The Dynamics of Allelochemicals and Phytotoxicity in Eisenia fetida during the Decomposition of Eucalyptus grandis Litter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Biodiversity Investigation
2.3. Leaf Litter and Soil Sampling
2.4. Earthworms
2.5. Identification of Secondary Metabolites
2.6. Acute Toxicity
2.7. Physiological Properties
2.8. Comet Assay
2.9. Statistical Analysis
3. Results
3.1. Soil Properties and Plant and Soil Biodiversity
3.2. Secondary Metabolites during E. grandis Litter Decomposition
3.3. Effects of Leaf Litter Extract on Earthworms
3.3.1. Survival Rate and Growth of E. fetida
3.3.2. Physiological Properties of E. fetida
3.4. Effect of E. grandis Leaf Litter Extract on DNA Damage in E. fetida
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.J.; Li, J.J.; Huang, Y.M.; Gao, S.; Zhang, J. Root-soil facilitation in mixed Eucalyptus grandis plantations including nitrogen-fixing species. For. Ecol. Manag. 2022, 516, 120215. [Google Scholar] [CrossRef]
- Massuque, J.; Roque Lima, M.D.; Müller Da Silva, P.H.; de Paula Protásio, T.; Trugilho, P.F. Potential of charcoal from non-commercial Corymbia and Eucalyptus wood for use in the steel industry. Renew. Energy 2023, 211, 179–187. [Google Scholar] [CrossRef]
- National Forestry and Grassland Administration. China Forest Resource Report; National Forestry and Grassland Administration: Beijing, China, 2020.
- Forrester, D.I.; Bauhus, J.; Cowie, A.L.; Vanclay, J.K. Mixed-species plantations of Eucalyptus with nitrogen-fixing trees: A review. For. Ecol. Manag. 2006, 233, 211–230. [Google Scholar] [CrossRef]
- Rodríguez-Loinaz, G.; Amezaga, I.; Onaindia, M. Use of native species to improve carbon sequestration and contribute towards solving the environmental problems of the timberlands in Biscay, northern Spain. J. Environ. Manag. 2013, 120, 18–26. [Google Scholar] [CrossRef]
- Ahmed, R.; Hoque, A.T.M.R.; Hossain, M.K. Allelopathic effects of leaf litters of Eucalyptus camaldulensis on some forest and agricultural crops. J. For. Res. 2008, 19, 19–24. [Google Scholar] [CrossRef]
- Zhang, D.J.; Zhang, J.; Yang, W.Q.; Wu, F.Z. Potential allelopathic effect of Eucalyptus grandis across a range of plantation ages. Ecol. Res. 2010, 25, 13–23. [Google Scholar] [CrossRef]
- Chu, C.J.; Mortimer, P.E.; Wang, H.C.; Wang, Y.F.; Liu, X.B.; Yu, S.X. Allelopathic effects of Eucalyptus on native and introduced tree species. For. Ecol. Manag. 2014, 323, 79–84. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Inderjit; Callaway, R.M. Experimental designs for the study of allelopathy. Plant Soil 2003, 256, 1–11. [Google Scholar] [CrossRef]
- Bonanomi, G.; Sicurezza, M.G.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578. [Google Scholar] [CrossRef]
- Chomel, M.; Fernandez, C.; Bousquet-Mélou, A.; Gers, C.; Monnier, Y.; Santonja, M.; Gauquelin, T.; Gros, R.; Lecareux, C.; Baldy, V. Secondary metabolites of Pinus halepensis alter decomposer organisms and litter decomposition during afforestation of abandoned agricultural zones. J. Ecol. 2014, 102, 411–424. [Google Scholar] [CrossRef]
- Kruidhof, H.M.; Gallandt, E.R.; Haramoto, E.R.; Bastiaans, L. Selective weed suppression by cover crop residues: Effects of seed mass and timing of species’ sensitivity. Weed Res. 2011, 51, 177–186. [Google Scholar] [CrossRef]
- Tang, Z.Q.; Zhang, J.; Yu, J.L.; Wang, C.Z.; Zhang, D.J. Allelopathic effects of volatile organic compounds from Eucalyptus grandis rhizosphere soil on Eisenia fetida assessed using avoidance bioassays, enzyme activity, and comet assays. Chemosphere 2017, 173, 307–317. [Google Scholar] [CrossRef]
- Molina, A.; Reigosa, M.J.; Carballeira, A. Release of allelochemical agents from litter, throughfall, and topsoil in plantations of Eucalyptus globulus Labill in Spain. J. Chem. Ecol. 1991, 17, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.Z.; Yu, S.X.; Wang, Y.F.; Qiu, X.; Cai, C.X.; Liu, S.P. Allelopathic effects of Eucalyptus urophylla on ten tree species in south China. Agrofor. Syst. 2009, 76, 401–408. [Google Scholar] [CrossRef]
- Mohamed, E.I.; Gad, E.K. Eucalyptus citriodora leaf extract as a source of allelochemicals for weed control in pea fields compared with some chemical herbicides. J. Plant Prot. Res. 2019, 59, 392–399. [Google Scholar] [CrossRef]
- Inderjit; Wardle, D.A.; Karban, R.; Callaway, R.M. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol. Evol. 2011, 26, 655–662. [Google Scholar] [CrossRef]
- Boeno, D.; Silva, R.F.; Almeida, H.S.; Rodrigues, A.C.; Vanzan, M.; Andreazza, R. Influence of eucalyptus development under soil fauna. Braz. J. Biol. 2020, 80, 345–353. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Dendooven, L.; Alvarez-Bernal, D.; Contreras-Ramos, S.M. Potential of earthworms to accelerate removal of organic contaminants from soil: A review. Appl. Soil Ecol. 2014, 79, 10–25. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, J.; Chen, X.F.; Li, H.H.; Lavelle, P. Effects of a native earthworm species (Amynthas morrisi) and Eisenia fetida on metal fractions in a multi-metal polluted soil from South China. Acta Oecologica 2020, 102, 103503. [Google Scholar] [CrossRef]
