BBX Genes of Cymbidium ensifolium Exhibited Intense Response to Blue Light in Meristem Induction through Artificial Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification and Tertiary Structures of BBX Genes
2.2. Examination of the Exon–Intron Architecture and Identification
2.3. Phylogenetic Analysis and Cis-Element Identification
2.4. Analyzing the Chromosome Position, Duplication Events, and Synteny of the CeBBX Gene in C. ensifolium
2.5. Plant Materials and Treatments
2.6. Extraction of RNA and Analysis Using Real-Time Quantitative PCR (RT-qPCR)
2.7. Subcellular Localization of BBX Proteins
3. Results
3.1. Identification and Characterization of BBX Proteins in C. ensifolium
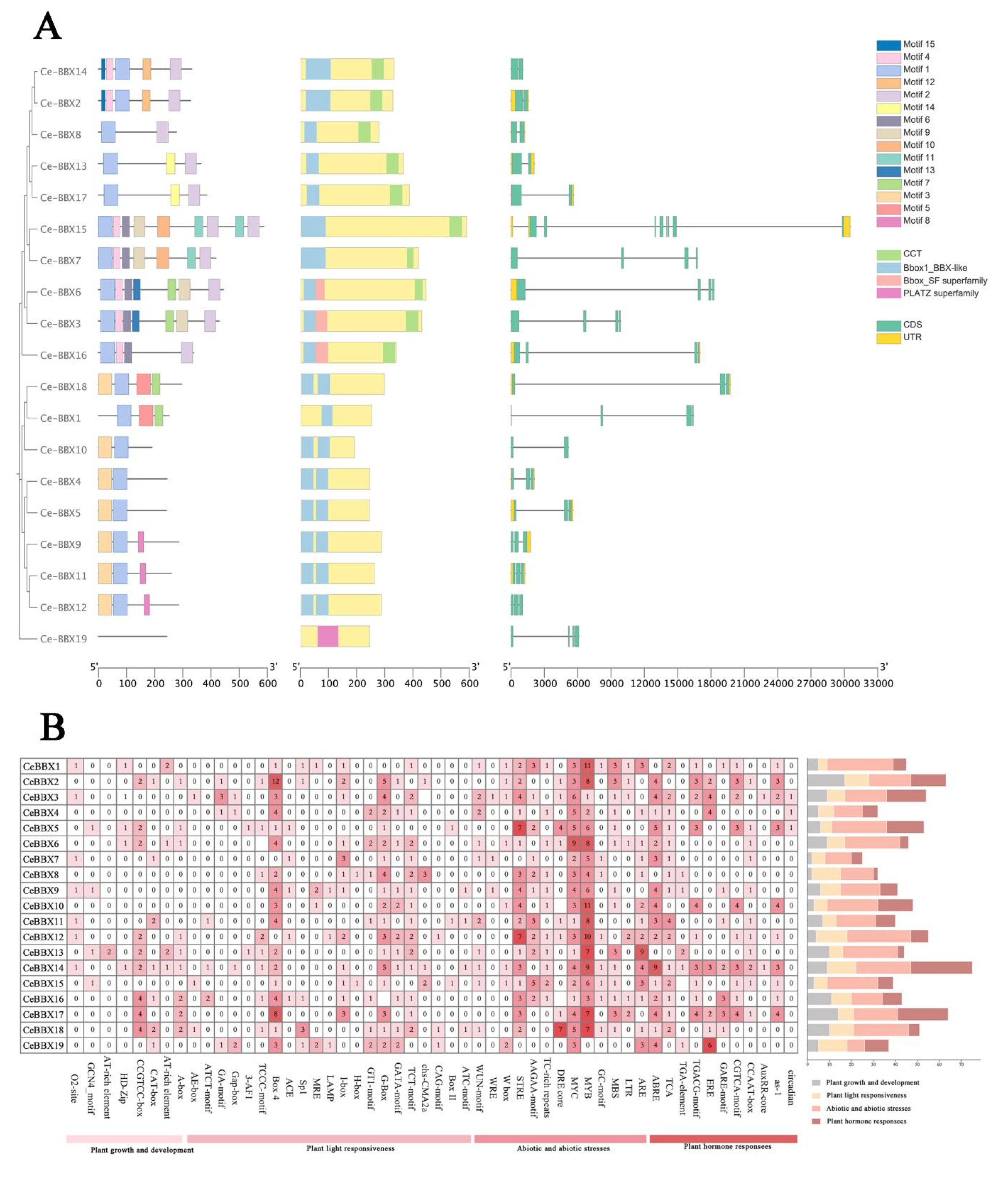
3.2. Gene Structures, Conserved Domains, and Motif Analysis
3.3. Cis-Element Analysis of CeBBXs
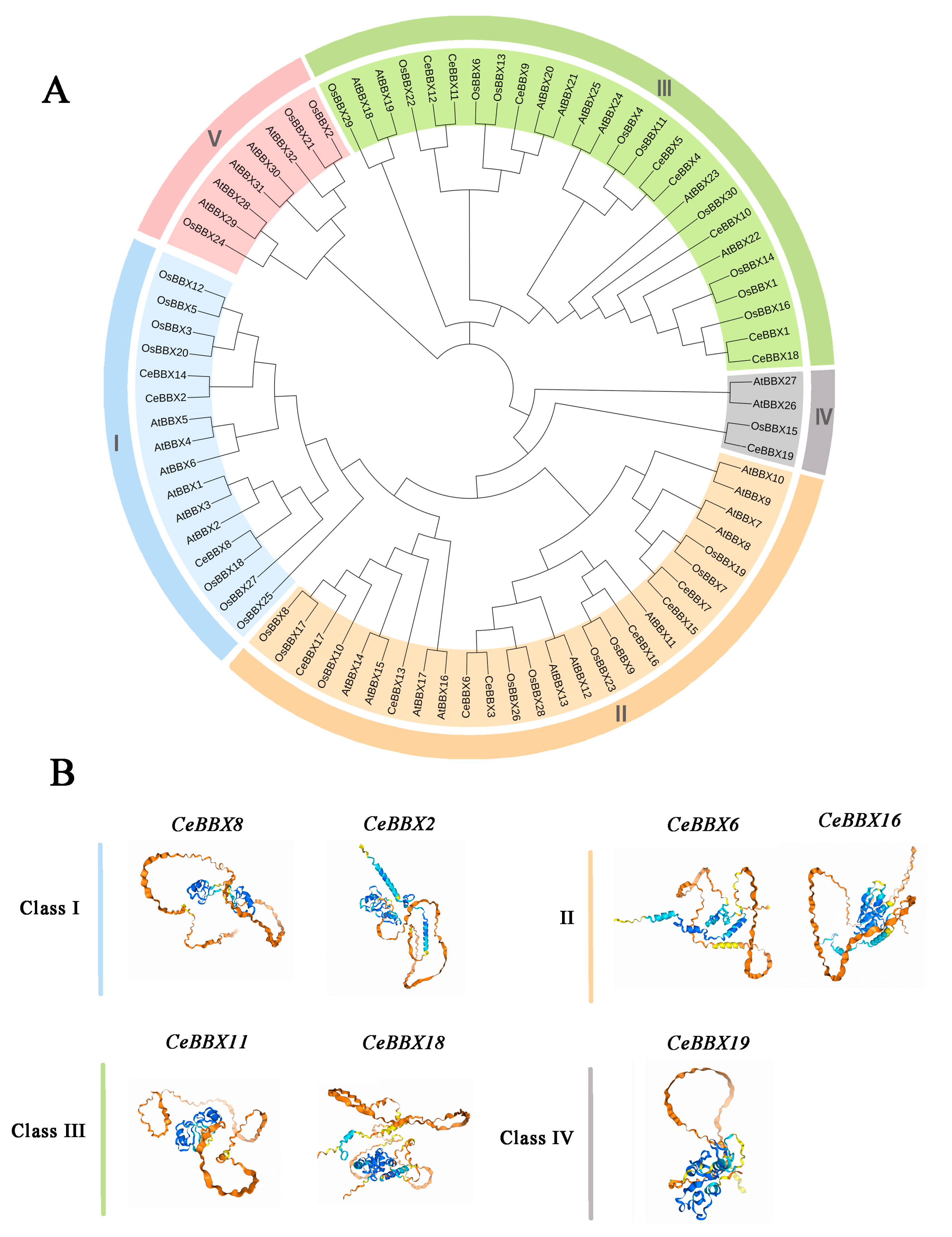
3.4. Phylogenetic Analysis and Tertiary Structure Analysis of CeBBXs
3.5. Chromosomal Location and Gene Duplication of CeBBX Gene
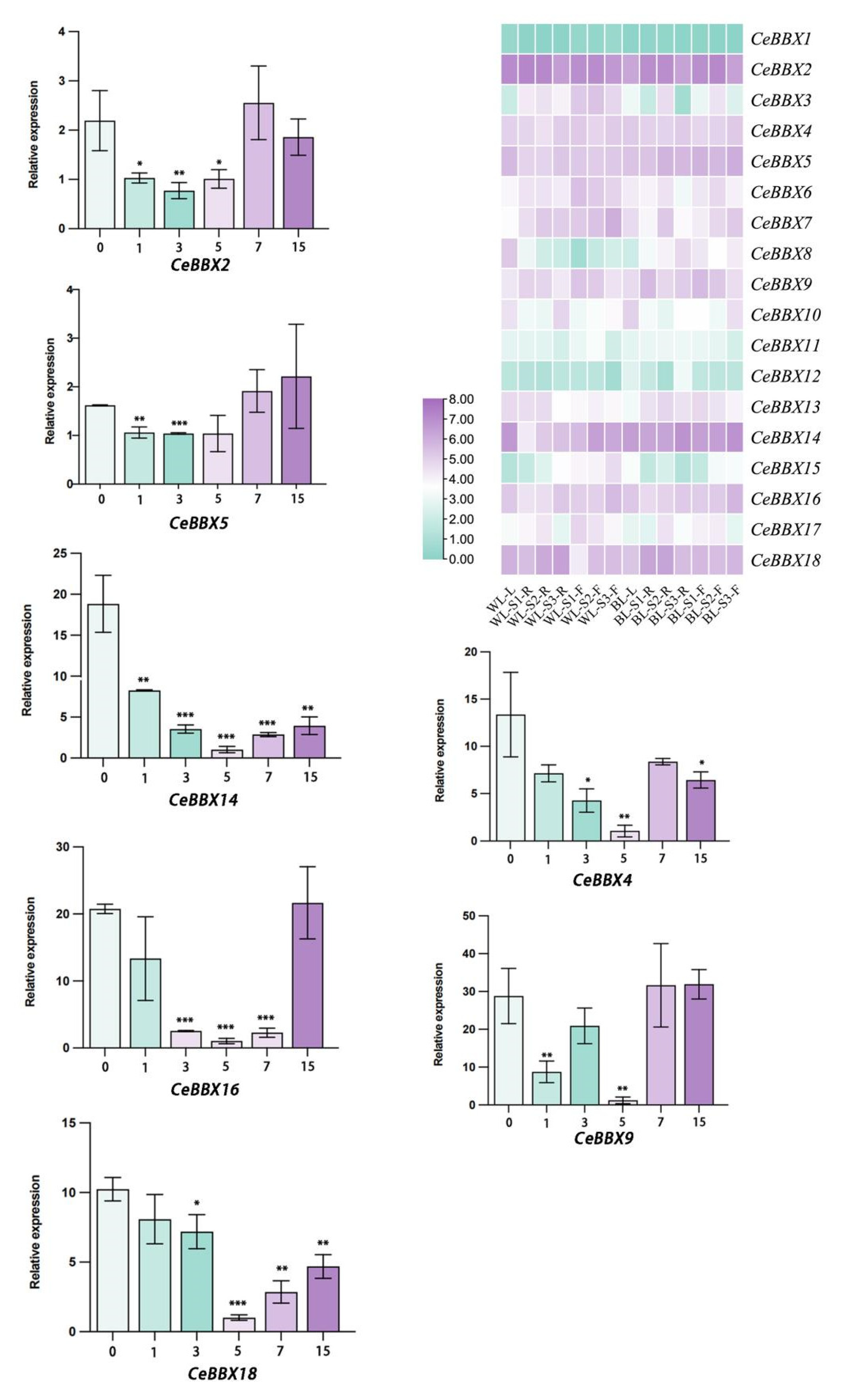
3.6. Expression Characteristics of CeBBX in C. ensifolium under Different Lights
3.7. Expression Profiles of Cymbidium ensifolium BBX Genes with Blue Light Treatment
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bula, R.J.; Morrow, R.C.; Tibbitts, T.W.; Barta, D.J.; Martin, T.S. Light-emitting Diodes as a Radiation Source for Plants. HortScience A Publ. Am. Soc. Hortic. Sci. 1991, 26, 203–205. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Zepka, L.Q.; Queiroz, M.I. Light-Emitting Diodes: Progress in Plant Micropropagation. InTech 2017, 6, 93–103. [Google Scholar]
- Reza, R.H.; Mahdi, B.; Mansour, G. The growth, nutrient uptake and fruit quality in four strawberry cultivars under different Spectra of LED supplemental light. BMC Plant Biol. 2024, 24, 179. [Google Scholar]
