Ecotones as Windows into Organismal-to-Biome Scale Responses across Neotropical Forests
Abstract
:1. Introduction: What Is an Ecotone?
2. Neotropical Ecotones: Our Current State of Understanding
3. Interesting Ecotones and Promising Research Questions
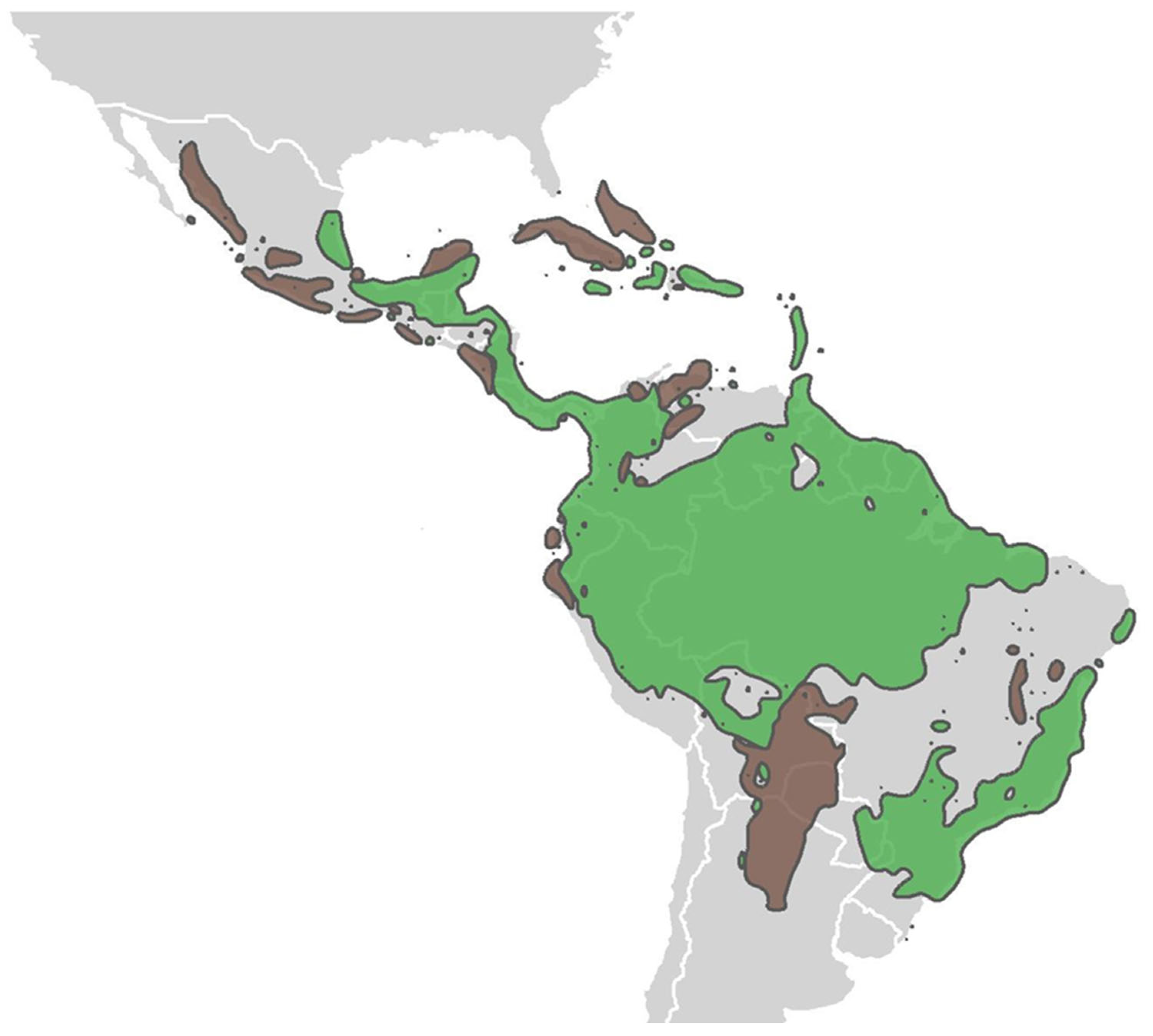
3.1. Mexico’s Pacific Dry Forests as a Biogeographical Bridge between Rainforests and Deserts
3.2. Cerrado and Atlantic Rainforest in Brazil: Linking Contemporary to Geological Change
3.3. Tropical Dry Forests and Rainforests: Ecological and Evolutionary Cousins
4. How to Study Ecotones
4.1. Scale
4.2. Remote Sensing and Sensor Networks
4.3. Vegetation Dynamics Plot Networks
4.4. Dispersal
4.5. Physiological Responses to Temperature and Drought
4.6. Environmental DNA Metabarcoding
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kark, S. Ecotones and Ecological Gradients. In Ecological Systems: Selected Entries from the Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2012; pp. 147–160. [Google Scholar]
- Clements, F.E. Plant Succession: An Analysis of the Development of Vegetation (No. 242); Carnegie Institution of Washington: Washington, DC, USA, 1916. [Google Scholar]
- Gleason, H.A. The Structure and Development of the Plant Association. Bull. Torrey Bot. Club 1917, 44, 463–481. [Google Scholar] [CrossRef]
- Huxley, J. Clines: An Auxiliary Taxonomic Principle. Nature 1938, 142, 219–220. [Google Scholar] [CrossRef]
- Hansen, A.J.; DiCastri, F. Landscape Boundaries: Consequences for Biotic Diversity and Ecological Flows; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 92. [Google Scholar]
- Neilson, R.P. Transient Ecotone Response to Climatic Change: Some Conceptual and Modelling Approaches. Ecol. Appl. 1993, 3, 385–395. [Google Scholar] [CrossRef]
- Smith, A.J.; Goetz, E.M. Climate Change Drives Increased Directional Movement of Landscape Ecotones. Landsc. Ecol. 2021, 36, 3105–3116. [Google Scholar] [CrossRef]
- Gosz, J.R. Fundamental Ecological Characteristics of Landscape Boundaries. In Ecotones: The Role of Landscape Boundaries in the Management and Restoration of Changing Environments; Springer: New York, NY, USA, 1991; pp. 8–30. [Google Scholar]
- Hufkens, K.; Scheunders, P.; Ceulemans, R. Ecotones in Vegetation Ecology: Methodologies and Definitions Revisited. Ecol. Res. 2009, 24, 977–986. [Google Scholar] [CrossRef]
- Delcourt, P.A.; Delcourt, H.R. Ecotone Dynamics in Space and Time. In Landscape Boundaries: Consequences for Biotic Diversity and Ecological Flows; Springer: New York, NY, USA, 1992; pp. 19–54. [Google Scholar]
- Kupfer, J.A.; Cairns, D.M. The Suitability of Montane Ecotones as Indicators of Global Climatic Change. Prog. Phys. Geogr. 1996, 20, 253–272. [Google Scholar] [CrossRef]
- Oliveras, I.; Malhi, Y. Many Shades of Green: The Dynamic Tropical Forest–Savannah Transition Zones. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150308. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, C.M.; Powers, J.S. Tropical Forest Composition and Function across Space and Time: Insights from Diverse Gradients in Área de Conservación Guanacaste. Biotropica 2019, 52, 1065–1075. [Google Scholar] [CrossRef]
- Leite, Y.L.; Costa, L.P.; Loss, A.C.; Rocha, R.G.; Batalha-Filho, H.; Bastos, A.C.; Pardini, R. Neotropical Forest Expansion during the Last Glacial Period Challenges Refuge Hypothesis. Proc. Natl. Acad. Sci. USA 2016, 113, 1008–1013. [Google Scholar] [CrossRef]
- Hooghiemstra, H.; Van der Hammen, T. Quaternary Ice-Age Dynamics in the Colombian Andes: Developing an Understanding of Our Legacy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 173–181. [Google Scholar] [CrossRef]
- Colinvaux, P.A.; De Oliveira, P.E.; Bush, M.B. Amazonian and Neotropical Plant Communities on Glacial Time-Scales: The Failure of the Aridity and Refuge Hypotheses. Quat. Sci. Rev. 2000, 19, 141–169. [Google Scholar] [CrossRef]
- Bachelet, D.; Neilson, R.P.; Lenihan, J.M.; Drapek, R.J. Climate Change Effects on Vegetation Distribution and Carbon Budget in the United States. Ecosystems 2001, 4, 164–185. [Google Scholar] [CrossRef]
- Tovar, C.; Carril, A.F.; Gutiérrez, A.G.; Ahrends, A.; Fita, L.; Zaninelli, P.; Hollingsworth, P.M. Understanding Climate Change Impacts on Biome and Plant Distributions in the Andes: Challenges and Opportunities. J. Biogeogr. 2022, 49, 1420–1442. [Google Scholar] [CrossRef]
- Brown, D.E.; Davis, R. One Hundred Years of Vicissitude: Terrestrial Bird and Mammal Distribution Changes in the American Southwest. In General Technical Report RM-GTR-264: Biodiversity and the Management of the Madrean Archipelago: The Sky Islands of Southwestern United States and Northwestern Mexico; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1995; pp. 231–244. [Google Scholar]