- Xie, X.C.; Qian, Y.; Wu, Y.X.; Yin, J.; Zhai, J.P. Effects of decabromodiphenyl ether (BDE-209) on the avoidance response, survival, growth and reproduction of earthworms (Eisenia fetida). Ecotoxicol. Environ. Saf. 2013, 90, 21–27. [Google Scholar] [CrossRef]
- Markert, B.; Breure, A.M.; Zechmeister, H.G. Bioindicators and Biomonitors: Principles, Concepts and Applications; Elsevier Science: New York, NY, USA, 2003. [Google Scholar]
- Gomez-Eyles, J.L.; Svendsen, C.; Lister, L.; Martin, H.; Hodson, M.E.; Spurgeon, D.J. Measuring and modelling mixture toxicity of imidacloprid and thiacloprid on Caenorhabditis elegans and Eisenia fetida. Ecotoxicol. Environ. Saf. 2009, 72, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Makkonen, M.; Berg, M.P.; van Logtestijn, R.S.P.; van Hal, J.R.; Aerts, R. Do physical plant litter traits explain non-additivity in litter mixtures? A test of the improved microenvironmental conditions theory. Oikos 2013, 122, 987–997. [Google Scholar] [CrossRef]
- Gartner, T.B.; Cardon, Z.G. Decomposition Dynamics in Mixed-Species Leaf Litter. Oikos 2004, 104, 230–246. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and Litter Decomposition in Terrestrial Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Wang, L.F.; Zhou, Y.; Chen, Y.M.; Xu, Z.F.; Zhang, J.; Liu, Y. Home-field advantage and ability alter labile and recalcitrant litter carbon decomposition in an alpine forest ecotone. Plant Soil 2023, 485, 213–225. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Vitousek, P.M. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol. Evol. 2000, 15, 238–243. [Google Scholar] [CrossRef]
- Coq, S.; Souquet, J.; Meudec, E.; Cheynier, V.; Hättenschwiler, S. Interspecific variation in leaf litter tannins drives decomposition in a tropical rain forest of French Guiana. Ecology 2010, 91, 2080–2091. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhang, D.J.; Qin, Y.; Zhou, Y.; Song, S.M.; Zhang, J. Characteristics of fungal community structure during the decomposition of mixed foliage litter from Pinus massoniana and broadleaved tree species in southwestern China. J. Plant Ecol. 2020, 13, 574–588. [Google Scholar] [CrossRef]
- Li, J.J.; Huang, Y.M.; Chen, L.H.; Gao, S.; Zhang, J.; Zhang, D.J. Understory plant diversity and phenolic allelochemicals across a range of Eucalyptus grandis plantation ages. J. For. Res. 2023, 34, 1577–1590. [Google Scholar] [CrossRef]
- An, M.; Pratley, J.E.; Haig, T. Phytotoxicity of Vulpia Residues: IV. Dynamics of Allelochemicals During Decomposition of Vulpia Residues and Their Corresponding Phytotoxicity. J. Chem. Ecol. 2001, 27, 395–409. [Google Scholar] [CrossRef]
- Bernhard-Reversat, F.; Main, G.; Holl, K.; Loumeto, J.; Ngao, J. Fast disappearance of the water-soluble phenolic fraction in eucalypt leaf litter during laboratory and field experiments. Appl. Soil Ecol. 2003, 23, 273–278. [Google Scholar] [CrossRef]
- Blum, U. Plant-Plant Allelopathic Interactions Ⅱ: Laboratory Bioassays for Water-Soluble Compounds with an Emphasis on Phenolic Acids; Springer Science and Business Media: Cham, Switzerland, 2014. [Google Scholar]
- Puig, C.G.; Gonçalves, R.F.; Valentão, P.; Andrade, P.B.; Reigosa, M.J.; Pedrol, N. The Consistency between Phytotoxic Effects and the Dynamics of Allelochemicals Release from Eucalyptus globulus Leaves Used as Bioherbicide Green Manure. J. Chem. Ecol. 2018, 44, 658–670. [Google Scholar] [CrossRef]
- Liu, S.; Qin, F.C.; Yu, S.X. Eucalyptus urophylla root-associated fungi can counteract the negative influence of phenolic acid allelochemicals. Appl. Soil Ecol. 2018, 127, 1–7. [Google Scholar] [CrossRef]
- Zhang, D.J.; Zhang, J.; Yang, W.Q.; Wu, F.Z. Effects of afforestation with Eucalyptus grandis on soil physicochemical and microbiological properties. Soil Res. 2012, 50, 167–176. [Google Scholar] [CrossRef]
- Zhang, D.J.; Zhang, J.; Yang, W.Q.; Wu, F.Z.; Huang, Y.M. Plant and soil seed bank diversity across a range of ages of Eucalyptus grandis plantations afforested on arable lands. Plant Soil 2014, 376, 307–325. [Google Scholar] [CrossRef]
- Zhang, D.J.; Zhang, J.; Yang, W.Q.; Wu, F.Z.; Huang, Y.M.; Zhang, Z.W.; Wang, X.; Wang, X.Q.; Zhu, L. Plant’s and soil organism’s diversity across a range of Eucalyptus grandis plantation ages. Acta Ecol. Sin. 2013, 33, 3947–3962. (In Chinese) [Google Scholar] [CrossRef]
- Research Group of Chinese Soil Taxonomy. Keys to Chinese Soil Taxonomy, 3rd ed.; Press of University of Science and Technology of China: Hefei, China, 2001. [Google Scholar]
- OECD. Guideline for Testing of Chemicals. No.222. Earthworm Reproduction Test (Eisenia fetida/andrei); Organization for Economic Cooperation and Development: Paris, France, 2004. [Google Scholar]
- ISO/DIS 17512-1.2; Soil Quality: Avoidance Test for Testing the Quality of Soils and Effects of Chemicals on Behavior—Part 1: Test with Earthworms (Eisenia fetida and Eisenia andrei). International Organization for Standardization: Geneva, Switzerland, 2008.
