Differential Responses of Bilberry (Vaccinium myrtillus) Phenology and Density to a Changing Environment: A Study from Western Carpathians
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
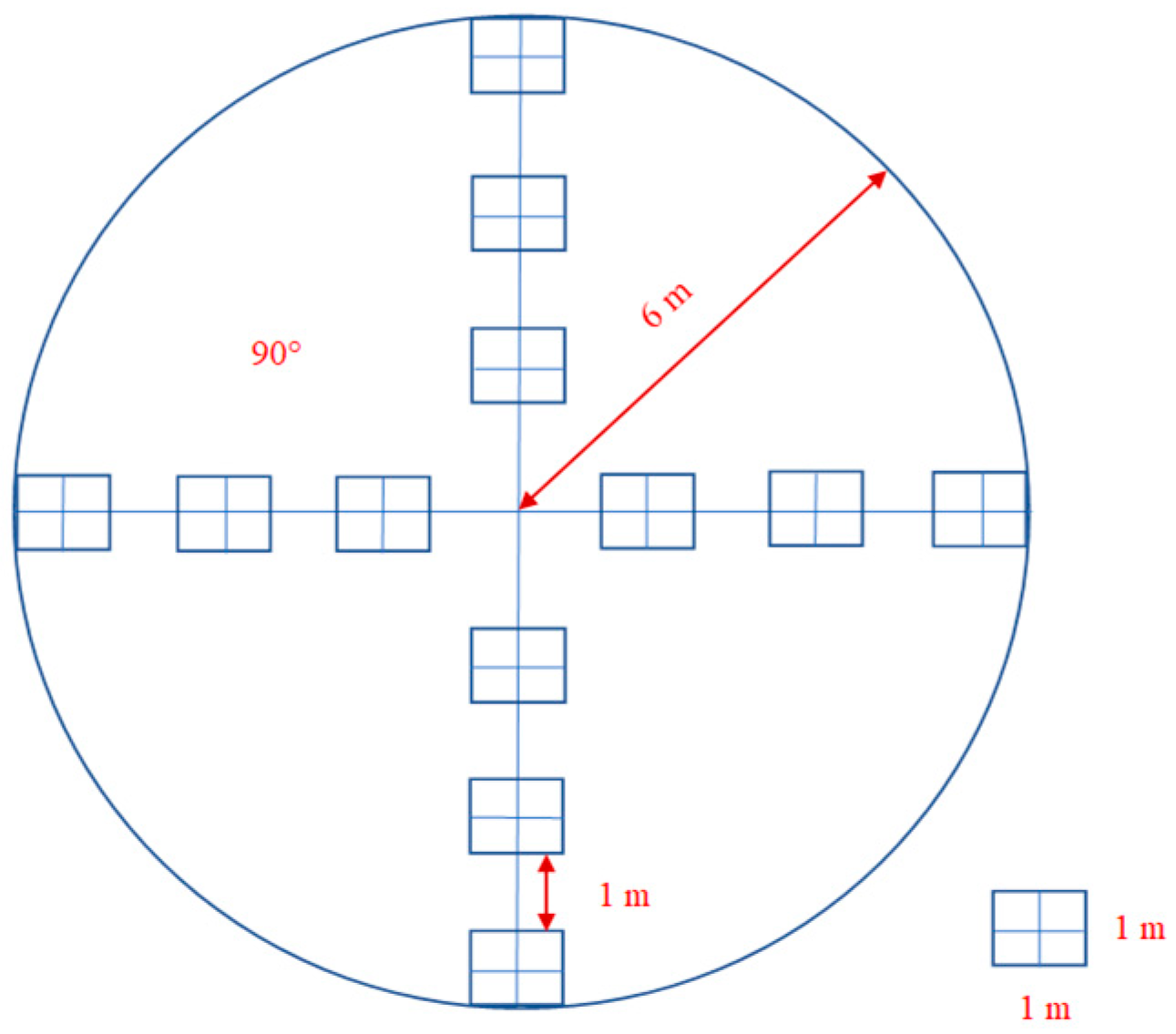
2.2. Sampling Design
2.3. Climate Data
2.4. Phenological Observations
- BBCH 07—Shoot expansion (Leaves are completely open)—vegetative stage
- BBCH 65—Full bloom (Flower petals are open for pollination)—generative stage
- BBCH 72—Petal fall (Petals drop, but the calyx and stamen remain)—generative stage
- BBCH 86—Blue fruit (Fruit is ripe, sugar content is high)—generative stage
- BBCH 92—Autumn leaf colouring (Leaves turn red and drop naturally)—vegetative stage
2.5. Statistical Analysis
3. Results
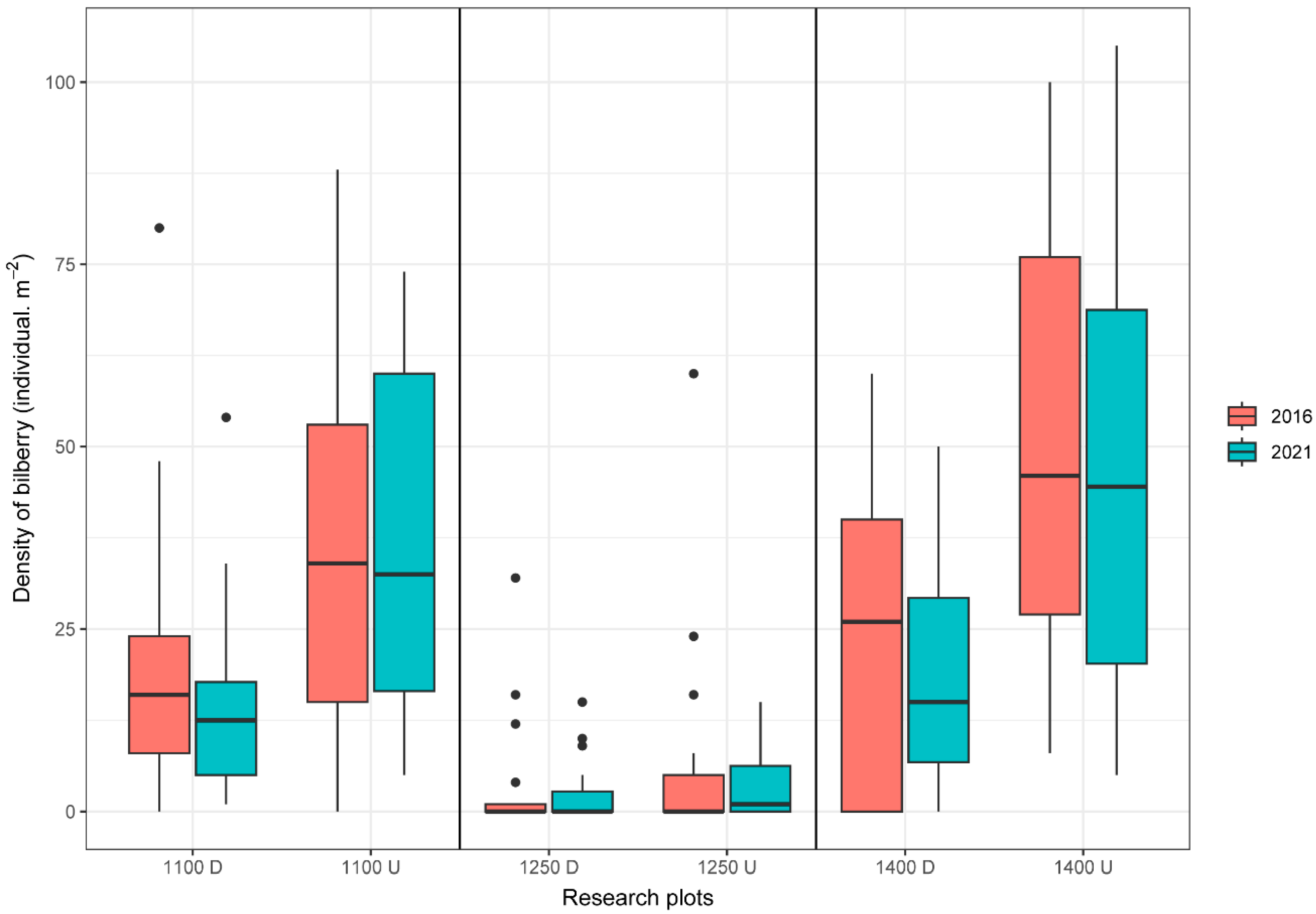
3.1. The Density of Bilberry
3.2. Phenology of Bilberry (2016–2021)
3.3. Effects of Environmental Factors in the Phenology
4. Discussion
4.1. Characteristics of Bilberry Population Growing in the Different Habitats
4.2. Phenology of Bilberry Population Growing in the Different Habitats
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Senf, C.; Seidl, R. Mapping the forest disturbance regimes of Europe. Nat. Sustain. 2021, 4, 63–70. [Google Scholar] [CrossRef]
- James, A. Reviewing the adaptability of pure and mixed Norway Spruce forests to climate change in Central Europe. J. Agric. Technol. 2022, 9, 70–74. [Google Scholar]
- Senf, C.; Seidl, R. Natural disturbances are spatially diverse but temporally synchronized across temperate forest landscapes in Europe. Glob. Chang. Biol. 2018, 24, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, P.H.W.; Müller, J.; Grégoire, J.C.; Gruppe, A.; Hagge, J.; Hammerbacher, A.; Hofstetter, R.W.; Kandasamy, D.; Kolarik, M.; Kostovcik, M.; et al. Bark Beetle population dynamics in the Anthropocene: Challenges and solutions. Trends Ecol. Evol. 2019, 34, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Loch, J.; Chwistek, K.; Wężyk, S.; Małek, S.; Pająk, M. Natural regeneration versus tree planting in the subalpine spruce forest Plagiothecio-Piceetum tatricum of the Gorce National Park (Southern Poland). Nat. Conserv. 2001, 58, 5–15. [Google Scholar]
- Cudmore, T.J.; Björklund, N.A.; Carroll, L.; Staffan, B.L. Climate change and range expansion of an aggressive bark beetle: Evidence of higher beetle reproduction in naïve host tree populations. J. Appl. Ecol. 2010, 47, 1036–1043. [Google Scholar] [CrossRef]
- Palm, K.; Vodde, F.; Tullus, T.; Engelhart, J.; Jõgiste, K. Impact of different storm severity levels and post-storm management on understory vegetation richness, diversity and composition 19–20 years after wind disturbance. For. Ecol. Manag. 2022, 524, 120506. [Google Scholar] [CrossRef]