- Cavanaugh, K.C.; Dangremond, E.M.; Doughty, C.L.; Williams, A.P.; Parker, J.D.; Hayes, M.A.; Feller, I.C. Climate-Driven Regime Shifts in a Mangrove–Salt Marsh Ecotone over the Past 250 Years. Proc. Natl. Acad. Sci. USA 2019, 116, 21602–21608. [Google Scholar] [CrossRef] [PubMed]
- Saintilan, N.; Rogers, K.; McKee, K.L. The Shifting Saltmarsh-Mangrove Ecotone in Australasia and the Americas. In Coastal Wetlands; Elsevier: Amsterdam, The Netherlands, 2019; pp. 915–945. [Google Scholar]
- Gonzalez, P. Desertification and a Shift of Forest Species in the West African Sahel. Clim. Res. 2001, 17, 217–228. [Google Scholar] [CrossRef]
- Dexter, K.G.; Pennington, R.T.; Oliveira-Filho, A.T.; Bueno, M.L.; Silva de Miranda, P.L.; Neves, D.M. Inserting Tropical Dry Forests into the Discussion on Biome Transitions in the Tropics. Front. Ecol. Evol. 2018, 6, 104. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Casassa, G.; Karoly, D.J.; Imeson, A.; Liu, C.; Menzel, A.; Rawlins, S.; Root, T.L.; Seguin, B.; Tryjanowski, P. Assessment of Observed Changes and Responses in Natural and Managed Systems. In Climate Change 2007: Impacts, Adaptation, and Vulnerability (Intergovernmental Panel on Climate Change); Cambridge University Press: Cambridge, UK, 2007; pp. 79–131. [Google Scholar]
- Mucina, L. Biome: Evolution of a Crucial Ecological and Biogeographical Concept. New Phytol. 2019, 222, 97–114. [Google Scholar] [CrossRef]
- Navarro, A.; Lee, G.; Martín, R.; Tapiador, F.J. Uncertainties in Measuring Precipitation Hinders Precise Evaluation of Loss of Diversity in Biomes and Ecotones. npj Clim. Atmos. Sci. 2024, 7, 35. [Google Scholar] [CrossRef]
- Wielgolaski, F.E.; Hofgaard, A.; Holtmeier, F.K. Sensitivity to Environmental Change of the Treeline Ecotone and Its Associated Biodiversity in European Mountains. Clim. Res. 2017, 73, 151–166. [Google Scholar] [CrossRef]
- Martin, P.H.; Fahey, T.J.; Sherman, R.E. Vegetation Zonation in a Neotropical Montane Forest: Environment, Disturbance, and Ecotones. Biotropica 2011, 43, 533–543. [Google Scholar] [CrossRef]
- Altamirano, T.A.; de Zwaan, D.R.; Ibarra, J.T.; Wilson, S.; Martin, K. Treeline Ecotones Shape the Distribution of Avian Species Richness and Functional Diversity in South Temperate Mountains. Sci. Rep. 2020, 10, 18428. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Segoviano, G.; Arenas-Navarro, M.; Villa-Galaviz, E.; Díaz-Infante, S.; del Coro Arizmendi, M. Hummingbird-Plant Interactions along an Altitudinal Gradient in Northwestern Mexico. Acta Oecol. 2021, 112, 103762. [Google Scholar] [CrossRef]
- Oommen, M.A.; Shanker, K. Elevational Species Richness Patterns Emerge from Multiple Local Mechanisms in Himalayan Woody Plants. Ecology 2005, 86, 3039–3047. [Google Scholar] [CrossRef]
- Gonçalves, G.R.; Santos, M.P.D.; Cerqueira, P.V.; Juen, L.; Bispo, A. The Relationship between Bird Distribution Patterns and Environmental Factors in an Ecotone Area of Northeast Brazil. J. Arid Environ. 2017, 140, 6–13. [Google Scholar] [CrossRef]
- Souza, C.R.; Paula, G.G.; Mendes, C.N.; Maia, V.A.; Aguiar-Campos, N.; Araújo, F.C.; Santos, R.M. Local-Scale Tree Community Ecotones Are Distinct Vegetation Types Instead of Mixed Ones: A Case Study from the Cerrado–Atlantic Forest Ecotonal Region in Brazil. Aust. J. Bot. 2020, 68, 153–164. [Google Scholar] [CrossRef]
- Ayres, J.M.; Da Fonseca, G.A.B.; Rylands, A.B.; Queiroz, H.L.; Pinto, L.P.; Masterson, D.; Cavalcanti, R.B. Os Corredores Ecológicos das Florestas Tropicais do Brasil. In Sociedade Civil Mamirauá; Instituto Mamirauá: Belém, Brazil, 2005. [Google Scholar]
- Banerjee, S.; Bora, S.; Thrall, P.H.; Richardson, A.E. Soil C and N as Causal Factors of Spatial Variation in Extracellular Enzyme Activity across Grassland-Woodland Ecotones. Appl. Soil Ecol. 2016, 105, 1–8. [Google Scholar] [CrossRef]
- Araujo, A.S.F.; de Souza Oliveira, L.M.; Melo, V.M.M.; Antunes, J.E.L.; Araujo, F.F.; Mendes, L.W. Distinct Taxonomic Composition of Soil Bacterial Community across a Native Gradient of Cerrado-Ecotone-Caatinga. Appl. Soil Ecol. 2021, 161, 103874. [Google Scholar] [CrossRef]
- Lourenço, G.M.; Soares, G.R.; Santos, T.P.; Dattilo, W.; Freitas, A.V.; Ribeiro, S.P. Equal but Different: Natural Ecotones Are Dissimilar to Anthropic Edges. PLoS ONE 2019, 14, e0213008. [Google Scholar] [CrossRef]
- Murcia, C. Edge Effects in Fragmented Forests: Implications for Conservation. Trends Ecol. Evol. 1995, 10, 58–62. [Google Scholar] [CrossRef]
- Laurance, W.F.; Camargo, J.L.; Fearnside, P.M.; Lovejoy, T.E.; Williamson, G.B.; Mesquita, R.C.; Laurance, S.G. An Amazonian Rainforest and Its Fragments as a Laboratory of Global Change. Biol. Rev. 2018, 93, 223–247. [Google Scholar] [CrossRef]