- Yu, J.L.; Zhang, J.; Tang, Z.Q.; Wang, C.Z.; Qian, L.Z.; Zhang, D.J. Variations of growth and physiological indicators of Eisenia foetida in soils across a chronosequence of Eucalyptus grandis plantations. Chin. J. Ecol. 2017, 36, 124–131. (In Chinese) [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Cortés-Ríos, J.; Zárate, A.M.; Figueroa, J.D.; Medina, J.; Fuentes-Lemus, E.; Rodríguez-Fernández, M.; Aliaga, M.; López-Alarcón, C. Protein quantification by bicinchoninic acid (BCA) assay follows complex kinetics and can be performed at short incubation times. Anal. Biochem. 2020, 608, 113904. [Google Scholar] [CrossRef]
- Ōyanagui, Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal. Biochem. 1984, 142, 290–296. [Google Scholar] [CrossRef]
- Kosugi, H.; Kikugawa, K. Thiobarbituric acid reaction of aldehydes and oxidized lipids in glacial acetic acid. Lipids 1985, 20, 915–921. [Google Scholar] [CrossRef]
- Gorun, V.; Proinov, I.; Băltescu, V.; Balaban, G.; Bârzu, O. Modified Ellman procedure for assay of cholinesterases in crude enzymatic preparations. Anal. Biochem. 1978, 86, 324–326. [Google Scholar] [CrossRef]
- Eyambe, G.S.; Goven, A.J.; Fitzpatrick, L.C.; Venables, B.J.; Cooper, E.L. A non-invasive technique for sequential collection of earthworm (Lumbricus terrestris) leukocytes during subchronic immunotoxicity studies. Lab. Anim. 1991, 25, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.; Sousa, J.P.; Nogueira, A.J.A.; Soares, A.M.V.M. Effect of Endosulfan and Parathion on Energy Reserves and Physiological Parameters of the Terrestrial Isopod Porcellio dilatatus. Ecotoxicol. Environ. Saf. 2001, 49, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Verdú, I.; Trigo, D.; Martínez-Guitarte, J.L.; Novo, M. Bisphenol A in artificial soil: Effects on growth, reproduction and immunity in earthworms. Chemosphere 2018, 190, 287–295. [Google Scholar] [CrossRef]
- Olvera-Velona, A.; Capowiez, Y.; Mascle, O.; Ortiz-Hernandez, L.; Benoit, P. Assessment of the toxicity of ethyl-parathion to earthworms (Aporrectodea caliginosa) using behavioural, physiological and biochemical markers. Appl. Soil Ecol. 2008, 40, 476–483. [Google Scholar] [CrossRef]
- Lummer, D.; Scheu, S.; Butenschoen, O. Connecting litter quality, microbial community and nitrogen transfer mechanisms in decomposing litter mixtures. Oikos 2012, 121, 1649–1655. [Google Scholar] [CrossRef]
- Wu, F.Z.; Peng, C.H.; Yang, W.Q.; Zhang, J.; Han, Y.; Mao, T. Admixture of alder (Alnus formosana) litter can improve the decomposition of eucalyptus (Eucalyptus grandis) litter. Soil Biol. Biochem. 2014, 73, 115–121. [Google Scholar] [CrossRef]
