Reproductive Morphology and Success in Annual versus Perennial Legumes: Evidence from Astragalus and the Fabeae (Papilionoideae)
Abstract
1. Introduction
2. Results
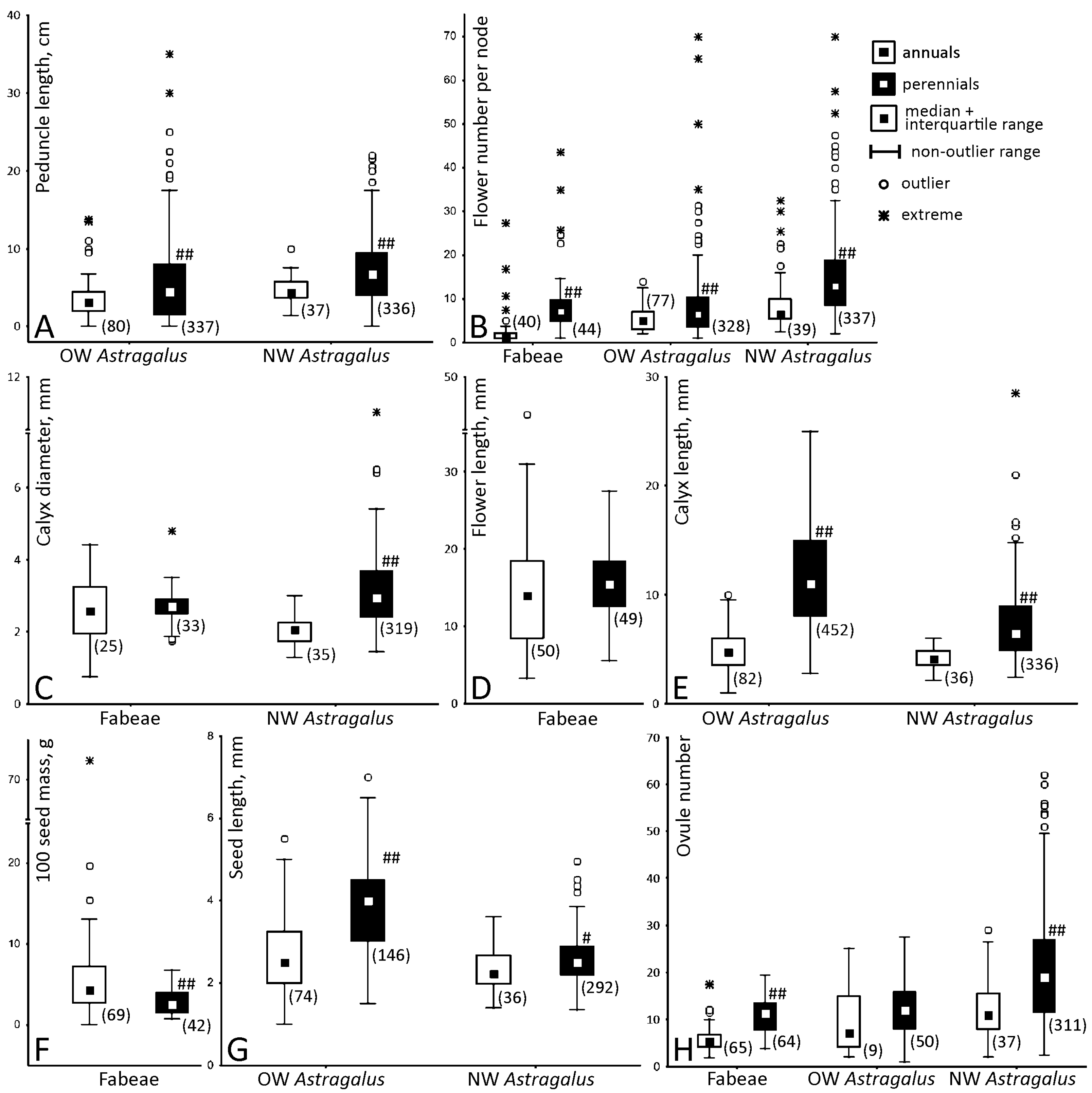
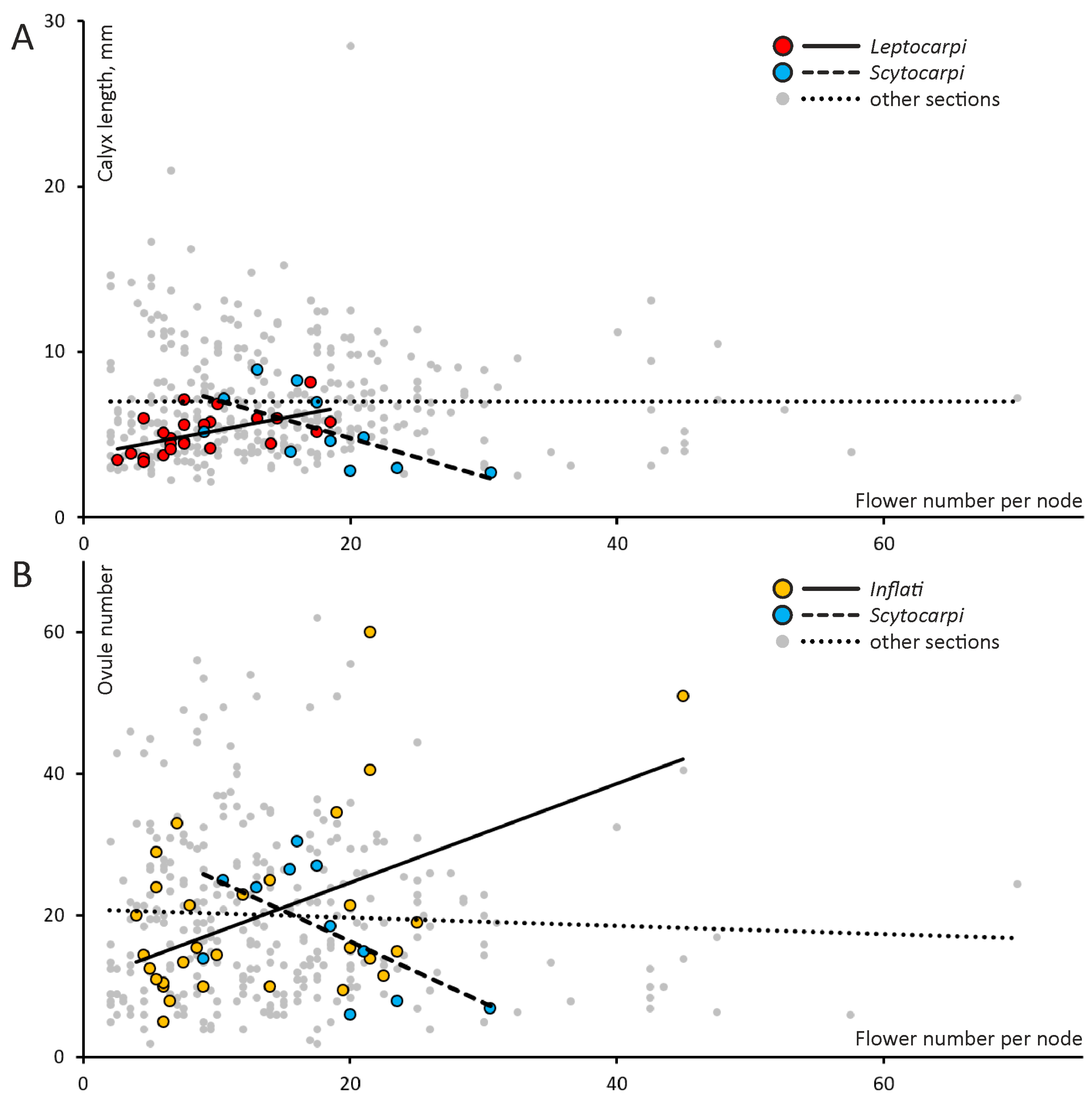
2.1. Quantitative Features of Reproductive Organs and Correlation between Them
2.2. The Real Productivity and Its Components
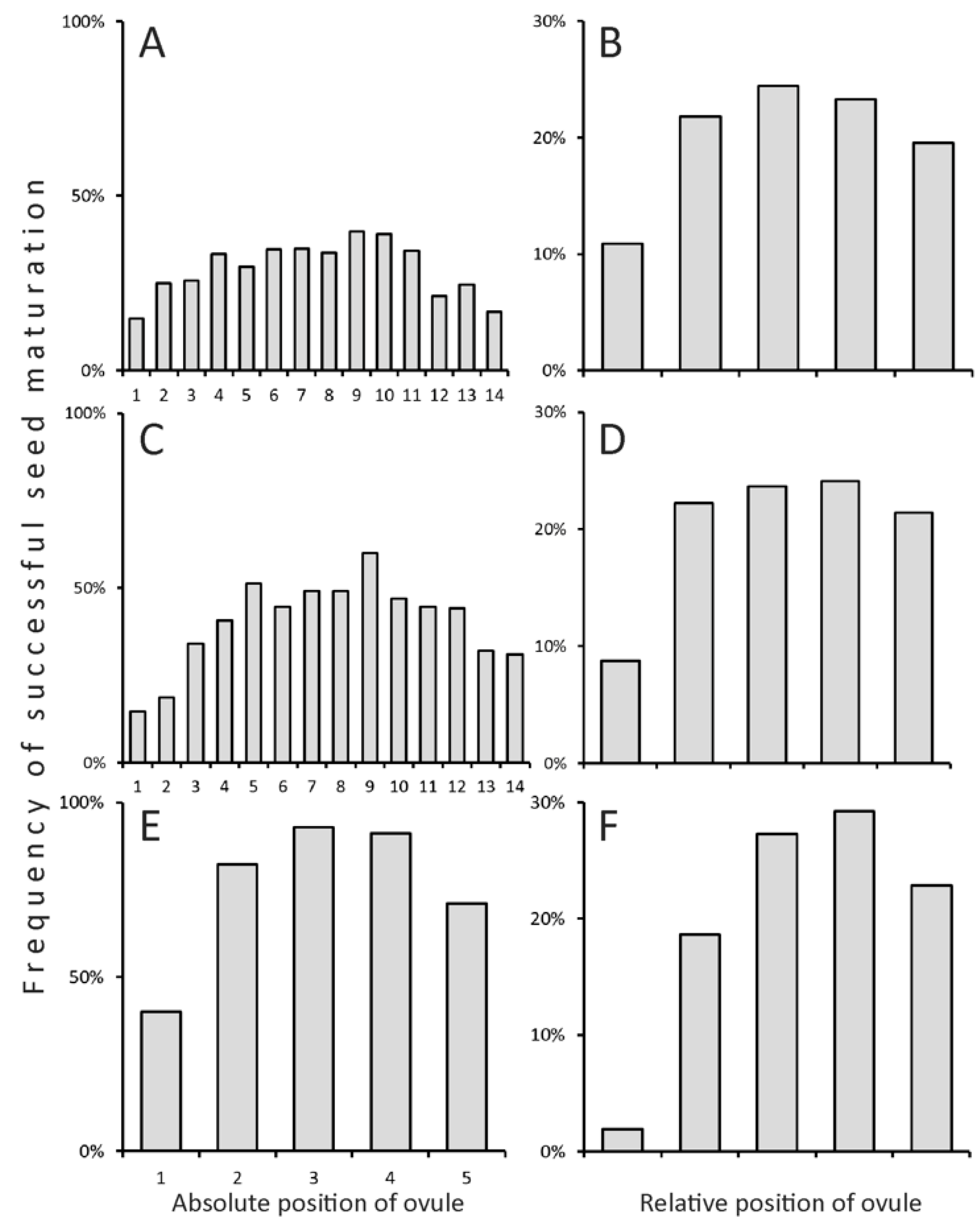
2.3. Non-Random Seed Abortion
3. Discussion
3.1. Variation and Correlation of Traits between Annuals and Perennials
3.2. Realization of the Potential Productivity Is Unequal between Taxa and Lifespans
3.3. A Trade-Off between Allocated Resources and the Resulting Productivity
4. Materials and Methods
4.1. Dataset
4.2. Data Acquisition and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harlan, J.R. Crops and Man, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1992; 284p. [Google Scholar]
- Wojciechowski, M.F.; Lavin, M.; Sanderson, M.J. A phylogeny of legumes (Leguminosae) based on analysis of the plastid matK gene resolves many well-supported subclades within the family. Am. J. Bot. 2004, 91, 1846–1862. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qu, L.; Dong, Y.; Han, L.; Liu, E.; Fang, S.; Zhang, Y.; Wang, T. A review of recent research progress on the Astragalus genus. Molecules 2014, 19, 18850–18880. [Google Scholar] [CrossRef] [PubMed]
- Barneby, R.C. Atlas of North American Astragalus. Mem. N. Y. Bot. Gard. 1964, 13, 1–1188. [Google Scholar] [CrossRef]
- Podlech, D.; Zarre, S. A Taxonomic Revision of the Genus Astragalus L. (Leguminosae) in the Old World; Naturhistorisches Museum: Vienna, Austria, 2013; pp. 1–2439. [Google Scholar]
- Kupicha, F. Vicieae. In Advances in Legume Systematics; Polhill, R.M., Raven, P.H., Eds.; Royal Botanical Gardens: Kew, UK, 1981; Volume 1, pp. 377–381. [Google Scholar]
- Kuznetzova, T.V. Morphology of the legume inflorescences 1. Types of the flower arrangement in the genus Astragalus L. Bull. Moscow Soc. Nat. Biol. Ser. 1989, 94, 103–113. [Google Scholar]
- Sinjushin, A.A.; Belyakova, A.S. Ontogeny, variation and evolution of inflorescence in tribe Fabeae (Fabaceae) with special reference to genera Lathyrus, Pisum and Vavilovia. Flora 2015, 211, 11–17. [Google Scholar] [CrossRef]
- Soltani, E.; Benakashani, F.; Baskin, J.M.; Baskin, C.C. Reproductive biology, ecological life history/demography and genetic diversity of the megagenus Astragalus (Fabaceae, Papilionoideae). Bot. Rev. 2021, 87, 55–106. [Google Scholar] [CrossRef]
- Hanelt, P.; Mettin, D. Biosystematics of the genus Vicia L. (Leguminosae). Ann. Rev. Ecol. Syst. 1989, 20, 199–223. [Google Scholar] [CrossRef]
- Potokina, E.K.; Aleksandrova, T.G. The pollination features in the annual species of the genus Vicia. Bot. Zhurn. 1996, 81, 74–79. [Google Scholar]
- Aarssen, L.W. Why are most selfers annuals? A new hypothesis for the fitness benefit of selfing. Oikos 2000, 89, 606–612. [Google Scholar] [CrossRef]
- Stebbins, G.L. Self fertilization and population variability in the higher plants. Am. Nat. 1957, 91, 337–354. [Google Scholar] [CrossRef]
- Azani, N.; Bruneau, A.; Wojciechowski, M.F.; Zarre, S. Molecular phylogenetics of annual Astragalus (Fabaceae) and its systematic implications. Bot. J. Linn. Soc. 2017, 184, 347–365. [Google Scholar] [CrossRef]
- Schaefer, H.; Hechenleitner, P.; Santos-Guerra, A.; de Sequeira, M.M.; Pennington, R.T.; Kenicer, G.; Carine, M.A. Systematics, biogeography, and character evolution of the legume tribe Fabeae with special focus on the middle-Atlantic island lineages. BMC Evol. Biol. 2012, 12, 250. [Google Scholar] [CrossRef]
