Strategies to Increase the Phosphorus Content in the Soil Profile of Vineyards Grown in Subtropical Climates
Abstract
:1. Introduction
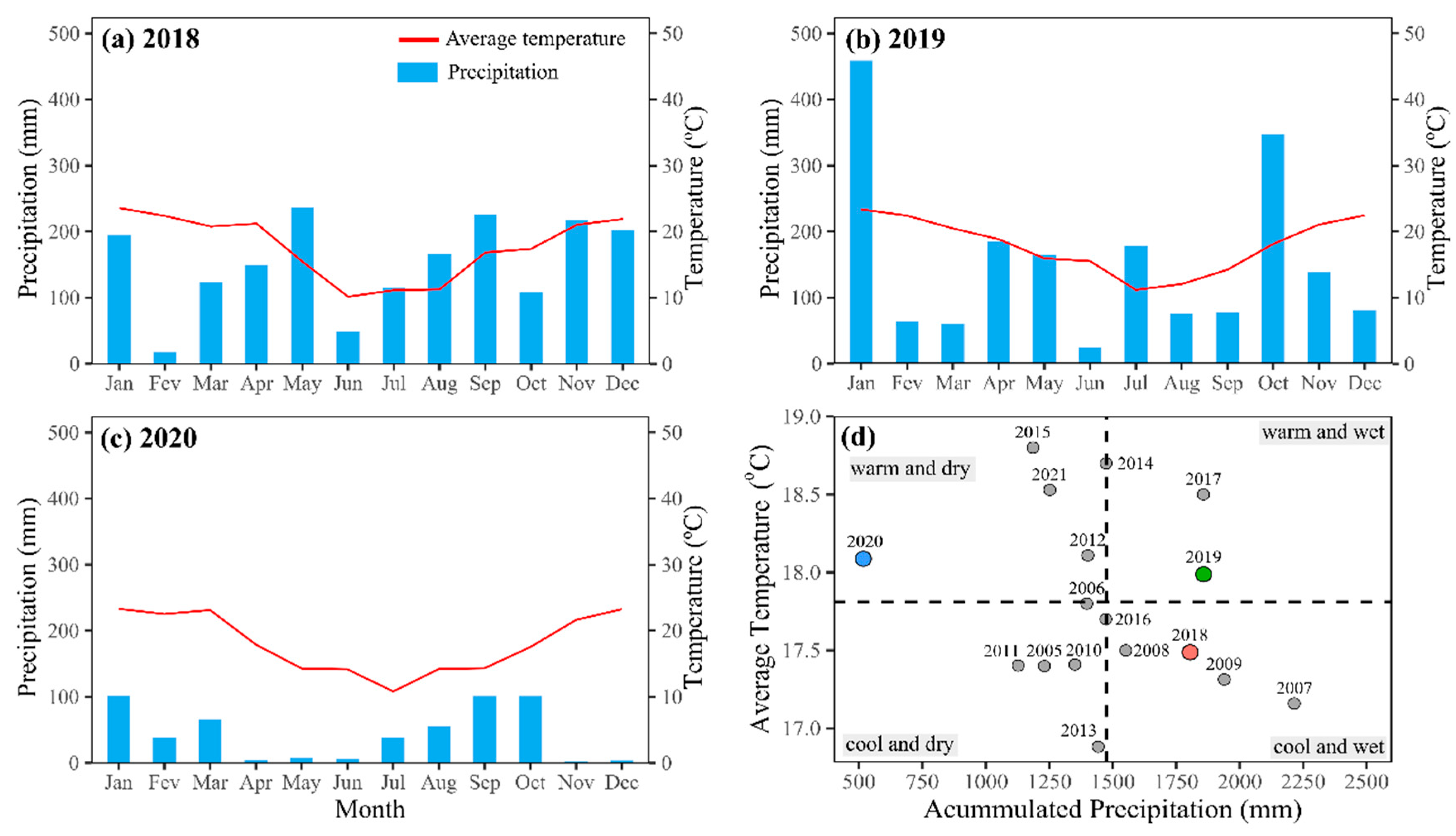
2. Results
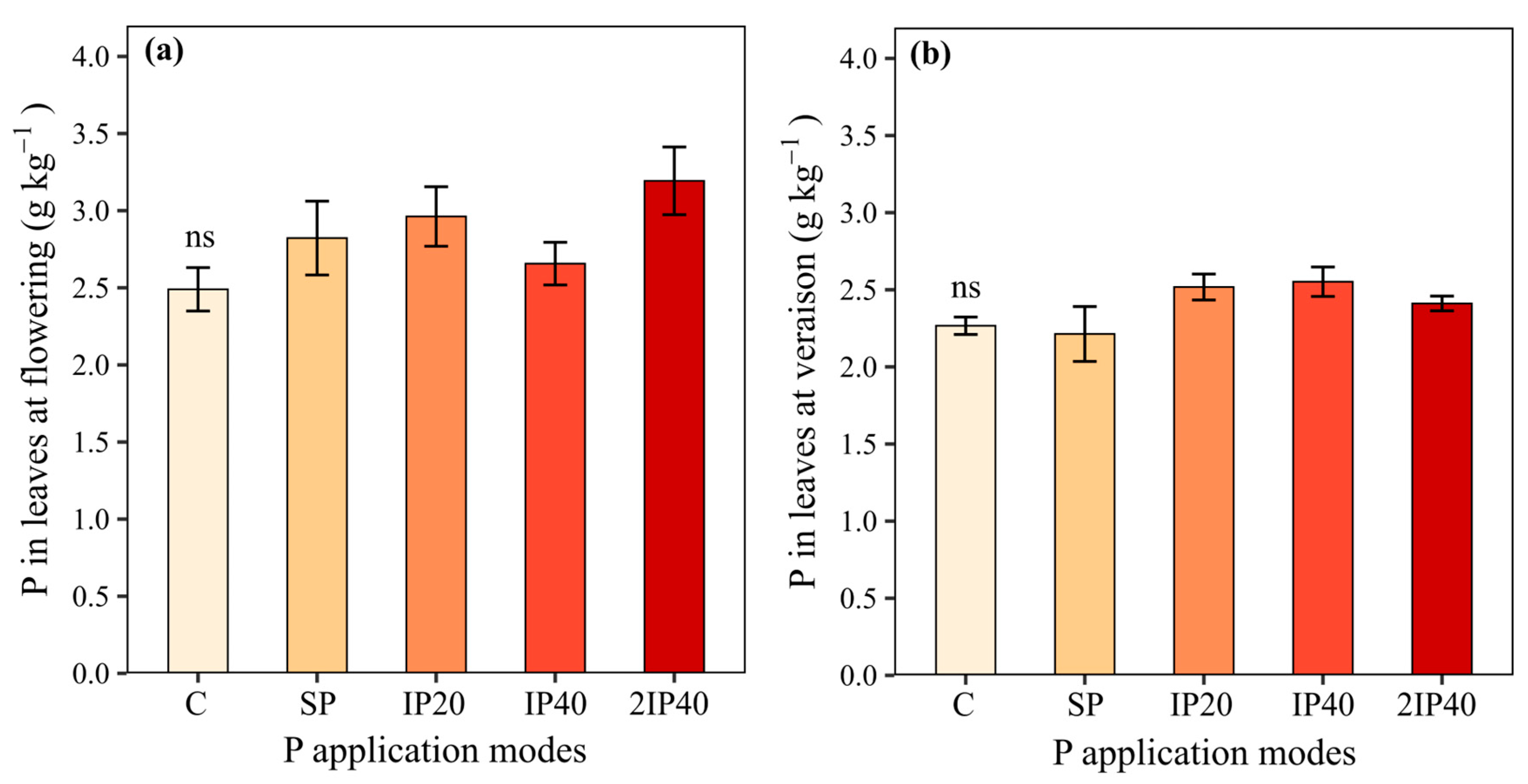
2.1. P Concentration in Leaves and Grape Production
2.2. Composition of the Grape Must
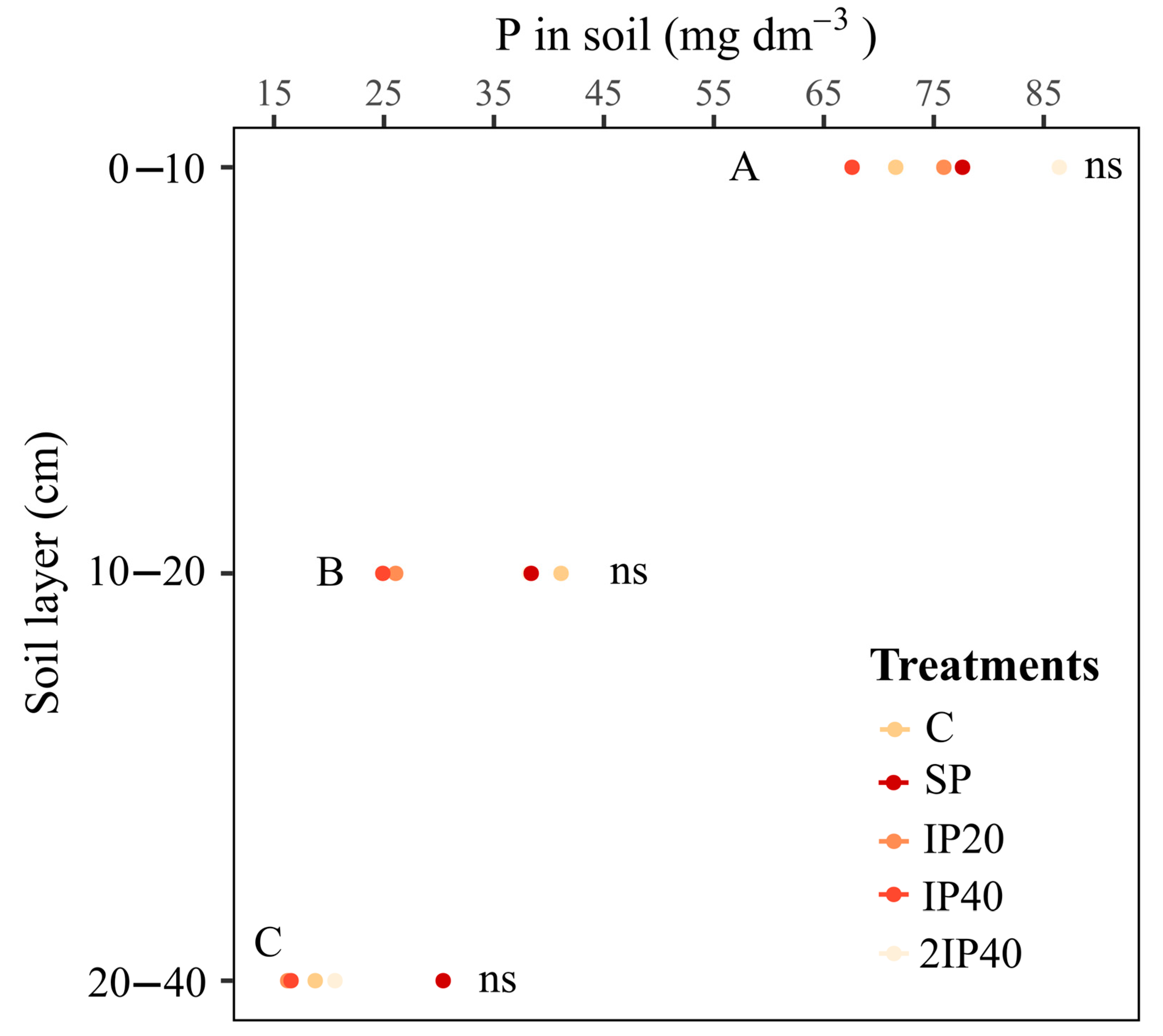
2.3. Phosphorus Content in the Soil Profile
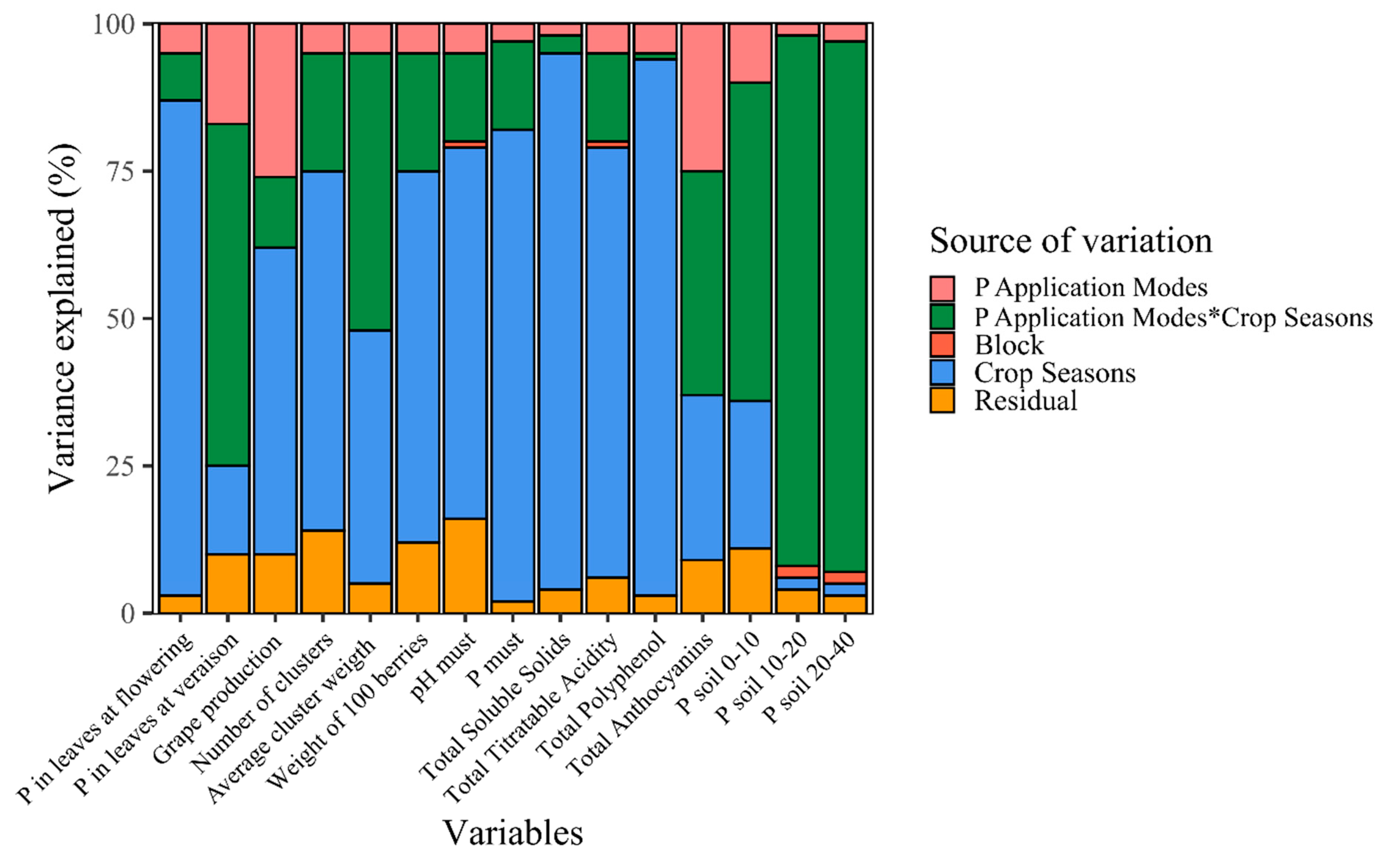
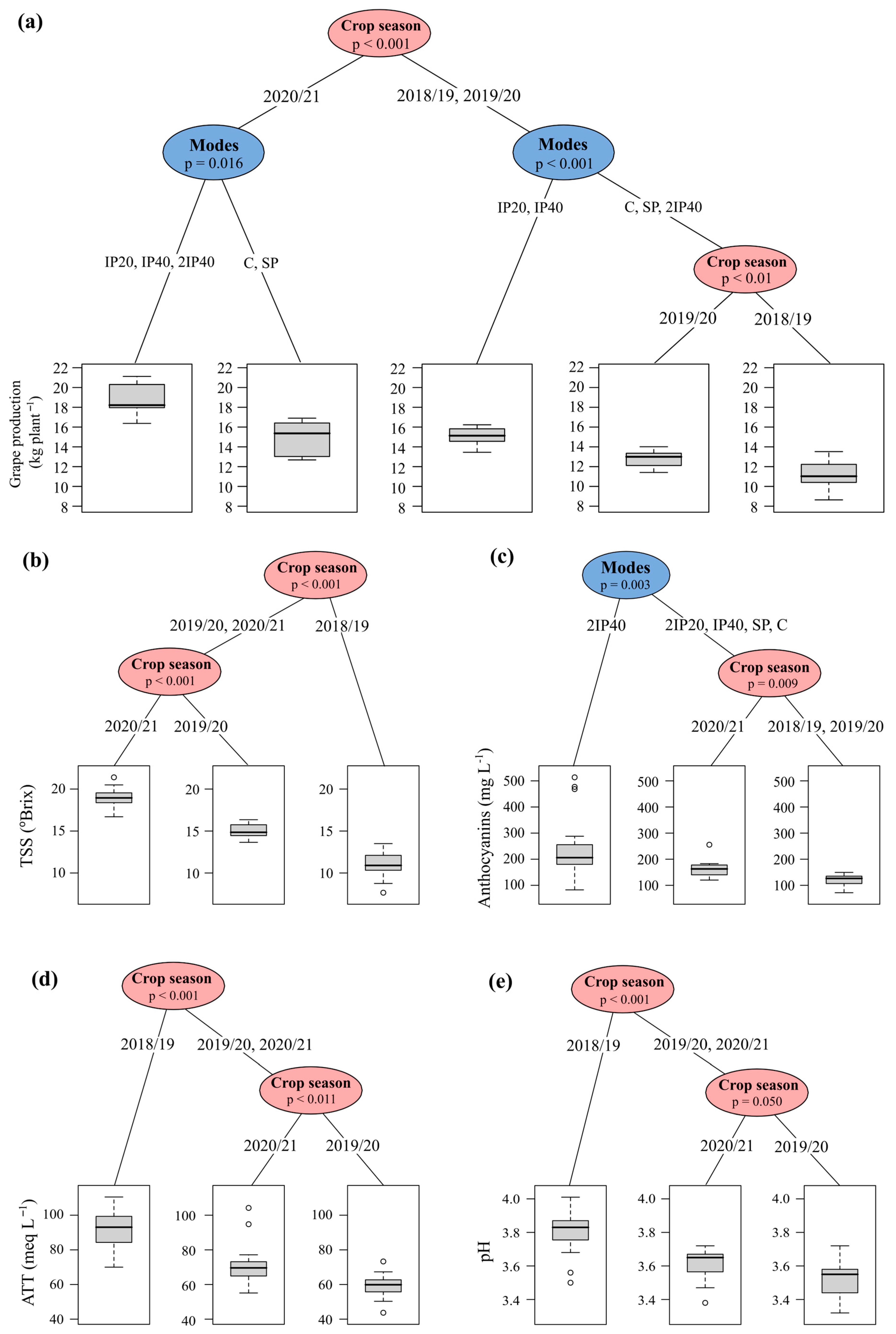
2.4. Variance Components and Inference Tree
2.5. Principal Component Analysis
3. Discussion
3.1. Phosphorus Concentration in Leaves and Soil
3.2. Grape Production and Chemical Parameters of the Grape Must
4. Materials and Methods
4.1. Experimental Layout and Treatments
4.2. Leaf Sampling and P Determination
4.3. Grape Production and Its Parameters
4.4. Chemical Analysis of the Grape Must
4.5. Soil Sampling and P Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Figueiredo, M.C.d.S.; Rosseto, V.; Barão, C.F.; Nunes, R.S.G.; Duarte, I.d.L.; Weber, M.A.; Vieira, F.C.B. Correlations among nutrient contents in soil, leaf, and fruit tissues and yield of Olea europaea L. cv. Arbequina in the South of Brazil. Res. Soc. Dev. 2022, 11, e489111033114. [Google Scholar] [CrossRef]