- de Carvalho, D.L.; Silva, S.M.; Sousa-Neves, T.; Gonçalves, G.S.R.; Silva, D.P.; Santos, M.P.D. Predicting the Future of Threatened Birds from a Neotropical Ecotone Area. Environ. Monit. Assess. 2024, 196, 61. [Google Scholar] [CrossRef]
- Dongmo, M.A.; Hanna, R.; Smith, T.B.; Fiaboe, K.K.M.; Fomena, A.; Bonebrake, T.C. Local Adaptation in Thermal Tolerance for a Tropical Butterfly across Ecotone and Rainforest Habitats. Biol. Open 2021, 10, bio058619. [Google Scholar] [CrossRef] [PubMed]
- Mayle, F.E.; Beerling, D.J.; Gosling, W.D.; Bush, M.B. Responses of Amazonian Ecosystems to Climatic and Atmospheric Carbon Dioxide Changes since the Last Glacial Maximum. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Mayle, F.E.; Langstroth, R.P.; Fisher, R.A.; Meir, P. Long-Term Forest–Savannah Dynamics in the Bolivian Amazon: Implications for Conservation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Condé, T.M.; Tonini, H.; Higuchi, N.; Higuchi, F.G.; Lima, A.J.N.; Barbosa, R.I.; Haas, M.A. Effects of Sustainable Forest Management on Tree Diversity, Timber Volumes, and Carbon Stocks in an Ecotone Forest in the Northern Brazilian Amazon. Land Use Policy 2022, 119, 106145. [Google Scholar] [CrossRef]
- Hill, J.; Black, S.; Soto, D.; Chavez, E.; Vos, V.; Mayle, F. Differing Local-Scale Responses of Bolivian Amazon Forest Ecotones to Middle Holocene Drought Based upon Multiproxy Soil Data. J. Quat. Sci. 2023, 38, 970–990. [Google Scholar] [CrossRef]
- Bush, M.B. Distributional Change and Conservation on the Andean Flank: A Palaeoecological Perspective. Glob. Ecol. Biogeogr. 2002, 11, 463–473. [Google Scholar] [CrossRef]
- Araujo-Murakami, A.; Doughty, C.E.; Metcalfe, D.B.; Silva-Espejo, J.E.; Arroyo, L.; Heredia, J.P.; Malhi, Y. The Productivity Allocation and Cycling of Carbon in Forests at the Dry Margin of the Amazon Forest in Bolivia. Plant Ecol. Divers. 2014, 7, 55–69. [Google Scholar] [CrossRef]
- Fadrique, B.; Báez, S.; Duque, Á.; Malizia, A.; Blundo, C.; Carilla, J.; Feeley, K.J. Widespread but Heterogeneous Responses of Andean Forests to Climate Change. Nature 2018, 564, 207–212. [Google Scholar] [CrossRef]
- Vargas-Rueda, A.F.; Rivera-Hernández, J.E.; Álvarez-Aquino, C.; Salas-Morales, S.H.; Alcántara-Salinas, G.; Pérez-Sato, J.A. Composición Florística del Bosque Mesófilo de Montaña Perturbado y Sus Ecotonos en el Parque Nacional Cañón del Río Blanco, Veracruz, México. Acta Bot. Mex. 2021, 128. [Google Scholar] [CrossRef]
- Ainsworth, A.B.; Drake, D.R. Hawaiian Treeline Ecotones: Implications for Plant Community Conservation under Climate Change. Plants 2023, 13, 123. [Google Scholar] [CrossRef]
- Peinetti, H.R.; Bestelmeyer, B.T.; Chirino, C.C.; Vivalda, F.L.; Kin, A.G. Thresholds and Alternative States in a Neotropical Dry Forest in Response to Fire Severity. Ecol. Appl. 2024, 34, e2937. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.L.; Dexter, K.G.; Pennington, R.T.; Pontara, V.; Neves, D.M.; Ratter, J.A.; de Oliveira-Filho, A.T. The Environmental Triangle of the Cerrado Domain: Ecological Factors Driving Shifts in Tree Species Composition between Forests and Savannas. J. Ecol. 2018, 106, 2109–2120. [Google Scholar] [CrossRef]
- Ribeiro Costa, T.; da Silva, L.A.; de Moura, C.C.; de Souto Azevedo, C.H.; Bueno, M.L.; Mucida, D.P.; Gonzaga, A.P.D. Vulnerability of the Cerrado–Atlantic Forest Ecotone in the Espinhaço Range Biosphere Reserve to Climate Change. Theor. Appl. Climatol. 2023, 151, 1151–1170. [Google Scholar] [CrossRef]
- Pouteau, R.; Giambelluca, T.W.; Ah-Peng, C.; Meyer, J.Y. Will Climate Change Shift the Lower Ecotone of Tropical Montane Cloud Forests Upwards on Islands? J. Biogeogr. 2018, 45, 1326–1333. [Google Scholar] [CrossRef]
- Urbina-Cardona, J.N.; Olivares-Pérez, M.; Reynoso, V.H. Herpetofauna Diversity and Microenvironment Correlates across a Pasture–Edge–Interior Ecotone in Tropical Rainforest Fragments in the Los Tuxtlas Biosphere Reserve of Veracruz, Mexico. Biol. Conserv. 2006, 132, 61–75. [Google Scholar] [CrossRef]
- Trujillo-Arias, N.; Rodríguez-Cajarville, M.J.; Sari, E.; Miyaki, C.Y.; Santos, F.R.; Witt, C.C.; Cabanne, G.S. Evolution between Forest Macrorefugia Is Linked to Discordance between Genetic and Morphological Variation in Neotropical Passerines. Mol. Phylogenet. Evol. 2020, 149, 106849. [Google Scholar] [CrossRef]
- Martin, P.H.; Sherman, R.E.; Fahey, T.J. Tropical Montane Forest Ecotones: Climate Gradients, Natural Disturbance, and Vegetation Zonation in the Cordillera Central, Dominican Republic. J. Biogeogr. 2007, 34, 1792–1806. [Google Scholar] [CrossRef]