- Poorter, L.; van der Sande, M.T.; Amissah, L.; Bongers, F.; Hordijk, I.; Kok, J.; Laurance, S.G.W.; Martínez-Ramos, M.; Matsuo, T.; Meave, J.A.; et al. A comprehensive framework for vegetation succession. Ecosphere 2024, 15, e4794. [Google Scholar] [CrossRef]
- Ulanova, N.G. The effects of windthrow on forest at different spatial scales: A review. For. Ecol. Manag. 2000, 135, 155–167. [Google Scholar] [CrossRef]
- Vodde, F.; Jõgiste, K.; Gruson, L.; Ilisson, T.; Köster, K.; Stanturf, J.A. Regeneration in windthrow areas in hemiboreal forests: The influence of microsite on the height growths of different tree species. J. For. Res. 2010, 15, 55–64. [Google Scholar] [CrossRef]
- Malík, K.; Remeš, J.; Vacek, S.; Štícha, V. Development and dynamics of mountain spruce (Picea abies [L.] Karsten) stand regeneration. J. For. Sci. 2014, 60, 61–69. [Google Scholar] [CrossRef]
- Jonášová, M.; Prach, K. Central-European mountain spruce (Picea abies (L.) Karst.) forests: Regeneration of tree species after a bark beetle outbreak. Ecol. Eng. 2004, 23, 15–27. [Google Scholar] [CrossRef]
- Vacek, S.; Nosková, I.; Bilek, L.; Vacek, Z.; Schwarz, O. Regeneration of forest stands on permanent research plots in the Krkonoše Mts. J. For. Sci. 2010, 56, 541–554. [Google Scholar] [CrossRef]
- Fischer, A. Long term vegetation development in Bavarian Mountain Forest ecosystems following natural destruction. Vegetatio 1992, 103, 93–104. [Google Scholar] [CrossRef]
- Holeksa, J. Relationship between field-layer vegetation and canopy openings in a Carpathian subalpine spruce forest. Plant Ecol. 2003, 168, 57–67. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Miechówka, A. The role of bilberry and Alpine lady-fern in soil formation within the Carpathian subalpine spruce forest stands. Geoderma 2017, 305, 162–172. [Google Scholar] [CrossRef]
- Nilsson, M.C.; Wardle, D. Understory vegetation as a forest ecosystem driver: Evidence from the northern Swedish boreal forest. Front. Ecol. Environ. 2005, 3, 421–428. [Google Scholar] [CrossRef]
- Kolari, P.; Pumpanen, J.; Kulmala, L.; Ilvesniemi, H.; Nikinmaa, E.; Grönholm, T.; Hari, P. Forest floor vegetation plays an important role in photosynthetic production of boreal forests. For. Ecol. Manag. 2006, 221, 241–248. [Google Scholar] [CrossRef]
- Michalová, Z.; Morrissey, R.C.; Wohlgemuth, T.; Bače, R.; Fleischer, P.; Svoboda, M. Salvage-logging after windstorm leads to structural and functional homogenization of understory layer and delayed spruce tree recovery in Tatra Mts., Slovakia. Forests 2017, 8, 88. [Google Scholar] [CrossRef]
- Jäderlund, A.; Norberg, G.; Zackrisson, O.; Dahlberg, A.; Teketay, D.; Dolling, A.; Nilsson, M.C. Control of bilberry vegetation by steam treatment—Effects on seeded Scots pine and associated mycorrhizal fungi. For. Ecol. Manag. 1998, 108, 275–285. [Google Scholar] [CrossRef]