- Azani, N.; Bruneau, A.; Wojciechowski, M.F.; Zarre, S. Miocene climate change as a driving force for multiple origins of annual species in Astragalus (Fabaceae, Papilionoideae). Mol. Phylogenet. Evol. 2019, 137, 210–221. [Google Scholar] [CrossRef]
- Vishnyakova, M.; Solovyova, A.; Sabitov, A.; Chebukin, P.; Burlyaeva, M. Economic value of perennial vetchlings (Lathyrus L.) species from the Russian Far East. Legume Perspect. 2014, 5, 32–33. [Google Scholar]
- Viko, G.; Manzoni, S.; Nkurunziza, L.; Murphy, K.; Weih, M. Trade-offs between seed output and life span—A quantitative comparison of traits between annual and perennial congeneric species. New Phytol. 2016, 209, 104–114. [Google Scholar]
- Stankevich, A.K.; Repjev, S.I. Flora of Cultivated Plants; Vetch. All-Russian Institute of Plant Industry (VIR): St. Petersburg, Russia, 1999; pp. 1–491. [Google Scholar]
- Herron, S.A.; Rubin, M.J.; Albrecht, M.A.; Long, Q.G.; Sandoval, M.C.; Miller, A.J. The role of genus and life span in predicting seed and vegetative trait variation and correlation in Lathyrus, Phaseolus, and Vicia. Am. J. Bot. 2021, 108, 2388–2404. [Google Scholar] [CrossRef] [PubMed]
- Sytin, A.K. On the relationship between biomorphs and taxa of annual astragali (Astragalus L., Fabaceae). Bull. Moscow Soc. Nat. Biol. Ser. 2023, 128, 40–51. [Google Scholar] [CrossRef]
- Snell, R.; Aarssen, L.W. Life history traits in selfing versus outcrossing annuals: Exploring the ‘time-limitation’ hypothesis for the fitness benefit of self-pollination. BMC Ecol. 2005, 5, 2. [Google Scholar] [CrossRef]
- Krizek, B.A.; Anderson, J.T. Control of flower size. J. Exp. Bot. 2013, 64, 1427–1437. [Google Scholar] [CrossRef]
- Sinjushin, A.A. The duration of the life cycle is associated with C-value and affects reproductive features in the Fabeae, the tribe with largest genomes in Fabaceae. Flora 2021, 285, 151954. [Google Scholar] [CrossRef]
- Gallardo, R.; Dominguez, E.; Muñoz, J.M. Pollen-ovule ratio, pollen size, and breeding system in Astragalus (Fabaceae) subgenus Epiglottis: A pollen and seed allocation approach. Am. J. Bot. 1994, 81, 1611–1619. [Google Scholar] [CrossRef]
- Mazer, S.J. Ecological, taxonomic, and life history correlates of seed mass among Indiana dune angiosperms. Ecol. Monogr. 1989, 59, 153–175. [Google Scholar] [CrossRef]
- Folk, R.A.; Charboneau, J.L.M.; Belitz, M.; Singh, T.; Kates, H.R.; Soltis, D.E.; Soltis, P.S.; Guralnick, R.P.; Siniscalchi, C.M. Anatomy of a mega-radiation: Biogeography and niche evolution in Astragalus. Am. J. Bot. 2024, 111, e16299. [Google Scholar] [CrossRef] [PubMed]
- Searle, A.B. Reproductive Success and Soil Seed Bank Characteristics of Astragalus ampullarioides and A. holmgreniorum (Fabaceae): Two Rare Endemics of Southwestern Utah. M.S. Thesis, Brigham Young University, Provo, UT, USA, 2011. [Google Scholar]
- Ehrlén, J. Proximate limits to seed production in a herbaceous perennial legume, Lathyrus vernus. Ecology 1992, 73, 1820–1831. [Google Scholar] [CrossRef]
- Charlesworth, D. Why do plants produce so many more ovules than seeds? Nature 1989, 338, 21–22. [Google Scholar] [CrossRef]
- Shamrov, I.I. Metabolite transport and possible factors of aberrant ovule formation. Bot. Zhurn. 2005, 90, 1651–1675. [Google Scholar]
- Kolyasnikova, N.L. The reproductive biology of some perennial species of Astragalus (Fabaceae). Bot. Zhurn. 2004, 89, 774–780. [Google Scholar]
- Karron, J.D. Breeding systems and levels of inbreeding depression in geographically restricted and widespread species of Astragalus (Fabaceae). Am. J. Bot. 1989, 76, 331–340. [Google Scholar] [CrossRef]
- Hossaert, M.; Valéro, M. Effect of ovule position in the pod on patterns of seed formation in two species of Lathyrus (Leguminosae: Papilionoideae). Am. J. Bot. 1988, 75, 1714–1731. [Google Scholar] [CrossRef]
