Heterologous Expression of Sunflower HaHPT and HaTMT Genes Enhances Rice-Grain Vitamin E Content
Abstract
:1. Introduction
2. Results
2.1. HPLC Analysis of Vitamin E Isomers
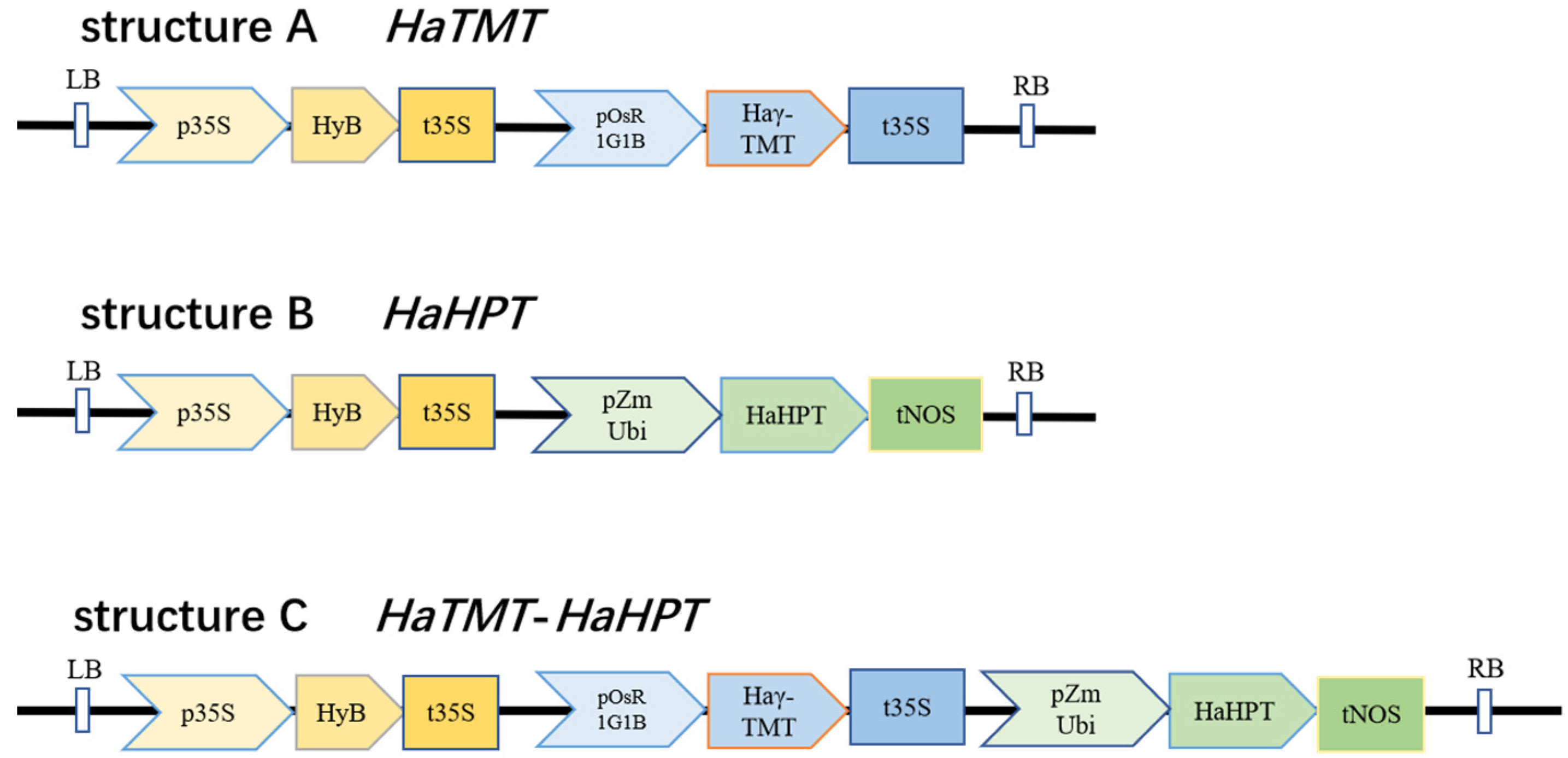
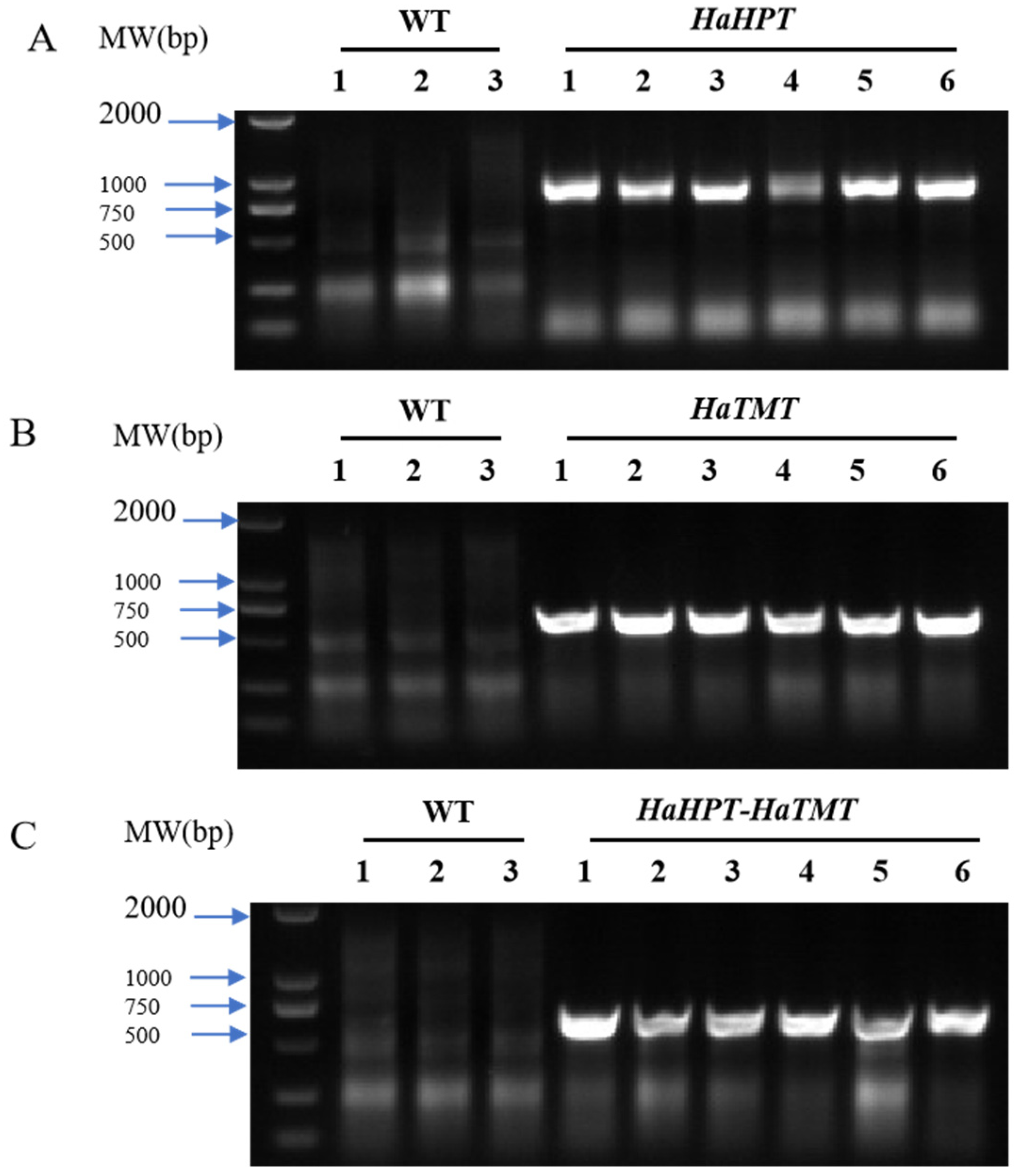
2.2. Acquisition and Identification of Transgenic Plants
2.3. Relative Expression Analysis of Transgenic Plants
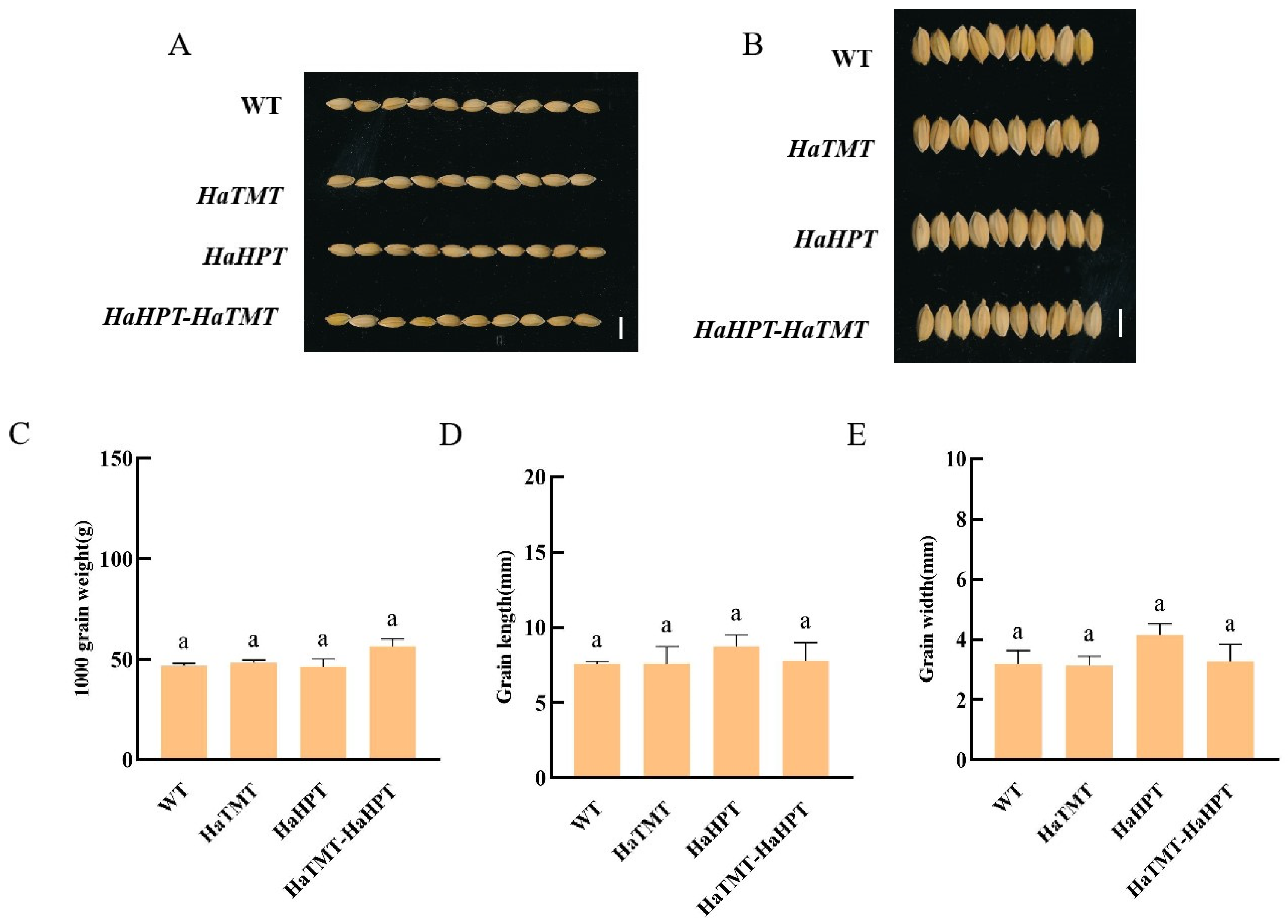
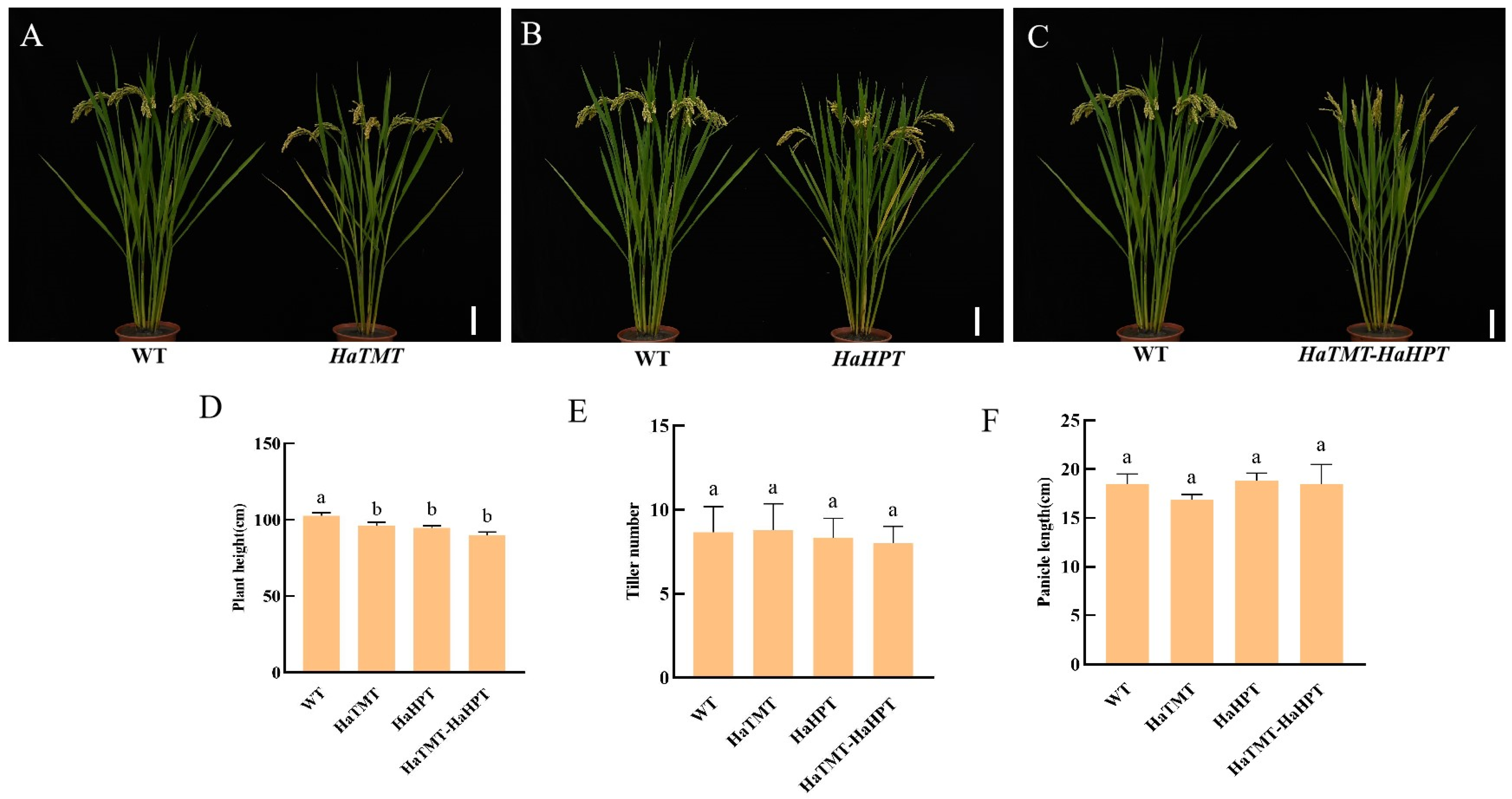
2.4. Changes of Main Agronomic Traits of Transgenic Plants
2.5. Vitamin E Content in Transgenic Plants
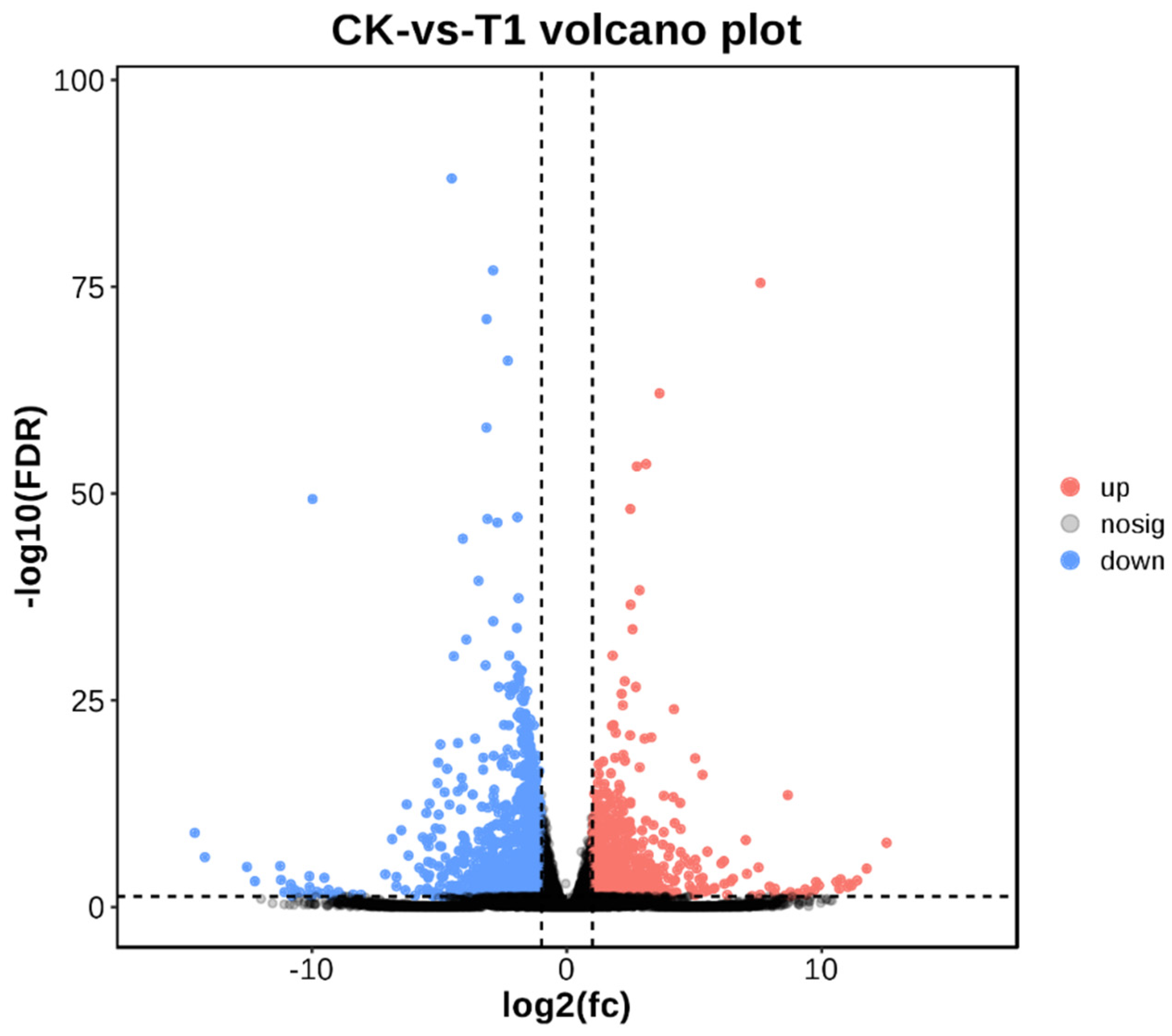
2.6. Transcriptome and Expression Analysis
2.6.1. Number of Differentially Express Genes (DEG) Statistics
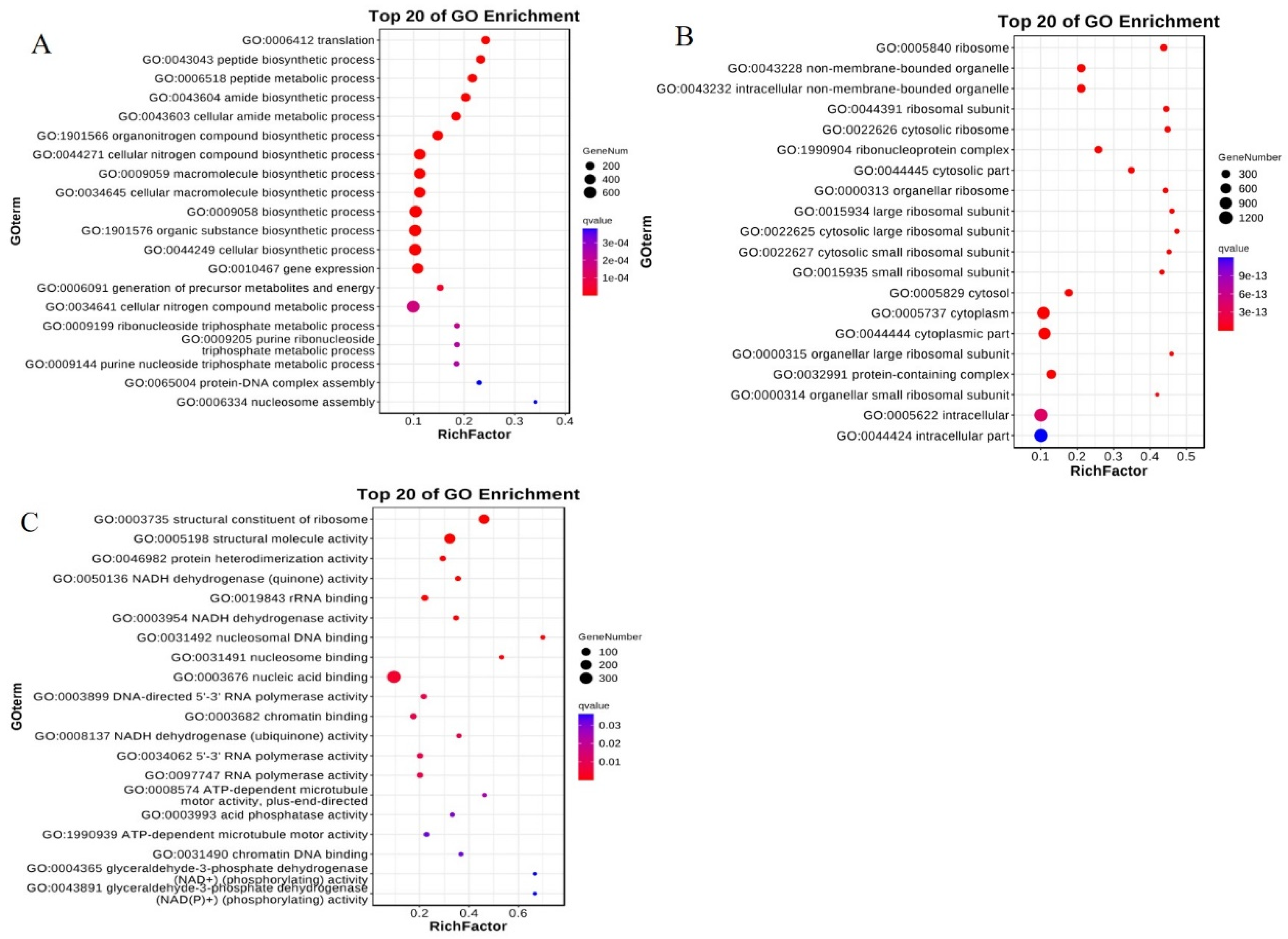
2.6.2. GO Enrichment Analysis of Differentially Expressed Genes
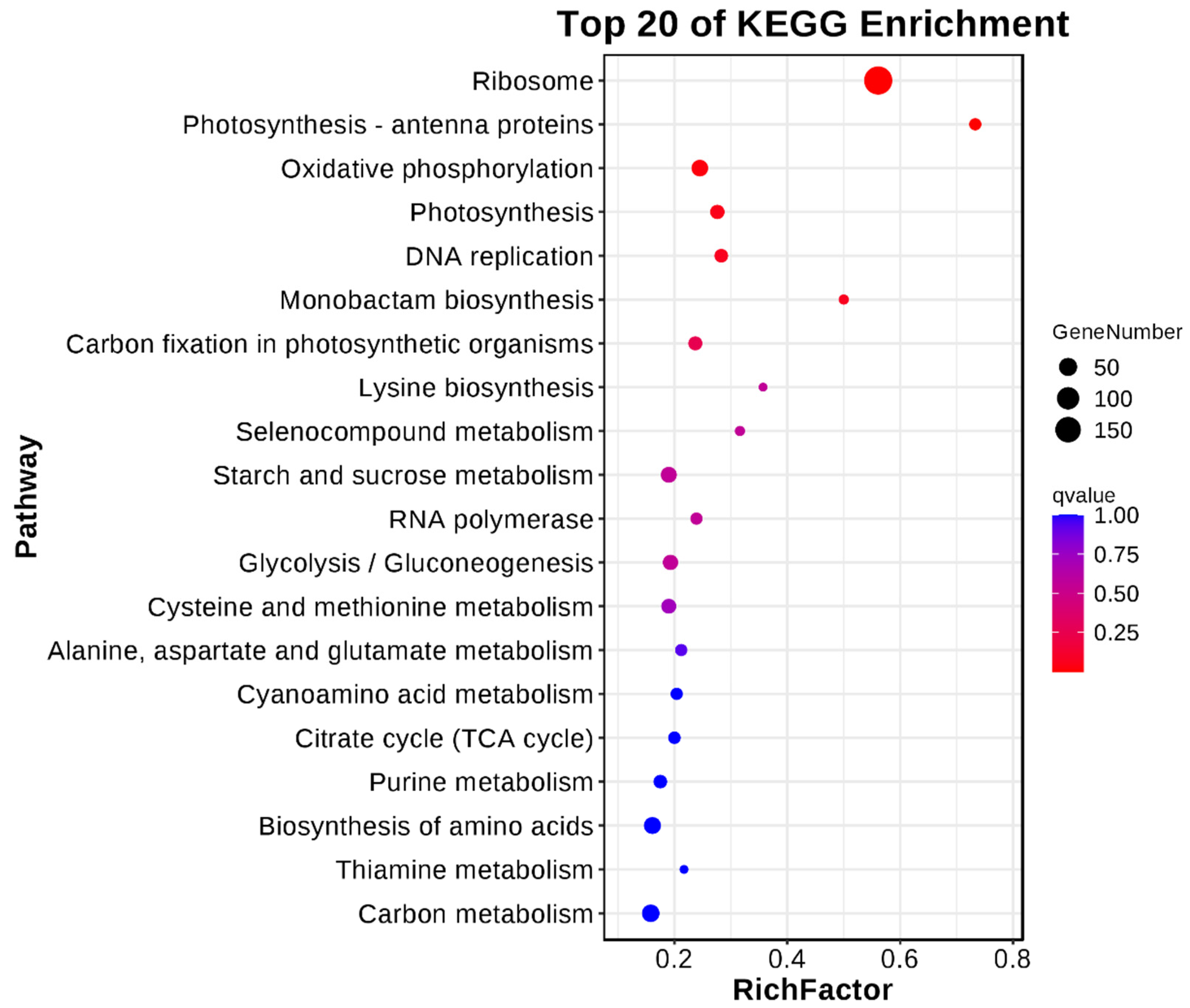
2.6.3. KEGG Enrichment Analysis of Differentially Expressed Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Strains, Carriers and Main Reagents
4.3. Primer Design
4.4. Vector Construction
4.5. Identification of Transgenic Rice
4.6. qRT-PCR Analysis
4.7. Extraction of Vitamin E
4.8. HPLC Analysis
4.9. Transcriptome Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, M.C.; Lin, Y.J.; Chen, H. Improving nutritional quality of rice for human health. Theor. Appl. Genet. 2020, 133, 1397–1413. [Google Scholar] [CrossRef]