- Maity, S.K.; Joy, V.C. Impact of antinutritional chemical compounds of leaf litter on detritivore soil arthropod fauna. J. Ecobiol. 1999, 11, 193–202. [Google Scholar]
- Hodge, A.; Stewart, J.; Robinson, D.; Griffiths, B.S.; Fitter, A.H. Root proliferation, soil fauna and plant nitrogen capture from nutrient-rich patches in soil. New Phytol. 1998, 139, 479–494. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Pazos-Malvido, E. Phytotoxic Effects of 21 Plant Secondary Metabolites on Arabidopsis thaliana Germination and Root Growth. J. Chem. Ecol. 2007, 33, 1456–1466. [Google Scholar] [CrossRef]
- Otte, B.A.; Rice, C.P.; Davis, B.W.; Schomberg, H.H.; Mirsky, S.B.; Tully, K.L. Phenolic acids released to soil during cereal rye cover crop decomposition. Chemoecology 2020, 30, 25–34. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Idbella, M.; Mazzoleni, S.; Abd-ElGawad, A.M. Microbiota modulation of allelopathy depends on litter chemistry: Mitigation or exacerbation? Sci. Total Environ. 2021, 776, 145942. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.T.; Yuan, Y.D.; Cai, M.; Wang, D.S.; Lv, W.G. Toxicity of isoprocarb to earthworms (Eisenia fetida): Oxidative stress, neurotoxicity, biochemical responses and detoxification mechanisms. Environ. Pollut. 2021, 290, 118038. [Google Scholar] [CrossRef]
- Chen, L.Y.; Bai, J.; Wan, J.; Song, Y.; Xiang, G.H.; Duan, R.Y.; Zheng, Y. Endocrine system, cell growth and death, and energy metabolism induced by Sb(III) exposure in earthworm (Pheretima guillemi) revealed by transcriptome and metabolome analysis. Environ. Pollut. 2024, 356, 124357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Shi, S.Y.; Zhao, H.; Guo, J.; Yang, Z.; Gao, H.S.; Lu, F.P. Proteomic analysis of the earthworm Eisenia fetida exposed to oxytetracycline in soil. RSC Adv. 2019, 9, 41628–41638. [Google Scholar] [CrossRef]
- Rong, H.; Wang, C.R.; Liu, H.T.; Zhang, M.; Yuan, Y.T.; Pu, Y.J.; Huang, J.; Yu, J.Y. Biochemical Toxicity and Potential Detoxification Mechanisms in Earthworms Eisenia fetida Exposed to Sulfamethazine and Copper. Bull. Environ. Contam. Toxicol. 2020, 105, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.F.; Liang, C.L.; Lv, H.J.; Liu, W.R.; Wang, Q.; Ding, J.; Li, X.X.; Wang, J. Expanding the insight of ecological risk on the novel chiral pesticide mefentrifluconazole: Mechanism of enantioselective toxicity to earthworms (Eisenia fetida). J. Hazard. Mater. 2024, 466, 133585. [Google Scholar] [CrossRef]
