Phenological Analysis of Grasses (Poaceae) in Comparison with Aerobiological Data in Moscow (Russia)
Abstract
:1. Introduction
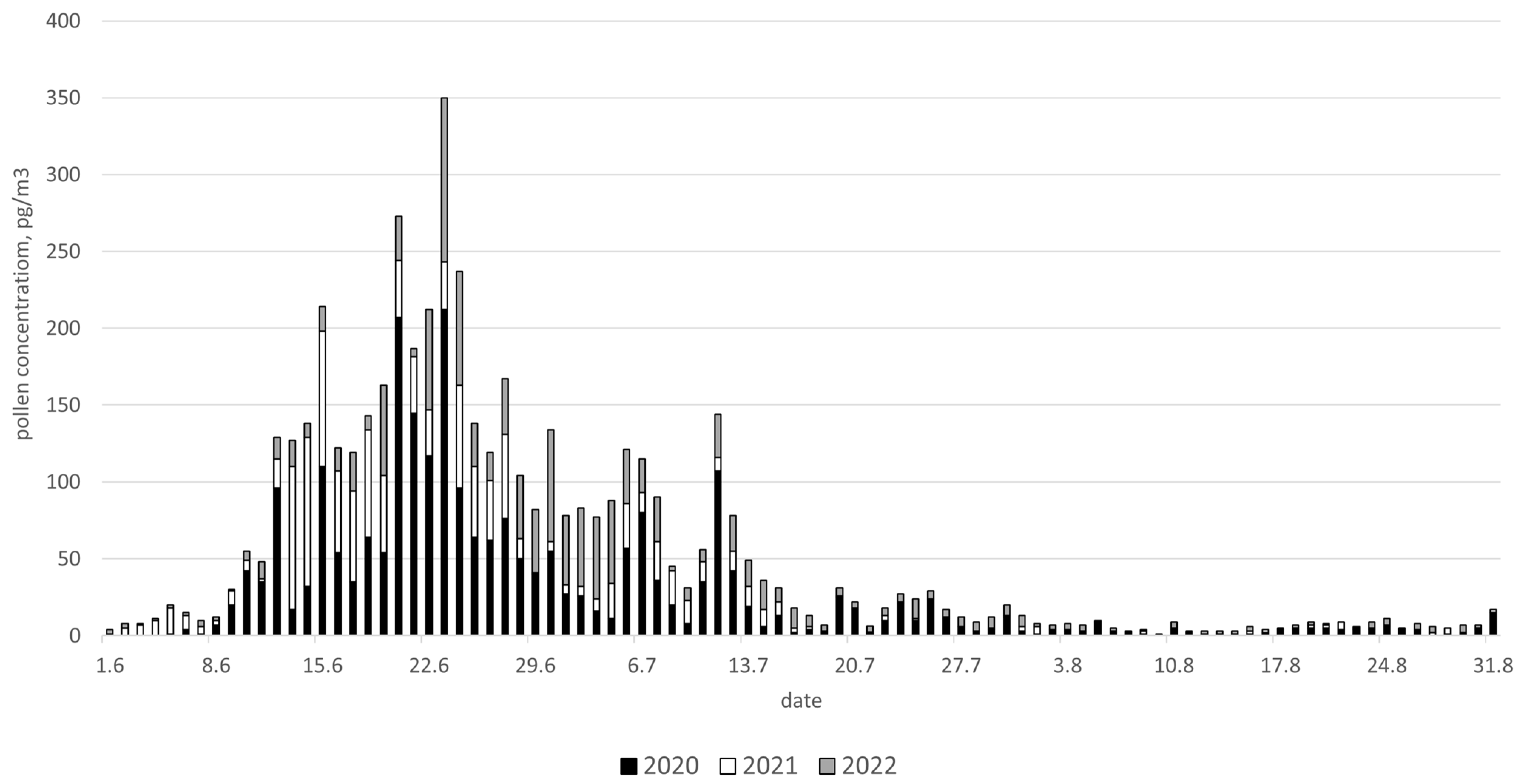
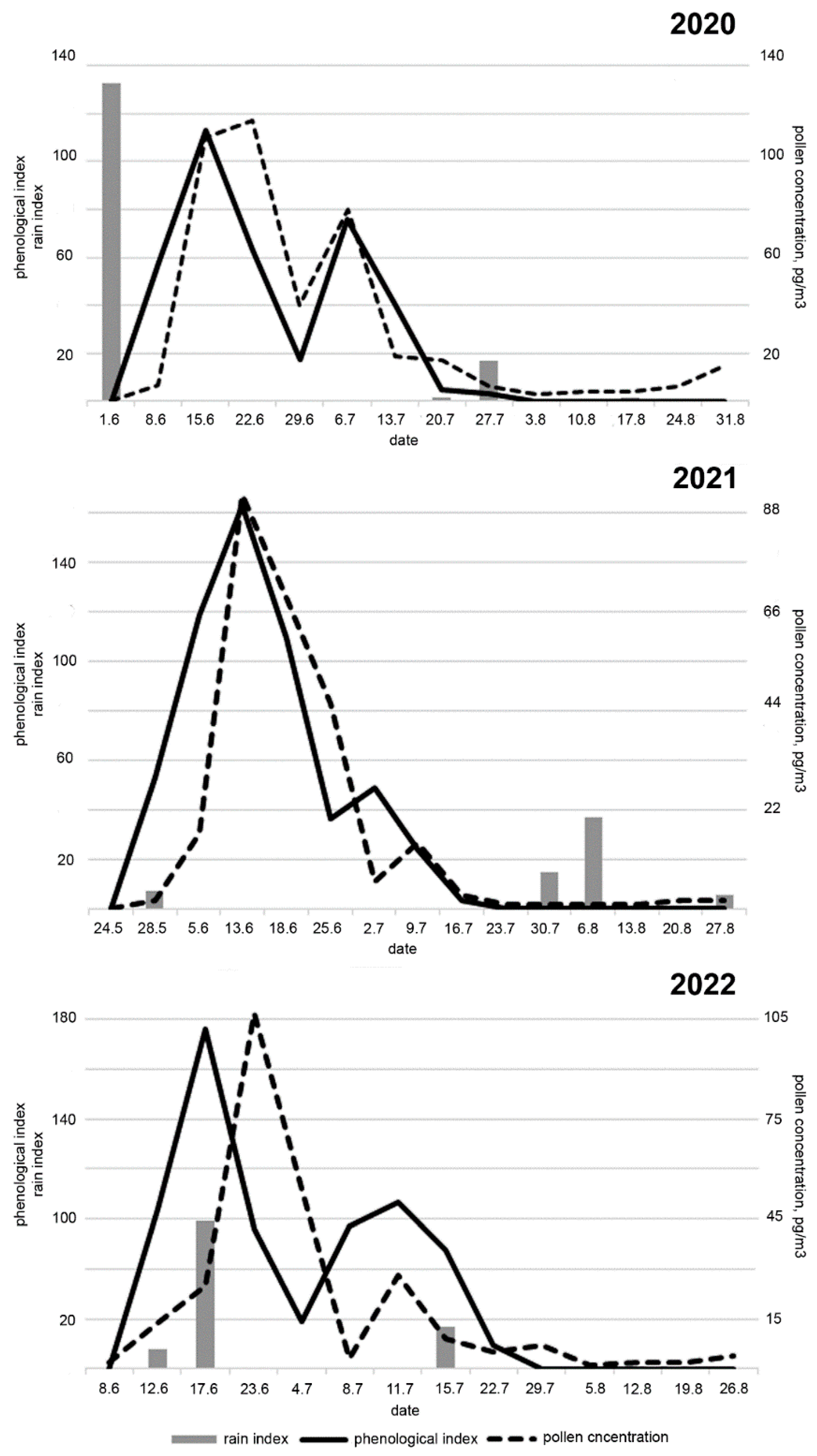
2. Results
3. Discussion
4. Material and Methods
4.1. Aerobiological Data
4.2. Meteorological Data
4.3. Phenological Data
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kellogg, E.A. Poaceae. In The Families and Genera of Vascular Plants; Springer: Berlin, Germany, 2015; Volume XIII, pp. 1–416. [Google Scholar]
- Soreng, R.J.; Peterson, P.M.; Zuloaga, F.O.; Romaschenko, K.; Clark, L.G.; Teisher, J.K.; Gillespie, L.J.; Barberá, P.; Welker, C.A.D.; Kellogg, E.A.; et al. A Worldwide Phylogenetic Classification of the Poaceae (Gramineae) III: An Update. J. Syst. Evol. 2022, 60, 476–521. [Google Scholar] [CrossRef]
- Watson, L. The Grass Family, Poaceae. In Reproductive Versatility in the Grasses; Cambridge University Press: New York, NY, USA, 1990; pp. 1–31. [Google Scholar]
- McKevith, B. Nutritional Aspects of Cereals. Nutr. Bull. 2004, 29, 111–142. [Google Scholar] [CrossRef]
- Laskowski, W.; Górska-Warsewicz, H.; Rejman, K.; Czeczotko, M.; Zwolińska, J. How Important Are Cereals and Cereal Products in the Average Polish Diet? Nutrients 2019, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Linder, H.P.; Rudall, P.J. Evolutionary History of Poales. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 107–124. [Google Scholar] [CrossRef]
- Connor, H.E. Breeding Systems in the Grasses: A Survey. N. Z. J. Bot. 1979, 17, 547–574. [Google Scholar] [CrossRef]
- Adams, D.E.; Perkins, W.E.; Estes, J.R. Pollination Systems in Paspalum dilatatum Poir. (Poaceae): An Example of Insect Pollination in a Temperate Grass. Am. J. Bot. 1981, 68, 389–394. [Google Scholar] [CrossRef]
- Smart, I.J.; Tuddenham, W.G.; Knox, R.B. Aerobiology of Grass Pollen in the City Atmosphere of Melbourne: Effects of Weather Parameters and Pollen Sources. Aust. J. Bot. 1979, 27, 333–342. [Google Scholar] [CrossRef]
