone Signalling. New Phytol. 2009, 181, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.S.; Ali, S. Possible Mechanisms for the Equilibrium of ACC and Role of ACC Deaminase-Producing Bacteria. Appl. Microbiol. Biotechnol. 2022, 106, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Guinel, F.C.; Glick, B.R. Rhizobium Leguminosarum Biovar Viciae 1-Aminocyclopropane-1-Carboxylate Deaminase Promotes Nodulation of Pea Plants. Appl. Environ. Microbiol. 2003, 69, 4396–4402. [Google Scholar] [CrossRef]
- Nukui, N.; Minamisawa, K.; Ayabe, S.-I.; Aoki, T. Expression of the 1-Aminocyclopropane-1-Carboxylic Acid Deaminase Gene Requires Symbiotic Nitrogen-Fixing Regulator Gene NifA2 in Mesorhizobium Loti MAFF303099. Appl. Environ. Microbiol. 2006, 72, 4964–4969. [Google Scholar] [CrossRef]
- Alemneh, A.A.; Zhou, Y.; Ryder, M.H.; Denton, M.D. Is Phosphate Solubilizing Ability in Plant Growth-promoting Rhizobacteria Isolated from Chickpea Linked to Their Ability to Produce ACC Deaminase? J. Appl. Microbiol. 2021, 131, 2416–2432. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Bacterial Modulation of Plant Ethylene Levels. Plant Physiol. 2015, 169, 13–22. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Maji, D.; Chanotiya, C.S.; Kalra, A. 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Deaminase-Containing Rhizobacteria Protect Ocimum sanctum Plants during Waterlogging Stress via Reduced Ethylene Generation. Plant Physiol. Biochem. 2012, 58, 227–235. [Google Scholar] [CrossRef]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of Plant Growth by ACC Deaminase-Producing Soil Bacteria. In New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research; Springer Netherlands: Dordrecht, The Netherlands, 2007; pp. 329–339. [Google Scholar]
- Pierik, R.; Tholen, D.; Poorter, H.; Visser, E.J.W.; Voesenek, L.A.C.J. The Janus Face of Ethylene: Growth Inhibition and Stimulation. Trends Plant Sci. 2006, 11, 176–183. [Google Scholar] [CrossRef]
- van Loon, L.C.; Geraats, B.P.J.; Linthorst, H.J.M. Ethylene as a Modulator of Disease Resistance in Plants. Trends Plant Sci. 2006, 11, 184–191. [Google Scholar] [CrossRef]
- Robison, M.M.; Griffith, M.; Pauls, K.P.; Glick, B.R. Dual Role for Ethylene in Susceptibility of Tomato to Verticillium Wilt. J. Phytopathol. 2001, 149, 385–388. [Google Scholar] [CrossRef]
- Glick, B.R.; Penrose, D.M.; Li, J. A Model For the Lowering of Plant Ethylene Concentrations by Plant Growth-Promoting Bacteria. J. Theor. Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef]
- Prayitno, J.; Rolfe, B.G.; Mathesius, U. The Ethylene-Insensitive Sickle Mutant of Medicago Truncatula Shows Altered Auxin Transport Regulation during Nodulation. Plant Physiol. 2006, 142, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Stearns, J.C.; Woody, O.Z.; McConkey, B.J.; Glick, B.R. Effects of Bacterial ACC Deaminase on Brassica Napus Gene Expression. Mol. Plant-Microbe Interact. 2012, 25, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Adie, B.; Chico, J.M.; Rubio-Somoza, I.; Solano, R. Modulation of Plant Defenses by Ethylene. J. Plant Growth Regul. 2007, 26, 160–177. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Navarro, L.; Bari, R.; Jones, J.D. Pathological Hormone Imbalances. Curr. Opin. Plant Biol. 2007, 10, 372–379. [Google Scholar] [CrossRef]
- Cao, H.; Li, X.; Dong, X. Generation of Broad-Spectrum Disease Resistance by Overexpression of an Essential Regulatory Gene in Systemic Acquired Resistance. Proc. Natl. Acad. Sci. USA 1998, 95, 6531–6536. [Google Scholar] [CrossRef]
- Koornneef, A.; Pieterse, C.M.J. Cross Talk in Defense Signaling. Plant Physiol. 2008, 146, 839–844. [Google Scholar] [CrossRef]