- Nestby, R.; Percival, D.; Martinussen, I. The European blueberry (Vaccinium myrtillus L.) and the potential for cultivation. A review. Eur. J. Plant Sci. Biotechnol. 2010, 5, 5–16. [Google Scholar]
- Hjältén, J.; Danell, K.; Ericson, L. Hare and vole browsing preferences during winter. Acta Theriol. (Warsz.) 2004, 49, 53–62. [Google Scholar] [CrossRef]
- Stenset, N.E.; Lutnæs, P.N.; Bjarnadóttir, V.; Dahle, B.R.; Fossum, K.H.I.; Jigsved, P.; Johansen, T.; Neumann, W.; Opseth, O.; Rønning, O.; et al. Seasonal and annual variation in the diet of brown bears Ursus arctos in the boreal forest of southcentral Sweden. Wildl. Biol. 2016, 22, 107–116. [Google Scholar] [CrossRef]
- Hodkinson, I.D. Terrestrial insects along elevation gradients: Species and community responses to altitude. Biol. Rev. Camb. Philos. Soc. 2005, 80, 489–513. [Google Scholar] [CrossRef] [PubMed]
- Delnevo, N.; Petraglia, A.; Carbognani, M.; Vandvik, V.; Halbritter, A.H. Plastic and genetic responses to shifts in snowmelt time affects the reproductive phenology and growth of Ranunculus acris. Perspect. Plant Ecol. Evol. Syst. 2018, 30, 62–70. [Google Scholar] [CrossRef]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Saunders, S.C.; Crow, T.R.; Naiman, R.J.; Brosofske, K.D.; Mroz, G.D.; Brookshire, B.L.; Franklin, J.F. Microclimate in Forest Ecosystem and Landscape Ecology: Variations in local climate can be used to monitor and compare the effects of different management regimes. BioScience 1999, 49, 288–297. [Google Scholar] [CrossRef]
- Hesslerová, P.; Huryna, H.; Pokorný, J.; Procházka, J. The effect of forest disturbance on landscape temperature. Ecol. Eng. 2018, 120, 345–354. [Google Scholar] [CrossRef]
- Kukla, J.; Kuklová, M. Growth of Vaccinium myrtillus L. (Ericaceae) in spruce forests damaged by air pollution. Pol. J. Ecol. 2008, 56, 149–155. [Google Scholar]
- Kuklová, M.; Kukla, J. Accumulation of macronutrients in soils and some herb species of spruce ecosystems. Cereal Res. Commun. 2008, 36, 1319–1322. [Google Scholar]
- Vaneková, Z.; Vanek, M.; Škvarenina, J.; Nagy, M. The influence of local habitat and microclimate on the levels of secondary metabolites in Slovak bilberry (Vaccinium myrtillus L.) fruits. Plants 2020, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Kubov, M.; Janík, R.; Tomes, J.; Schieber, B. Nutrient content in biomass of bilberry (Vaccinium myrtillus L.) in different habitats of protected areas of Inner Western Carpathians. J. For. Sci. 2024, 70, 161–175. [Google Scholar] [CrossRef]
- Budzáková, M.; Galvánek, D.; Littera, P.; Šibík, J. The wind and fire disturbance in central European mountain spruce forests: The regeneration after four years. Acta Soc. Bot. Pol. Pol. Tow Bot. 2013, 82, 13–24. [Google Scholar] [CrossRef]
