Prasiolopsis wulf-kochii (Prasiolales, Trebouxiophyceae), a New Species Occurring in Hairs of the Sloth Bradypus tridactylus
Abstract
:1. Introduction
2. Results
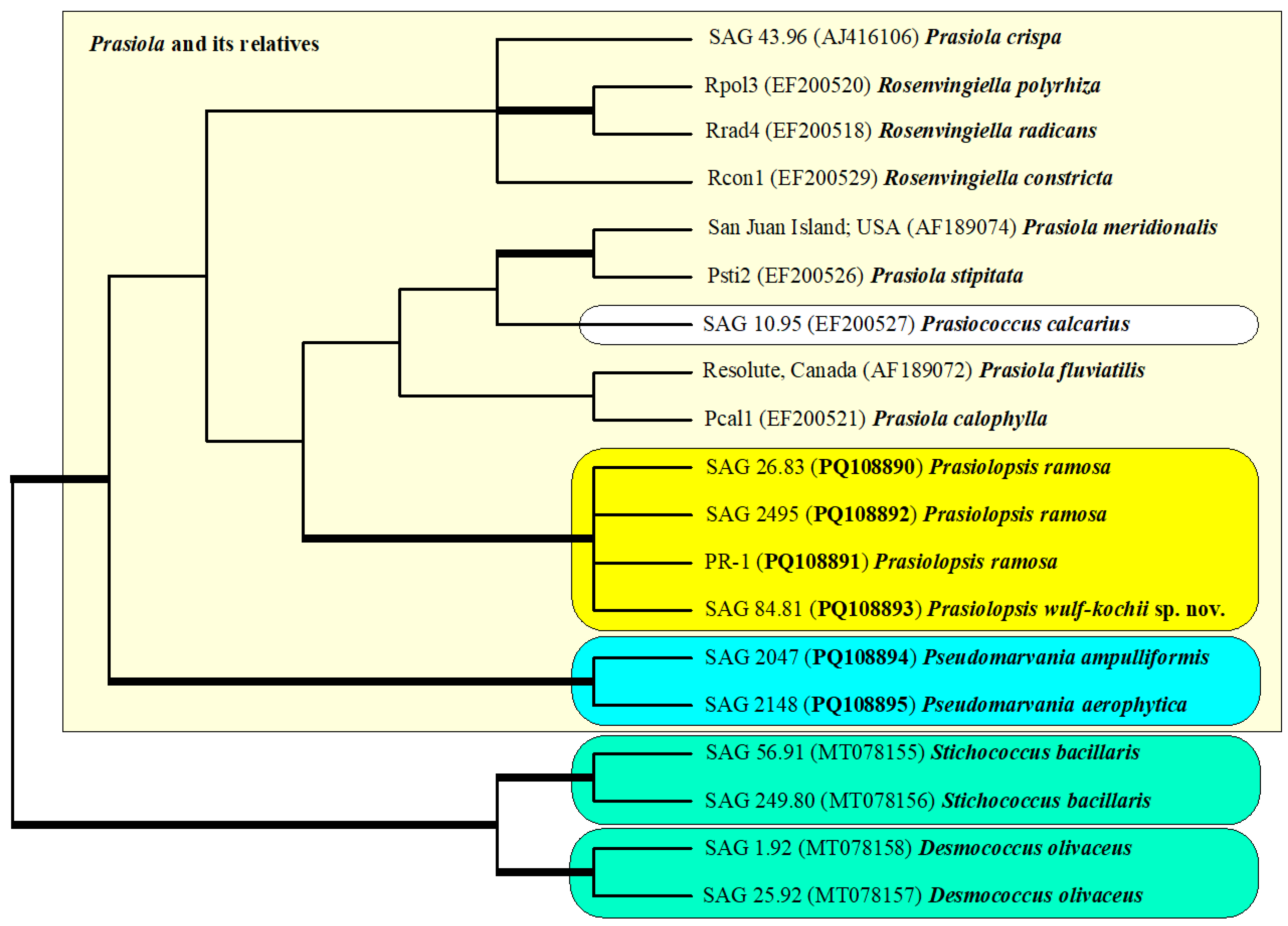
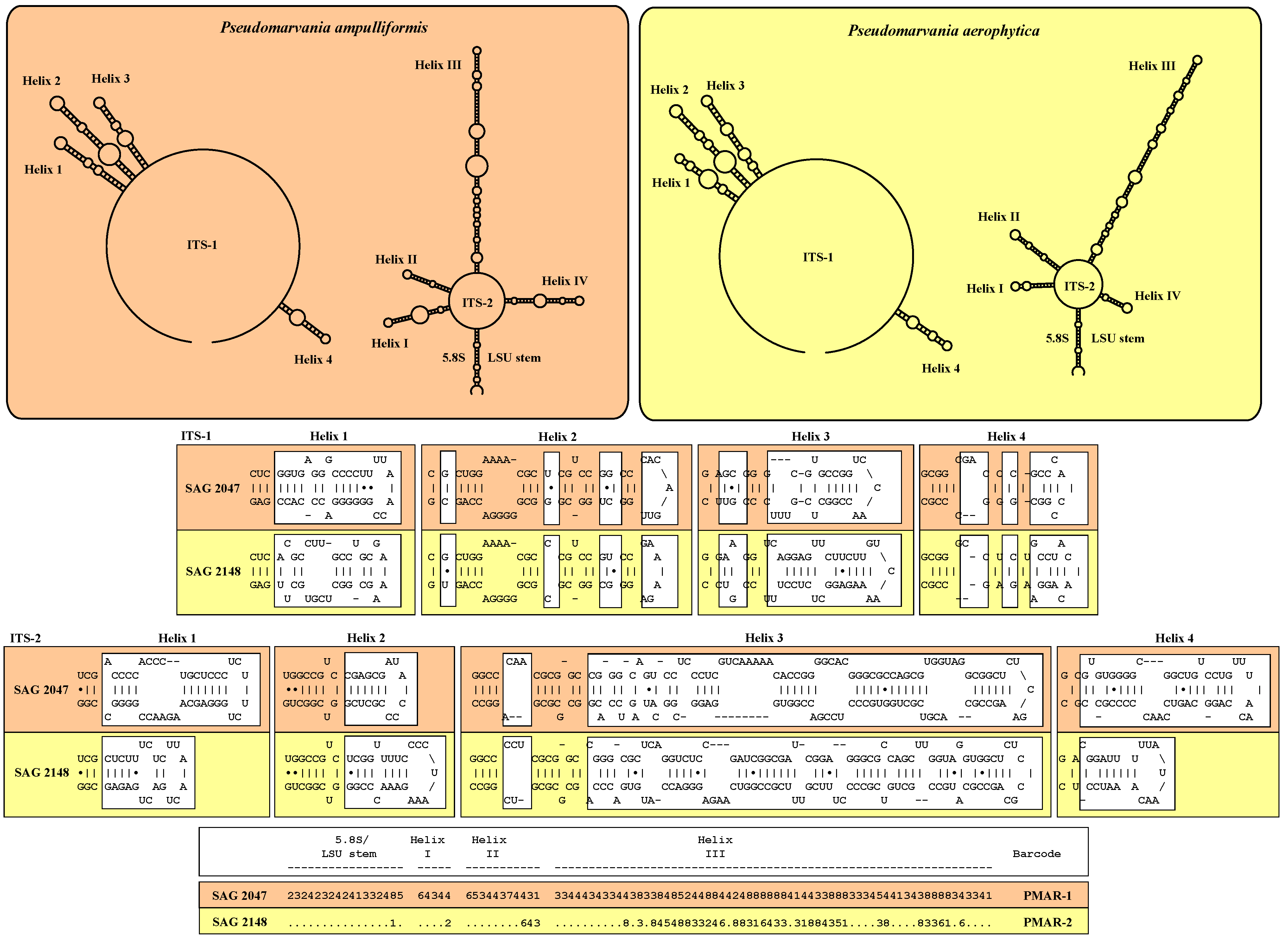