- Walter, J.A.; Atkins, J.W.; Hulshof, C.M. Climate and Topography Shape Variation in the Tropical Dry Forest-Rainforest Ecotone. Ecology 2024. under review. [Google Scholar]
- Williams-Linera, G. Vegetation Structure and Environmental Conditions of Forest Edges in Panama. J. Ecol. 1990, 78, 356–373. [Google Scholar] [CrossRef]
- Farias, H.L.S.; Silva, W.R.; de Oliveira Perdiz, R.; Citó, A.C.; da Silva Carvalho, L.C.; Barbosa, R.I. Dataset on Wood Density of Trees in Ecotone Forests in Northern Brazilian Amazonia. Data Brief 2020, 30, 105378. [Google Scholar] [CrossRef]
- Farias, H.L.S.; Pequeno, P.A.C.L.; Silva, W.R.; Melo, V.F.; Carvalho, L.C.S.; Perdiz, R.O.; Barbosa, R.I. Amazon Forest Biomass: Intra-and Interspecific Variability in Wood Density Drive Divergences in Brazil’s Far North. iForest 2023, 16, 95. [Google Scholar] [CrossRef]
- Swenson, N.G.; Howard, D.J. Clustering of Contact Zones, Hybrid Zones, and Phylogeographic Breaks in North America. Am. Nat. 2005, 166, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, G.; Döbert, T.; Dobbie, L.; Hashim, N.; Bastow Wilson, J. Functional Traits Shed New Light on the Nature of Ecotones: A Study across a Bog-to-Forest Sequence. Community Ecol. 2013, 14, 31–40. [Google Scholar] [CrossRef]
- Ramírez, L.A.; Llambí, L.D.; Azocar, C.J.; Fernandez, M.; Torres, J.E.; Bader, M.Y. Patterns in Climate and Seedling Establishment at a Dry Tropical Treeline. Plant Ecol. 2022, 223, 1047–1068. [Google Scholar] [CrossRef]
- Poorter, L. Are Species Adapted to Their Regeneration Niche, Adult Niche, or Both? Am. Nat. 2007, 169, 433–442. [Google Scholar] [CrossRef]
- Frei, E.R.; Bianchi, E.; Bernareggi, G.; Bebi, P.; Dawes, M.A.; Brown, C.D.; Rixen, C. Biotic and Abiotic Drivers of Tree Seedling Recruitment across an Alpine Treeline Ecotone. Sci. Rep. 2018, 8, 10894. [Google Scholar] [CrossRef]
- Poorter, L.; Markesteijn, L. Seedling Traits Determine Drought Tolerance of Tropical Tree Species. Biotropica 2008, 40, 321–331. [Google Scholar] [CrossRef]
- Issifu, H.; Ametsitsi, G.K.; De Vries, L.J.; Djagbletey, G.D.; Adu-Bredu, S.; Vergeer, P.; Veenendaal, E. Variation in Vegetation Cover and Seedling Performance of Tree Species in a Forest-Savanna Ecotone. J. Trop. Ecol. 2019, 35, 74–82. [Google Scholar] [CrossRef]
- Liautaud, K.; Barbier, M.; Loreau, M. Ecotone Formation through Ecological Niche Construction: The Role of Biodiversity and Species Interactions. Ecography 2020, 43, 714–723. [Google Scholar] [CrossRef]
- Malanson, G.P.; Resler, L.M.; Tomback, D.F. Ecotone Response to Climatic Variability Depends on Stress Gradient Interactions. Clim. Change Responses 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Ploughe, L.W.; Jacobs, E.M.; Frank, G.S.; Greenler, S.M.; Smith, M.D.; Dukes, J.S. Community Response to Extreme Drought (CRED): A Framework for Drought-Induced Shifts in Plant–Plant Interactions. New Phytol. 2019, 222, 52–69. [Google Scholar] [CrossRef]
- Williams, S.E.; Marsh, H.; Winter, J. Spatial Scale, Species Diversity, and Habitat Structure: Small Mammals in Australian Tropical Rain Forest. Ecology 2002, 83, 1317–1329. [Google Scholar] [CrossRef]
- Rojas Alvarado, C.; Valverde González, R.; Somerville, S.; Rollins, A.W.; Stephenson, S.L. Myxomycetes within Ecotones in Temperate and Tropical Forests. Uniciencia 2021, 35, 299–311. [Google Scholar] [CrossRef]
- Becerra, J.X. Timing the Origin and Expansion of the Mexican Tropical Dry Forest. Proc. Natl. Acad. Sci. USA 2005, 102, 10919–10923. [Google Scholar] [CrossRef] [PubMed]
- Rzedowski, J. Vegetación de México; Editorial Limusa: Mexico City, Mexico, 1978; Volume 432. [Google Scholar]
- Pérez-García, E.A.; Meave, J.A.; Cevallos-Ferriz, S.R. Flora and Vegetation of the Seasonally Dry Tropics in Mexico: Origin and Biogeographical Implications. Acta Bot. Mex. 2012, 100, 149–193. [Google Scholar]
- Van Devender, T.R. The Deep History of the Sonoran Desert. In A Natural History of the Sonoran Desert; Phillips, S.J., Wentworth Comus, P., Eds.; Arizona-Sonora Desert Museum Press: Tucson, AZ, USA, 2000; pp. 61–70. [Google Scholar]
- Lott, E.J.; Atkinson, T.H. Biodiversidad y Fitogeografía de Chamela-Cuitxmala, Jalisco. In Historia Natural de Chamela; Nogera, F.A., Vega, J., Quesada, M., Eds.; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2002; pp. 83–97. [Google Scholar]
- Díaz-Castellanos, A.; Meave, J.A.; Vega-Ramos, F.; Pineda-García, F.; Bonfil, C.; Paz, H. The Above–Belowground Functional Space of Tropical Dry Forest Communities Responds to Local Hydric Habitats. Biotropica 2022, 54, 1003–1014. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.; Underwood, E.C.; Kassem, K.R. Terrestrial Ecoregions of the World: A New Map of Life on Earth. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Schimper, A.F.W. Plant-Geography upon a Physiological Basis; Fisher, W.R., Translator, Groom, P., Balfour, I.B., Eds.; Clarendon Press: Oxford, UK, 1903. [Google Scholar]