- Griebeler, S.R.; Gonzatto, M.P.; Schwarz, S.F.; Böettcher, G.N.; Pauletti, G.F.; Rota, R.D. Nutritional diagnosis of ‘Montenegrina’ mandarin orchards at the southern Brazil. Rev. Ceres 2023, 70, 69–77. [Google Scholar] [CrossRef]
- Schreiner, R.P.; Osborne, J. Defining phosphorus requirements for Pinot Noir grapevines. Am. J. Enol. Vitic. 2018, 69, 351–359. [Google Scholar] [CrossRef]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of phosphate solubilizing microorganisms from managing soil phosphorus deficiency to mediating biogeochemical P cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef]
- Schmitt, D.E.; Comin, J.J.; Gatiboni, L.C.; Tiecher, T.; Lorensini, F.; Melo, G.W.B.; Girotto, E.; Guardini, R.; Heinzen, J.; Brunetto, G. Phosphorus fractions in sandy soils of vineyards in southern Brazil. Rev. Bras. Cien. Solo 2013, 37, 472–481. [Google Scholar] [CrossRef]
- Grando, D.L.; Gatiboni, L.C.; Mumbach, G.L.; Dall’Orsoletta, D.J.; de Souza Junior, A.A.; Schmitt, D.E. Phosphorus in the runoff of soils with contrasting textures influenced by soil slope and pig slurry application. Agric. Water Manag. 2021, 258, 107178. [Google Scholar] [CrossRef]