2.1. Molecular Phylogeny of Prasiolopsis and Its Relatives
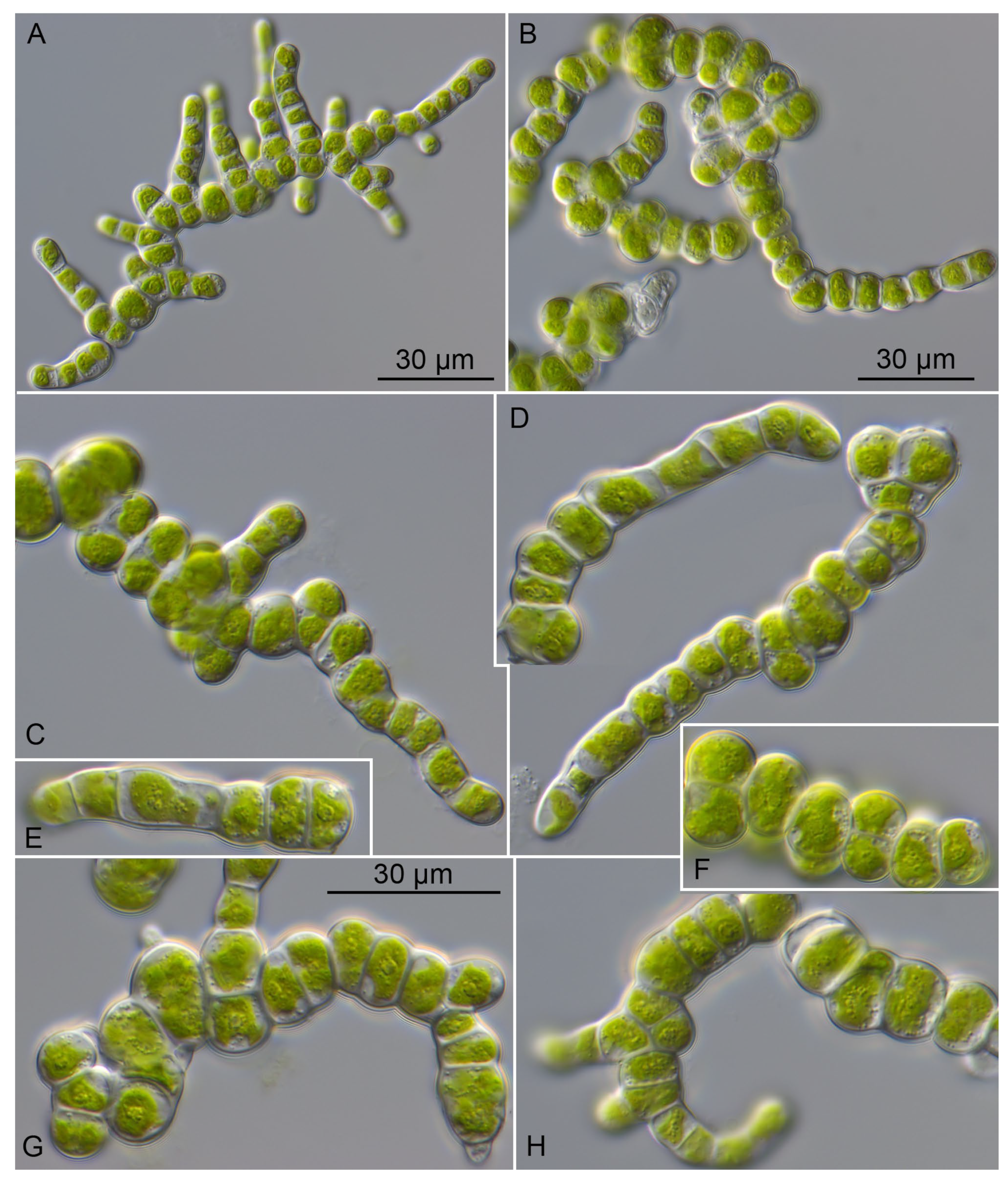
2.2. Morphology and Phenotypic Plasticity of the Investigated Prasiolopsis Strains
3. Discussion
3.1. Systematics, Ecology and Geographical Distribution of the Genus Prasiolopsis
3.2. The Mystery of Trichophilus Welckeri—A New View on an Old Story
4. Materials and Methods
4.1. Cultures and Light Microscopy
- SAG 26.83: Switzerland, Basel, bark of a fruit tree (47°28′52″ N, 7°51′7″ E); authentic strain of Prasiolopsis ramosa [1].