- Lown, N.M. The global challenge of hidden hunger: Perspectives from the field. Proc. Nutr. Soc. 2021, 80, 283–289. [Google Scholar]
- Weffort, V.R.S.; Lamounier, J.A. Hidden hunger—A narrative review. J. Pediatr. (Rio. J.) 2024, 100, S10–S17. [Google Scholar] [CrossRef] [PubMed]
- Mène-Saffrané, L. Vitamin E biosynthesis and its regulation in plants. Antioxidants 2018, 7, 2. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and tocotrienols—Bioactive dietary compounds; what is certain, what is doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef]
- Straeten, D.V.D.; Bhullar, N.K.; Steur, H.D.; Gruissem, W.; MacKenzie, D.; Pfeiffer, W.; Qaim, M.; Slamet-Loedin, I.; Strobbe, S.; Tohme, J.; et al. Multiplying the efficiency and impact of biofortification through metabolic engineering. Nat. Commun. 2020, 11, 5203. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Zeng, D.C.; Yu, S.Z.; Cui, C.J.; Li, J.M.; Li, H.Y.; Chen, J.Y.; Zhang, R.Z.; Zhao, X.C.; Chen, L.T.; et al. From golden rice to aSTARice: Bioengineering astaxanthin biosynthesis in rice endosperm. Mol. Plant 2018, 11, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- DellaPenna, D.; Pogson, B.J. Vitamin synthesis in plants: Tocopherols and carotenoids. Annu. Rev. Plant Biol. 2006, 57, 711–738. [Google Scholar] [CrossRef] [PubMed]
