The Impact of Changing Climate on an Endangered Epiphytic Orchid (Pleione formosana) in a Montane Cloud Forest and the Conservation Challenge Ahead
Abstract
:1. Introduction
2. Material and Methods
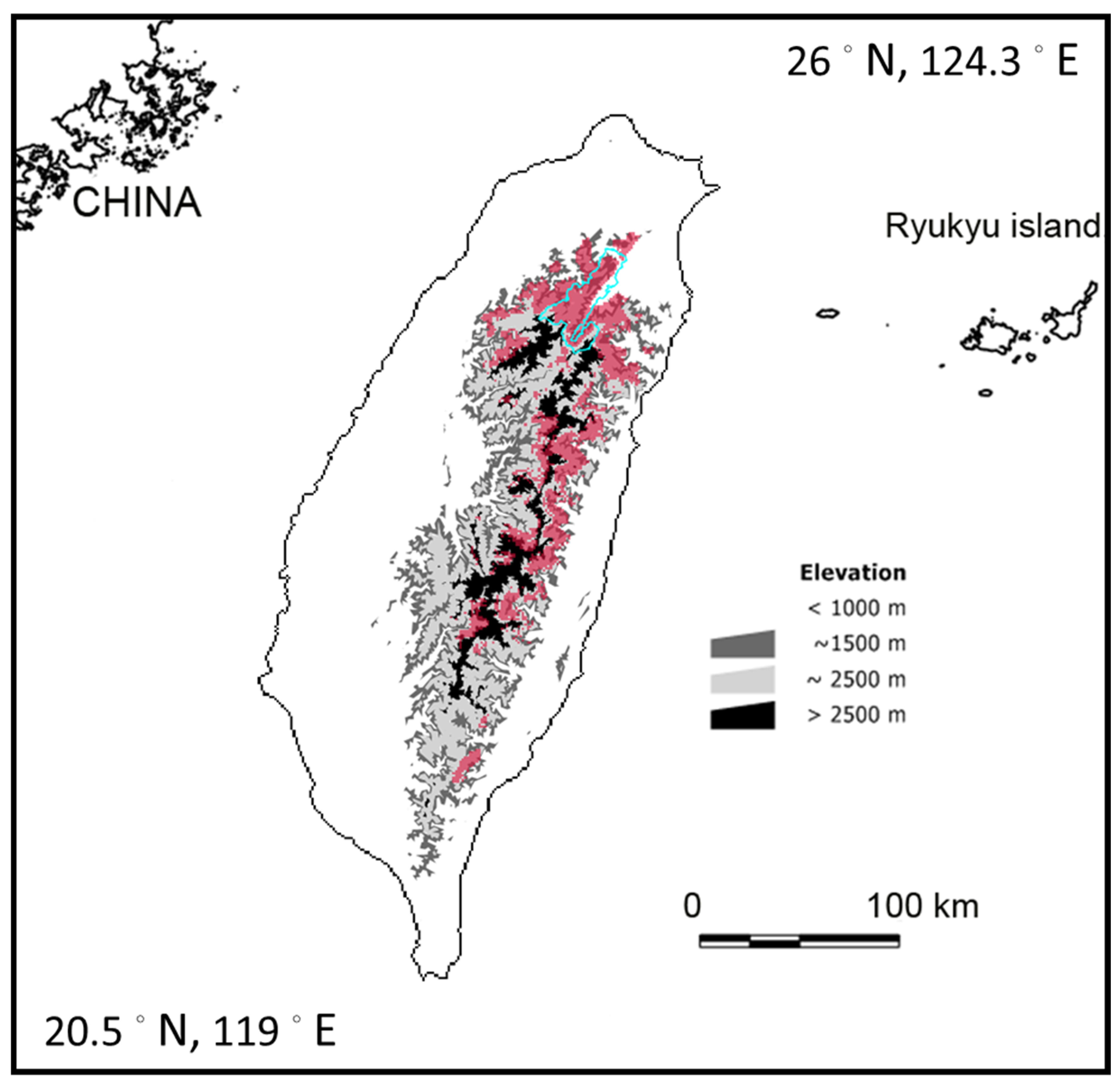
2.1. Study Area and Species
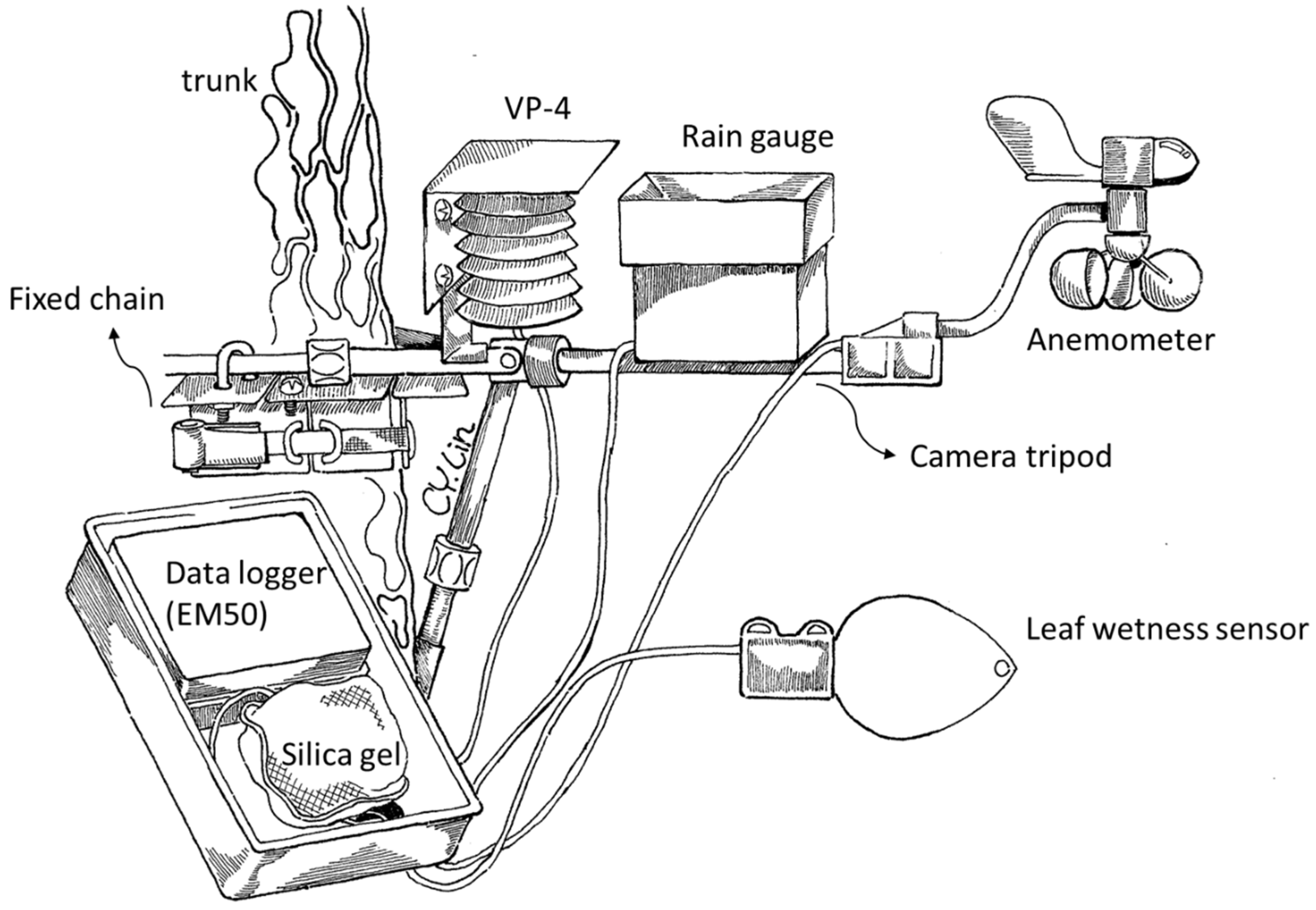
2.2. Canopy Microclimate Measurement
2.3. In Vitro Germination and Reintroduction
2.4. Data Analysis
3. Results
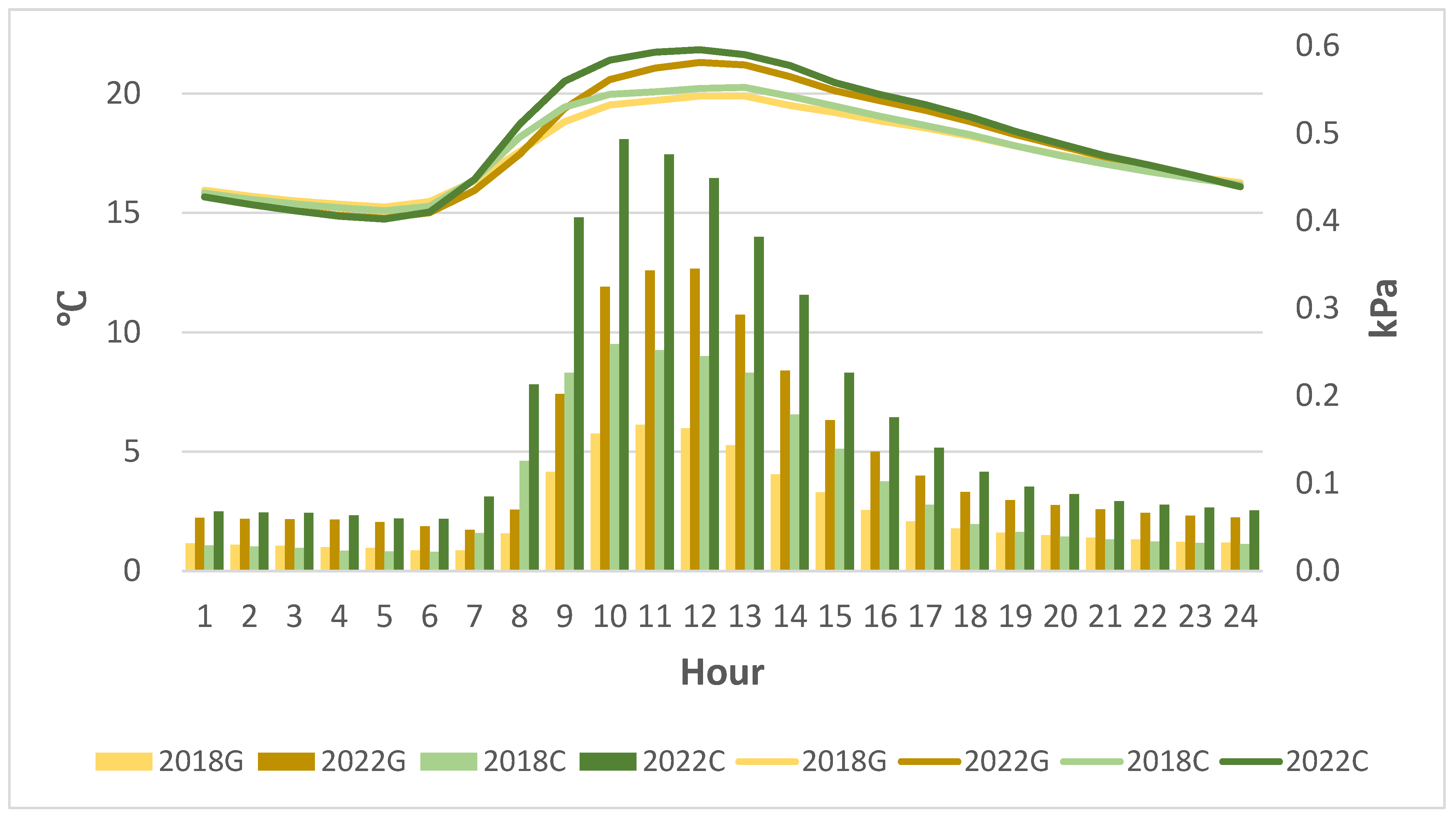
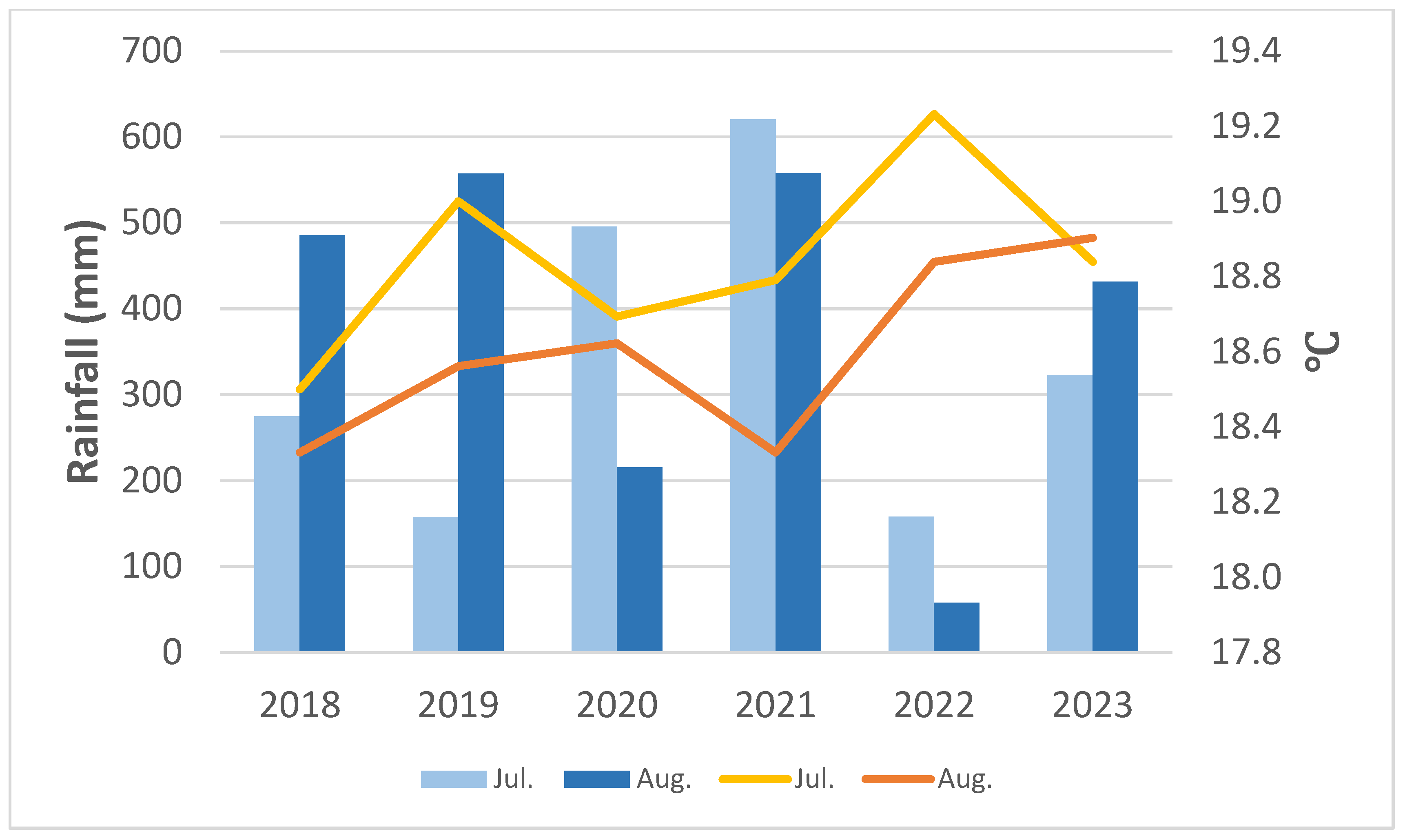
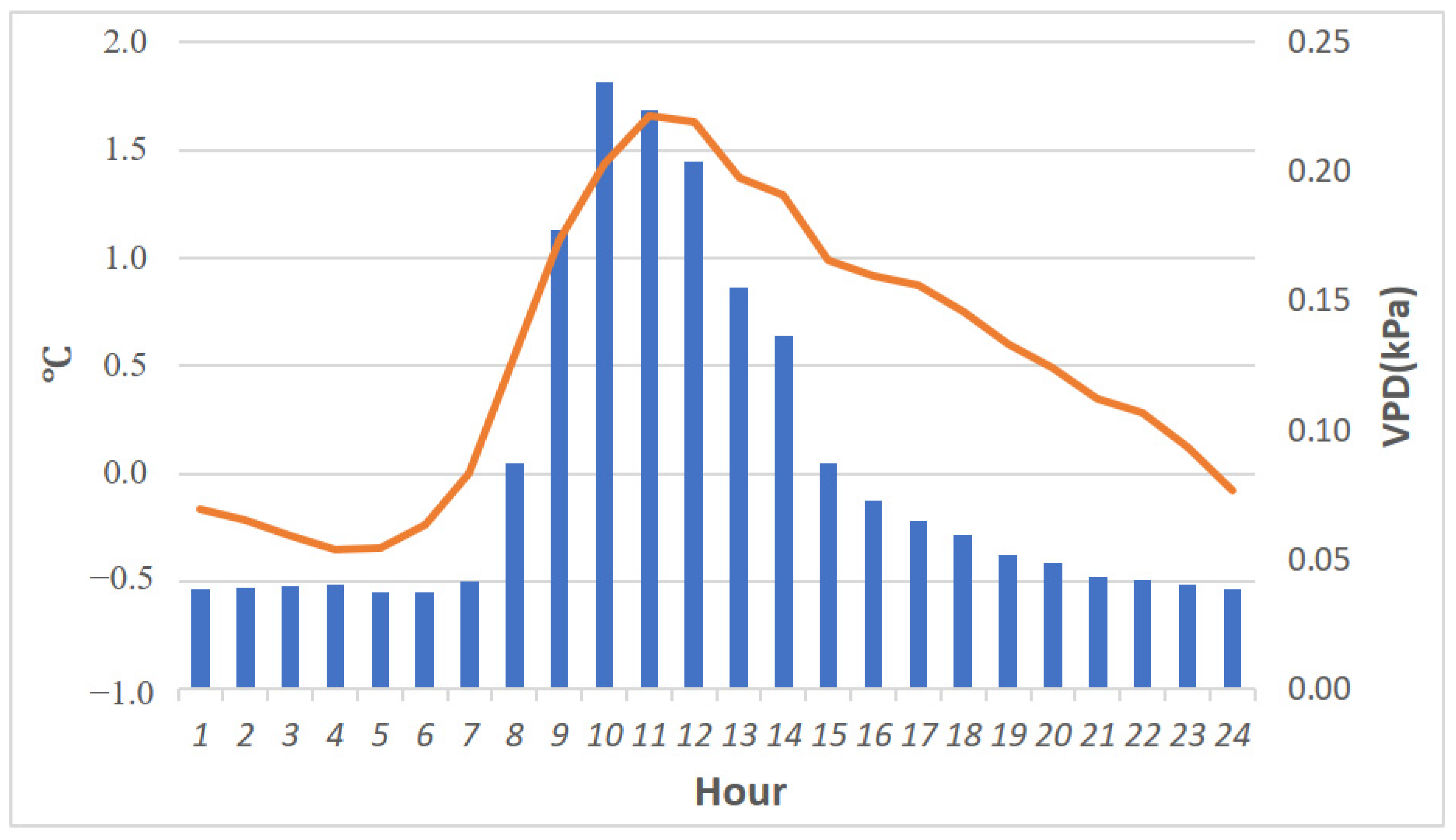
3.1. Climate Recorded on Site
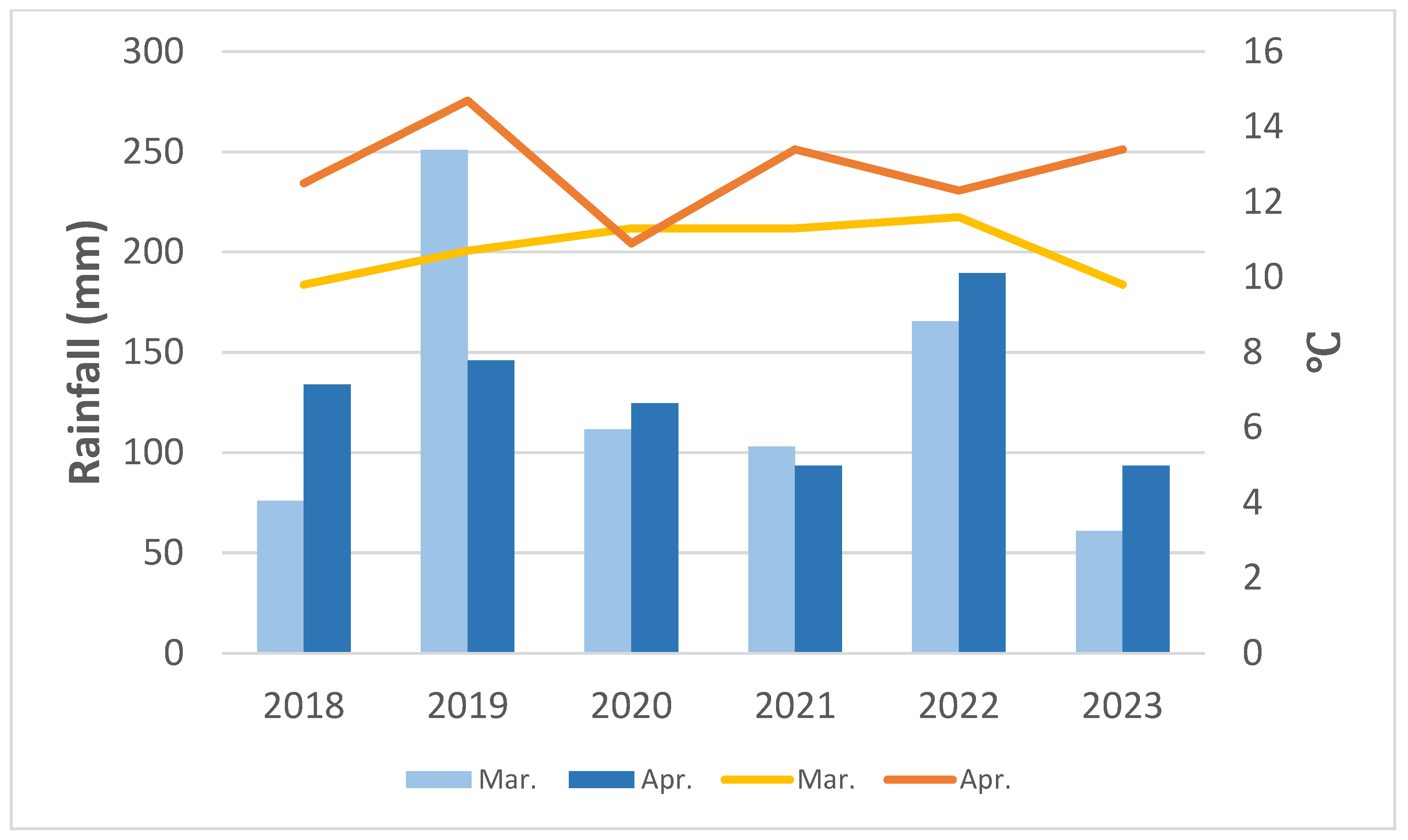
3.1.1. Temperature and Rainfall from 2018
3.1.2. Canopy Sensors
3.2. Observations on P. formosana Phenology and Field Collection
3.3. Growth of Seedlings and Reintroduction
4. Discussion
4.1. Key Climate Factors for P. formosana Survival
4.2. Potential Threats from Changing Climate to P. formosana Survival
4.3. Implications for Re-Establishment of P. formosana
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Foster, P. The Potential Negative Impacts of Global Climate Change on Tropical Montane Cloud Forests. Earth Sci. Rev. 2001, 55, 73–106. [Google Scholar] [CrossRef]