- Kang, H. Position effects on abortion of reproductive characters in Vicia cracca (Leguminosae). Korean J. Ecol. 1996, 19, 107–123. [Google Scholar]
- Sinjushin, A.A. Seed propagation in four perennial species of Astragalus (Leguminosae, Papilionoideae, Galegeae): Productivity, non-random seed abortion and germination rate. Wulfenia 2021, 28, 211–222. [Google Scholar]
- Kupicha, F.K. The infrageneric structure of Vicia. Notes Roy. Bot. Gard. Edinb. 1974, 34, 287–326. [Google Scholar]
- Sargent, R.D.; Goodwillie, C.; Kalisz, S.; Ree, R.H. Phylogenetic evidence for a flower size and number trade-off. Am. J. Bot. 2007, 94, 2059–2062. [Google Scholar] [CrossRef]
- Gambín, B.L.; Borrás, L. Resource distribution and the trade-off between seed number and seed weight: A comparison across crop species. Ann. Appl. Biol. 2010, 156, 91–102. [Google Scholar] [CrossRef]
- Uhlarik, A.; Ćeran, M.; Živanov, D.; Grumeza, R.; Skøt, L.; Sizer-Coverdale, E.; Lloyd, D. Phenotypic and genotypic characterization and correlation analysis of pea (Pisum sativum L.) diversity panel. Plants 2022, 11, 1321. [Google Scholar] [CrossRef] [PubMed]
- Gnan, S.; Priest, A.; Kover, P.X. The genetic basis of natural variation in seed size and seed number and their trade-off using Arabidopsis thaliana MAGIC lines. Genetics 2014, 198, 1751–1758. [Google Scholar] [CrossRef]
- Shreter, I.A. Some biological peculiarities of Astragalus propinquus Schischk. for cultivation in middle region of Russia. Probl. Biol. Med. Pharm. Chem. 2012, 7, 36–37. [Google Scholar]
- Richards, K.W. Pollination requirements of cicer milkvetch, Astragalus cicer L. Rangel. Ecol. Manag. 1986, 39, 457–459. [Google Scholar] [CrossRef]
- Pankova, O.; Melnychuk, O.; Kubinska, L. Seed production and quality of seeds of the genus Astragalus L. in the conditions of Kremenets Botanical Garden. Molod. Vchenyi (Young Sci.) 2022, 2, 12–16. [Google Scholar]
- Kornievskaya, T.V.; Mikhaylova, S.I. Seed productivity and quality of seeds of an Astragalus cicer milkvetch in the South of Western Siberia. Acta Biol. Sib. 2016, 2, 5–10. [Google Scholar] [CrossRef][Green Version]
- Nemirova, E.; Martynov, N. On seed productivity of some species of Astragalus in Smolensk region. Bull. Mosc. Reg. State Univ. Nat. Sci. Ser. 2012, 1, 53–57. [Google Scholar]
- Safonova, O.N. Biological features of fruiting and seed quality in different species of Astragalus L. during introduction to the Central Chernozem Region. In Contemporary Problems of Introduction and Plant Biodiversity Conservation, Proceedings of the International Conference Devoted to 70th Anniversary of the Botanical Garden, Voronezh, Russia; Voronezh State University: Voronezh, Russia, 2007. [Google Scholar]
- Abdushaeva, Y.M. Ecological Assessment and Resource Potential of Fabaceae Lindl. in Landscapes of Novgorod Region. Ph.D. Thesis, Moscow Timiryazev Agricultural Academy, Moscow, Russia, 20 March 2013. [Google Scholar]
- Mamedova, Z.D. Seed productivity of some leguminous plants studied at the level of coenopopulation in Azerbaijan. Bull. Sci. Pract. 2020, 6, 58–65. [Google Scholar] [CrossRef]
- Chubirko, M.M. Embryological study of Astragalus glycyphyllus L. Biol. Nauk. 1989, 4, 62–66. [Google Scholar]
- Belkovskaya, T.P. On anthecology of some relict and endemic species of Astragalus of Kungur wooded steppe. In Ecology of Plant Pollination; Perm University: Perm, Russia, 1984; Volume 8, pp. 34–49. [Google Scholar]
- Mamatkulov, O.I. Potential seed productivity of some types of the genus Astragalus L. domining the territory of the Alai ridge. Sci. New Technol. Innov. Kyrg. 2019, 6, 68–72. [Google Scholar]