- Piccin, R.; Couto, R.R.; Bellinaso, R.J.S.; Gatiboni, L.C.; De Conti, L.; Rodrigues, L.A.T.; Somavilla, L.M.; Kulmann, M.S.S.; Brunetto, G. Phosphorus forms in leaves and their relationships with must composition and yield in grapevines. Pesqui. Agropecu. Bras. 2017, 52, 319–327. [Google Scholar] [CrossRef]
- Botelho, R.V.; Müller, M.M.L. Nutrient redistribution in fruit crops: Physiological implications. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 33–46. [Google Scholar] [CrossRef]
- Zhu, J.; Gou, F.; Rossouw, G.; Begum, F.; Henke, M.; Johnson, E.; Holzapfel, B.; Field, S.; Seleznyova, A. Simulating organ biomass variability and carbohydrate distribution in perennial fruit crops: A comparison between the common assimilate pool and phloem carbohydrate transport models. In Silico Plants 2021, 3, diab024. [Google Scholar] [CrossRef]
- Vittal, H.; Sharma, N.; Shivran, M. Impact of rootstock via carbohydrate metabolism and nutrients on bearing habit of fruit crops. Mysore J. Agric. Sci. 2023, 57, 1–15. [Google Scholar]
- Penn, C.J.; Camberato, J.J.; Wiethorn, M.A. How much phosphorus uptake is required for achieving maximum maize grain yield? Part 1: Luxury consumption and implications for yield. Agronomy 2023, 13, 95. [Google Scholar] [CrossRef]
- Nachtigall, G.R.; Dechen, A.R. Seasonality of nutrients in leaves and fruits of apple trees. Sci. Agric. 2006, 63, 493–501. [Google Scholar] [CrossRef]