- Grant, M.; Lamb, C. Systemic Immunity. Curr. Opin. Plant Biol. 2006, 9, 414–420. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- van Wees, S.C.; Luijendijk, M.; Smoorenburg, I.; van Loon, L.C.; Pieterse, C.M. Rhizobacteria-Mediated Induced Systemic Resistance (ISR) in Arabidopsis Is Not Associated with a Direct Effect on Expression of Known Defense-Related Genes but Stimulates the Expression of the Jasmonate-Inducible Gene Atvsp upon Challenge. Plant Mol. Biol. 1999, 41, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; van Wees, S.C.M.; van Pelt, J.A.; Knoester, M.; Laan, R.; Gerrits, H.; Weisbeek, P.J.; van Loon, L.C. A Novel Signaling Pathway Controlling Induced Systemic Resistance in Arabidopsis. Plant Cell 1998, 10, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Ahn, I.-P.; Lee, S.-W.; Suh, S.-C. Rhizobacteria-Induced Priming in Arabidopsis Is Dependent on Ethylene, Jasmonic Acid, and NPR1. Mol. Plant-Microbe Interact. 2007, 20, 759–768. [Google Scholar] [CrossRef]
- Tran, H.; Ficke, A.; Asiimwe, T.; Höfte, M.; Raaijmakers, J.M. Role of the Cyclic Lipopeptide Massetolide A in Biological Control of Phytophthora Infestans and in Colonization of Tomato Plants by Pseudomonas fluorescens. New Phytol. 2007, 175, 731–742. [Google Scholar] [CrossRef]
- Verhagen, B.W.M.; Glazebrook, J.; Zhu, T.; Chang, H.-S.; van Loon, L.C.; Pieterse, C.M.J. The Transcriptome of Rhizobacteria-Induced Systemic Resistance in Arabidopsis. Mol. Plant-Microbe Interact. 2004, 17, 895–908. [Google Scholar] [CrossRef]
- Cartieaux, F.; Contesto, C.; Gallou, A.; Desbrosses, G.; Kopka, J.; Taconnat, L.; Renou, J.-P.; Touraine, B. Simultaneous Interaction of Arabidopsis thaliana with Bradyrhizobium Sp. Strain ORS278 and Pseudomonas syringae Pv. Tomato DC3000 Leads to Complex Transcriptome Changes. Mol. Plant-Microbe Interact. 2008, 21, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Reddy, M.S.; Ryu, C.-M.; McInroy, J.A.; Wilson, M.; Kloepper, J.W. Induced Systemic Protection Against Tomato Late Blight Elicited by Plant Growth-Promoting Rhizobacteria. Phytopathology® 2002, 92, 1329–1333. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Djavaheri, M.; Bakker, P.A.H.M.; Höfte, M. Pseudomonas Fluorescens WCS374r-Induced Systemic Resistance in Rice against Magnaporthe Oryzae Is Based on Pseudobactin-Mediated Priming for a Salicylic Acid-Repressible Multifaceted Defense Response. Plant Physiol. 2008, 148, 1996–2012. [Google Scholar] [CrossRef]
- Segarra, G.; Van der Ent, S.; Trillas, I.; Pieterse, C.M.J. MYB72, a Node of Convergence in Induced Systemic Resistance Triggered by a Fungal and a Bacterial Beneficial Microbe. Plant Biol. 2009, 11, 90–96. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting Mechanisms of Defense Against Biotrophic and Necrotrophic Pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Van der Ent, S.; Van Wees, S.C.M.; Pieterse, C.M.J. Jasmonate Signaling in Plant Interactions with Resistance-Inducing Beneficial Microbes. Phytochemistry 2009, 70, 1581–1588. [Google Scholar] [CrossRef]
- Glick, B.R.; Jacobson, C.B.; Schwarze, M.M.K.; Pasternak, J.J. 1-Aminocyclopropane-1-Carboxylic Acid Deaminase Mutants of the Plant Growth Promoting Rhizobacterium Pseudomonas putida GR12-2 Do Not Stimulate Canola Root Elongation. Can. J. Microbiol. 1994, 40, 911–915. [Google Scholar] [CrossRef]
- Cheng, Z.; Duncker, B.P.; McConkey, B.J.; Glick, B.R. Transcriptional Regulation of ACC Deaminase Gene Expression in Pseudomonas putida UW4. Can. J. Microbiol. 2008, 54, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Müller, K.M.; Charles, T.C.; Vesely, S.; Glick, B.R. 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase Genes in Rhizobia from Southern Saskatchewan. Microb. Ecol. 2009, 57, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Bouffaud, M.-L.; Renoud, S.; Dubost, A.; Moënne-Loccoz, Y.; Muller, D. 1-Aminocyclopropane-1-Carboxylate Deaminase Producers Associated to Maize and Other Poaceae Species. Microbiome 2018, 6, 114. [Google Scholar] [CrossRef]