- Gáfriková, J.; Zvarík, M.; Hanajík, P.; Súlovský, M.; Vykouková, I. Impact of natural disturbance, forest management and vegetation cover on topsoil biochemical characteristics of Tatra Mts. (Slovakia). J. Mt. Sci. 2020, 17, 1294–1309. [Google Scholar] [CrossRef]
- Koutecký, T.; Ujházy, K.; Volařík, D.; Ujházyová, M.; Máliš, F.; Gömöryová, E.; Bače, R.; Ehrenbergerová, L.; Glončák, P.; Hofmeister, J.; et al. Disturbance history drives current compositional and diversity patterns of primary Picea abies (L.) Karst. forest vegetation. For. Ecol. Manag. 2022, 520, 120387. [Google Scholar] [CrossRef]
- Höcke, C.E.; Spiegelhalter, J.; Gärtner, S.M.; Reif, A. The influence that Picea abies Karst. and Fagus sylvatica L. have on the vitality of Vaccinium myrtillus L. in montane mixed forests of central Europe on silicate bedrock. Waldokologie 2016, 15, 43–56. [Google Scholar]
- Špulák, O.; Souček, J.; Dušek, D. Quality of organic and upper mineral horizons of mature mountain beech stands with respect to herb layer species. J. For. Sci. 2016, 62, 163–174. [Google Scholar] [CrossRef]
- Homolová, Z.; Fleischer, P. Tatry ako objekt dlhodobého ekologického výskumu prírodných disturbancií. Životné Prostr. 2016, 50, 40–43. [Google Scholar]
- Jamnická, G.; Konôpková, A.; Fleischer, P.; Kurjak, D.; Petrík, P.; Petek, A.; Húdoková, H.; Fleischer, P.; Homolová, Z.; Ježík, M.; et al. Physiological vitality of norway spruce (Picea abies L.) stands along an altitudinal gradient in Tatra national park. Cent. Eur. For. J. 2020, 66, 227–242. [Google Scholar] [CrossRef]
- Tomes, J.; Fleischer, P.; Kubov, M.; Fleischer, P. Soil respiration after bark beetle infestation along a vertical transect in mountain spruce forest. Forests 2024, 15, 611. [Google Scholar] [CrossRef]
- Dostál, J. Nová Květena ČSSR 1–2; Academia Praha: Praha, Czech Republic, 1989. [Google Scholar]
- Braslavská, O.; Kamenský, L. Phenological Observation of Forest Plants; Methodical Prescription; SHMI: Bratislava, Slovakia, 1996; p. 22. [Google Scholar]
- Meier, U. BBCH-Monograph: Growth Stages of Mono- and Dicotyledonous Plants, 2nd ed.; Technical Report; Federal Biological Research Centre for Agriculture and Forestry: Bonn, Germany, 2001. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.project.org/ (accessed on 10 December 2021).
- Evans, J.D. Straightforward statistics for the behavioral sciences. Am. Stat. Assoc. 1996, 91, 1750–1751. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Eldegard, K.; Scholten, J.; Stokland, J.N.; Granhus, A.; Lie, M. The influence of stand density on bilberry (Vaccinium myrtillus L.) cover depends on stand age, solar irradiation, and tree species composition. For. Ecol. Manag. 2019, 432, 582–590. [Google Scholar] [CrossRef]