- Beard, J.S. Climax Vegetation in Tropical America. Ecology 1944, 25, 127–158. [Google Scholar] [CrossRef]
- Bews, J.W. Studies in the Ecological Evolution of the Angiosperms (continued). New Phytol. 1927, 26, 209–248. [Google Scholar] [CrossRef]
- Axelrod, D.I. Origin of Deciduous and Evergreen Habits in Temperate Forests. Evolution 1966, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zanne, A.E.; Tank, D.C.; Cornwell, W.K.; Eastman, J.M.; Smith, S.A.; FitzJohn, R.G.; Beaulieu, J.M. Three Keys to the Radiation of Angiosperms into Freezing Environments. Nature 2014, 506, 89–92. [Google Scholar] [CrossRef]
- Edwards, E.J.; Chatelet, D.S.; Chen, B.C.; Ong, J.Y.; Tagane, S.; Kanemitsu, H.; Donoghue, M.J. Convergence, Consilience, and the Evolution of Temperate Deciduous Forests. Am. Nat. 2017, 190, S87–S104. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Vázquez, I. El Clima de la Selva Baja Caducifolia en México. Investig. Geogr. 1999, 39, 40–52. [Google Scholar] [CrossRef]
- Bojórquez, A.; Álvarez-Yépiz, J.C.; Búrquez, A.; Martínez-Yrízar, A. Understanding and Predicting Frost-Induced Tropical Tree Mortality Patterns. Glob. Change Biol. 2019, 25, 3817–3828. [Google Scholar] [CrossRef]
- Davis, C.C.; Lyra, G.M.; Park, D.S.; Asprino, R.; Maruyama, R.; Torquato, D.; Ellison, A.M. New Directions in Tropical Phenology. Trends Ecol. Evol. 2022, 37, 683–693. [Google Scholar] [CrossRef]
- Vazquez, G.J.A.; Givnish, T.J. Altitudinal Gradients in Tropical Forest Composition, Structure, and Diversity in the Sierra de Manantlán. J. Ecol. 1998, 86, 999–1020. [Google Scholar]
- Salas-Morales, S.H.; Meave, J.A. Elevational Patterns in the Vascular Flora of a Highly Diverse Region in Southern Mexico. Plant Ecol. 2012, 213, 1209–1220. [Google Scholar] [CrossRef]
- Killeen, T.J.; Douglas, M.; Consiglio, T.; Jørgensen, P.M.; Mejia, J. Dry Spots and Wet Spots in the Andean Hotspot. J. Biogeogr. 2007, 34, 1357–1373. [Google Scholar] [CrossRef]
- Sterck, F.; Markesteijn, L.; Toledo, M.; Schieving, F.; Poorter, L. Sapling Performance along Resource Gradients Drives Tree Species Distributions within and across Tropical Forests. Ecology 2014, 95, 2514–2525. [Google Scholar] [CrossRef]
- Janzen, D.H. How Moths Pass the Dry Season in a Costa Rican Dry Forest. Int. J. Trop. Insect Sci. 1987, 8, 489–500. [Google Scholar] [CrossRef]
- Hunt, J.H.; Brodie, R.J.; Carithers, T.P.; Goldstein, P.Z.; Janzen, D.H. Dry Season Migration by Costa Rican Lowland Paper Wasps to High Elevation Cold Dormancy Sites. Biotropica 1999, 31, 192–196. [Google Scholar] [CrossRef]
- Janzen, H.H. Carbon Cycling in Earth Systems—A Soil Science Perspective. Agric. Ecosyst. Environ. 2004, 104, 399–417. [Google Scholar] [CrossRef]
- Smith, M.A.; Hallwachs, W.; Janzen, D.H. Diversity and Phylogenetic Community Structure of Ants along a Costa Rican Elevational Gradient. Ecography 2014, 37, 720–731. [Google Scholar] [CrossRef]
- Allen, K.; Dupuy, J.M.; Gei, M.G.; Hulshof, C.; Medvigy, D.; Pizano, C.; Powers, J.S. Will Seasonally Dry Tropical Forests Be Sensitive or Resistant to Future Changes in Rainfall Regimes? Environ. Res. Lett. 2017, 12, 023001. [Google Scholar] [CrossRef]
- Powers, J.S.; Vargas, G.G.; Brodribb, T.J.; Schwartz, N.B.; Pérez-Aviles, D.; Smith-Martin, C.M.; Medvigy, D. A Catastrophic Tropical Drought Kills Hydraulically Vulnerable Tree Species. Glob. Change Biol. 2020, 26, 3122–3133. [Google Scholar] [CrossRef]
- McFadden, I.R.; Bartlett, M.K.; Wiegand, T.; Turner, B.L.; Sack, L.; Valencia, R.; Kraft, N.J. Disentangling the Functional Trait Correlates of Spatial Aggregation in Tropical Forest Trees. Ecology 2019, 100, e02591. [Google Scholar] [CrossRef]
- Renner, S. Plant Dispersal across the Tropical Atlantic by Wind and Sea Currents. Int. J. Plant Sci. 2004, 165, S23–S33. [Google Scholar] [CrossRef]
- Cavelier, J.; Aide, T.; Santos, C.; Eusse, A.; Dupuy, J. The Savannization of Moist Forests in the Sierra Nevada de Santa Marta, Colombia. J. Biogeogr. 1998, 25, 901–912. [Google Scholar] [CrossRef]
- Strewe, R.; Navarro, C. New and Noteworthy Records of Birds from the Sierra Nevada de Santa Marta Region, North-Eastern Colombia. Bull. Br. Ornithol. Club 2004, 124, 38–50. [Google Scholar]
- Mayle, F.E. The Late Quaternary Biogeographical History of South American Seasonally Dry Tropical Forests: Insights from Palaeo-Ecological Data. In Neotropical Savannas and Seasonally Dry Forests; CRC Press: Boca Raton, FL, USA, 2006; pp. 395–416. [Google Scholar]