- Kornievskaya, T.V.; Silantieva, M.M. Biological peculiarities of seed productivity and quality of seed of Astragalus onobrychis L. in conditions of dry steppe zone of Kulunda. In Problems of Study of Vegetation of Siberia, Proceedings of the 6th International Conference Devoted to 100th Anniversary of Prof. Antonina Polozhii, Tomsk, Russia, 24–26 October 2017; Tomsk State University: Tomsk, Russia, 2017; pp. 211–213. [Google Scholar]
- Bakulin, S.D. Some aspects of seed propagation of selected plant species of Fabaceae included into Red List of Rostov Region. In Investigation, Conservation, and Restoration of Natural Landscapes, Proceedings of the 7th All-Russian Conference of, Volgograd, Russia, 9–13 October 2017; Planeta: Moscow, Russia, 2017; pp. 18–25. [Google Scholar]
- Shmaraeva, A.N.; Shishlova, Z.N.; Fedyaeva, V.V. The condition of Astragalus ponticus Pall. population on the territory of nature monument «Salskaya steppe» (Rostov-on-Don province, Russia). Zhivye I Biokosnye Sist. 2014, 6, 4. [Google Scholar]
- Kuzmenko, I.P.; Shmaraeva, A.N. The experience of introduction of Astragalus ponticus Pall. in the botanical garden of Southern Federal University. Nov. Nauk. V APK 2019, 1, 81–86. [Google Scholar]
- Kucherevsky, V.V.; Sirenko, T.V.; Baranets, M.O.; Scholl, G.N. Chorologic, ecologicenotic, biomorphological and population studies of Astragalus ponticus Pall. in the Dnipropetrovsk region. Nauk. Visnyk NLTU Ukr. 2016, 26, 105–112. [Google Scholar] [CrossRef]
- Zhmud’, E.V.; Elisafenko, T.V.; Verhozina, A.V.; Krivenko, D.A.; Zviagina, N.S.; Dorogina, O.V. State of population of the endemic species Astragalus olchonensis (Fabaceae) on the Olkhon Island (Baikal Lake). Bot. Zhurn. 2011, 96, 245–255. [Google Scholar]
- Ivanova, M.M.; Semenova, G.P. Astragalus olkhonensis, the endemic species of Olkhon Island. Bull. Glavn. Bot. Sada 1988, 151, 44–47. [Google Scholar]
- Becker, T.; Voss, N.; Durka, W. Pollen limitation and inbreeding depression in an ‘old rare’ bumblebee-pollinated grassland herb. Plant Biol. 2011, 13, 857–864. [Google Scholar] [CrossRef]
- Martínez-Sánchez, J.J.; Segura, F.; Aguado, M.; Franco, J.A.; Vicente, M.J. Life history and demographic features of Astragalus nitidiflorus, a critically endangered species. Flora 2011, 206, 423–432. [Google Scholar] [CrossRef]
- Kudo, G.; Molau, U. Variations in reproductive traits at inflorescence and flower levels of an arctic legume, Astragalus alpinus L.: Comparisons between a subalpine and an alpine population. Plant Species Biol. 1999, 14, 181–191. [Google Scholar] [CrossRef]
- Tepedino, V.J. Reproduction and Pollination of Two Rare Species of Astragalus from Washington County, Southern Utah: A. holmgreniorum and A. ampullarioides; USDA-ARS Bee Biology and Systematics Laboratory, Department of Biology, Utah State University: Logan, UT, USA, 2005. [Google Scholar]
- Subaşı, Ü. Conservation and ecological interactions of Astragalus bozakmanii Podlech (Fabaceae). Hoehnea 2021, 48, e1302020. [Google Scholar] [CrossRef]
- Atasagun, B.; Aksoy, A.; Güllü, I.B.; Albayrak, S. Reproductive Biology of Astragalus argaeus (Fabaceae), a critically endangered endemic species. An. Acad. Bras. Cienc. 2021, 93 (Suppl. S3), e20201613. [Google Scholar] [CrossRef]
- Tomilova, L.I.; Koroleva, E.F. Flowering, pollination, and seed productivity of endemic Ural species of Astragalus in conditions of introduction. In Ecology of Flowering and Pollination of Plants; University of Perm: Perm, Russia, 1989; pp. 6–18. [Google Scholar]
- Sandanov, D.V.; Selyutina, I.Y.; Dulepova, N.A. Structure of plant communities and cenopopulations of Astragalus serioceocanus Gontsch. on the shore of Lake Baikal. Contemp. Probl. Ecol. 2014, 7, 237–245. [Google Scholar] [CrossRef]