- Vanwalleghem, T.; Meentemeyer, R.K. Predicting forest microclimate in heterogeneous landscapes. Ecosystems 2009, 12, 1158–1172. [Google Scholar] [CrossRef]
- Von Arx, G.; Dobbertin, M.; Rebetez, M. Spatio-temporal effects of forest canopy on understory microclimate in a long-term experiment in Switzerland. Agric. For. Meteorol. 2012, 166–167, 144–155. [Google Scholar] [CrossRef]
- Uleberg, E.; Rohloff, J.; Jaakola, L.; Trôst, K.; Junttila, O.; Häggman, H.; Martinussen, I. Effects of temperature and photoperiod on yield and chemical composition of northern and southern clones of bilberry (Vaccinium myrtillus L.). J. Agric. Food Chem. 2012, 60, 10406–10414. [Google Scholar] [CrossRef] [PubMed]
- Elisabetta, B.; Flavia, G.; Paolo, F.; Giorgio, L.; Attilio, S.G.; Fiorella, L.S.; Juri, N. Nutritional profile and productivity of bilberry (Vaccinium myrtillus L.) in different habitats of a protected area of the Eastern Italian Alps. J. Food Sci. 2013, 78, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Hertel, A.G.; Steyaert, S.M.; Zedrosser, A.; Mysterud, A.; Lodberg-Holm, H.K.; Gelink, H.W.; Kindberg, J.; Swenson, J.E. Bears and berries: Species-specific selective foraging on a patchily distributed food resource in a human-altered landscape. Behav. Ecol. Sociobiol. 2016, 70, 831–842. [Google Scholar] [CrossRef]
- Storch, I. Habitat selection by Capercaillie in summer and autumn: Is bilberry important? Oecologia 1993, 95, 257–265. [Google Scholar] [CrossRef]
- Coudun, C.; Gégout, J.C. Quantitative prediction of the distribution and abundance of Vaccinium myrtillus with climatic and edaphic factors. J. Veg. Sci. 2007, 18, 517. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Turrill, N.L. Herbaceous layer cover and biomass in a young versus a mature stand of a central Appalachian hardwood forest. Bull. Torrey Bot. Club 1993, 120, 445–450. [Google Scholar] [CrossRef]
- Légaré, S.; Bergeron, Y.; Paré, D. Influence of forest composition on understory cover in boreal mixed-wood forests of western Quebec. Silva Fenn. 2002, 36, 353–366. [Google Scholar] [CrossRef]
- Mäkipää, R. Response patterns of Vaccinium myrtillus and V. vitis-idaea along nutrient gradients in boreal forest. J. Veg. Sci. 1999, 10, 17–26. [Google Scholar] [CrossRef]
- Wagner, M.; Kahmen, A.; Schlumprecht, H.; Audorff, V.; Perner, J.; Buchmann, N.; Weisser, W.W. Prediction of herbage yield in grassland: How well do Ellenberg N-values perform? Appl. Veg. Sci. 2007, 10, 15. [Google Scholar] [CrossRef]
- Soto, D.P.; Donoso, P.J.; Salas, C.; Puettmann, K. Light availability and soil compaction influence the growth of underplanted Nothofagus following partial shelterwood harvest and soil scarification. Can. J. For. Res. 2015, 45, 998–1005. [Google Scholar] [CrossRef]