- Still, C.J.; Foster, P.N.; Schneider, S.H. Simulating the Effects of Climate Change on Tropical Montane Cloud Forests. Nature 1999, 398, 608–610. [Google Scholar] [CrossRef]
- Guzmán, Q.J.A.; Hamann, H.F.; Sánchez-Azofeifa, G.A. Multi-Decadal Trends of Low-Clouds at the Tropical Montane Cloud Forests. Ecol. Indic. 2024, 158, 111599. [Google Scholar] [CrossRef]
- Hu, T.; Liu, W.Y.; Wen, H.D.; Song, L.; Zhang, T.T.; Chen, Q.; Liu, S. Vascular Epiphyte Populations with Higher Leaf Nutrient Concentrations Showed Weaker Resilience to an Extreme Drought in a Montane Cloud Forest. Plant Biol. 2023, 25, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.C.; Oostermeijer, J.G.; Wolf, J.D. Adaptation of a Widespread Epiphytic Fern to Simulated Climate Change Conditions. Plant Ecol. 2014, 215, 889–897. [Google Scholar] [CrossRef]
- El-Madany, T.S.; Walk, J.B.; Deventer, M.J.; Degefie, D.T.; Chang, S.; Juang, J.; Griessbaum, F.; Klemm, O. Canopy-atmosphere Interactions under Foggy Condition—Size-resolved Fog Droplet Fluxes and Their Implications. J. Geophys. Res. Biogeosci. 2016, 121, 796–808. [Google Scholar] [CrossRef]