- SAG 2495: Germany, Hamburg, surface of a marble monument (53°33′43″ N, 9°59′27″ E); assigned as Prasiolopsis sp. [25].
- SAG 84.81: Brazil, Amazonia, from hairs of the sloth Bradypus tridactylus; assigned as Trichophilus welckeri.
- Strain PR-1: Austria, Vienna, Türkenschanzpark, wall near Dänenstrasse (48°14′8″ N, 16°20′2″ E); assigned as Prasiolopsis ramosa.
- For comparison, two strains of the sister genus Pseudomarvania were investigated:
4.2. DNA Extraction, PCR, Sequencing and Phylogenetic Analyses
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vischer, W. Über primitivste Landpflanzen. Ber. Schweiz. Bot. Ges. 1953, 63, 169–193. [Google Scholar]
- Chodat, R. Etude Critique et Expérimentale sur le Polymorphisme des Algues; Librairie Georg & Co.: Geneva, Switzerland, 1909; p. 165. [Google Scholar]
- Brand, F. Analyse der aerophilen Grünalgenanflüge, insbesondere der proto-pleurococcoiden Formen. Arch. Protistenkd. 1925, 52, 265–355. [Google Scholar]
- Schaffner, J.H. The classification of plants XII. Ohio J. Sci. 1922, 22, 129–139. [Google Scholar]
- Knebel, G. Monographie der Algenreihe der Prasiolales, insbesondere von Prasiola crispa. Hedwigia 1935, 75, 1–120. [Google Scholar]
- Silva, P.C. Notes on Pacific marine algae. Madroño 1957, 14, 41–51. [Google Scholar]
- Friedmann, I. Structure, life-history and sex determination of Prasiola stipitata Suhr. Ann. Bot. 1959, 23, 571–594. [Google Scholar] [CrossRef]
- Friedmann, I. Geographic and environmental factors controlling life history and morphology in Prasiola stipitata Suhr. Österr. Bot. Z. 1969, 116, 203–225. [Google Scholar] [CrossRef]
- Friedmann, I.; Manton, I. Gametes, fertilization and zygote development in P. stipitata. Nova Hedwigia 1960, 1, 333–344. [Google Scholar]
- Kornmann, P.; Sahling, P.H. Prasiolales (Chlorophyta) von Helgoland. Helgol. Wiss. Meeresunters. 1974, 26, 99–133. [Google Scholar] [CrossRef]
- Pröschold, T.; Darienko, T. The green puzzle Stichococcus (Trebouxiophyceae, Chlorophyta): New generic and species concept among this widely distributed genus. Phytotaxa 2020, 441, 113–142. [Google Scholar] [CrossRef]
- O’Kelly, C.J.; Garbary, D.J.; Floyd, G.L. Flagellar apparatus of male gametes and other aspects of gamete and zygote ultrastructure in Prasiola and Rosenvingiella (Chlorophyta, Prasiolales) from British Columbia. Can. J. Bot. 1989, 67, 505–514. [Google Scholar] [CrossRef]
- Sherwood, A.R.; Garbary, D.J.; Sheath, R.G. Assessing the phylogenetic position of the Prasiolales (Chlorophyta) using rbcL and 18S rRNA sequence data. Phycologia 2000, 39, 139–146. [Google Scholar] [CrossRef]
- Karsten, U.; Friedl, T.; Schumann, R.; Hoyer, K.; Lembcke, S. Mycosporine-like amino acids and phylogenies in green algae: Prasiola and its relatives from the Trebouxiophyceae (Chlorophyta). J. Phycol. 2005, 41, 557–566. [Google Scholar] [CrossRef]
- Rindi, F.; McIvor, L.; Sherwood, A.R.; Friedl, T.; Guiry, M.D.; Sheath, R.G. Molecular phylogeny of the green algal order Prasiolales (Trebouxiophyceae, Chlorophyta). J. Phycol. 2007, 43, 811–822. [Google Scholar] [CrossRef]
- Heesch, S.; Pažoutová, M.; Moniz, M.B.J.; Rindi, F. Prasiolales (Trebouxiophyceae, Chlorophyta) of the Svalbard Archipelago: Diversity, biogeography and description of the new genera Prasionella and Prasionema. Eur. J. Phycol. 2016, 51, 171–187. [Google Scholar] [CrossRef]