- Shao, Y.T.; Wang, J.H.; Wang, J.; Du, Z.K.; Li, B.; Zhu, L.S.; Juhasz, A.; Liu, X.Y.; Xu, Y.Q.; Li, W.X. Oxidative stress and genotoxic effects in earthworms induced by five imidazolium bromide ionic liquids with different alkyl chains. Chemosphere 2019, 227, 570–579. [Google Scholar] [CrossRef]
- Song, P.P.; Gao, J.P.; Li, X.X.; Zhang, C.; Zhu, L.S.; Wang, J.H.; Wang, J. Phthalate induced oxidative stress and DNA damage in earthworms (Eisenia fetida). Environ. Int. 2019, 129, 10–17. [Google Scholar] [CrossRef]
- Shi, Z.M.; Yan, J.H.; Ren, X.N.; Wen, M.; Zhao, Y.H.; Wang, C.Y. Effects of biochar and thermally treated biochar on Eisenia fetida survival, growth, lysosomal membrane stability and oxidative stress. Sci. Total Environ. 2021, 770, 144778. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wang, Z.J.; Liu, L.; Wang, D.W.; Gu, Y.T.; Chen, L.; Chen, X.J.; Meng, Z.Y. The combined effects of azoxystrobin and different aged polyethylene microplastics on earthworms (Eisenia fetida): A systematic evaluation based on oxidative damage and intestinal function. Sci. Total Environ. 2024, 923, 171494. [Google Scholar] [CrossRef] [PubMed]
- Gautam, K.; Dwivedi, S.; Verma, R.; Vamadevan, B.; Patnaik, S.; Anbumani, S. Combined effects of polyethylene microplastics and carbendazim on Eisenia fetida: A comprehensive ecotoxicological study. Environ. Pollut. 2024, 348, 123854. [Google Scholar] [CrossRef]
- He, F.L.; Liu, R.T. Mechanistic insights into phenanthrene-triggered oxidative stress-associated neurotoxicity, genotoxicity, and behavioral disturbances toward the brandling worm (Eisenia fetida) brain: The need for an ecotoxicological evaluation. J. Hazard. Mater. 2023, 450, 131072. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.M.; Abdel-Aty, B.; El-Sayed, W.; Mariy, F.M.; Hegazy, G.M.; Mohamed, R.A.; Zoghly, H.M. Imidacloprid effects on acetylcholinesterase and nicotinic acetylcholine receptor in Apis mellifera. Experimental and molecular modeling approaches. Chemosphere 2024, 356, 141899. [Google Scholar] [CrossRef]
- Zhao, S.L.; Wang, Y.L.; Duo, L. Biochemical toxicity, lysosomal membrane stability and DNA damage induced by graphene oxide in earthworms. Environ. Pollut. 2021, 269, 116225. [Google Scholar] [CrossRef]