- Panfili, G.; Fratianni, A.; Irano, M. Normal phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. J. Agric. Food Chem. 2003, 51, 3940–3944. [Google Scholar] [CrossRef]
- Britz, S.J.; Prasad, P.V.V.; Moreau, R.A. Influence of growth temperature on the amounts of tocopherols, tocotrienols, and γ-Oryzanol in brown rice. J. Agric. Food Chem. 2007, 55, 7559–7565. [Google Scholar] [CrossRef]
- Grilo, E.C.; Costa, P.N.; Gurgel, C.S.S. Alpha-tocopherol and gamma-tocopherol concentration in vegetable oils. Food Sci. Technol. 2014, 34, 379–385. [Google Scholar] [CrossRef]
- Sattler, S.E.; Gilliland, L.U.; Magallanes-Lundback, M.; Pollard, M.; DellaPenna, D. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 2004, 16, 1419–1432. [Google Scholar] [CrossRef]
- Muñoz, P.; Munné-Bosch, S. Vitamin E in plants: Biosynthesis, transport, and function. Trends Plant Sci. 2019, 24, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Somsri, A.; Chu, S.H.; Nawade, B.; Lee, C.Y.; Park, Y.J. Harnessing γ-TMT genetic variations and haplotypes for vitamin E diversity in the Korean rice collection. Antioxidants 2024, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- MèneSaffrané, L.; Pellaud, S. Current strategies for vitamin E biofortification of crops. Curr. Opin. Biotechnol. 