- Prieto-Baena, J.C.; Hidalgo, P.J.; Domínguez, E.; Galán, C. Pollen Production in the Poaceae Family. Grana 2003, 42, 153–159. [Google Scholar] [CrossRef]
- Piotrowska, K. Pollen Production in Selected Species of Anemophilous Plants. Acta Agrobot. 2008, 61, 41–52. [Google Scholar] [CrossRef]
- Aboulaich, N.; Bouziane, H.; Kadiri, M.; Mar Trigo, M.; Riadi, H.; Kazzaz, M.; Merzouki, A. Pollen Production in Anemophilous Species of the Poaceae Family in Tetouan (NW Morocco). Aerobiologia 2009, 25, 27–38. [Google Scholar] [CrossRef]
- Tormo-Molina, R.; Maya-Manzano, J.-M.; Silva-Palacios, I.; Fernández-Rodríguez, S.; Gonzalo-Garijo, Á. Flower Production and Phenology in Dactylis glomerata. Aerobiologia 2015, 31, 469–479. [Google Scholar] [CrossRef]
- Severova, E.; Kopylov-Guskov, Y.; Selezneva, Y.; Karaseva, V.; Yadav, S.R.; Sokoloff, D. Pollen Production of Selected Grass Species in Russia and India at the Levels of Anther, Flower and Inflorescence. Plants 2022, 11, 285. [Google Scholar] [CrossRef]
- D’Amato, G.; Cecchi, L.; Bonini, S.; Nunes, C.; Annesi-Maesano, I.; Behrendt, H.; Liccardi, G.; Popov, T.; Van Cauwenberge, P. Allergenic Pollen and Pollen Allergy in Europe. Allergy 2007, 62, 976–990. [Google Scholar] [CrossRef]
- García-Mozo, H. Poaceae Pollen as the Leading Aeroallergen Worldwide: A Review. Allergy 2017, 72, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.; Lidholm, J. Characteristics and Immunobiology of Grass Pollen Allergens. Int. Arch. Allergy Immunol. 2003, 130, 87–107. [Google Scholar] [CrossRef]
- Hrabina, M.; Peltre, G.; Van Ree, R.; Moingeon, P. Grass Pollen Allergens. Clin. Exp. Allergy Rev. 2008, 8, 7–11. [Google Scholar] [CrossRef]
- Bauchau, V.; Durham, S.R. Prevalence and Rate of Diagnosis of Allergic Rhinitis in Europe. Eur. Respir. J. 2004, 24, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, P.-J.; Chinn, S.; Janson, C.; Kogevinas, M.; Burney, P.; Jarvis, D. Geographical Variation in the Prevalence of Positive Skin Tests to Environmental Aeroallergens in the European Community Respiratory Health Survey I. Allergy 2007, 62, 301–309. [Google Scholar] [CrossRef]