- Jang, Y.; Shen, S.; Juang, J.; Huang, C.; Lo, M. Discontinuity of Diurnal Temperature Range along Elevated Regions. Geophys. Res. Lett. 2022, 49, e2021GL097551. [Google Scholar] [CrossRef]
- Hamilton, L.S.; Juvik, J.O.; Scatena, F.N. Tropical Montane Cloud Forests; Springer: New York, NY, USA, 1995; ISBN 0387943234. [Google Scholar]
- Benzing, D.H. Vulnerabilities of Tropical Forests to Climate Change: The Significance of Resident Epiphytes. Clim. Change 1998, 39, 519–540. [Google Scholar] [CrossRef]
- Hsu, R.C.C.; Wolf, J.H.D.; Tamis, W.L.M. Regional and Elevational Patterns in Vascular Epiphyte Richness on an East Asian Island. Biotropica 2014, 46, 549–555. [Google Scholar] [CrossRef]
- Hsu, R.C.C.; Tamis, W.L.M.; Raes, N.; de Snoo, G.R.; Wolf, J.H.D.; Oostermeijer, G.; Lin, S.-H. Simulating Climate Change Impacts on Forests and Associated Vascular Epiphytes in a Subtropical Island of East Asia. Divers. Distrib. 2012, 18, 334–347. [Google Scholar] [CrossRef]