2017, 44, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Mène-Saffrané, L.; Jones, A.D.; DellaPenna, D. Plastochromanol-8 and tocopherols are essential lipid-soluble antioxidants during seed desiccation and quiescence in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 17815–17820. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.; Kumar, V.; Kumar, A.; Bansal, N.; Vinutha, T.; Ali, K.; Sachdev, A.; Kumari, S.; Dahuja, A. Molecular cloning and functional analysis of the promoter of γ-Tocopherol Methyl Transferase (γ-TMT) gene of soybean (Glycine max). 3 Biotech 2018, 8, 325. [Google Scholar] [CrossRef]
- Collakova, E.; DellaPenna, D. Homogentisate phytyltransferase activity is limiting for tocopherol biosynthesis in Arabidopsis. Plant Physiol. 2003, 131, 632–642. [Google Scholar] [CrossRef]
- Collakova, E.; DellaPenna, D. The role of homogentisate phytyltransferase and other tocopherol pathway enzymes in the regu lation of tocopherol synthesis during abiotic stress. Plant Physiol. 2003, 133, 930–940. [Google Scholar] [CrossRef]
- Alberta, E.; Kima, S.; Magallanes-Lundbacka, M.; Bao, Y.; Deason, N.; Danilo, B.; Wu, D.; Li, X.W.; Wood, J.C.; Bornowski, N.; et al. Genome-wide association identifies a missing hydrolase for tocopherol synthesis in plants. Proc. Natl. Acad. Sci. USA 2022, 119, e2113488119. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, W.; Ren, G.; Li, D.L.; Cahoon, R.E.; Chen, M.; Zhou, Y.M.; Yu, B.; Cahoon, E.B. Chlorophyll synthase under epigenetic surveillance is critical for vitamin E synthesis, and altered expression affects tocopherol levels in Arabidopsis. Plant Physiol. 2015, 168, 1503–1511. [Google Scholar] [CrossRef]
- Diepenbrock, C.H.; Kandianis, C.B.; Lipka, A.E.; Vaillancourt, B.; Wallace, J.G.; Cepela, J.; Dellapenna, D. Novel loci underlie natural variation in vitamin E levels in maize grain. Plant Cell. 2017, 29, 2374–2392. [Google Scholar] [CrossRef]
- Rey, F.; Zacarias, L.; Rodrigo, M.J. Regulation of tocopherol biosynthesis during fruit maturation of different citrus species. Front. Plant Sci. 2021, 12, 743993. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.N.; Zhang, Z.F.; Hu, Y.; Fang, C.; Xu, X.D.; Yuan, J.; Zhang, J.S.; Tian, Z.X.; Wang, G.D. Genome-wide scan for oil quality reveals a coregulation mechanism of tocopherols and fatty acids in soybean seeds. Plant Commun. 2023, 4, s2590–s3462. [Google Scholar] [CrossRef]
- Jon, F.; Mario, B.; Anke, S.F.; Braun, S.; Krupinska, K. Characterization of transplastomic tobacco plants with a plastid localized barley 4-hydroxy phenylpyruvate dioxygenase. J. Plant Physiol. 2005, 162, 738–742. [Google Scholar]
- Cela, J.; Chang, C.; Munne-Bosch, S. Accumulation of γ-rather than α-tocopherol alters ethylene signaling gene expression in the vte4 mutant of Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 1389–1400. [Google Scholar] [CrossRef]