- Balandin, S.V.; Ladygin, I.V. Condition of population of Astragalus kungurensis (Fabaceae), a restricted endemic of Urals. Bot. Zhurn. 2008, 93, 1715–1724. [Google Scholar]
- Karshibaev, J.K. Reproduction characteristics of annual Astragalus (Fabaceae) in the semi-desert zone of Uzbekistan. Rastit. Resur. 2013, 49, 10–20. [Google Scholar]
- Wiens, D. Ovule survivorship, brood size, life history, breeding systems, and reproductive success in plants. Oecologia 1984, 64, 47–53. [Google Scholar] [CrossRef]
- Allphin, L.; Brian, N.; Matheson, T. Reproductive success and genetic divergence among varieties of the rare and endangered Astragalus cremnophylax (Fabaceae) from Arizona, USA. Conserv. Genet. 2005, 6, 803–821. [Google Scholar] [CrossRef]
- Kožuharova, E.; Firmage, D.H. On the pollination ecology of Astragalus alopecurus Pallas (Fabaceae) in Bulgaria. C. R. Acad. Bulg. Sci. 2007, 60, 863–870. [Google Scholar]
- Green, T.W.; Bohart, G.E. The pollination ecology of Astragalus cibarius and Astragalus utahensis (Leguminosae). Am. J. Bot. 1975, 62, 379–386. [Google Scholar] [CrossRef]
- Kožuharova, E.; Firmage, D.H. Notes on the reproductive biology of Astragalus dasyanthus Pall. (Fabaceae) a rare plant for Bulgaria. C. R. Acad. Bulg. Sci. 2009, 62, 1079–1088. [Google Scholar]
- Shugayeva, E.V.; Perebeynos, L.I. Peculiarities of flowering and fertilization process in Astragalus dasyanthus Pall. In Problems of Reproduction of Flowering Plants (Applied Aspects); Perm State University: Perm, Russia, 1987; pp. 97–98. [Google Scholar]
- Rundel, P.W.; Huggins, T.R.; Prigge, B.A.; Sharifi, M.R. Community Dynamics and Soil Seed Bank Ecology of the Lane Mountain milkvetch (Astagalus jaegerianus Munz); Final report; U.S. Army Research Office: Research Triangle Park, NC, USA, 2012. [Google Scholar]
- Mazer, S.J.; Travers, S. Baseline Monitoring Study of a Rare Plant, the Fish Slough milkvetch (Astragalus lentiginosus var. piscinensis [Fabaceae]): Sources of Variation in Reproduction, Herbivory and Growth; A grant report; Department of Biological Sciences of the University of California: Santa Barbara, CA, USA, 1992. [Google Scholar]
- Geer, S.M.; Tepedino, V.J. Breeding systems of the rare heliotrope milkvetch (Astragalus montii Welsh: Fabaceae) and two common congeners. In Southwestern Rare and Endangered Plants, Proceedings of the Southwestern Rare and Endangered Plant Conference; New Mexico Forestry and Resources, Conservation Division: Santa Fe, NM, USA, 1992; pp. 334–344. [Google Scholar]
- Baskin, C.C.; Baskin, J.M.; Quarterman, E. Observations on the ecology of Astragalus tennesseensis. Am. Midl. Nat. 1972, 88, 167–182. [Google Scholar] [CrossRef]
- Beregovaya, Y.S. Seed productivity and germination of seeds Astragalus ionae Palibin. Sovrem. Nauchnye Issledovniya I Razrab. 2018, 5, 644–646. [Google Scholar]
- Karshibaev, J.K. The reproductive strategy of Astragalus globiceps (Fabaceae) in the semidesert zone of Uzbekistan. Rastit. Resur. 2015, 51, 336–344. [Google Scholar]
- Boe, A.; Fluharty, K. Reproductive biology of a Canada milk-vetch population from eastern South Dakota. Prairie Nat. 1993, 25, 65–72. [Google Scholar]
- Meinke, R.; Meyers, S.; Amsberry, K.; Wilson, C.; Groberg, M.; Woolverton, R.; Brown, J. Assessing the Population Genetics, Taxonomy, Reproductive Ecology, and Life History Traits of Humboldt milkvetch (Astragalus agnicidus) in Relation to Conservation and Management: A Report Prepared for the California Department of Fish and Wildlife; Eureka Field Office: Eureka, CA, USA, 2013. [Google Scholar]
- Kaye, T.N. From flowering to dispersal: Reproductive ecology of an endemic plant, Astragalus australis var. olympicus (Fabaceae). Am. J. Bot. 1999, 86, 1248–1256. [Google Scholar] [CrossRef]