- Hsu, R.C.-C.; Lin, C.; Chen, C. Topography-Induced Local Climatic Variations as the Decisive Factor in the Shaping of Epiphyte Distributions in Chilan, Northeastern Taiwan. Forests 2023, 14, 358. [Google Scholar] [CrossRef]
- Hayata, B. Materials for a Flora of Formosa: Supplementary Notes to the Enumeratio Plantarum Formosanarum and Flora Montana Formosae. J. Coll. Sci. Imp. Univ. Tokyo Jpn. 1911, 30, 1–471. [Google Scholar]
- Fukuyama, N. Neue Orchideen von Formosa. Trans. Nat. Hist. Soc. Formosa 1933, 22, 413–416. [Google Scholar]
- Chao, W.-C.; Liu, Y.-C.; Jiang, M.-T.; Wu, S.-S.; Fang, C.-L.; Ho, J.-F.; Huang, C.-L. Genetic Diversity, Population Genetic Structure and Conservation Strategies for Pleione Formosana (Orchideace). Taiwania 2021, 66, 20–30. [Google Scholar]
- Hsu, R.C.-C. Modelling Spatial Patterns of Rare Orchids for Conservation Priority in Taiwan. In Proceedings of the 20th World Orchid Conference, Singapore, 13–20 November 2011. [Google Scholar]
- Hsu, R.C.-C. Using Spatially Autocorrelated Environmental Conditions in Habitats to Project Potential Distributions of Rare Orchids. Taiwan J. For. Sci. 2015, 30, 97–107. [Google Scholar]