- Darienko, T.; Gustavs, L.; Pröschold, T. Species concept and nomenclatural changes within the genera Elliptochloris and Pseudochlorella (Trebouxiophyceae) based on an integrative approach. J. Phycol. 2016, 52, 1125–1145. [Google Scholar] [CrossRef]
- Hodac, L.; Hallmann, C.; Spitzer, K.; Elster, J.; Faßhauer, F.; Brinkmann, N.; Lepka, D.; Diwan, V.; Friedl, T. Widespread green algae Chlorella and Stichococcus exhibit polar-temperate and tropical-temperate biogeography. FEMS Microbiol. Ecol. 2016, 93, fiw122. [Google Scholar]
- Lemieux, C.; Otis, C.; Turmel, M. Chloroplast phylogenomic analysis resolves deep-level relationships within the green algal class Trebouxiophyceae. BMC Evol. Biol. 2014, 14, 211. [Google Scholar] [CrossRef]
- Weber-van-Bosse, A. Étude sur les algues parasites des Paresseux. Natuurk. Verh. Holl. Maatsch. Wetensch. Haarlem III 1887, 5, 1–23. [Google Scholar]
- Suutari, M.; Majaneva, M.; Fewer, D.P.; Voirin, B.; Aiello, A.; Friedl, T.; Chiarello, A.G.; Blomster, J. Molecular evidence for a diverse green algal community growing in the hair of sloths and a specific association with Trichophilus welckeri (Chlorophyta, Ulvophyceae). BMC Evol. Biol. 2010, 10, 86. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Coleman, A.W.; Mai, J.C. Ribosomal DNA ITS-1 and ITS-2 sequence comparisons as a tool for predicting genetic relatedness. J. Mol. Evol. 1997, 45, 168–177. [Google Scholar] [CrossRef]
- Mai, J.C.; Coleman, A.W. The internal transcribed spacer 2 exhibits a common secondary structure in green algae and flowering plants. J. Mol. Evol. 1997, 44, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, C.; Rüdrich, J.; Enseleit, M.; Friedl, T.; Hoppert, M. Microbial diversity on a marble monument: A case study. Environ. Earth Sci. 2011, 63, 1701–1711. [Google Scholar] [CrossRef]
- Mikhailyuk, T.I. Terrestrial Algae from the Granite Outcrops of River Valleys of the Ukraine. Internat. J. Algae 2013, 15, 313–332. [Google Scholar] [CrossRef]
- Das, S.K. Prasiolopsis ramosa Vischer—New addition to the algal flora of Asia. J. New Biol. Rep. 2015, 4, 157–158. [Google Scholar]
- Handa, S.; Nakahara, M.; Tsubota, H.; Deguchi, H.; Nakano, T. A new aerial alga, Stichococcus ampulliformis sp. nov. (Trebouxiophyceae, Chlorophyta) from Japan. Phycol. Res. 2003, 51, 203–210. [Google Scholar] [CrossRef]
- Elias, M.; Neustupa, J. Pseudomarvania, gen. nov. (Chlorophyta, Trebouxiophyceae), a new genus for “budding” subaerial green algae Marvania aerophytica Neustupa et Sejnohova and Stichococcus ampulliformis Handa. Fottea 2009, 9, 169–177. [Google Scholar] [CrossRef]
- Neustupa, J.; Sejnohova, L. Marvania aerophytica sp. nov., a new subaerial tropical green alga. Biologia 2003, 58, 503–507. [Google Scholar]
- Lagerheim, G. Trichophilus neniae Lagerh. n. sp., eine neue epizoische Alge. Ber. Dt. Bot. Ges. 1892, 10, 514–517. [Google Scholar]
- Yang, H.; Genot, B.; Duhamel, S.; Kerney, R.; Burns, J.A. Organismal and cellular interactions in vertebrate–alga symbioses. Biochem. Soc. Trans. 2022, 50, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Kaup, M.; Trull, S.; Hom, E.F.Y. On the move: Sloths and their epibionts as model mobile ecosystems. Biol. Rev. 2021, 96, 2638–2660. [Google Scholar] [CrossRef] [PubMed]