- Jha, B.; Gontia, I.; Hartmann, A. The Roots of the Halophyte Salicornia brachiata Are a Source of New Halotolerant Diazotrophic Bacteria with Plant Growth-Promoting Potential. Plant Soil 2012, 356, 265–277. [Google Scholar] [CrossRef]
- Sorty, A.M.; Ntana, F.; Hansen, M.; Stougaard, P. Plant-Root Exudate Analogues Influence Activity of the 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase Gene in Pseudomonas hormoni G20-18T. Microorganisms 2023, 11, 2504. [Google Scholar] [CrossRef]
- Vega-Celedón, P.; Bravo, G.; Velásquez, A.; Cid, F.P.; Valenzuela, M.; Ramírez, I.; Vasconez, I.-N.; Álvarez, I.; Jorquera, M.A.; Seeger, M. Microbial Diversity of Psychrotolerant Bacteria Isolated from Wild Flora of Andes Mountains and Patagonia of Chile towards the Selection of Plant Growth-Promoting Bacterial Consortia to Alleviate Cold Stress in Plants. Microorganisms 2021, 9, 538. [Google Scholar] [CrossRef]
- Heydarian, Z.; Gruber, M.; Coutu, C.; Glick, B.R.; Hegedus, D.D. Gene Expression Patterns in Shoots of Camelina Sativa with Enhanced Salinity Tolerance Provided by Plant Growth Promoting Bacteria Producing 1-Aminocyclopropane-1-Carboxylate Deaminase or Expression of the Corresponding AcdS Gene. Sci. Rep. 2021, 11, 4260. [Google Scholar] [CrossRef]
- Guo, D.-J.; Singh, R.K.; Singh, P.; Li, D.-P.; Sharma, A.; Xing, Y.-X.; Song, X.-P.; Yang, L.-T.; Li, Y.-R. Complete Genome Sequence of Enterobacter roggenkampii ED5, a Nitrogen Fixing Plant Growth Promoting Endophytic Bacterium With Biocontrol and Stress Tolerance Properties, Isolated From Sugarcane Root. Front. Microbiol. 2020, 11, 580081. [Google Scholar] [CrossRef]
- Fernández-Llamosas, H.; Ibero, J.; Thijs, S.; Imperato, V.; Vangronsveld, J.; Díaz, E.; Carmona, M. Enhancing the Rice Seedlings Growth Promotion Abilities of Azoarcus Sp. CIB by Heterologous Expression of ACC Deaminase to Improve Performance of Plants Exposed to Cadmium Stress. Microorganisms 2020, 8, 1453. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Simultaneous Detection and Quantification of Indole-3-Acetic Acid (IAA) and Indole-3-Butyric Acid (IBA) Produced by Rhizobacteria from l-Tryptophan (Trp) Using HPTLC. J. Microbiol. Methods 2015, 110, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Arund, J.; Luman, M.; Uhlin, F.; Tanner, R.; Fridolin, I. Is Fluorescence Valid to Monitor Removal of Protein Bound Uremic Solutes in Dialysis? PLoS ONE 2016, 11, e0156541. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, M.; Foster, J.W. EXPERIMENTS WITH SOME MICROORGANISMS WHICH UTILIZE ETHANE AND HYDROGEN. J. Bacteriol. 1958, 75, 592–603. [Google Scholar] [CrossRef]
- Honma, M.; Shimomura, T. Metabolism of 1-Aminocyclopropane-1-Carboxylic Acid. Agric. Biol. Chem. 1978, 42, 1825–1831. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. How Do Plant Growth-Promoting Bacteria Use Plant Hormones to Regulate Stress Reactions? Plants 2024, 13, 2371. https://doi.org/10.3390/plants13172371
Timofeeva AM, Galyamova MR, Sedykh SE. How Do Plant Growth-Promoting Bacteria Use Plant Hormones to Regulate Stress Reactions? Plants. 2024; 13(17):2371. https://doi.org/10.3390/plants13172371
Chicago/Turabian StyleTimofeeva, Anna M., Maria R. Galyamova, and Sergey E. Sedykh. 2024. "How Do Plant Growth-Promoting Bacteria Use Plant Hormones to Regulate Stress Reactions?" Plants 13, no. 17: 2371. https://doi.org/10.3390/plants13172371
APA StyleTimofeeva, A. M., Galyamova, M. R., & Sedykh, S. E. (2024). How Do Plant Growth-Promoting Bacteria Use Plant Hormones to Regulate Stress Reactions? Plants, 13(17), 2371. https://doi.org/10.3390/plants13172371