- Watrous, K.M.; Cane, J.H. Breeding biology of the threadstalk milkvetch, Astragalus filipes (Fabaceae), with a review of the genus. Am. Midl. Nat. 2011, 165, 225–240. [Google Scholar] [CrossRef]
- Melgoza-Castillo, A. Ecology of Locoweed (Astragalus mollissimus) in Chihuahua, Mexico. Ph.D. Dissertation, New Mexico State University, Las Cruces, Mexico, 1995. [Google Scholar]
- Martin, E.F. Reproduction, Demography, and Habitat Characterization of Astragalus peckii (Fabaceae), a Rare Central Oregon Endemic. Ph.D. Dissertation, Oregon State University, Corvallis, OR, USA, 17 August 2010. [Google Scholar]
- Crone, E.E.; Lesica, P. Pollen and water limitation in Astragalus scaphoides, a plant that flowers in alternate years. Oecologia 2006, 150, 40–49. [Google Scholar] [CrossRef]
- Schurr, L.; Affre, L.; Flacher, F.; Tatoni, T.; Pecheux, L.L.M.; Geslin, B. Pollination insights for the conservation of a rare threatened plant species, Astragalus tragacantha (Fabaceae). Biodivers. Conserv. 2019, 28, 1389–1409. [Google Scholar] [CrossRef]
- Martínez-Fernández, V.; Martínez-García, F.; Pérez-García, F. Census, reproductive biology, and germination of Astragalus gines-lopezii (Fabaceae), a narrow and endangered endemic species of SW Spain. Turk. J. Bot. 2014, 38, 686–695. [Google Scholar] [CrossRef]
- Khabibov, A.D. Yearly dynamics of variation of seed productivity of Astragalus lehmannianus Bge. on Sarykum sand dune. Tr. Instituta Geol. Dagest. Nauchnogo Tsentra RAN 2015, 65, 142–146. [Google Scholar]
- Shamurin, V.F.; Tikhmenev, E.A. Flowering and fructification in Leguminosae and Scrophulariaceae in the Wrangel Island. Bot. Zhurn. 1971, 56, 403–413. [Google Scholar]
- Prokopyev, A.S.; Kataeva, T.N. On the state of coenopopulations of some rare plant species in Tomsk region. Rastit. Resur. 2017, 53, 220–237. [Google Scholar]
- Kotukhov, Y.A.; Danilova, A.N.; Anufrieva, O.A. The Current State of Populations of Rare and Endangered Plants of Eastern Kazakhstan. V. 2; Tethys: Almaty, Kazakhstan, 2007; pp. 105–134. [Google Scholar]
- Kotukhov, Y.A.; Danilova, A.N.; Anufrieva, O.A. The Current State of Populations of Rare and Endangered Plants of Eastern Kazakhstan. V. 3; Media-Alliance: Ust-Kamenogorsk, Kazakhstan, 2017; pp. 144–175. [Google Scholar]
- Kolyasnikova, N.L.; Gladkov, T.G. Studies of coenopopulations of Astragalus permiensis C.F.Mey. ex Rupr. In Individual and Population—A Strategy of Life, Proceedings of the 9th All-Russian Seminar on Population Biology, V. 1, Ufa, Russia, 2–6 October 2006; Villi Oksler: Ufa, Russia, 2006; pp. 119–122. [Google Scholar]
- Zamaletdinov, R.I.; Okulova, S.M.; Gavrilova, E.A.; Zakhvatova, A.A.; Mingaliev, R.R. Materials on the study of seed productivity of some leguminous plants species (Fabaceae Lind., 1836) in the conditions of habitat urbanization. Ad Alta 2017, 7, 47–50. [Google Scholar]
- Gulenkova, M.A.; Egorova, V.N. Lathyrus pratensis. In Biological Flora of the Moscow Region; Rabotnov, T.A., Ed.; Moscow University Press: Moscow, Russia, 1978; Volume 4, pp. 127–137. [Google Scholar]
- Gurusamy, C.; Bal, A.K. Effect of seed abortion on the pattern of seed formation in Lathyrus maritimus and L. sativus (Fabaceae). Phyton 2000, 40, 223–238. [Google Scholar]
- Zimnitskaya, S.A.; Betekhtina, A.A. Features of Reproductive Biology of Legumes in Different Parts of Their Natural Habitats. In Botanical Studies in Asiatic Russia, Proceedings of the 11th Meeting of the Russian Botanical Society, Novosibirsk, Barnaul, Russia, 18–22 August 2003. V. 2; Azbuka: Barnaul, Russia, 2003; pp. 142–144. [Google Scholar]
- Seijo, G.J.; Neffa, V.G.S. Cytogenetic studies in the rare South American Lathyrus hasslerianus Burk. (Leguminosae). Cytologia 2006, 71, 11–19. [Google Scholar] [CrossRef]