- Hirst, J. An Automatic Volumetric Spore Trap. Ann. Appl. Biol. 1952, 39, 257–265. [Google Scholar] [CrossRef]
- Driessen, M.N.B.M.; Willemse, M.T.M.; Van Luijn, J.A.G. Grass Pollen Grain Determination by Light- and UV-Microscopy. Grana 1989, 28, 115–122. [Google Scholar] [CrossRef]
- Weber, R.W. Patterns of Pollen Cross-Allergenicity. J. Allergy Clin. Immunol. 2003, 112, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Lockey, R.F.; Bukantz, S.C.; Bousquet, J. Allergens and Allergen Immunotherapy; Marcel Dekker: New York, NY, USA, 2004; ISBN 0-8247-5650-9. [Google Scholar]
- Weger, L.A.; Beerthuizen, T.; Gast-Strookman, J.M.; Plas, D.T.; Terreehorst, I.; Hiemstra, P.S.; Sont, J.K. Difference in Symptom Severity between Early and Late Grass Pollen Season in Patients with Seasonal Allergic Rhinitis. Clin. Transl. Allergy 2011, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.; Hawranek, T.; Gruber, P.; Wopfner, N.; Mari, A. Allergic Cross-reactivity: From Gene to the Clinic. Allergy 2004, 59, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Johansen, N.; Weber, R.W.; Ipsen, H.; Barber, D.; Broge, L.; Hejl, C. Extensive IgE Cross-Reactivity towards the Pooideae Grasses Substantiated for a Large Number of Grass-Pollen-Sensitized Subjects. Int. Arch. Allergy Immunol. 2009, 150, 325–334. [Google Scholar] [CrossRef]
- Frenguelli, G.; Passalacqua, G.; Bonini, S.; Fiocchi, A.; Incorvaia, C.; Marcucci, F.; Tedeschini, E.; Canonica, G.W.; Frati, F. Bridging Allergologic and Botanical Knowledge in Seasonal Allergy: A Role for Phenology. Ann. Allergy. Asthma. Immunol. 2010, 105, 223–227. [Google Scholar] [CrossRef]
- Ghitarrini, S.; Galán, C.; Frenguelli, G.; Tedeschini, E. Phenological Analysis of Grasses (Poaceae) as a Support for the Dissection of Their Pollen Season in Perugia (Central Italy). Aerobiologia 2017, 33, 339–349. [Google Scholar] [CrossRef]