- Mota, M.; Martins, M.J.; Policarpo, G.; Sprey, L.; Pastaneira, M.; Almeida, P.; Maurício, A.; Rosa, C.; Faria, J.; Martins, M.B.; et al. Nutrient content with different fertilizer management and influence on yield and fruit quality in apple cv. Gala. Horticulturae 2022, 8, 713. [Google Scholar] [CrossRef]
- Ljavić, D.; Radović, M.; Kulina, M.; Zejak, D.; Spalević, V.; Kader, S.; Dudic, B.; Michael, R.N.; Campbell, J.; Jauferi, L.; et al. Influence of cultivar and fertilization treatment on the yield and leaf nutrients content of apple (Malus domestica Borkh.). Heliyon 2023, 9, e16321. [Google Scholar] [CrossRef]
- Yu, X.; Wang, B.; Zhang, C.; Xu, W.; He, J.; Zhu, L.; Wang, S. Effect of root restriction on nitrogen levels and glutamine synthetase activity in ‘Kyoho’ grapevines. Sci. Hortic. 2012, 137, 156–163. [Google Scholar] [CrossRef]
- Schmitt, D.E.; Borghezan, M.; Ambrosini, V.G.; Comin, J.J.; Trapp, T.; Brunetto, G. Yield and must composition of grapevines subjected to phosphate fertilization in Southern Brazil. Pesqui. Agropecu. Bras. 2020, 55, e01167. [Google Scholar] [CrossRef]