- Yang, J.; Song, J.; Jeong, B.R. The flowering of SDP chrysanthemum in response to intensity of supplemental or night-interruptional blue light is modulated by both photosynthetic carbon assimilation and photoreceptor-mediated regulation. Front. Plant Sci. 2022, 13, 981143. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Runkle, E.S. The role of blue light in night-interruption lighting of petunia. Acta Hortic. 2015, 1107, 101–106. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Low-intensity blue light in night-interruption lighting does not influence flowering of herbaceous ornamentals. Sci. Hortic. 2015, 186, 230–238. [Google Scholar] [CrossRef]
- Rantanen, M.; Kurokura, T.; Mouhu, K.; Pinho, P.; Tetri, E.; Halonen, L.; Palonen, P.; Elomaa, P.; HytöNen, T. Light quality regulates flowering in FvFT1/FvTFL1 dependent manner in the woodland strawberry Fragaria vesca. Front. Plant Sci. 2014, 5, 271. [Google Scholar] [CrossRef]
- Yoshida, H.; Hikosaka, S.; Goto, E.; Takasuna, H.; Kudou, T. Effects of light quality and light period on flowering of everbearing strawberry in a closed plant production system. Acta Hortic. 2012, 956, 107–112. [Google Scholar] [CrossRef]
- Jeong, S.W.; Park, S.; Jin, J.S.; Seo, O.N.; Kim, G.-S.; Kim, Y.-H.; Bae, H.; Lee, G.; Kim, S.T.; Lee, W.S.; et al. Influences of Four Different Light-Emitting Diode Lights on Flowering and Polyphenol Variations in the Leaves of Chrysanthemum (Chrysanthemum morifolium). J. Agric. Food Chem. 2012, 60, 9793–9800. [Google Scholar] [CrossRef]
- Laity, J.H.; Lee, B.M.; Wright, P.E. Zinc finger proteins: New insights into structural and functional diversity. Curr. Opin. Struct. Biol. 2001, 11, 39–46. [Google Scholar] [CrossRef]
- Deng, Z.; Yang, Z.; Liu, X.; Dai, X.; Zhang, J.; Deng, K. Genome-Wide Identification and Expression Analysis of C3H Zinc Finger Family in Potato (Solanum tuberosum L.). Int. J. Mol. Sci. 2023, 24, 1288. [Google Scholar] [CrossRef] [PubMed]
- Gangappa, S.N.; Botto, J.F. The BBX family of plant transcription factors. Trends Plant Sci. 2014, 19, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Bian, Y.; Liu, J.; Sun, Y.; Xu, D. B-box proteins: Pivotal players in light-mediated development in plants. J. Integr. Plant Biol. 2020, 62, 1293–1309. [Google Scholar] [CrossRef]