- Kraaijeveld, K.; de Weger, L.A.; Ventayol García, M.; Buermans, H.; Frank, J. Hiemstra 452 PS, Den Dunnen JT. Efficient and Sensitive Identification and Quantification of 453 Airborne Pollen Using next-Generation DNA Sequencing. Mol. Ecol. Resour. 2015, 15, 8–16. [Google Scholar]
- Behzad, H.; Gojobori, T.; Mineta, K. Challenges and Opportunities of Airborne Metagenomics. Genome Biol. Evol. 2015, 7, 1216–1226. [Google Scholar] [CrossRef]
- Bell, K.L.; De Vere, N.; Keller, A.; Richardson, R.T.; Gous, A.; Burgess, K.S.; Brosi, B.J. Pollen DNA Barcoding: Current Applications and Future Prospects. Genome 2016, 59, 629–640. [Google Scholar] [CrossRef]
- Ghitarrini, S.; Pierboni, E.; Rondini, C.; Tedeschini, E.; Tovo, G.R.; Frenguelli, G.; Albertini, E. New Biomolecular Tools for Aerobiological Monitoring: Identification of Major Allergenic Poaceae Species through Fast Real-time PCR. Ecol. Evol. 2018, 8, 3996–4010. [Google Scholar] [CrossRef]
- Leontidou, K.; Vernesi, C.; De Groeve, J.; Cristofolini, F.; Vokou, D.; Cristofori, A. Metabarcoding Airborne Pollen from Subtropical and Temperate Eastern Australia over Multiple Years Reveals Pollen Aerobiome Diversity and Complexity. Aerobiologia 2018, 34, 63–74. [Google Scholar] [CrossRef]
- Brennan, G.L.; Potter, C.; De Vere, N.; Griffith, G.W.; Skjøth, C.A.; Osborne, N.J.; Wheeler, B.W.; McInnes, R.N.; Clewlow, Y.; Barber, A. Temperate Airborne Grass Pollen Defined by Spatio-Temporal Shifts in Community Composition. Nat. Ecol. Evol. 2019, 3, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.; Al Kouba, J.; Timbrell, V.; Noor, M.J.; Massel, K.; Gilding, E.K.; Angel, N.; Kemish, B.; Hugenholtz, P.; Godwin, I.D. Tracking Seasonal Changes in Diversity of Pollen Allergen Exposure: Targeted Metabarcoding of a Subtropical Aerobiome. Sci. Total Environ. 2020, 747, 141189. [Google Scholar] [CrossRef] [PubMed]
- Polling, M.; Sin, M.; de Weger, L.A.; Speksnijder, A.G.C.L.; Koenders, M.J.F.; de Boer, H.; Gravendeel, B. DNA Metabarcoding Using nrITS2 Provides Highly Qualitative and Quantitative Results for Airborne Pollen Monitoring. Sci. Total Environ. 2022, 806, 150468. [Google Scholar] [CrossRef] [PubMed]
