Taxonomic Revision of Genus Ephedra Tourn. ex L. in Egypt with Intra-Gender Diversity in Morphometric Traits and Fatty Acid Composition
Abstract
:1. Introduction
2. Results and Discussion
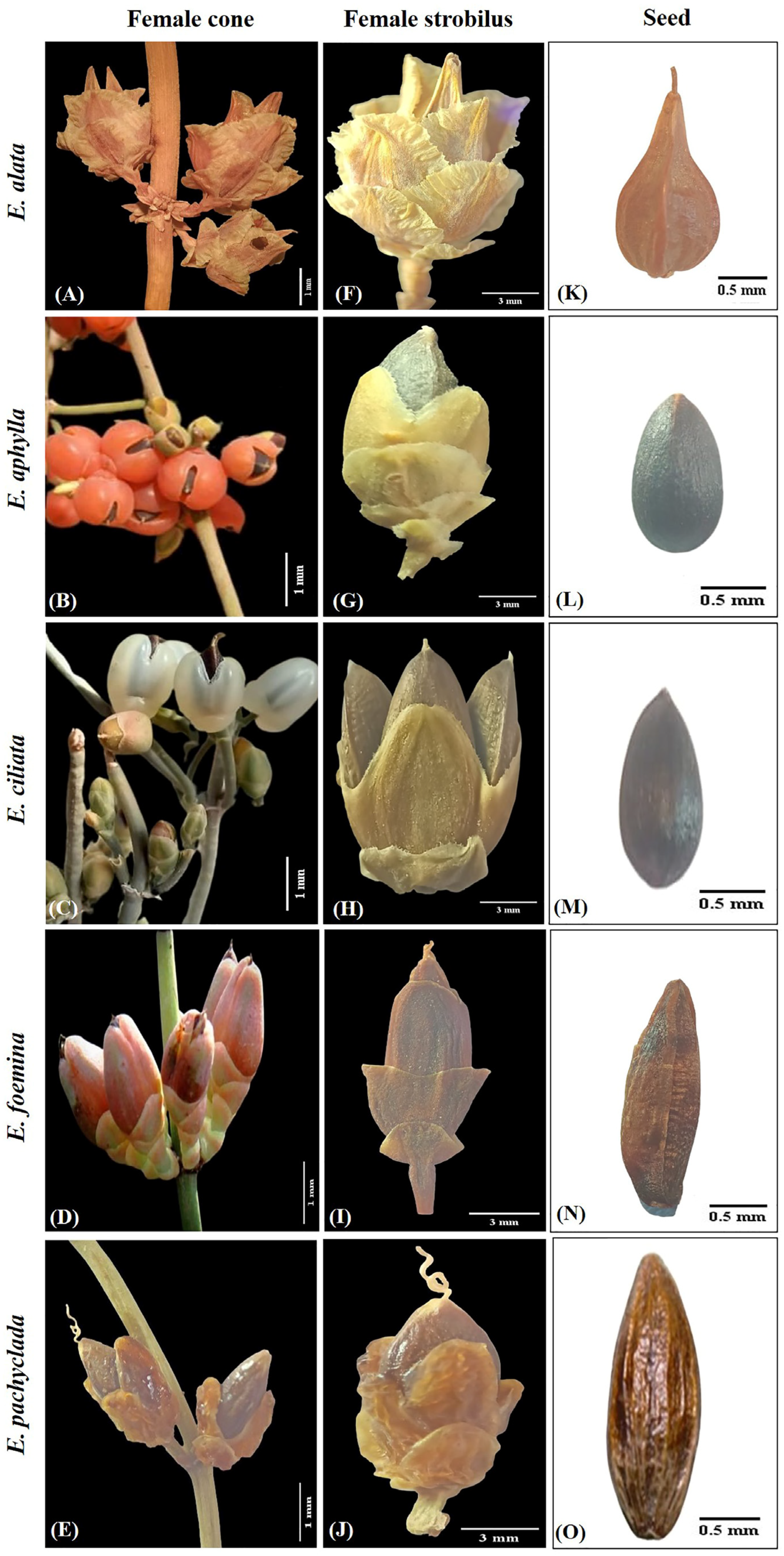
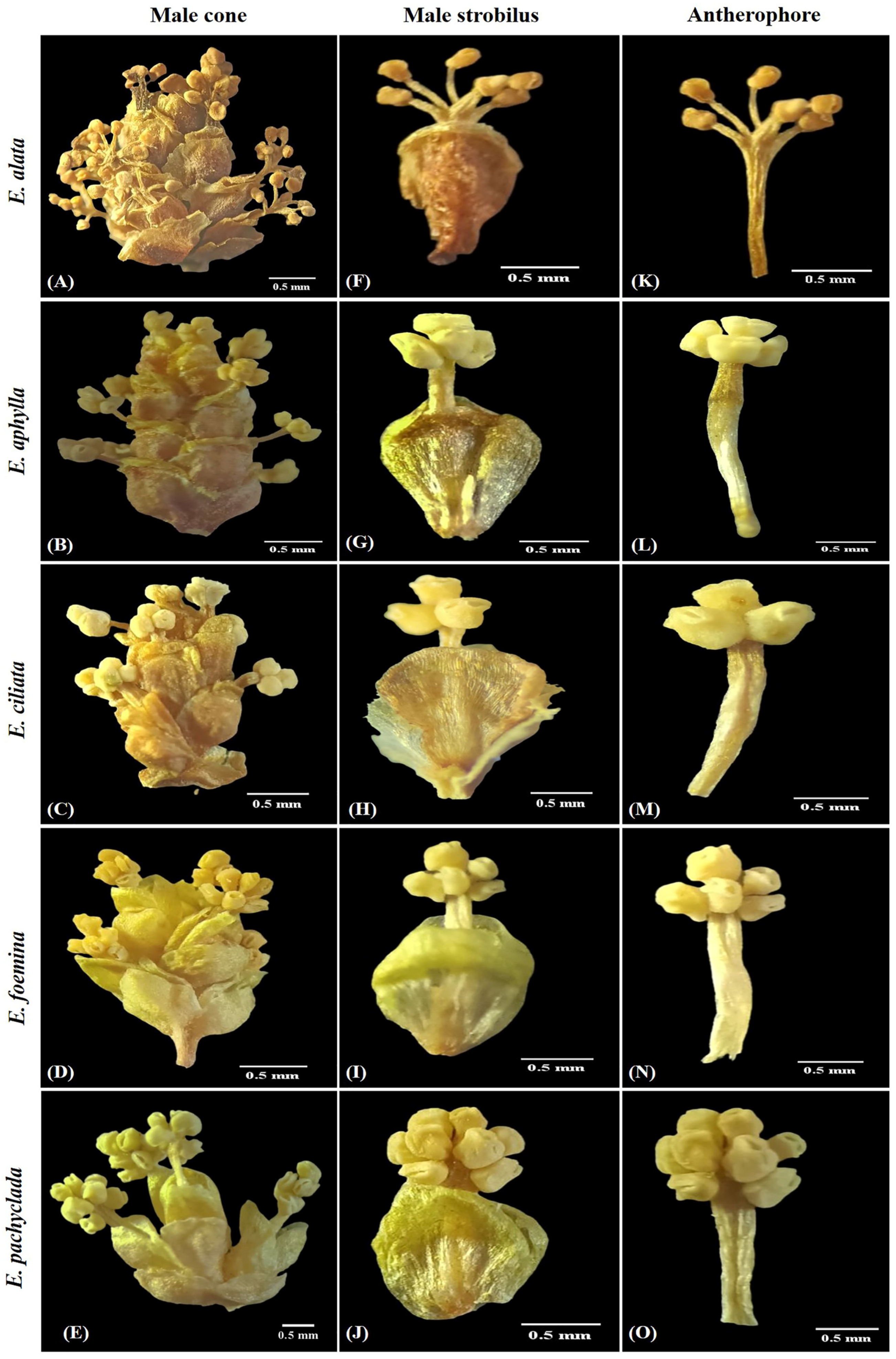
2.1. Taxonomic Investigation
2.1.1. Ephedra alata Decne. (Section Alatae) Ann. Sci. Nat., Bot. sér. 2, 2: 239 (1834)
2.1.2. Ephedra aphylla Forssk. (Section Ephedra), Fl. Aegypt.-Arab.: 170 (1775)
2.1.3. Ephedra ciliata Fisch. & C.A.Mey. (Section Ephedra), Bull. Cl. Phys.-Math. Acad. Imp. Sci. Saint-Pétersbourg 5: 36 (1845)
2.1.4. Epherda foeminea Forssk. (Section Ephedra), Fl. Aegypt.-Arab.: 219 (1775)
2.1.5. Ephedra pachyclada Boiss. (Section Ephedra), Fl. Orient. 5: 713 (1884)
2.2. Species and Gender Key Based on Morpho-Taxonomic Traits
- I.
- Margins of cone bracts and leaf sheath glabrous ……………………….………… II
- +
- Margins of cone bracts and leaf sheath ciliated……...…………………………….III
- II.
- Stem erect, much branched at nodes, branches whitish-green, stiff, thickened upright ………………………………………………………………...…………….. E. pachyclada