- Torok, M.; Etkin, L.D. Two B or not two B? Overview of the rapidly expanding B-box family of proteins. Differentiation 2001, 67, 63–71. [Google Scholar] [CrossRef]
- Kwan, Y.S.; Sook, C.K.; Joonki, K.; Hwan, L.J.; Myun, H.S.; Jeon, Y.S.; Yeon, Y.S.; Seob, L.J.; Hoon, A.J. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiol. 2005, 139, 770–778. [Google Scholar]
- Crocco, C.D.; Botto, J.F. BBX proteins in green plants: Insights into their evolution, structure, feature and functional diversification. Gene 2013, 531, 44–52. [Google Scholar] [CrossRef]
- Holm, M.; Hardtke, C.S.; Gaudet, R.; Deng, X.W. Identification of a structural motif that confers specific interaction with the WD40 repeat domain of Arabidopsis COP1. EMBO J. 2001, 20, 118–127. [Google Scholar] [CrossRef]
- Turck, F.; Fornara, F.; Coupland, G. Regulation and Identity of Florigen: FLOWERING LOCUS T Moves Center Stage. Annu. Rev. Plant Biol. 2008, 59, 573–594. [Google Scholar] [CrossRef]
- Federico, V. CONSTANS and the evolutionary origin of photoperiodic timing of flowering. J. Exp. Bot. 2011, 62, 2453–2463. [Google Scholar]
- Datta, S.; Hettiarachchi, G.H.C.M.; Deng, X.W.; Holm, M. Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell 2006, 18, 70–84. [Google Scholar] [CrossRef]
- Zhang, X.; Shang, F.; Huai, J.; Xu, G.; Tang, W.; Jing, Y.; Lin, R. A PIF1/PIF3-HY5-BBX23 transcription factor cascade affects photomorphogenesis. Plant Physiol. 2017, 174, 2487–2500. [Google Scholar] [CrossRef]
- Job, N.; Yadukrishnan, P.; Bursch, K.; Datta, S.; Johansson, H. Two B-box proteins regulate photomorphogenesis by oppositely modulating HY5 through their diverse C-terminal domains. Plant Physiol. 2018, 176, 2963–2976. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jiang, Y.; Li, J.; Lin, F.; Deng, X.W. BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc. Natl. Acad. Sci. USA 2016, 113, 7655. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jiang, Y.; Li, J.; Holm, M.; Deng, X. The B-Box Domain Protein BBX21 Promotes Photomorphogenesis1. Plant Physiol. 2017, 176, 2365–2375. [Google Scholar] [CrossRef]