- Markesteijn, L.; Iraipi, J.; Bongers, F.; Poorter, L. Seasonal Variation in Soil and Plant Water Potentials in a Bolivian Tropical Moist and Dry Forest. J. Trop. Ecol. 2010, 26, 497–508. [Google Scholar] [CrossRef]
- Wiens, J.A. Spatial Scaling in Ecology. Funct. Ecol. 1989, 3, 385–397. [Google Scholar] [CrossRef]
- Levin, S.A. The Problem of Pattern and Scale in Ecology: The Robert H. MacArthur Award Lecture. Ecology 1992, 73, 1943–1967. [Google Scholar] [CrossRef]
- Gardner, R.H.; Turner, M.G.; O’Neill, R.V.; Lavorel, S. Simulation of the Scale-Dependent Effects of Landscape Boundaries on Species Persistence and Dispersal. In Ecotones: The Role of Landscape Boundaries in the Management and Restoration of Changing Environments; Springer: New York, NY, USA, 1991; pp. 76–89. [Google Scholar]
- Ellison, A.M.; Mukherjee, B.B.; Karim, A. Testing Patterns of Zonation in Mangroves: Scale Dependence and Environmental Correlates in the Sundarbans of Bangladesh. J. Ecol. 2000, 88, 813–824. [Google Scholar] [CrossRef]
- Soto-Medina, E.; Londoño-Lemos, V.; Díaz-Escandón, D. Epiphytes from a Forest Type Transition Zone in the Choco Biogeographic Region, Valle del Cauca, Colombia. Rev. Biol. Trop. 2015, 63, 915–926. [Google Scholar] [CrossRef]
- Dodoo, D.N.A.; Antwi-Agyei, P.; Baidoo, E.; Logah, V.; Abubakari, A.; Adarkwa, B.O. Soil Carbon Stock and Nutrient Characteristics of Forest–Savanna Transition: Estimates from Four Land Use Systems in Ghana. Sustain. Environ. 2023, 9, 2262684. [Google Scholar] [CrossRef]
- Morales, L.V.; Sevillano-Rios, C.S.; Fick, S.; Young, T.P. Differential Seedling Regeneration Patterns across Forest–Grassland Ecotones in Two Tropical Treeline Species (Polylepis spp.). Austral. Ecol. 2018, 43, 514–526. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, K.; Zang, R.; Ding, Y. Environmental Filtering of Species with Different Functional Traits into Plant Assemblages across a Tropical Coniferous-Broadleaved Forest Ecotone. Plant Soil 2014, 380, 361–374. [Google Scholar] [CrossRef]
- Hirota, M.; Holmgren, M.; Van Nes, E.H.; Scheffer, M. Global Resilience of Tropical Forest and Savanna to Critical Transitions. Science 2011, 334, 232–235. [Google Scholar] [CrossRef]
- Baker, P.A.; Fritz, S.C.; Battisti, D.S.; Dick, C.W.; Vargas, O.M.; Asner, G.P.; Prates, I. Beyond Refugia: New Insights on Quaternary Climate Variation and the Evolution of Biotic Diversity in Tropical South America. In Neotropical Diversification: Patterns and Processes; Springer: Cham, Switzerland, 2020; pp. 51–70. [Google Scholar]
- Kark, S. Effects of Ecotones on Biodiversity. Encycl. Biodivers. 2013, 142, 1. [Google Scholar]
- Chhetri, P.K.; Thai, E. Remote Sensing and Geographic Information Systems Techniques in Studies on Treeline Ecotone Dynamics. J. For. Res. 2019, 30, 1543–1553. [Google Scholar] [CrossRef]
- Ratana, P.; Huete, A.R.; Shimabukuro, Y.E.; da Rocha, H.R.; Saleska, S.R. Variability in Amazon Phenology across the Transitional Rainforest-Cerrado Ecotone. In Spatial and Temporal Amazon Vegetation Dynamics and Phenology Using Time Series Satellite Data; The University of Arizona: Tucson, AZ, USA, 2006; p. 58. [Google Scholar]
- Danby, R.K. Monitoring Forest–Tundra Ecotones at Multiple Scales. Geogr. Compass 2011, 5, 623–640. [Google Scholar] [CrossRef]
- Staal, A.; Flores, B.M. Sharp Ecotones Spark Sharp Ideas: Comment on “Structural, Physiognomic and Above-Ground Biomass Variation in Savanna–Forest Transition Zones on Three Continents–How Different Are Co-Occurring Savanna and Forest Formations?” by Veenendaal et al. (2015). Biogeosciences 2015, 12, 5563–5566. [Google Scholar] [CrossRef]
- Morley, P.J.; Donoghue, D.N.; Chen, J.C.; Jump, A.S. Integrating Remote Sensing and Demography for More Efficient and Effective Assessment of Changing Mountain Forest Distribution. Ecol. Inform. 2018, 43, 106–115. [Google Scholar] [CrossRef]
- Comesaña-Cebral, L.; Martínez-Sánchez, J.; Lorenzo, H.; Arias, P. Individual Tree Segmentation Method Based on Mobile Backpack LiDAR Point Clouds. Sensors 2021, 21, 6007. [Google Scholar] [CrossRef]
- Karan, M.; Liddell, M.; Prober, S.M.; Arndt, S.; Beringer, J.; Boer, M.; Wardlaw, T. The Australian SuperSite Network: A Continental, Long-Term Terrestrial Ecosystem Observatory. Sci. Total Environ. 2016, 568, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.E.; Clayton, M.K.; Townsend, P.A.; Berg, S.; Elza, H.; Mladenoff, D.J. Identifying Ecotone Location Using the Co-Occurrence Property. J. Veg. Sci. 2021, 32, e12929. [Google Scholar] [CrossRef]
- Phillips, O.L. Sensing Forests Directly: The Power of Permanent Plots. Plants 2023, 12, 3710. [Google Scholar] [CrossRef] [PubMed]