- Omelchenko, D.O.; Krinitsina, A.A.; Kasianov, A.S.; Speranskaya, A.S.; Chesnokova, O.V.; Polevova, S.V.; Severova, E.E. Assessment of ITS1, ITS2, 5′-ETS, and trnL-F DNA Barcodes for Metabarcoding of Poaceae Pollen. Diversity 2022, 14, 191. [Google Scholar] [CrossRef]
- Latorre, F.; Bianchi, M.M. Relationships between Flowering Development of Ulmus pumila and Fraxinus excelsior and Their Airborne Pollen. Grana 1998, 37, 233–238. [Google Scholar] [CrossRef]
- Jato, V.; Rodríguez-Rajo, F.J.; Seijo, M.C.; Aira, M.J. Poaceae Pollen in Galicia (NW Spain): Characterisation and Recent Trends in Atmospheric Pollen Season. Int. J. Biometeorol. 2009, 53, 333–344. [Google Scholar] [CrossRef]
- Orlandi, F.; Ruga, L.; Romano, B.; Fornaciari, M. An Integrated Use of Aerobiological and Phenological Data to Analyse Flowering in Olive Groves. Grana 2005, 44, 51–56. [Google Scholar] [CrossRef]
- León-Ruiz, E.; Alcázar, P.; Domínguez-Vilches, E.; Galán, C. Study of Poaceae Phenology in a Mediterranean Climate. Which Species Contribute Most to Airborne Pollen Counts? Aerobiologia 2011, 27, 37–50. [Google Scholar] [CrossRef]
- Tormo, R.; Silva, I.; Gonzalo, Á.; Moreno, A.; Pérez, R.; Fernández, S. Phenological Records as a Complement to Aerobiological Data. Int. J. Biometeorol. 2011, 55, 51–65. [Google Scholar] [CrossRef]
- Cebrino, J.; Galán, C.; Domínguez-Vilches, E. Aerobiological and Phenological Study of the Main Poaceae Species in Córdoba City (Spain) and the Surrounding Hills. Aerobiologia 2016, 32, 595–606. [Google Scholar] [CrossRef]
- Kmenta, M.; Bastl, K.; Kramer, M.F.; Hewings, S.J.; Mwange, J.; Zetter, R.; Berger, U. The Grass Pollen Season 2014 in Vienna: A Pilot Study Combining Phenology, Aerobiology and Symptom Data. Sci. Total Environ. 2016, 566–567, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Office of the Federal State Statistics Service for Moscow and the Moscow Region. Available online: https://mosstat.gks.ru/ (accessed on 16 December 2022).