- Holtan, H.E.; Bandong, S.; Marion, C.M.; Adam, L.; Tiwari, S.; Shen, Y.; Maloof, J.N.; Maszle, D.R.; Ohto, M.A.; Preuss, S. BBX32, an Arabidopsis B-Box protein, functions in light signaling by suppressing HY5-regulated gene expression and interacting with STH2/BBX21. Plant Physiol. 2011, 156, 2109–2123. [Google Scholar] [CrossRef]
- Gangappa, S.N.; Crocco, C.D.; Johansson, H.; Datta, S.; Hettiarachchi, C.; Holm, M.; Botto, J.F. The Arabidopsis B-BOX Protein BBX25 Interacts with HY5, Negatively Regulating BBX22 Expression to Suppress Seedling Photomorphogenesis. Plant Cell 2013, 25, 1243–1257. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Jiang, Y.; Li, J.; Yan, T.; Fan, L.; Liang, J.; Chen, Z.J.; Xu, D. Deng B-BOX DOMAIN PROTEIN28 Negatively Regulates Photomorphogenesis by Repressing the Activity of Transcription Factor HY5 and Undergoes COP1-Mediated Degradation. Plant Cell 2018, 30, 2006–2019. [Google Scholar] [CrossRef]
- Yadav, A.; Bakshi, S.; Yadukrishnan, P.; Lingwan, M.; Dolde, U.; Wenkel, S.; Masakapalli, S.K.; Datta, S. The B-Box-Containing MicroProtein miP1a/BBX31 Regulates Photomorphogenesis and UV-B Protection. Plant Physiol. 2019, 179, 1876–1892. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Jaina, M.; Sara, C.; Lowri, W.; Matloob, Q.; Gustavoa, S.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Lisanna, P.; Shriya, R.; Richardson, L.J. Pfam: The protein families database in 2021. Nucleic Acids Res. 2020, 49, D412–D419. [Google Scholar]
- Putterill, J.; Robson, F.; Lee, K.; Simon, R.; Coupland, G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 1995, 80, 847. [Google Scholar] [CrossRef]
- Cheng, X.F.; Wang, Z.Y. Overexpression of COL9, a CONSTANS-LIKE gene, delays flowering by reducing expression of CO and FT in Arabidopsis thaliana. Plant J. 2010, 43, 758–768. [Google Scholar] [CrossRef]