- Lai, I.-L.; Chang, S.-C.; Lin, P.-H.; Chou, C.-H.; Wu, J.-T. Climatic Characteristics of the Subtropical Mountainous Cloud Forest at the Yuanyang Lake Long-Term Ecological Research Site, Taiwan. Taiwania 2006, 51, 317–329. [Google Scholar]
- Chang, S.-C.; Lai, I.-L.; Wu, J.-T. Estimation of Fog Deposition on Epiphytic Bryophytes in a Subtropical Montane Forest Ecosystem in Northeastern Taiwan. Atmos. Res. 2002, 64, 159–167. [Google Scholar] [CrossRef]
- Jiang, M.-T.; Chao, W.-C.; Huang, C.-L.; Lan, S.-R.; Liu, Z.-J.; Wu, S.-S. The Complete Chloroplast Genome of Pleione Formosana (Orchidaceae). Mitochondrial DNA Part B 2019, 4, 1044–1046. [Google Scholar] [CrossRef]
- Chiang, Y.L.; Chen, Y.-R. Observations on Pleione formosana Hayata. Taiwania 1968, 14, 271–301. [Google Scholar]
- Lu, M.-C. High Frequency Plant Regeneration from Callus Culture of Pleione Formosana Hayata. Plant Cell Tissue Organ. Cult. 2004, 78, 93–96. [Google Scholar] [CrossRef]