- Seijo, G. Spontaneous cytomixis in the microsporogenesis of sweet pea Lathyrus odoratus L.(Leguminosae). Cytologia 1996, 61, 189–195. [Google Scholar] [CrossRef]
- Akhundova, V.A.; Morozova, Z.A.; Murashev, V.V.; Sedova, E.A.; Turkova, E.V. Morphogenesis and Productivity of Plants; Lomonosov Moscow State University: Moscow, Russian, 1994; pp. 97–130. [Google Scholar]
- Donskaya, M.V.; Donskoj, M.M.; Naumkin, V.P. The study of promising varieties of Indian pea by the complex of signs. Zernobobovye I Krupyanye Kul’tury 2018, 4, 113–120. [Google Scholar]
- Navasardyan, E.M.; Melikova, T.S. Autogamy of annual species Lathyrus inconspicuus L. (Fabaceae) of Armenian flora. In Actual Problems of Botany in Armenia; Institute of Botany NAS RA: Yerevan, Armenia, 2008; pp. 137–140. [Google Scholar]
- Navasardyan, E.M.; Ananyan, K.V.; Melikova, T.S. Self-compatibility in annual species of genus Lathyrus (Fabaceae): L. hirsutus L. and L. cassius Boiss. Biol. J. Armen. 2003, 55, 98–101. [Google Scholar]
- Navasardyan, E.M.; Melikova, T.S. Evaluation of annual species of genus Lathyrus (Fabaceae) of Armenia with respect to level of self-compatibility. In Botanical Studies in Asiatic Russia, Proceedings of the 11th Meeting of the Russian Botanical Society, Novosibirsk, Barnaul, Russia, 18–22 August 2003. V. 2; Azbuka: Barnaul, Russia, 2003; pp. 155–156. [Google Scholar]
- Navasardyan, E.M.; Melikova, T.S. Self-compatibility of annual species of Lathyrus (Fabaceae): L. cicera L. and L. nissolia L. Flora Rastit. I Rastit. Resur. Armen. 2004, 15, 71–73. [Google Scholar]
- Romanchuk, E.I.; Khusnidinov, S.K.; Zamashchikov, R.V. Fructification features of Tangier pea (Lathyrus tingitanus L.) in condition of Predbaikalie. Vestn. IrGSKhA 2014, 62, 25–33. [Google Scholar]
- Ortega-Olivencia, A.; Devesa, J. Seed set and germination in some wild species of Vicia from SW Europe (Spain). Nord. J. Bot. 1997, 17, 639–648. [Google Scholar] [CrossRef]
- Kolyasnikova, N.L. Reproductive Biology of Cultivated and Wild-Growing Legumes with Reference to Low Seed Productivity. Ph.D. Thesis, Perm State University, Perm, Russia, 17 November 2005. [Google Scholar]
- Izmaiłow, R. Reproduction of Vicia cracca L. in the polluted environment of the Legnica-Głogów copper basin (Poland). Acta Biol. Cracoviensia Ser. Bot. 2000, 42, 125–133. [Google Scholar]
- Makarova, Y.A.; Zamaletdinov, R.I. Monitoring of ecological conditions of urbanized territories using reproductive parameters of legumes in Kazan city. In Proceedings of the 8th International Symposium of Young Scientists on Management, Economics, and Finance, Kazan, Russia, 28–29 November 2019; Kazan State University: Kazan, Russia, 2019; pp. 210–213. [Google Scholar]
- Egorova, V.N. Vicia cracca. In Biological Flora of the Moscow Region; Rabotnov, T.A., Ed.; Moscow University Press: Moscow, Russia, 1978; Volume 4, pp. 114–126. [Google Scholar]
- Gonyan, G.G. Biological features of Vicia variabilis Fr. et Sint. Proc. Acad. Sci. Armen. SSR 1961, 14, 3–15. [Google Scholar]
- Vishnyakova, M.A.; Kilikovskaya, T.V.; Tikhmenev, E.A. Seed reproduction of Vicia multicaulis Ledeb. (Fabaceae) in the Okhotsk-Kolyma Upland. Russ. J. Ecol. 2000, 31, 123–125. [Google Scholar] [CrossRef]
- Dyuryagina, G.P.; Ivanova, M.M. Characterization of rare species of flora of Buryatia. Bot. Zhurn. 1985, 70, 1529–1538. [Google Scholar]
- Boikov, T.G.; Sutkin, A.V. Ecophytocenotic features of Vicia tsydenii Malysch. in Southern Transbaikalia. Russ. J. Ecol. 2012, 43, 367–372. [Google Scholar] [CrossRef]
- Semenova, E.V. Reproductive Biology of Faba bona Medik. and Causes of Decrease of Its Seed Productivity. Ph.D. Thesis, Vavilov Institute of Plant Industry, Leningrad, Russia, 1988. [Google Scholar]