- Cavender-Bares, J.; Schneider, F.D.; Santos, M.J.; Armstrong, A.; Carnaval, A.; Dahlin, K.M.; Wilson, A.M. Integrating Remote Sensing with Ecology and Evolution to Advance Biodiversity Conservation. Nat. Ecol. Evol. 2022, 6, 506–519. [Google Scholar] [CrossRef]
- Dyer, M.I.; Di Castri, F.; Hansen, A.J. Geosphere-Biosphere Observatories: Their Definition and Design for Studying Global Change. In Biology International; IUBS: Paris, France, 1988. [Google Scholar]
- Anderson-Teixeira, K.J.; Davies, S.J.; Bennett, A.C.; Gonzalez-Akre, E.B.; Muller-Landau, H.C.; Wright, S.J.; Zimmerman, J. CTFS-Forest GEO: A Worldwide Network Monitoring Forests in an Era of Global Change. Glob. Change Biol. 2015, 21, 528–549. [Google Scholar] [CrossRef]
- Lin, L.; Cao, M. Edge Effects on Soil Seed Banks and Understory Vegetation in Subtropical and Tropical Forests in Yunnan, SW China. For. Ecol. Manag. 2009, 257, 1344–1352. [Google Scholar] [CrossRef]
- Tingstad, L.; Olsen, S.L.; Klanderud, K.; Vandvik, V.; Ohlson, M. Temperature, Precipitation and Biotic Interactions as Determinants of Tree Seedling Recruitment across the Tree Line Ecotone. Oecologia 2015, 179, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Větrovský, T.; Morais, D.; Kohout, P.; Lepinay, C.; Algora Gallardo, C.; Awokunle Hollá, S.D.; Baldrian, P. GlobalFungi: A Global Database of Fungal Occurrences from High-Throughput-Sequencing Metabarcoding Studies. Sci. Data 2020, 7, 228. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Shi, X.; Yi, X.; Ma, J. Rodent-Mediated Seed Dispersal Shapes Species Composition and Recruitment Dynamics in Ecotones. Front. Plant Sci. 2018, 9, 1911. [Google Scholar] [CrossRef]
- Corlett, R.T.; Westcott, D.A. Will Plant Movements Keep Up with Climate Change? Trends Ecol. Evol. 2013, 28, 482–488. [Google Scholar] [CrossRef]
- Malanson, G.P.; Rodriguez, N. Traveling Waves and Spatial Patterns from Dispersal on Homogeneous and Gradient Habitats. Ecol. Complex. 2018, 33, 57–65. [Google Scholar] [CrossRef]
- Wright, S.J.; Trakhtenbrot, A.; Bohrer, G.; Detto, M.; Katul, G.G.; Horvitz, N.; Nathan, R. Understanding Strategies for Seed Dispersal by Wind under Contrasting Atmospheric Conditions. Proc. Natl. Acad. Sci. USA 2008, 105, 19084–19089. [Google Scholar] [CrossRef]
- Janzen, D.H. Management of Habitat Fragments in a Tropical Dry Forest: Growth. Ann. Mo. Bot. Gard. 1988, 75, 105–116. [Google Scholar] [CrossRef]
- Trakhtenbrot, A.; Katul, G.G.; Nathan, R. Mechanistic Modeling of Seed Dispersal by Wind over Hilly Terrain. Ecol. Model. 2014, 274, 29–40. [Google Scholar] [CrossRef]
- Peterson, M.L.; Doak, D.F.; Morris, W.F. Incorporating Local Adaptation into Forecasts of Species’ Distribution and Abundance under Climate Change. Glob. Change Biol. 2019, 25, 775–793. [Google Scholar]
- Pérez-Méndez, N.; Jordano, P.; Valido, A. Persisting in Defaunated Landscapes: Reduced Plant Population Connectivity after Seed Dispersal Collapse. J. Ecol. 2018, 106, 936–947. [Google Scholar] [CrossRef]
- Hanski, I.; Mononen, T. Eco-evolutionary Dynamics of Dispersal in Spatially Heterogeneous Environments. Ecol. Lett. 2011, 14, 1025–1034. [Google Scholar] [CrossRef]
- Sieger, C.S.; Hovestadt, T. The Effect of Landscape Structure on the Evolution of Two Alternative Dispersal Strategies. Ecol. Process. 2021, 10, 1–13. [Google Scholar] [CrossRef]
- Dieckmann, U.; O’Hara, B.; Weisser, W. The Evolutionary Ecology of Dispersal. Trends Ecol. Evol. 1999, 14, 88–90. [Google Scholar] [CrossRef]
- Duputié, A.; Massol, F. An Empiricist’s Guide to Theoretical Predictions on the Evolution of Dispersal. Interface Focus 2013, 3, 20130028. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The Determinants of Leaf Turgor Loss Point and Prediction of Drought Tolerance of Species and Biomes: A Global Meta-Analysis. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef]
- Johnson, D.M.; Domec, J.C.; Carter Berry, Z.; Schwantes, A.M.; McCulloh, K.A.; Woodruff, D.R.; Jackson, R.B. Co-occurring Woody Species Have Diverse Hydraulic Strategies and Mortality Rates during an Extreme Drought. Plant Cell Environ. 2018, 41, 576–588. [Google Scholar] [CrossRef]
- Koepke, D.F.; Kolb, T.E.; Adams, H.D. Variation in Woody Plant Mortality and Dieback from Severe Drought among Soils, Plant Groups, and Species within a Northern Arizona Ecotone. Oecologia 2010, 163, 1079–1090. [Google Scholar] [CrossRef]
- Mitchell, R.M.; Wright, J.P.; Ames, G.M. Intraspecific Variability Improves Environmental Matching but Does Not Increase Ecological Breadth along a Wet-to-Dry Ecotone. Oikos 2017, 126, 988–995. [Google Scholar] [CrossRef]