- Hilmers, T.; Hilmers, T.; Friess, N.; Bässler, C.; Heurich, M.; Brandl, R.; Pretzsch, H.; Müller, J. Biodiversity along temperate forest succession. J. Appl. Ecol. 2018, 55, 2756–2766. [Google Scholar] [CrossRef]
- Štursová, M.; Šnajdr, J.; Cajthaml, T.; Bárta, J.; Šantrůčková, H.; Baldrian, P. When the forest dies: The response of forest soil fungi to a bark beetle-induced tree dieback. ISME J. 2014, 8, 1920–1931. [Google Scholar] [CrossRef]
- Čerevková, A.; Renčo, M.; Cagáň, Ľ. Short-term effects of forest disturbances on soil nematode communities in European mountain spruce forests. J. Helminthol. 2013, 87, 376–385. [Google Scholar] [CrossRef]
- Davis, T.S.; Rhoades, P.R.; Mann, A.J.; Griswold, T. Bark beetle outbreak enhances biodiversity and foraging habitat of native bees in alpine landscapes of the southern Rocky mountains. Sci. Rep. 2020, 10, 16400. [Google Scholar] [CrossRef]
- Zavitkovski, J. Ground vegetation biomass, production, and efficiency of energy utilization in some northern Wisconsin forest ecosystems. Ecology 1976, 57, 694–706. [Google Scholar] [CrossRef]
- Kneitel, J.M.; Perrault, D. Disturbance-induced changes in community composition increase species invasion success. Community Ecol. 2006, 7, 245–252. [Google Scholar] [CrossRef]
- Häntzschel, J.; Goldberg, V.; Bernhofer, C. GIS-based regionalisation of radiation, temperature and coupling measures in complex terrain for low mountain ranges. Meteorol. Appl. 2005, 12, 33–42. [Google Scholar] [CrossRef]
- Bartík, M.; Jančo, M.; Střelcová, K.; Škvareninová, J.; Škvarenina, J.; Mikloš, M.; Vido, J.; Dagsson Waldhauserová, P. Rainfall interception in a disturbed montane spruce (Picea abies) stand in the West Tatra Mountains. Biologia 2016, 71, 1002–1008. [Google Scholar] [CrossRef]
- Ørbæk, H.V.B. Reproduction and Pollination in Bilberry (Vaccinium myrtillus) along Two Elevational Gradients in Western Norway. Master’s Thesis, University of Bergen, Bergen, Norway, 2022. [Google Scholar]
- Selås, V. Seed production of a masting dwarf shrub, Vaccinium myrtillus, in relation to previous reproduction and weather. Can. J. Bot. 2000, 78, 423–429. [Google Scholar]
- Wipf, S.; Rixen, C.; Mulder, C.P.H. Advanced snowmelt causes shift towards positive neighbour interactions in a subarctic tundra community. Glob. Chang. Biol. 2006, 12, 1496–1506. [Google Scholar] [CrossRef]
- Wipf, S.; Stoeckli, V.; Bebi, P. Winter climate change in alpine tundra: Plant responses to changes in snow depth and snowmelt timing. Clim. Chang. 2009, 94, 105–121. [Google Scholar] [CrossRef]
- Boscutti, F.; Casolo, V.; Beraldo, P.; Braidot, E.; Zancani, M.; Rixen, C. Shrub growth and plant diversity along an elevation gradient: Evidence of indirect effects of climate on alpine ecosystems. PLoS ONE 2018, 13, e0196653. [Google Scholar] [CrossRef]