- Li, X.X.; Jiang, N.; Zhang, J.; Yao, X.F.; Liu, W.R.; Wang, Q.; Ding, J.; Hu, Z.R.; Zhu, L.S.; Wang, J.H.; et al. Soil health hazards of di(2-ethylhexyl) phthalate: New perspectives on earthworms from different ecological niches DNA damage, gut microbial disruption and soil enzyme changes. J. Hazard. Mater. 2024, 467, 133700. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Reyes, G.; Costilla-Salazar, R.; Pérez-Vázquez, F.J.; González-Mille, D.J.; Flores-Ramírez, R.; Del Carmen Cuevas-Díaz, M.; Medellin-Garibay, S.E.; Ilizaliturri-Hernández, C.A. DNA damage in earthworms by exposure of Persistent Organic Pollutants in low basin of Coatzacoalcos River, Mexico. Sci. Total Environ. 2019, 651, 1236–1242. [Google Scholar] [CrossRef]
- Yan, X.J.; Wang, J.H.; Zhu, L.S.; Wang, J.; Li, S.Y.; Kim, Y.M. Oxidative stress, growth inhibition, and DNA damage in earthworms induced by the combined pollution of typical neonicotinoid insecticides and heavy metals. Sci. Total Environ. 2021, 754, 141873. [Google Scholar] [CrossRef]
- Xue, Y.N.; Li, Z.G.; Liu, C.; Liu, D.M.; Wang, J.H.; Liu, C.; Xia, X.M. Effect of different exposure times and doses of cyantraniliprole on oxidative stress and genotoxicity in earthworms (Eisenia fetida). Chemosphere 2023, 319, 138023. [Google Scholar] [CrossRef]
- Lin, W.D.; Li, J.M. Comparative Analysis of the Main Metabolites in Tender and Matured Leaves of Cyclocarya Paliurus Based on Metabolomic Data. J. Taizhou Univ. 2021, 43, 55–62. (In Chinese) [Google Scholar] [CrossRef]
- Wang, C.Z.; Zhang, D.J.; Yu, J.L.; Tang, Z.Q.; Zhang, J. Soil Microbial Community Diversity and Composition across a Range of Eucalyptus grandis Plantations of Different Ages. Int. J. Agric. Biol. 2019, 21, 527–537. [Google Scholar] [CrossRef]
- Kuiters, A.T.; Sarink, H.M. Leaching of phenolic compounds from leaf and needle litter of several deciduous and coniferous trees. Soil Biol. Biochem. 1986, 18, 475–480. [Google Scholar] [CrossRef]
- Ormeño, E.; Fernandez, C.; Mévy, J. Plant coexistence alters terpene emission and content of Mediterranean species. Phytochemistry 2007, 68, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.; Armstrong, W. Phragmites die-back: Toxic effects of propionic, butyric and caproic acids in relation to pH. New Phytol. 1999, 142, 201–217. [Google Scholar] [CrossRef]
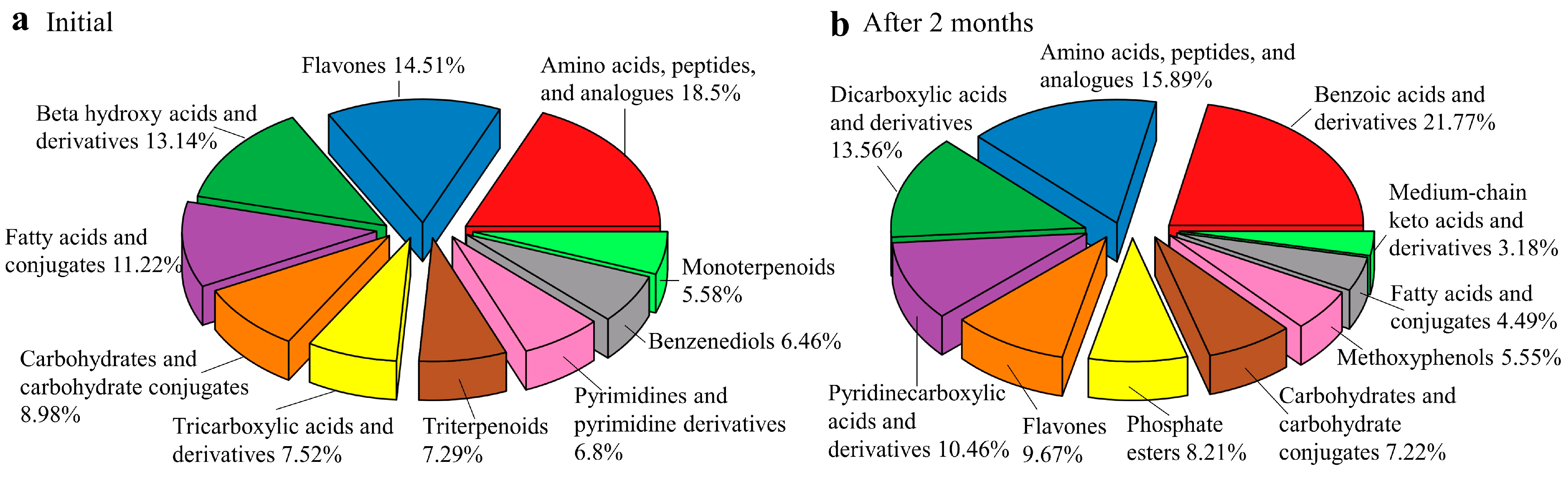
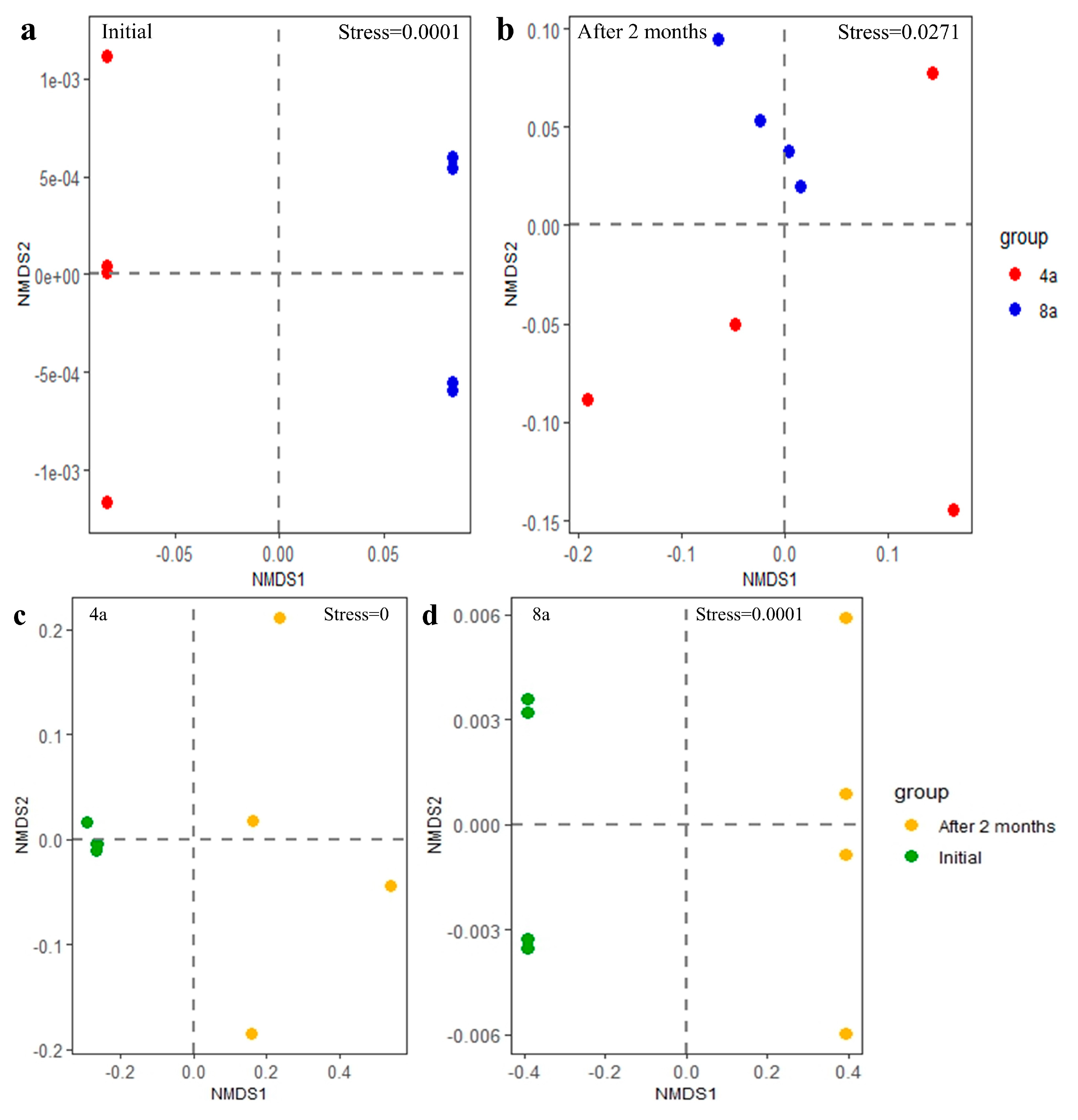
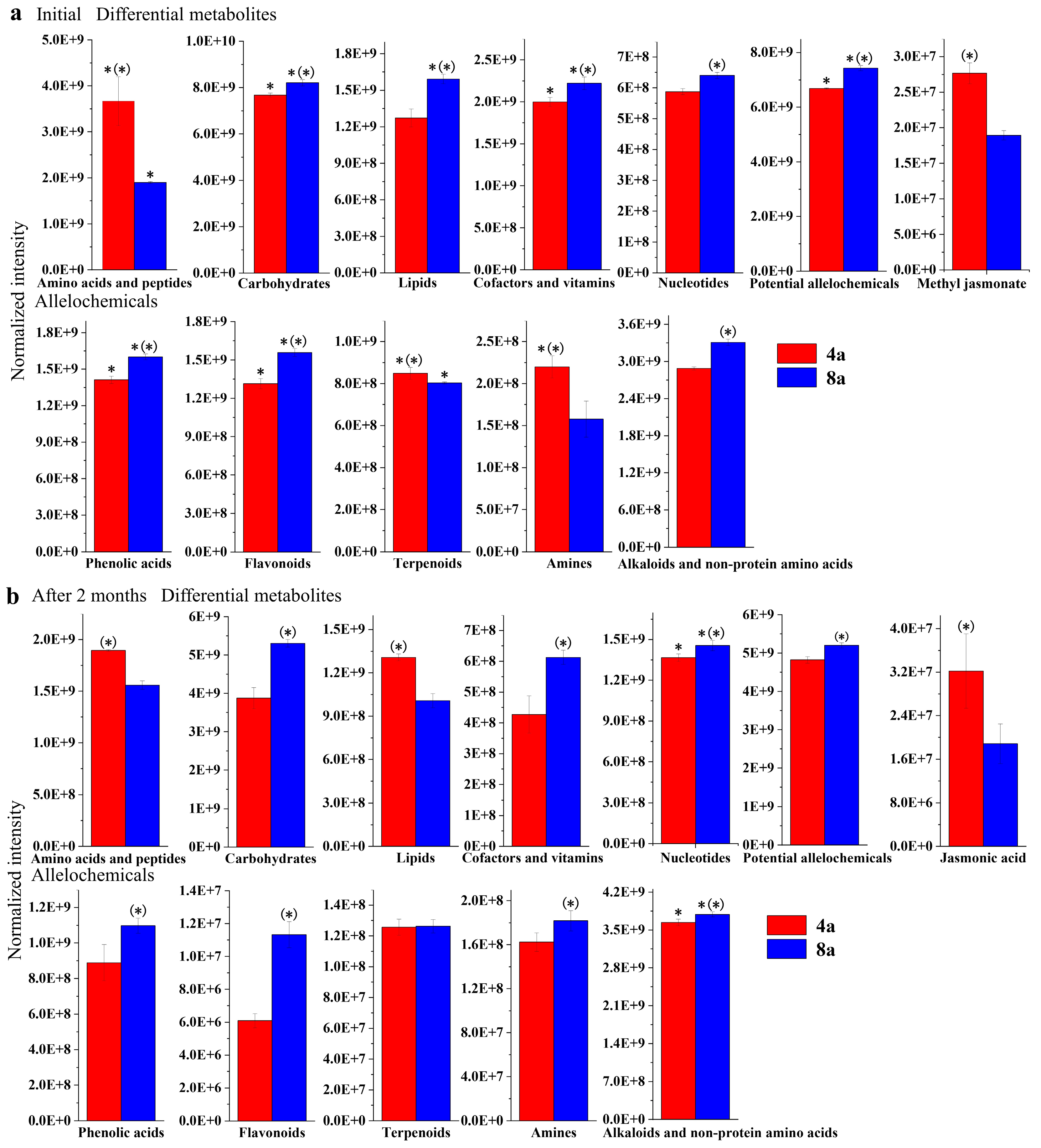
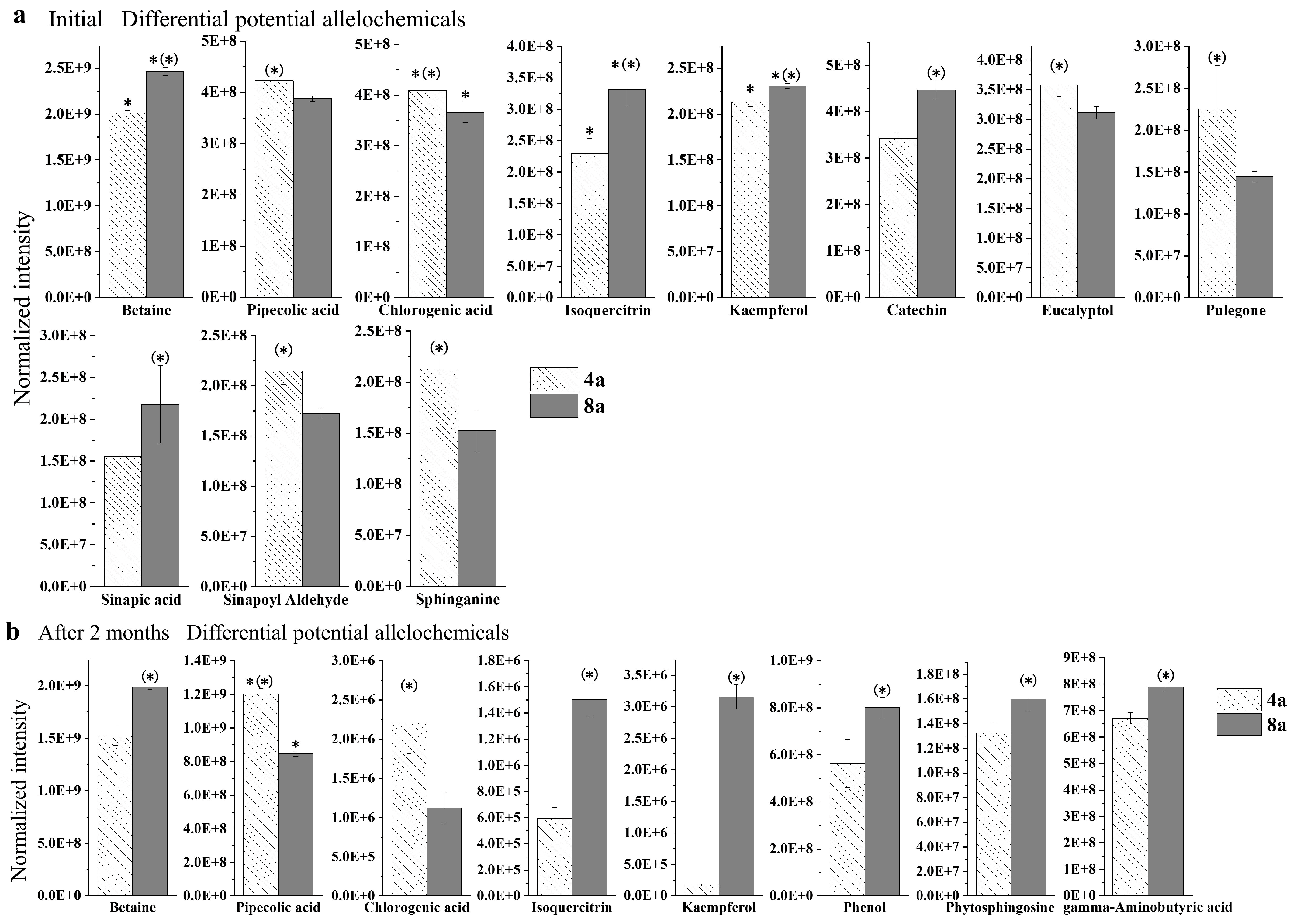
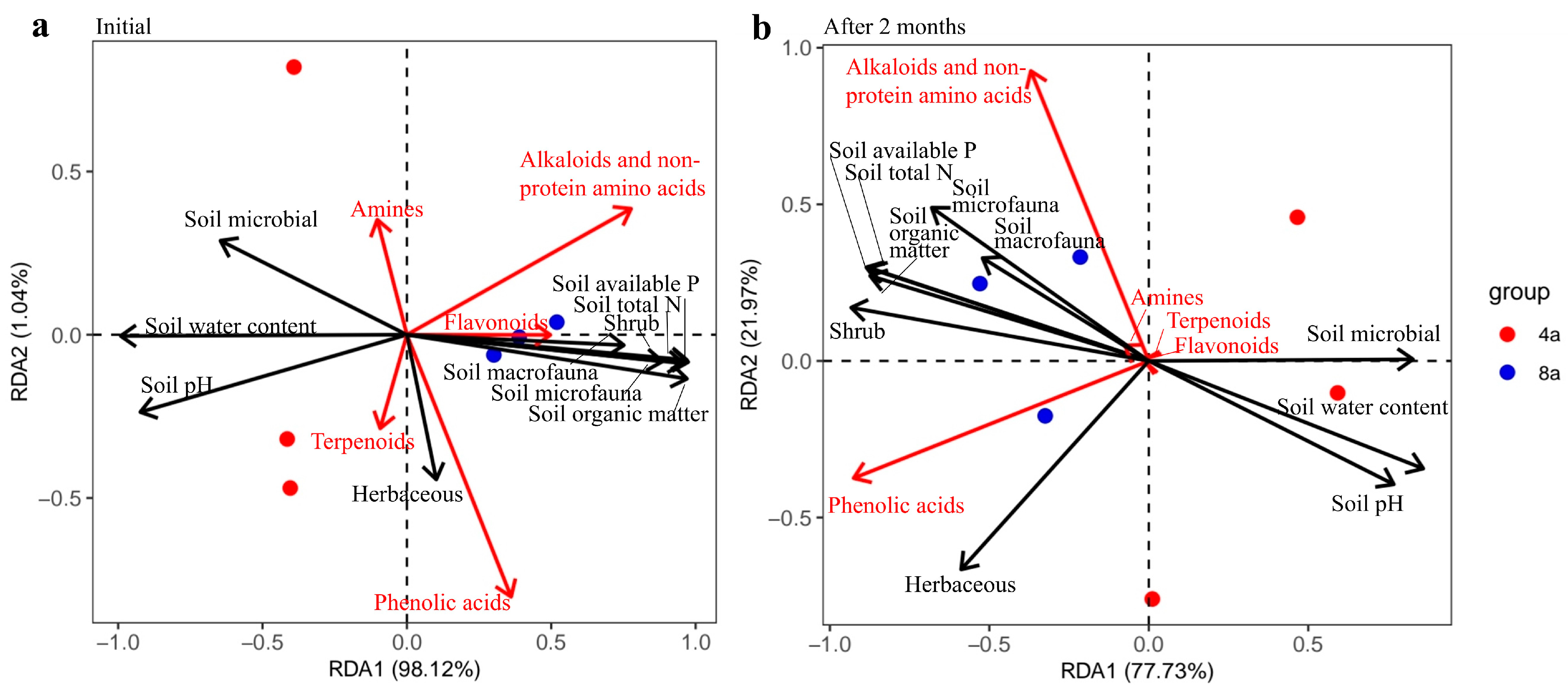
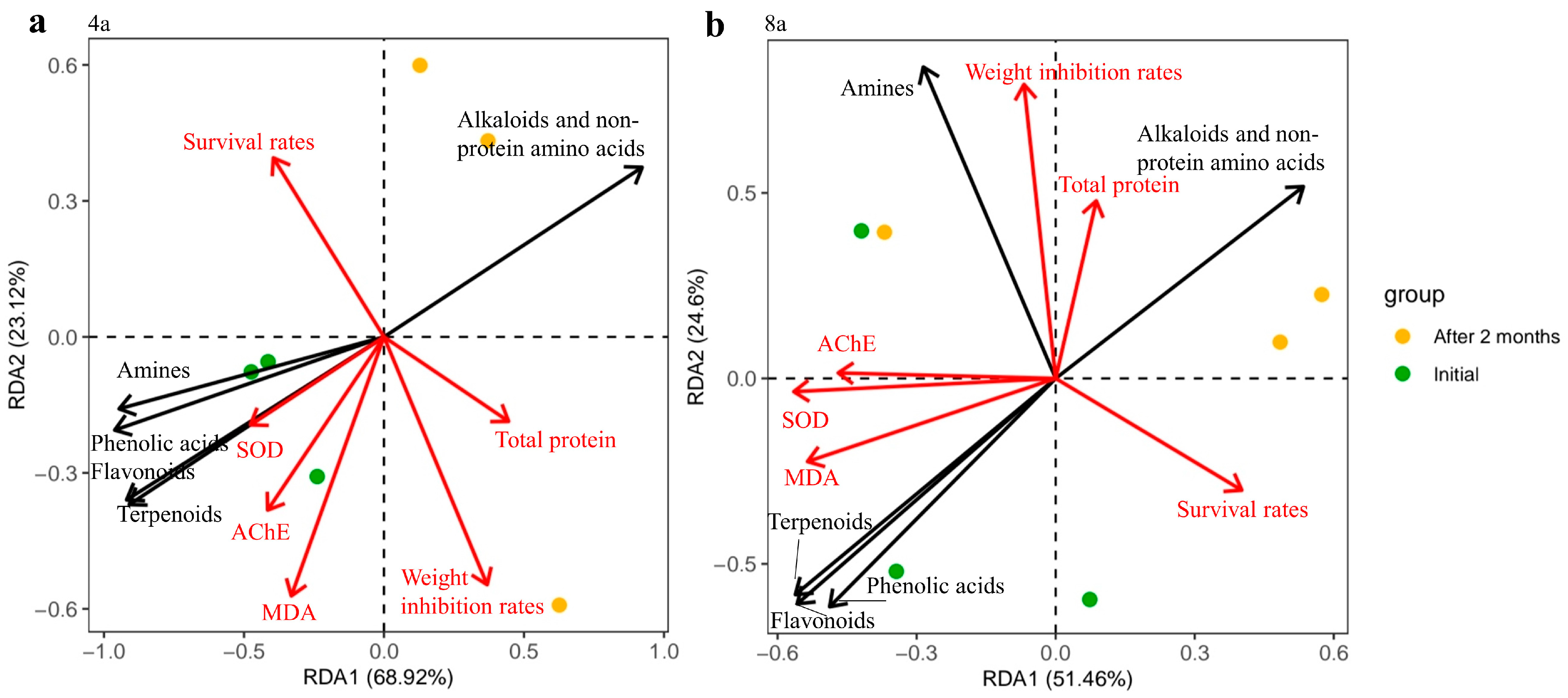
| Stand | Soil Bulk Density | Soil pH | Soil Water Content | Soil Organic Matter | Soil Total N | Soil Available p |
|---|---|---|---|---|---|---|
| 4a | 1.33 ± 0.08 | 3.99 ± 0.03 * | 29.38 ± 0.01 *** | 15.90 ± 0.88 | 0.27 ± 0.03 | 2.54 ± 0.24 |
| 8a | 1.22 ± 0.12 | 3.90 ± 0.03 | 28.99 ± 0.02 | 24.56 ± 0.58 *** | 0.91 ± 0.06 *** | 6.89 ± 0.26 *** |