- Apgaua, D.M.; Tng, D.Y.; Forbes, S.J.; Ishida, Y.F.; Vogado, N.O.; Cernusak, L.A.; Laurance, S.G. Elevated Temperature and CO2 Cause Differential Growth Stimulation and Drought Survival Responses in Eucalypt Species from Contrasting Habitats. Tree Physiol. 2019, 39, 1806–1820. [Google Scholar] [CrossRef]
- Mena, J.L.; Yagui, H.; Tejeda, V.; Bonifaz, E.; Bellemain, E.; Valentini, A.; Lyet, A. Environmental DNA Metabarcoding as a Useful Tool for Evaluating Terrestrial Mammal Diversity in Tropical Forests. Ecol. Appl. 2021, 31, e02335. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Stewart, K.A.; Dey, G.; Antognazza, C.M.; Sharma, R.K.; Maity, J.P.; Saha, S.; Doi, H.; de Vere, N.; Chan, M.W.Y.; et al. Environmental DNA Analysis as an Emerging Non-Destructive Method for Plant Biodiversity Monitoring: A Review. AoB Plants 2022, 14, plac031. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.W.; Mielke, L.; Stefanski, A.; Bermudez, R.; Hobbie, S.E.; Montgomery, R.A.; Kennedy, P.G. Climate Change-Induced Stress Disrupts Ectomycorrhizal Interaction Networks at the Boreal–Temperate Ecotone. Proc. Natl. Acad. Sci. USA 2023, 120, e2221619120. [Google Scholar] [CrossRef]
- Muniz, A.C.; Pimenta, R.J.G.; Cruz, M.V.; Rodrigues, J.G.; Buzatti, R.S.D.O.; Heuertz, M.; Lovato, M.B. Hybrid Zone of a Tree in a Cerrado/Atlantic Forest Ecotone as a Hotspot of Genetic Diversity and Conservation. Ecol. Evol. 2022, 12, e8540. [Google Scholar] [CrossRef]
- Andres, K.J.; Lodge, D.M.; Sethi, S.A.; Andrés, J. Detecting and Analysing Intraspecific Genetic Variation with eDNA: From Population Genetics to Species Abundance. Mol. Ecol. 2023, 32, 4118–4132. [Google Scholar] [CrossRef] [PubMed]

| Country | Region | Relevant Studies |
|---|---|---|
| Mexico | Sierra de Manantlán Biosphere Reserve, central Mexico; the southern slope of the Sierra Madre del Sur in Oaxaca | [90,91] |
| Costa Rica | Área de Conservación Guanacaste, northwestern Costa Rica | [13,58,97] |
| Colombia | La Sierra Nevada de Santa Marta; Cauca-Patía Basin; Magdalena River Basin | [102,103,104] |
| Bolivia | Santa Cruz, Bolivia | [93,105] |
| Brazil | Caatinga-Cerrado-Atlantic ranforest ecotones, northeastern Brazil | [23,33] |
| Scale | Focus | Example Studies and Key Findings |
|---|---|---|
| Stand | Impact of microhabitat on species composition | The transition zone between tropical moist and dry forests significantly influenced epiphyte composition (vascular and non-vascular), but microsite conditions affected only non-vascular epiphytes [110]. |
| Plant trait variation | Functional trait diversity showed a mosaicity pattern in the ecotone, indicating that the spatial heterogeneity of functional traits within transition zones played a crucial role in defining the ecological dynamics of bog-forest ecotones [63]. | |
| Landscape | Soil carbon dynamics | Microbial activity and nutrient availability were key drivers of soil carbon dynamics in a forest–savanna ecotone in Ghana [111]. |
| Edge effects on plant recruitment | The negative edge effect on seedling recruitment in a forest and grassland ecotone was attributed to reduced seed availability, unfavorable post-dispersal conditions for germination, and seedling establishment [112]. | |
| Environmental filters | Plant community trait values shifted in response to soil and light variation. Low soil nutrients and water in the coniferous zone were major constraints for most lowland rainforest species with acquisitive traits [113]. | |
| Region | ||
| Disturbance | Tropical forests and savannas worldwide may represent alternative stable states, with their resilience universally varying based on precipitation. Tree cover responded non-linearly to changes in precipitation [114]. | |
| Speciation | Quaternary climate variations influenced population divergence including genetic differentiation due to forest contraction and biome separation between Amazonia and Atlantic Forests. These climate-driven divergences, occurring in recent times, also explained speciation and evolutionary radiation over longer timescales [115]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Colin, P.; Hulshof, C.M. Ecotones as Windows into Organismal-to-Biome Scale Responses across Neotropical Forests. Plants 2024, 13, 2396. https://doi.org/10.3390/plants13172396
Ortiz-Colin P, Hulshof CM. Ecotones as Windows into Organismal-to-Biome Scale Responses across Neotropical Forests. Plants. 2024; 13(17):2396. https://doi.org/10.3390/plants13172396
Chicago/Turabian StyleOrtiz-Colin, Perla, and Catherine M. Hulshof. 2024. "Ecotones as Windows into Organismal-to-Biome Scale Responses across Neotropical Forests" Plants 13, no. 17: 2396. https://doi.org/10.3390/plants13172396