- Petraitytė, N.; Sliesaravičius, A.; Dastikaitė, A. Potential reproduction and real seed productivity of Vicia villosa L. Biologija 2007, 53, 48–51. [Google Scholar]
- Zolotarev, V.N. Influence of sowing date on biotypic content and seed productivity of Vicia villosa Roth. ‘Lugovskaya 2’. In Innovations of the Agricultural Sector: Reserves of Cost Saving and Increase of Product Quality, Proceedings of the International Conference, Tulovo, Belarus, 12–13 July 2018; Belaruskaya Navuka: Minsk, Belarus, 2018; pp. 22–31. [Google Scholar]
- Fırıncıoğlu, K.H.; Ünal, S.; Doğruyol, L. Phenotypic variation of Vicia pannonica Crantz (var. pannonica and var. purpurascens) in central Turkey. J. Cent. Eur. Agric. 2011, 12, 82–91. [Google Scholar]

| Flower Number | Peduncle Length | Flower Size a | Ovule Number | ||
|---|---|---|---|---|---|
| Peduncle length | OW | 0.65 ** (282) | |||
| NW | 0.39 ** (367) | ||||
| F | 0.76 ** (36) | ||||
| Flower size a | OW | 0.14 (403) | 0.18 (389) | ||
| NW | 0.01 (368) | 0.18 ** (364) | |||
| F | –0.03 (104) | 0.09 (36) | |||
| Ovule number | OW | –0.07 (50) | 0.28 (48) | –0.01 (59) | |
| NW | –0.00 (344) | 0.19 ** (340) | 0.66 ** (335) | ||
| F | 0.03 (74) | – | – | ||
| Seed size b | OW | –0.05 (170) | –0.18 (185) | 0.79 ** (220) | –0.13 (24) |
| NW | 0.08 (324) | 0.24 ** (322) | 0.35 ** (315) | 0.35 ** (303) | |
| F | –0.10 (30) | –0.14 (21) c | 0.53 ** (57) c | –0.35 (63) | |
| –0.08 (55) c | |||||
| Fruit Number | Flower Number | F/F Ratio | Seed Number | Ovule Number | S/O Ratio | ||
|---|---|---|---|---|---|---|---|
| Flower number | A | 0.83 ** (40) | |||||
| LC | 0.80 ** (18) | ||||||
| F | 0.57 ** (32) | ||||||
| F/F ratio | A | 0.74 ** (41) | 0.38 ** (52) | ||||
| LC | 0.74 ** (18) | 0.35 (29) | |||||
| F | –0.11 (32) | –0.81 ** (33) | |||||
| Seed number | A | –0.48 ** (45) | –0.45 ** (28) | –0.24 (64) | |||
| LC | –0.40 (22) | –0.44 * (27) | 0.12 (32) | ||||
| F | –0.30 (29) | –0.21 (32) | 0.19 (33) | ||||
| Ovule number | A | –0.32 (25) | –0.31 (24) | –0.12 (41) | 0.64 ** (42) | ||
| LC | 0.30 (5) | 0.30 (5) | 0.00 (16) | 0.58 * (14) | |||
| F | 0.12 (28) | 0.33 (30) | –0.46 ** (33) | 0.54 ** (72) | |||
| S/O ratio | A | –0.34 (25) | –0.32 (24) | –0.05 (48) | 0.79 ** (50) | 0.20 (45) | |
| LC | –0.90 * (5) | –0.90 * (5) | 0.42 (19) | 0.81 ** (20) | 0.37 (17) | ||
| F | –0.40 * (29) | –0.64 ** (31) | 0.60 ** (32) | 0.11 (71) | –0.67 ** (71) | ||
| Seed size a | A | 0.11 (34) | 0.07 (35) | 0.10 (67) | –0.13 (58) | 0.03 (30) | –0.35 * (46) |
| LC | 0.19 (20) | 0.17 (21) | 0.01 (45) | –0.12 (38) | 0.02 (17) | –0.40 * (28) | |
| F | –0.27 (26) | –0.36 (30) | 0.10 (40) | –0.12 (65) | –0.18 (63) | 0.07 (63) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinjushin, A.; Ploshinskaya, M.; Sytin, A. Reproductive Morphology and Success in Annual versus Perennial Legumes: Evidence from Astragalus and the Fabeae (Papilionoideae). Plants 2024, 13, 2380. https://doi.org/10.3390/plants13172380
Sinjushin A, Ploshinskaya M, Sytin A. Reproductive Morphology and Success in Annual versus Perennial Legumes: Evidence from Astragalus and the Fabeae (Papilionoideae). Plants. 2024; 13(17):2380. https://doi.org/10.3390/plants13172380
Chicago/Turabian StyleSinjushin, Andrey, Maria Ploshinskaya, and Andrey Sytin. 2024. "Reproductive Morphology and Success in Annual versus Perennial Legumes: Evidence from Astragalus and the Fabeae (Papilionoideae)" Plants 13, no. 17: 2380. https://doi.org/10.3390/plants13172380
APA StyleSinjushin, A., Ploshinskaya, M., & Sytin, A. (2024). Reproductive Morphology and Success in Annual versus Perennial Legumes: Evidence from Astragalus and the Fabeae (Papilionoideae). Plants, 13(17), 2380. https://doi.org/10.3390/plants13172380