- Volkova, O.; Severova, E. Poaceae Pollen Season and Associations with Meteorological Parameters in Moscow, Russia, 1994–2016. Aerobiologia 2019, 35, 73–84. [Google Scholar] [CrossRef]
- Jäger, S.; Spieksma, E.T.M.; Nolard, N. Fluctuations and Trends in Airborne Concentrations of Some Abundant Pollen Types, Monitored at Vienna, Leiden, and Brussels. Grana 1991, 30, 309–312. [Google Scholar] [CrossRef]
- Spieksma, F.M.; Nikkels, A.H. Airborne Grass Pollen in Leiden, The Netherlands: Annual Variations and Trends in Quantities and Season Starts over 26 Years. Aerobiologia 1998, 14, 347–358. [Google Scholar] [CrossRef]
- Emberlin, J.; Mullins, J.; Corden, J.; Jones, S.; Millington, W.; Brooke, M.; Savage, M. Regional Variations in Grass Pollen Seasons in the UK, Long-Term Trends and Forecast Models. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 1999, 29, 347–356. [Google Scholar] [CrossRef]
- Detandt, M.; Nolard, N. The Fluctuations of the Allergenic Pollen Content of the Air in Brussels (1982 to 1997). Aerobiologia 2000, 16, 55–61. [Google Scholar] [CrossRef]
- Voltolini, S.; Minale, P.; Troise, C.; Bignardi, D.; Modena, P.; Arobba, D.; Negrini, A.C. Trend of Herbaceous Pollen Diffusion and Allergic Sensitisation in Genoa, Italy. Aerobiologia 2000, 16, 245–249. [Google Scholar] [CrossRef]
- Spieksma, F.T.M.; Corden, J.M.; Detandt, M.; Millington, W.M.; Nikkels, H.; Nolard, N.; Schoenmakers, C.H.H.; Wachter, R.; De Weger, L.A.; Willems, R. Quantitative Trends in Annual Totals of Five Common Airborne Pollen Types (Betula, Quercus, Poaceae, Urtica, and Artemisia), at Five Pollen-Monitoring Stations in Western Europe. Aerobiologia 2003, 19, 171–184. [Google Scholar] [CrossRef]
- Emberlin, J.; Savage, M.; Jones, S. Annual Variations in Grass Pollen Seasons in London 1961–1990: Trends and Forecast Models. Clin. Exp. Allergy 1993, 23, 911–918. [Google Scholar] [CrossRef]
- Green, B.J.; Dettmann, M.; Yli-Panula, E.; Rutherford, S.; Simpson, R. Atmospheric Poaceae Pollen Frequencies and Associations with Meteorological Parameters in Brisbane, Australia: A 5-Year Record, 1994–1999. Int. J. Biometeorol. 2004, 48, 172–178. [Google Scholar] [CrossRef]
- Myszkowska, D. Poaceae Pollen in the Air Depending on the Thermal Conditions. Int. J. Biometeorol. 2014, 58, 975–986. [Google Scholar] [CrossRef]