- Filippi, A.; Braidot, E.; Petrussa, E.; Fabro, M.; Vuerich, M.; Boscutti, F. Plant growth shapes the effects of elevation on the content and variability of flavonoids in subalpine bilberry stands. Plant Biol. 2021, 23, 241–249. [Google Scholar] [CrossRef]
- Hertel, A.G.; Bischof, R.; Langval, O.; Mysterud, A.; Kindberg, J.; Swenson, J.E.; Zedrosser, A. Berry production drives bottom–up effects on body mass and reproductive success in an omnivore. Oikos 2018, 127, 197–207. [Google Scholar] [CrossRef]
- Vicens, N.; Bosch, J. Weather-dependent pollinator activity in an apple orchard, with special reference to Osmia cornuta and Apis mellifera (Hymenoptera: Megachilidae and Apidae). Environ. Entomol. 2000, 29, 413–420. [Google Scholar] [CrossRef]
- Peat, J.; Goulson, D. Effects of experience and weather on foraging rate and pollen versus nectar collection in the bumblebee, Bombus terrestris. Behav. Ecol. Sociobiol. 2005, 58, 152–156. [Google Scholar] [CrossRef]
- Price, M.V.; Waser, N.M. Effects of experimental warming on plant reproductive phenology in a subalpine meadow. Ecology 1998, 79, 1261–1271. [Google Scholar] [CrossRef]
- Tolvanen, A.; Laine, K.; Pakonen, T.; Saari, E.; Havas, P. Responses to harvesting intensity in a clonal dwarf shrub, the bilberry (Vaccinium myrtillus L.). Vegetatio 1994, 110, 163–169. [Google Scholar] [CrossRef]
- Lönnberg, K.; Ekbohm, G. Skogsskötselmetodernas inverkan på blåbær och lingon. Resultat av en tioårig försöksserie. SLU Rapp. 1990, 47, 1–30. [Google Scholar]


| Habitat | Plot | Alt (m a.s.l.) | WGS (N, E) | Soil | Surface | Slope (°) | Exp | Age (Years) | Stocking |
|---|---|---|---|---|---|---|---|---|---|
| Undisturbed | 1100 U | 1100 | 49°10′29.33′ N, 20°14′45.12′ E | Dystric Cambisol | Stony | 10 | SE | 100 | 0.8 |
| Undisturbed | 1250 U | 1250 | 49°10′36.91′ N, 20°14′32.59′ E | Dystric Cambisol | Stony | 35 | SE | 165 | 0.4 |
| Undisturbed | 1400 U | 1400 | 49°10′51.03′ N, 20°14′24.14′ E | Podzol | Boulder | 35 | SE | 165 | 0.4 |
| Disturbed | 1100 D | 1100 | 49°10′28.03′ N, 20°14′43.08′ E | Dystric Cambisol | Stony | 10 | SE | - | - |
| Disturbed | 1250 D | 1250 | 49°10′34.59′ N, 20°14′31.05′ E | Dystric Cambisol | Stony | 35 | SE | - | - |
| Disturbed | 1400 D | 1400 | 49°10′52.82′ N, 20°14′26.67′ E | Podzol | Boulder | 35 | SE | - | - |
| Mean Air Temperature of April (°C) | Mean Air Temperature of May (°C) | Mean Air Temperature of GS * (°C) | Mean Annual Air Temperature (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Altitude (m a.s.l.) | 1100 | 1250 | 1400 | 1100 | 1250 | 1400 | 1100 | 1250 | 1400 | 1100 | 1250 | 1400 |
| 2016 | 5.7 | 4.9 | 4.1 | 9.6 | 8.6 | 7.6 | 11.5 | 9.6 | 9.9 | 5.4 | 4.8 | 4.1 |