- Fountain, E.D.; Pauli, J.N.; Mendoza, J.E.; Carlson, J.; Peery, M.Z. Cophylogenetics and biogeography reveal a coevolved relationship between sloths and their symbiont algae. Mol. Phylogen. Evol. 2017, 110, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Pauli, J.N.; Mendoza, J.E.; Steffan, S.A.; Carey, C.C.; Weimer, P.J.; Peery, M.Z. A syndrome of mutualism reinforces the lifestyle of a sloth. Proc. R. Soc. B 2022, 281, 20133006. [Google Scholar] [CrossRef]
- Schlösser, U.G. Additions to the culture collections of algae since 1994. Bot. Acta 1997, 110, 424–429. [Google Scholar] [CrossRef]
- Darienko, T.; Rad Menéndez, C.; Campbell, C.; Pröschold, T. Are there any true marine Chlorella species? Molecular phylogenetic assessment and ecology of marine Chlorella-like organisms, including description of Droopiella gen. nov. Syst. Biodivers. 2019, 17, 811–829. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP* Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002.
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Jow, H.; Hudelot, C.; Rattray, M.; Higgs, P. Bayesian phylogenetics using an RNA substitution model applied to early mammalian evolution. Mol. Biol. Evol. 2002, 19, 1591–1601. [Google Scholar] [CrossRef]
- Higgs, P.; Jameson, D.; Jow, H.; Rattray, M. The evolution of tRNA-Leu genes in animal mitochondrial genomes. J. Mol. Evol. 2003, 57, 435–445. [Google Scholar] [CrossRef]
- Hudelot, C.; Gowri-Shankar, V.; Jow, H.; Rattray, M.; Higgs, P. RNA-based phylogenetic methods: Application to mammalian mitochondrial RNA sequences. Mol. Phylogen. Evol. 2003, 28, 241–252. [Google Scholar] [CrossRef]
- Gibson, A.; Gowri-Shankar, V.; Higgs, P.; Rattray, M. A comprehensive analysis of mammalian mitochondrial genome base composition and improved phylogenetic methods. Mol. Biol. Evol. 2005, 22, 251–264. [Google Scholar] [CrossRef]
- Telford, M.J.; Wise, M.J.; Gowri-Shankar, V. Consideration of RNA secondary structure significantly improves likelihood-based estimates of phylogeny: Examples from the bilateria. Mol. Biol. Evol. 2005, 22, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acid Res. 2003, 31, 3406–3615. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.; Han, K. PseudoViewer3: Generating planar drawings of large-scale RNA structures with pseudoknots. Bioinformatics 2009, 25, 1435–1437. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darienko, T.; Pröschold, T. Prasiolopsis wulf-kochii (Prasiolales, Trebouxiophyceae), a New Species Occurring in Hairs of the Sloth Bradypus tridactylus. Plants 2024, 13, 2405. https://doi.org/10.3390/plants13172405
Darienko T, Pröschold T. Prasiolopsis wulf-kochii (Prasiolales, Trebouxiophyceae), a New Species Occurring in Hairs of the Sloth Bradypus tridactylus. Plants. 2024; 13(17):2405. https://doi.org/10.3390/plants13172405
Chicago/Turabian StyleDarienko, Tatyana, and Thomas Pröschold. 2024. "Prasiolopsis wulf-kochii (Prasiolales, Trebouxiophyceae), a New Species Occurring in Hairs of the Sloth Bradypus tridactylus" Plants 13, no. 17: 2405. https://doi.org/10.3390/plants13172405
APA StyleDarienko, T., & Pröschold, T. (2024). Prasiolopsis wulf-kochii (Prasiolales, Trebouxiophyceae), a New Species Occurring in Hairs of the Sloth Bradypus tridactylus. Plants, 13(17), 2405. https://doi.org/10.3390/plants13172405