- Subiza, J.; Masiello, J.M.; Subiza, J.L.; Jerez, M.; Hinojosa, M.; Subiza, E. Prediction of Annual Variations in Atmospheric Concentrations of Grass Pollen. A Method Based on Meteorological Factors and Grain Crop Estimates. Clin. Exp. Allergy 1992, 22, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Garnier, E.; Lavorel, S.; Ansquer, P.; Castro, H.; Cruz, P.; Dolezal, J.; Eriksson, O.; Fortunel, C.; Freitas, H.; Golodets, C. Assessing the Effects of Land-Use Change on Plant Traits, Communities and Ecosystem Functioning in Grasslands: A Standardized Methodology and Lessons from an Application to 11 European Sites. Ann. Bot. 2007, 99, 967–985. [Google Scholar] [CrossRef] [PubMed]
- Severova, E.; Volkova, O. Sampling Height in Aerobiological Monitoring. In Proceedings of the 11th International Congress on Aerobiology, Parma, Italy, 3–7 September 2018; Programme & Abstract book. p. 74. [Google Scholar]
- Dmitriev, A.; Bessonov, N. (Eds.) Climate of Moscow; Hydrometeoizdat: Leningrad, Russia, 1969. [Google Scholar]
- Petersen, A.; Becker, W.-M.; Schlaak, M. Characterization of Grass Group I Allergens in Timothy Grass Pollen. J. Allergy Clin. Immunol. 1993, 92, 789–796. [Google Scholar] [CrossRef]
- Ghunaim, N.; Grönlund, H.; Kronqvist, M.; Grönneberg, R.; Söderström, L.; Ahlstedt, S.; Van Hage-Hamsten, M. Antibody Profiles and Self-reported Symptoms to Pollen-related Food Allergens in Grass Pollen-allergic Patients from Northern Europe. Allergy 2005, 60, 185–191. [Google Scholar] [CrossRef]
- Galán, C.; Smith, M.; Thibaudon, M.; Frenguelli, G.; Oteros, J.; Gehrig, R.; Berger, U.; Clot, B.; Brandao, R.; EAS QC Working Group. Minimum Requirements and Reproducibility of Analysis. Aerobiologia 2014, 30, 385–395. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants; Blackwell Wissenschafts: Berlin, Germany, 1997; ISBN 3-8263-3152-4. [Google Scholar]
- Baumann, U.; Juttner, J.; Bian, X.; Langridge, P. Self-Incompatibility in the Grasses. Ann. Bot. 2000, 85, 203–209. [Google Scholar] [CrossRef]
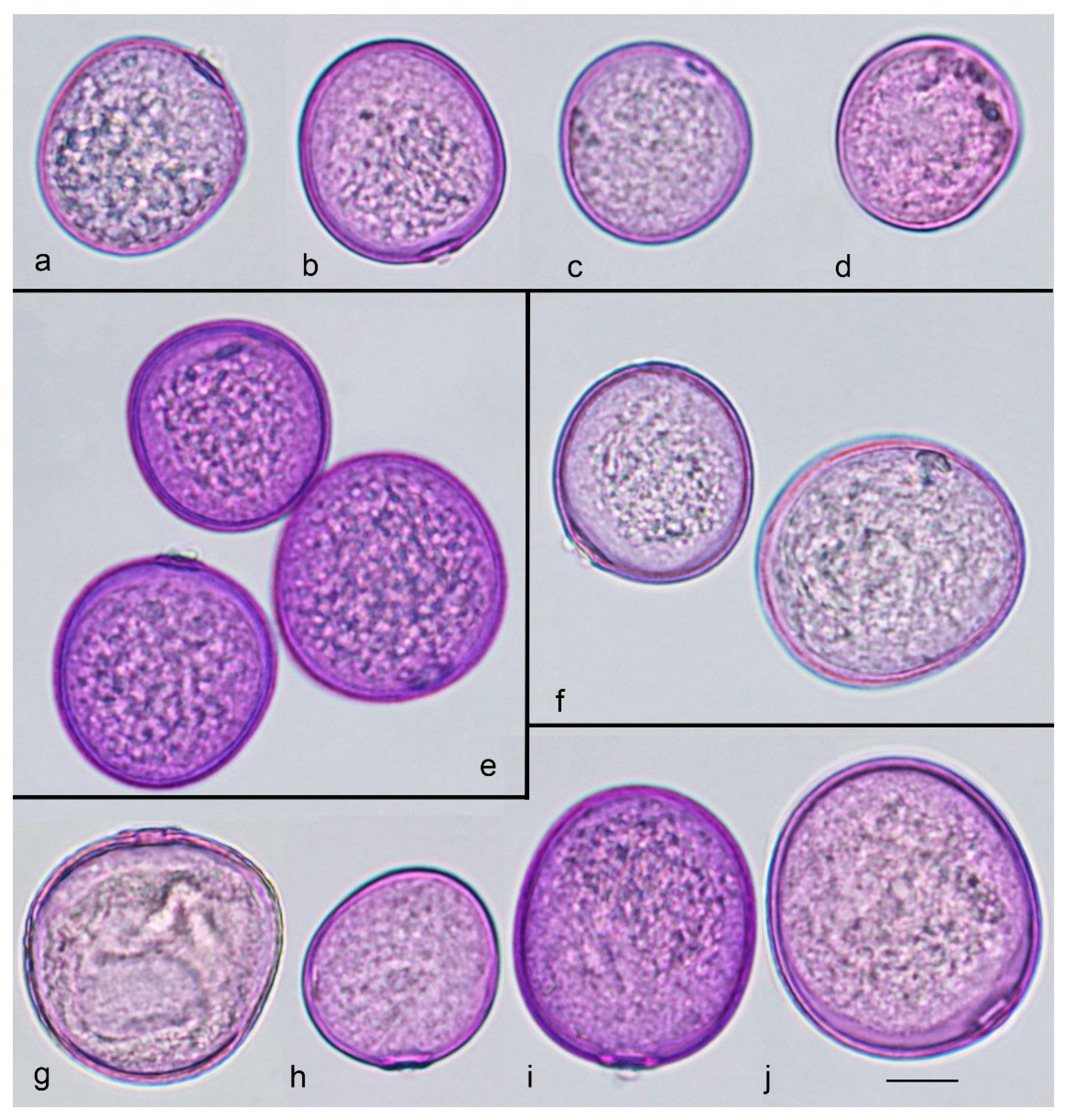


| Taxon | Contribution to the Cumulative Phenological Index, % | ||
|---|---|---|---|
| 2020 | 2021 | 2022 | |
| Festuca pratensis | 11 | 12 | |
| Dactylis glomerata | 18 | 33 | 24 |