- Quadrana, L.; Almeida, J.; Asıs, R.; Duffy, T.; Conti, G.; Peralta, I.E.; Colot, V. Natural occurring epialleles determine vitamin E accumulation in tomato fruits. Nat. Commun. 2014, 5, 4027. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.E.; Ahn, J.W.; Kwon, S.J.; Kim, J.B.; Kim, S.H.; Kang, S.Y.; Kim, D.S. Selection and molecular characterization of a high tocopherol accumulation rice mutant line induced by gamma irradiation. Mol. Biol. Rep. 2014, 41, 7671–7681. [Google Scholar] [CrossRef] [PubMed]
- Harish, M.C.; Dachinamoorthy, P.; Balamurugan, S. Overexpression of homogentisate phytyltransferase (HPT) and to copherol cyclase (TC) enhances α-tocopherol content in transgenic tobacco. Biol. Plantarum. 2013, 57, 395–400. [Google Scholar] [CrossRef]
- Harish, M.C.; Dachinamoorthy, P.; Balamurugan, S. Enhancement of a-tocopherol content through transgenic and cell suspension culture systems in tobacco. Acta Physiol. Planta. 2013, 35, 1121–1130. [Google Scholar] [CrossRef]
- Sathish, S.; Preethy, K.S.; Venkatesh, R.; Sathishkumar, R. Rapid enhancement of a-tocopherol content in Nicotiana benthamiana by transient expression of Arabidopsis thaliana tocopherol cyclase and homogentisatephytyl transferase genes. 3 Biotech 2018, 8, 485. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, S.; Rajendran, V.; Sivakumar, H.P.; Nayeem, S.; Chandra, H.M.; Sharma, A.; Ramalingam, S. Enhanced vitamin E content in an Indica rice cultivar harbouring two transgenes from Arabidopsis thaliana involved in tocopherol biosynthesis pathway. Plant Biotechnol. J. 2021, 19, 1083–1085. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.Z.; Liu, B.; Zhang, L.; Zhang, W.; Chen, R.M.; Wang, L. Overexpression of the maize γ-tocopherol methyltransferase gene (ZmTMT) increases α-tocopherol content in transgenic Arabidopsis and maize seeds. Transgenic Res. 2020, 29, 95–104. [Google Scholar] [CrossRef]
- Liu, N.N.; Du, Y.H.; Yan, S.J.; Chen, W.; Deng, M.; Xu, S.T.; Wang, H.; Zhan, W.; Huang, W.J.; Yin, Y.; et al. The light and hypoxia induced gene ZmPORB1 determines tocopherol content in the maize kernel. Sci. China Life Sci. 2024, 67, 435–448. [Google Scholar] [CrossRef]
- Weber, E.; Engler, C.; Gruetzner, R.; Werner, S.; Marillonnet, S. A modular cloning system for standardized assembly of multigene constructs. PLoS ONE 2011, 6, e16765. [Google Scholar] [CrossRef]
- Hiei, Y.; Ohta, S.; Komari, T.; Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994, 6, 271–282. [Google Scholar] [CrossRef]
- Li, J.L.; Wang, S.; Yu, J.; Wang, J. A modified CTAB protocol for plant DNA extraction. Chin. Bull. Bot. 2013, 48, 72–78. [Google Scholar]