| 2017 | 3.4 | 2.5 | 1.5 | 10.1 | 9.2 | 8.4 | 11.2 | 10.3 | 9.5 | 5.6 | 5.3 | 4.9 |
| 2018 | 9.9 | 9.0 | 8.2 | 12.3 | 11.5 | 10.6 | 13.0 | 11.4 | 11.3 | 6.1 | 5.5 | 4.9 |
| 2019 | 5.7 | 4.8 | 3.8 | 7.5 | 6.6 | 5.7 | 11.5 | 10.3 | 10.0 | 6.2 | 5.6 | 5.1 |
| 2020 | 5.3 | 4.4 | 3.6 | 7.1 | 6.1 | 5.1 | 11.1 | 9.6 | 9.5 | 5.9 | 5.4 | 4.9 |
| 2021 | 1.5 | 0.6 | −0.3 | 7.6 | 6.6 | 5.7 | 10.6 | 9.0 | 8.9 | 4.8 | 4.2 | 3.6 |
| Mean | 5.3 | 4.4 | 3.5 | 9.0 | 8.1 | 7.2 | 11.5 | 10.0 | 9.6 | 5.7 | 5.1 | 4.6 |
| BBCH 07 | BBCH 65 | BBCH 72 | BBCH 86 | BBCH 92 | |
|---|---|---|---|---|---|
| 1100 D | 127 ± 8.80 | 143 ± 10.50 | 153 ± 13.52 | 232 ± 7.26 | 276 ± 10.34 |
| 1250 D | 130 ± 8.90 | 147 ± 10.09 | 154 ± 9.69 | 234 ± 6.86 | 274 ± 10.93 |
| 1400 D | 134 ± 10.63 | 154 ± 8.24 | 160 ± 7.90 | 237 ± 4.99 | 268 ± 12.75 |
| 1100 U | 128 ± 8.65 | 146 ± 10.28 | 158 ± 11.72 | 234 ± 5.98 | 278 ± 12.75 |
| 1250 U | 130 ± 8.28 | 148 ± 10.14 | 159 ± 9.62 | 235 ± 6.50 | 275 ± 12.78 |
| 1400 U | 135 ± 10.30 | 155 ± 8.68 | 162 ± 7.60 | 239 ± 4.22 | 268 ± 11.30 |
| Best Model | |||||
|---|---|---|---|---|---|
| BBCH 07 | BBCH 65 | BBCH 72 | BBCH 86 | BBCH 92 | |
| Intercept | 110.48 *** | 72.05 *** | 128.29 *** | 236.14 *** | 353.28 *** |
| T2 | 6.8 ** | −1.61 | |||
| T3 | −5.88 * | 6.61 *** | |||
| T6 | −1.89 | ||||
| T7 | 0.8 | −6.14 ** | |||
| T8 | −5.46 * | ||||
| T9 | 13.08 *** | ||||
| P1 | −0.54 ** | ||||
| P2 | 0.05 | 0.29 *** | 0.1 | ||
| P3 | 0.60 * | 0.77 *** | −0.07 | ||
| P4 | 0.05 | ||||
| P7 | −0.04 | −0.16 ** | |||
| P8 | 0.06 | ||||
| alt | 0.02 *** | 0.02 *** | 0.02 | 0.02 * | −0.02 * |
| Habitat [U] | 3.5 | −5.57 ** | 0.38 | 3.71 | −0.44 |
| R2M | 0.516 | 0.716 | 0.252 | 0.129 | 0.829 |
| R2C | 0.968 | 0.977 | 0.405 | 0.917 | 0.909 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubov, M.; Fleischer, P., Sr.; Tomes, J.; Mukarram, M.; Janík, R.; Turyasingura, B.; Fleischer, P., Jr.; Schieber, B. Differential Responses of Bilberry (Vaccinium myrtillus) Phenology and Density to a Changing Environment: A Study from Western Carpathians. Plants 2024, 13, 2406. https://doi.org/10.3390/plants13172406
Kubov M, Fleischer P Sr., Tomes J, Mukarram M, Janík R, Turyasingura B, Fleischer P Jr., Schieber B. Differential Responses of Bilberry (Vaccinium myrtillus) Phenology and Density to a Changing Environment: A Study from Western Carpathians. Plants. 2024; 13(17):2406. https://doi.org/10.3390/plants13172406
Chicago/Turabian StyleKubov, Martin, Peter Fleischer, Sr., Jakub Tomes, Mohammad Mukarram, Rastislav Janík, Benson Turyasingura, Peter Fleischer, Jr., and Branislav Schieber. 2024. "Differential Responses of Bilberry (Vaccinium myrtillus) Phenology and Density to a Changing Environment: A Study from Western Carpathians" Plants 13, no. 17: 2406. https://doi.org/10.3390/plants13172406