| Species Number | Density (Ind m−2) | Shannon– Wiener | Pielou | Berger–Parker | ||
|---|---|---|---|---|---|---|
| Plant’s diversity | Shrub (4a) | 3.00 ± 0.87 | 0.51 ± 0.18 | 0.75 ± 0.14 | 0.76 ± 0.08 | 0.68 ± 0.03 *** |
| Shrub (8a) | 6.50 ± 0.43 ** | 1.11 ± 0.18 * | 1.65 ± 0.05 *** | 0.82 ± 0.02 | 0.35 ± 0.00 | |
| Herbaceous (4a) | 7.50 ± 2.18 | 48.00 ± 18.99 | 1.57 ± 0.21 | 0.73 ± 0.06 | 0.40 ± 0.03 | |
| Herbaceous (8a) | 7.75 ± 1.30 | 54.25 ± 4.63 | 1.64 ± 0.20 | 0.79 ± 0.07 | 0.39 ± 0.03 | |
| Soil faunal diversity | Macrofauna (4a) | 6.42 ± 0.14 | 54.00 ± 11.27 | 1.58 ± 0.09 | 0.80 ± 0.05 | 0.40 ± 0.05 * |
| Macrofauna (8a) | 7.50 ± 1.30 | 62.33 ± 19.73 | 1.79 ± 0.09 * | 0.86 ± 0.07 | 0.31 ± 0.02 | |
| Microfauna (4a) | 9.08 ± 0.95 | 8441.67 ± 1203.21 | 1.66 ± 0.02 | 0.64 ± 0.07 | 0.40 ± 0.02 | |
| Microfauna (8a) | 10.67 ± 0.14 | 12141.67 ± 2546.61 | 1.79 ± 0.07 * | 0.59 ± 0.05 | 0.37 ± 0.04 | |
| OTUs | Shannon–Wiener | Simpson | ACE | Chao1 | ||
| Soil microbial diversity | (4a) | 1623.08 ± 20.12 | 5.94 ± 0.07 | 0.99 ± 0.00 | 2474.72 ± 35.69 | 2312.38 ± 16.72 |
| (8a) | 1537.58 ± 91.82 | 5.72 ± 0.17 | 0.98 ± 0.01 | 2398.02 ± 127.48 | 2208.14 ± 145.15 |
| Effects | R and Significance | Main Contributive Compounds for Variation |
|---|---|---|
| Two-way ANOSIM | ||
| Forest age | (1) *** | Betaine, pipecolic acid, phenol, gamma-aminobutyric acid, catechin, chlorogenic acid, eucalyptol, isoquercitrin, kaempferol, pulegone, sinapic acid, sinapoyl aldehyde, and sphinganine |
| Decomposition time | (1) *** | Gamma-aminobutyric acid, phenol, pipecolic acid, betaine, catechin, chlorogenic acid, eucalyptol, isoquercitrin, kaempferol, sinapoyl aldehyde, pulegone, sinapic acid, sphinganine, and phytosphingosine |
| One-way ANOSIM of forest age effects | ||
| Initial | (1) * | Betaine, methyleugenol, catechin, isoquercitrin, pulegone, sinapic acid, Sphinganine, 4-hydroxycinnamic acid, eucalyptol, Taraxerol, chlorogenic acid, sinapoyl aldehyde, and pipecolic acid |
| After 2 months | (1) * | Betaine, pipecolic acid, phenol, gamma-aminobutyric acid, and saccharopine |
| One-way ANOSIM of decomposition time effects | ||
| 4 years | (1) * | Betaine, 2-aminophenol, Taraxerol, gamma-aminobutyric acid, phenol, pipecolic acid, chlorogenic acid, eucalyptol, catechin, isoquercitrin, and pulegone |
| 8 years | (1) * | Betaine, 2-aminophenol, phenol, gamma-aminobutyric acid, Taraxerol, catechin, pipecolic acid, chlorogenic acid, isoquercitrin, and eucalyptol |