- Lu, M.-C. Tissue Culture of Pleione Formosana Hayata. Seed Nurs. 2004, 6, 60–71. (In Chinese) [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473. [Google Scholar] [CrossRef]
- Huang, J. A Simple Accurate Formula for Calculating Saturation Vapor Pressure of Water and Ice. J. Appl. Meteorol. Clim. 2018, 57, 1265–1272. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Chang, S.-T. The cultivation of Pleione formosana; Bulletin of Taoyuan District Agricultural Research and Extension Station: Taoyuan City, Taiwan, 2001; pp. 1–8. (In Chinese) [Google Scholar]
- Gotsch, S.G.; Davidson, K.; Murray, J.G.; Duarte, V.J.; Draguljić, D. Vapor Pressure Deficit Predicts Epiphyte Abundance across an Elevational Gradient in a Tropical Montane Region. Am. J. Bot. 2017, 104, 1790–1801. [Google Scholar] [CrossRef]
- Mangelsdorff, R.; Piepenbring, M.; Perdomo-Sánchez, O. Correlation of Diversity of Rust Fungi and Their Host Plants with Disturbance and Conservation of Vegetation in Western Panama: A Case Study in Western Panama Focused on Orchidaceae and Pteridophytes as Host Plants. Biodivers. Conserv. 2012, 21, 2323–2339. [Google Scholar] [CrossRef]