- Moon, Y.H. EMF1 Interacts with EIP1, EIP6 or EIP9 Involved in the Regulation of Flowering Time in Arabidopsis. Plant Cell Physiol. 2011, 52, 1376–1388. [Google Scholar]
- Talar, U.; Kiebowicz-Matuk, A. Beyond Arabidopsis: BBX Regulators in Crop Plants. Int. J. Mol. Sci. 2021, 22, 2906. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liu, Y.; Li, X.; Chen, Q.; Zhang, Y.; Luo, Y.; Liu, Z.; Wang, Y.; Lin, Y.; Zhang, Y.; et al. Transcriptome Profile Analysis of Strawberry Leaves Reveals Flowering Regulation under Blue Light Treatment. Int. J. Genom. 2021, 2021, 5572076. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, F.; Jin, L.; Jackson, A.; Ma, X.; Shu, X.; Wu, D.; Jin, G. Characterization and comparative profiling of the small RNA transcriptomes in two phases of flowering in Cymbidium ensifolium. BMC Genom. 2015, 16, 622. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.; Lian, H. Studies on Cymbidium ensifolium susin clonal propagation and floral bud differentiation by means of tissue culture. Hortic. Res. 1988, 8, 15. [Google Scholar]
- Poole, R.L. The TAIR Database; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar]
- Chandran, A.K.N.; Jung, K. Resources for systems biology in rice. J. Plant Biol. 2014, 57, 80–92. [Google Scholar] [CrossRef]
- Ai, Y.; Li, Z.; Sun, W.H.; Chen, J.; Zhang, D.; Ma, L.; Zhang, Q.H.; Chen, M.K.; Zheng, Q.D.; Liu, J.F. The Cymbidium genome reveals the evolution of unique morphological traits. Hortic. Res. 2021, 8, 15. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Elisabeth, G.; Alexandre, G.; Christine, H.; Ivan, I.; Appel, R.D.; Amos, B. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar]
- Yu, C.S.; Hwang, J.K. Prediction of Protein Subcellular Localizations. In Proceedings of the Intelligent Systems Design and Applications, Kaohsiung, Taiwan, 26–28 November 2008. [Google Scholar]
- Peng, Y.; Zhao, K.; Zheng, R.; Chen, J.; Zhu, X.; Xie, K.; Huang, R.; Zhan, S.; Su, Q.; Shen, M.; et al. A Comprehensive Analysis of Auxin Response Factor Gene Family in Melastoma dodecandrum Genome. Int. J. Mol. Sci. 2024, 25, 806. [Google Scholar] [CrossRef]