- CQFS-RS/SC (Comissão de Química e Fertilidade do Solo—RS/SC). Manual de Calagem e Adubação para os Estados do Rio Grande do Sul e de Santa Catarina; Silva, L.S., Gatiboni, L.C., Ibanor, A., Souza, R.O., Eds.; Sociedade Brasileira de Ciência do Solo-Núcleo Regional Sul: Viçosa, Brazil, 2016; 376p, ISBN 9788566301809. [Google Scholar]
- Brunetto, G.; Lorensini, F.; Ceretta, C.A.; Gatiboni, L.C.; Trentin, G.; Girotto, E.; Miotto, A.; Lourenzi, C.R.; Melo, G.W.B. Soil phosphorus fractions in a sandy typic Hapludaft as affected by phosphorus fertilization and grapevine cultivation period. Commun. Soil Sci. Plant Anal. 2013, 44, 1937–1950. [Google Scholar] [CrossRef]
- July, P.; Djodjic, F.; Bergstrom, L.; Ulen, B.; Shirmohammadi, A. Mode of transport of surface-applied phosphorus-33 through a clay and sandy soil. J. Environ. Qual. 1999, 1282, 1273–1282. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, W.; Zhao, Z.; Jiang, W.; Zhang, P.; Sun, X.; Xue, Q. Soil compaction and maize root distribution under subsoiling tillage in a wheat–maize double cropping system. Agronomy 2023, 13, 394. [Google Scholar] [CrossRef]
- Jezek, M.; Zörb, C.; Merkt, N.; Geilfus, C.M. Anthocyanin management in fruits by fertilization. J. Agric. Food Chem. 2018, 66, 753–764. [Google Scholar] [CrossRef]