- Jain, A.; Sarsaiya, S.; Chen, J.; Wu, Q.; Lu, Y.; Shi, J. Changes in Global Orchidaceae Disease Geographical Research Trends: Recent Incidences, Distributions, Treatment, and Challenges. Bioengineered 2021, 12, 13–29. [Google Scholar] [CrossRef]
- Kang, H.; Kang, K.W.; Kim, D.H.; Sivanesan, I. In Vitro Propagation of Gastrochilus matsuran (Makino) Schltr., an Endangered Epiphytic Orchid. Plants 2020, 9, 524. [Google Scholar] [CrossRef]
- Gautam, H.R.; Bhardwaj, M.L.; Kumar, R. Climate Change and Its Impact on Plant Diseases. Curr. Sci. 2013, 105, 1685–1691. [Google Scholar]







| Year | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 |
| Flowering Date 1 | +1.1 | −0.5 | 0 | 0 | 0 | +0.7 |
| Flowers | Average | Average | Few | Few | Average | Few |
| Fruiting Date 1 | +3.3 | −2 | 0 | 0 | 0 | +1.1 |
| Fruits | Average | Average | Few | Few | Abv. average | Few |
| Spring | ||||||
| RAINFALL 2 | 210 | 397 | 236 | 197 | 355 | 154 |
| TEMPERATURE 3 | −1.7/21.2 | 1.6/25.5 | −0.5/21.3 | 2.2/21.2 | −0.8/21.4 | −1.8/24.2 |
| Summer | ||||||
| RAINFALL 2 | 761 | 715 | 711 | 1178 | 216 | 755 |