- Bailey, T.L.; Mikael, B.; Buske, F.A.; Martin, F.; Grant, C.E.; Luca, C.; Jingyuan, R.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Kohli, D.K.; Bachhawat, A.K. CLOURE: Clustal Output Reformatter, a program for reformatting ClustalX/ClustalW outputs for SNP analysis and molecular systematics. Nucleic Acids Res. 2003, 31, 3501–3502. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G. Building Phylogenetic Trees from Molecular Data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
- Lescot, M. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, G.Z.; Huang, J.; Liu, D.K.; Xue, F.; Chen, X.L.; Chen, S.Q.; Liu, C.G.; Liu, H.; Ma, H. The Cymbidium goeringii genome provides insight into organ development and adaptive evolution in orchids. Ornam. Plant Res. 2021, 1, 10. [Google Scholar] [CrossRef]
- Chen, G.Z.; Huang, J.; Zhou, X.Q.; Hao, Y.; Chen, J.L.; Zhou, Y.Z.; Ahmad, S.; Lan, S.; Liu, Z.J.; Peng, D.H. Comprehensive Analysis for GRF Transcription Factors in Sacred Lotus (Nelumbo nucifera). Int. J. Mol. Sci. 2022, 23, 6673. [Google Scholar] [CrossRef]
- Shi, Z.; Zhao, W.; Li, C.; Tan, W.; Zhu, Y.; Han, Y.; Ai, P.; Li, Z.; Wang, Z. Overexpression of the Chrysanthemum lavandulifolium ROS1 gene promotes flowering in Arabidopsis thaliana by reducing the methylation level of CONSTANS. Plant Sci. Int. J. Exp. Plant Biol. 2024, 342, 112019. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, X.; Aiwaili, P.; Mu, X.; Zhao, M.; Zhao, J.; Cheng, L.; Ma, C.; Gao, J.; Hong, B. A zinc finger protein BBX19 interacts with ABF3 to affect drought tolerance negatively in chrysanthemum. Plant J. 2020, 103, 1783–1795. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, X.; Weng, X.; Wang, L.; Xie, W. The Rice B-Box Zinc Finger Gene Family: Genomic Identification, Characterization, Expression Profiling and Diurnal Analysis. PLoS ONE 2012, 7, e48242. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Sheng, F.; Peng, J.; Guofang, L.; Izhar, M.; Youmei, L.; Rahat, S.; Feng, D.; Xiya, Z.; Ke, L. Genome Identification of B-BOX Gene Family Members in Seven Rosaceae Species and Their Expression Analysis in Response to Flower Induction in Malus domestica. Molecules 2018, 23, 1763. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, M.; Qiao, X.; Cheng, Y.; Li, X.; Zhang, H.; Wu, J. A New Insight into the Evolution and Functional Divergence of SWEET Transporters in Chinese White Pear (Pyrus bretschneideri). Plant Cell Physiol. 2017, 58, 839–850. [Google Scholar] [CrossRef]
- Unruh, S.A.; McKain, M.R.; Lee, Y.-I.; Yukawa, T.; McCormick, M.K.; Shefferson, R.P.; Smithson, A.; Leebens-Mack, J.H.; Pires, J.C. Phylotranscriptomic analysis and genome evolution of the Cypripedioideae (Orchidaceae). Am. J. Bot. 2018, 105, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, G.; Yin, C.; Fang, Y. B-box transcription factor 28 regulates flowering by interacting with constans. Sci. Rep. 2020, 10, 17789. [Google Scholar] [CrossRef]
- Xian, H. Chinese Rose CONSTANS (RoCO) Gene Cloning and Function Identification. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2017. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Niu, M.; Wu, X.; Peng, Y.; Zheng, R.; Cheng, M.; Zhao, K.; Zhou, Y.; Peng, D. BBX Genes of Cymbidium ensifolium Exhibited Intense Response to Blue Light in Meristem Induction through Artificial Control. Plants 2024, 13, 2375. https://doi.org/10.3390/plants13172375
Chen X, Niu M, Wu X, Peng Y, Zheng R, Cheng M, Zhao K, Zhou Y, Peng D. BBX Genes of Cymbidium ensifolium Exhibited Intense Response to Blue Light in Meristem Induction through Artificial Control. Plants. 2024; 13(17):2375. https://doi.org/10.3390/plants13172375
Chicago/Turabian StyleChen, Xiuming, Muqi Niu, Xiaopei Wu, Yukun Peng, Ruiyue Zheng, Mengya Cheng, Kai Zhao, Yuzhen Zhou, and Donghui Peng. 2024. "BBX Genes of Cymbidium ensifolium Exhibited Intense Response to Blue Light in Meristem Induction through Artificial Control" Plants 13, no. 17: 2375. https://doi.org/10.3390/plants13172375