| Survival Rate | Weight Inhibition Rate | Total Protein Content | SOD Activity | MDA Content | AChE Activity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |
| Decomposition time | 17.476 | 0.000 *** | 5.498 | 0.000 *** | 41.199 | 0.000 *** | 69.155 | 0.000 *** | 29.708 | 0.000 *** | 43.625 | 0.000 *** |
| Age | 0.129 | 0.720 | 0.088 | 0.767 | 5.130 | 0.026 * | 10.673 | 0.001 *** | 2.596 | 0.110 | 2.815 | 0.097 |
| Dose | 0.587 | 0.673 | 4.680 | 0.002 ** | 3.034 | 0.021 * | 7.769 | 0.000 *** | 2.708 | 0.034 * | 6.349 | 0.000 *** |
| Exposure time | 134.20 | 0.000 *** | 420.00 | 0.000 *** | 22.767 | 0.000 *** | 2.395 | 0.125 | 94.713 | 0.000 *** | 8.834 | 0.004 ** |
| Age × Dose | 1.863 | 0.123 | 2.372 | 0.057 | 0.816 | 0.518 | 2.345 | 0.060 | 0.532 | 0.712 | 1.100 | 0.361 |
| Age × Decomposition time | 0.662 | 0.620 | 2.455 | 0.051 | 0.905 | 0.464 | 0.865 | 0.488 | 1.065 | 0.378 | 3.667 | 0.008 ** |
| Dose | Tail DNA (%) | Tail Moment | Olive Tail Moment | Tail Length | ||||
|---|---|---|---|---|---|---|---|---|
| 4a | 8a | 4a | 8a | 4a | 8a | 4a | 8a | |
| CK | 1.51 ± 0.81 | 1.51 ± 0.81 (ab) | 0.19 ± 0.12 | 0.19 ± 0.12 (ab) | 0.46 ± 0.21 | 0.46 ± 0.21 (ab) | 7.46 ± 2.31 | 7.46 ± 2.31 |
| C1 | 1.63 ± 0.48 | 1.17 ± 0.29 (b) | 0.34 ± 0.2 | 0.12 ± 0.03 (b) | 0.50 ± 0.17 | 0.29 ± 0.07 (b) | 6.28 ± 0.69 | 5.02 ± 0.49 |
| C2 | 2.16 ± 0.87 | 1.52 ± 0.13 (ab) | 0.52 ± 0.32 | 0.52 ± 0.18 (ab) | 0.75 ± 0.33 | 0.56 ± 0.04 (ab) | 9.04 ± 4.48 | 7.98 ± 1.44 |
| C3 | 1.50 ± 0.54 | 2.84 ± 1.32 (ab) | 0.24 ± 0.1 | 0.79 ± 0.56 (a) | 0.46 ± 0.21 | 0.97 ± 0.55 (a) | 6.85 ± 2.02 | 11.75 ± 6.19 |
| C4 | 1.03 ± 0.24 | 3.20 ± 0.74 (a)(*) | 0.12 ± 0.07 | 0.82 ± 0.58 (a) | 0.27 ± 0.08 | 1.04 ± 0.27 (a)(*) | 5.33 ± 1.34 | 11.70 ± 3.28 (*) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Lv, C.; Fan, S.; Huang, Y.; Kang, N.; Gao, S.; Chen, L. The Dynamics of Allelochemicals and Phytotoxicity in Eisenia fetida during the Decomposition of Eucalyptus grandis Litter. Plants 2024, 13, 2415. https://doi.org/10.3390/plants13172415
Zhang D, Lv C, Fan S, Huang Y, Kang N, Gao S, Chen L. The Dynamics of Allelochemicals and Phytotoxicity in Eisenia fetida during the Decomposition of Eucalyptus grandis Litter. Plants. 2024; 13(17):2415. https://doi.org/10.3390/plants13172415
Chicago/Turabian StyleZhang, Danju, Chaoyu Lv, Shaojun Fan, Yumei Huang, Na Kang, Shun Gao, and Lianghua Chen. 2024. "The Dynamics of Allelochemicals and Phytotoxicity in Eisenia fetida during the Decomposition of Eucalyptus grandis Litter" Plants 13, no. 17: 2415. https://doi.org/10.3390/plants13172415