| TEMPERATURE 3 | 11.1/24.6 | 13.1/25.4 | - | 12.3/25.6 | 12/26.5 | 12.9/26.8 |
| Extreme Weather Event | Snow event | Drought | Foehn wind | |||
| Seed Collection Date | 4 7 November | 1 10 September | 2 * 9 October | 6 ** 23 September | 6 *** 21 June and 22 September | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, R.C.-C.; Chen, Y.-C.; Lin, C. The Impact of Changing Climate on an Endangered Epiphytic Orchid (Pleione formosana) in a Montane Cloud Forest and the Conservation Challenge Ahead. Plants 2024, 13, 2414. https://doi.org/10.3390/plants13172414
Hsu RC-C, Chen Y-C, Lin C. The Impact of Changing Climate on an Endangered Epiphytic Orchid (Pleione formosana) in a Montane Cloud Forest and the Conservation Challenge Ahead. Plants. 2024; 13(17):2414. https://doi.org/10.3390/plants13172414
Chicago/Turabian StyleHsu, Rebecca C.-C., Yi-Chiann Chen, and Chienyu Lin. 2024. "The Impact of Changing Climate on an Endangered Epiphytic Orchid (Pleione formosana) in a Montane Cloud Forest and the Conservation Challenge Ahead" Plants 13, no. 17: 2414. https://doi.org/10.3390/plants13172414