| Calamagrostis epigeios | 30 | 12 | 28 |
| Poa pratensis | 12 | 18 | |
| Phleum pratense | 11 | ||
| Sample Plot | Type of Vegetation | Taxa of Grasses Identified in the Phenological Survey (Prevalent Species in Bold) | Distance from the Trap Location |
|---|---|---|---|
| 1 | Lawn | Dactylis glomerata Festuca pratensis Lolium perenne Phleum pratense Poa pratensis | 160 m |
| 2 | Waste ground | Calamagrostis epigeios Dactylis glomerata Elymus repens Festuca pratensis Phleum pratense Poa pratensis Poa trivialis | 60 m |
| 3 | Waste ground | Calamagrostis epigeios Elymus repens Poa pratensis Poa trivialis | 80 m |
| 4 | Lawn | Festuca pratensis Lolium perenne Phleum pratense Poa pratensis | 36 m |
| 5 | Lawn | Arrhenatherum elatius Dactylis glomerata Elymus repens Festuca pratensis Lolium perenne Phleum pratense Poa pratensis | 10 m |
| 6 | Unmown area in the Moscow University Botanical Garden | Arrhenatherum elatius Bromopsis inermis Calamagrostis epigeios Dactylis glomerata Elymus repens Festuca pratensis Lolium perenne Phleum pratense Poa pratensis Poa trivialis | 200 m |
| 7 | Lawn | Arrhenatherum elatius Dactylis glomerata Festuca pratensis Poa pratensis Poa trivialis | 530 m |
| 8 | Waste ground | Bromopsis inermis Calamagrostis epigeios Dactylis glomerata Elymus repens Festuca pratensis Poa pratensis Poa trivialis | 890 m |
| 9 | Lawn | Bromopsis inermis Dactylis glomerata Elymus repens Festuca pratensis Lolium perenne Phleum pratense Poa pratensis | 14.5 km |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Severova, E.E.; Karaseva, V.S.; Selezneva, Y.M.; Polevova, S.S. Phenological Analysis of Grasses (Poaceae) in Comparison with Aerobiological Data in Moscow (Russia). Plants 2024, 13, 2384. https://doi.org/10.3390/plants13172384
Severova EE, Karaseva VS, Selezneva YM, Polevova SS. Phenological Analysis of Grasses (Poaceae) in Comparison with Aerobiological Data in Moscow (Russia). Plants. 2024; 13(17):2384. https://doi.org/10.3390/plants13172384
Chicago/Turabian StyleSeverova, Elena E., Vera S. Karaseva, Yulia M. Selezneva, and Svetlana S. Polevova. 2024. "Phenological Analysis of Grasses (Poaceae) in Comparison with Aerobiological Data in Moscow (Russia)" Plants 13, no. 17: 2384. https://doi.org/10.3390/plants13172384