- Zheng, H.Z.; Weil, H.; Guo, S.H.; Yang, X.; Feng, M.X.; Jin, X.Q.; Fang, Y.L.; Zhang, Z.W.; Xu, T.F.; Meng, J.F. Nitrogen and phosphorus co-starvation inhibits anthocyanin synthesis in the callus of grape berry skin. Plant Cell Tissue Organ Cult. 2020, 142, 313–325. [Google Scholar] [CrossRef]
- Pradubsuk, S.; Davenport, J.R. Seasonal uptake and partitioning of macronutrients in mature ‘Concord’ grape. J. Am. Soc. Hortic. Sci. 2010, 135, 474–483. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J.; et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Embrapa Solos Embrapa: Brasília, Brazil, 2018; 356p. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-NRCS: Washington, DC, USA, 2014; 372p. [Google Scholar]
- CQFS-RS/SC (Comissão de Química e Fertilidade do Solo—RS/SC). Manual de Adubação e de Calagem para os Estados do Rio Grande do Sul e de Santa Catarina, 10th ed.; Sociedade Brasileira de Ciência do Solo. Comissão de Química e Fertilidade do Solo: Porto Alegre, Brazil, 2004; 400p. [Google Scholar]
- Miller, R.O. Nitric-Perchloric wet acid digestion in an open vessel. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Washington, DC, USA, 1998; pp. 57–68. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Baltimore, MD, USA, 2005; 1750p. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley, Sons: New York, NY, USA, 2001; Volume 1, pp. F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- R Core Team. Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org/ (accessed on 15 November 2023).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tassinari, A.; Stefanello, L.; Moura-Bueno, J.M.; Siqueira, G.N.d.; Peripolli, G.Z.; Dias, B.G.; Grando, D.L.; Natale, W.; Ceretta, C.A.; Brunetto, G. Strategies to Increase the Phosphorus Content in the Soil Profile of Vineyards Grown in Subtropical Climates. Plants 2024, 13, 2434. https://doi.org/10.3390/plants13172434
Tassinari A, Stefanello L, Moura-Bueno JM, Siqueira GNd, Peripolli GZ, Dias BG, Grando DL, Natale W, Ceretta CA, Brunetto G. Strategies to Increase the Phosphorus Content in the Soil Profile of Vineyards Grown in Subtropical Climates. Plants. 2024; 13(17):2434. https://doi.org/10.3390/plants13172434
Chicago/Turabian StyleTassinari, Adriele, Lincon Stefanello, Jean Michel Moura-Bueno, Gustavo Nogara de Siqueira, Guilherme Zanon Peripolli, Bianca Goularte Dias, Douglas Luiz Grando, William Natale, Carlos Alberto Ceretta, and Gustavo Brunetto. 2024. "Strategies to Increase the Phosphorus Content in the Soil Profile of Vineyards Grown in Subtropical Climates" Plants 13, no. 17: 2434. https://doi.org/10.3390/plants13172434