- Liu, L.L.; You, J.; Zhu, Z.; Chen, K.Y.; Hu, M.M.; Gu, H.; Liu, Z.W.; Wang, Z.Y.; Wang, Y.H.; Liu, S.J.; et al. WHITE STRIPE LEAF8, encoding a deoxyribonucleoside kinase, is involved in chloroplast development in rice. Plant Cell Rep. 2020, 39, 19–33. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.82-2016; China Food and Drug Administration. National Food Safety Standards—Determination of Vitamins A, D, and E in Food. China Health and Family Planning Commission: Beijing, China, 2016.
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Li, H.; Lin, S.; Dong, X.; Tian, R.; Wu, Z.; Li, Q.; Li, M.; Zhang, K.; Liu, X.; et al. Heterologous Expression of Sunflower HaHPT and HaTMT Genes Enhances Rice-Grain Vitamin E Content. Plants 2024, 13, 2392. https://doi.org/10.3390/plants13172392
Song S, Li H, Lin S, Dong X, Tian R, Wu Z, Li Q, Li M, Zhang K, Liu X, et al. Heterologous Expression of Sunflower HaHPT and HaTMT Genes Enhances Rice-Grain Vitamin E Content. Plants. 2024; 13(17):2392. https://doi.org/10.3390/plants13172392
Chicago/Turabian StyleSong, Shuang, Hang Li, Shaoyan Lin, Xiaoou Dong, Ruiping Tian, Zewan Wu, Qing Li, Mingyi Li, Keying Zhang, Xi Liu, and et al. 2024. "Heterologous Expression of Sunflower HaHPT and HaTMT Genes Enhances Rice-Grain Vitamin E Content" Plants 13, no. 17: 2392. https://doi.org/10.3390/plants13172392