- Seed 1–(2) ovules/cone, micropylar tubule curved………………………... Female
- Anthers whorled 6–8, sessile and unbranched above, 4–6 strobili/cone ….. Male
- +
- Stem climbing and prostrate, branched all over, branches dark green, thin, wiry ………………………..……………………………………………………....E. foemina
- Seed 1–2 ovules/cone, micropylar tubule straight ……………..…………...Female
- Anthers clustered 4–7, sessile and unbranched above, 7–8 strobili per cone...Male
- III.
- Cone bracts free with membranous edge……………………………….…... E. alata
- Fruit creamy, cone-like, seed (1)–2 ovules/cone…………………...………...... Female
- Anthers umbellate 3–6, stalked and branched above, 7–12 strobili/cone....… Male
- +
- Cone bract fused without membranous edges…………………………………...IV
- IV.
- Origin of female cone terminal……………………………………………... E. ciliata
- Fruit white-red, berry-like, seed 1–3 ovules/cone………………………..…Female
- Anthers opposite 3, sessile and unbranched above, 7–10 strobili/cone …......Male
- +
- Origin of female cone axillary………………………….…………….….....E. aphylla
- Fruit red, seed 1–2 ovules/cone………………………….………….………Female
- Anthers clustered 3–4, sessile and unbranched above, 7–10 strobili/cone…….Male
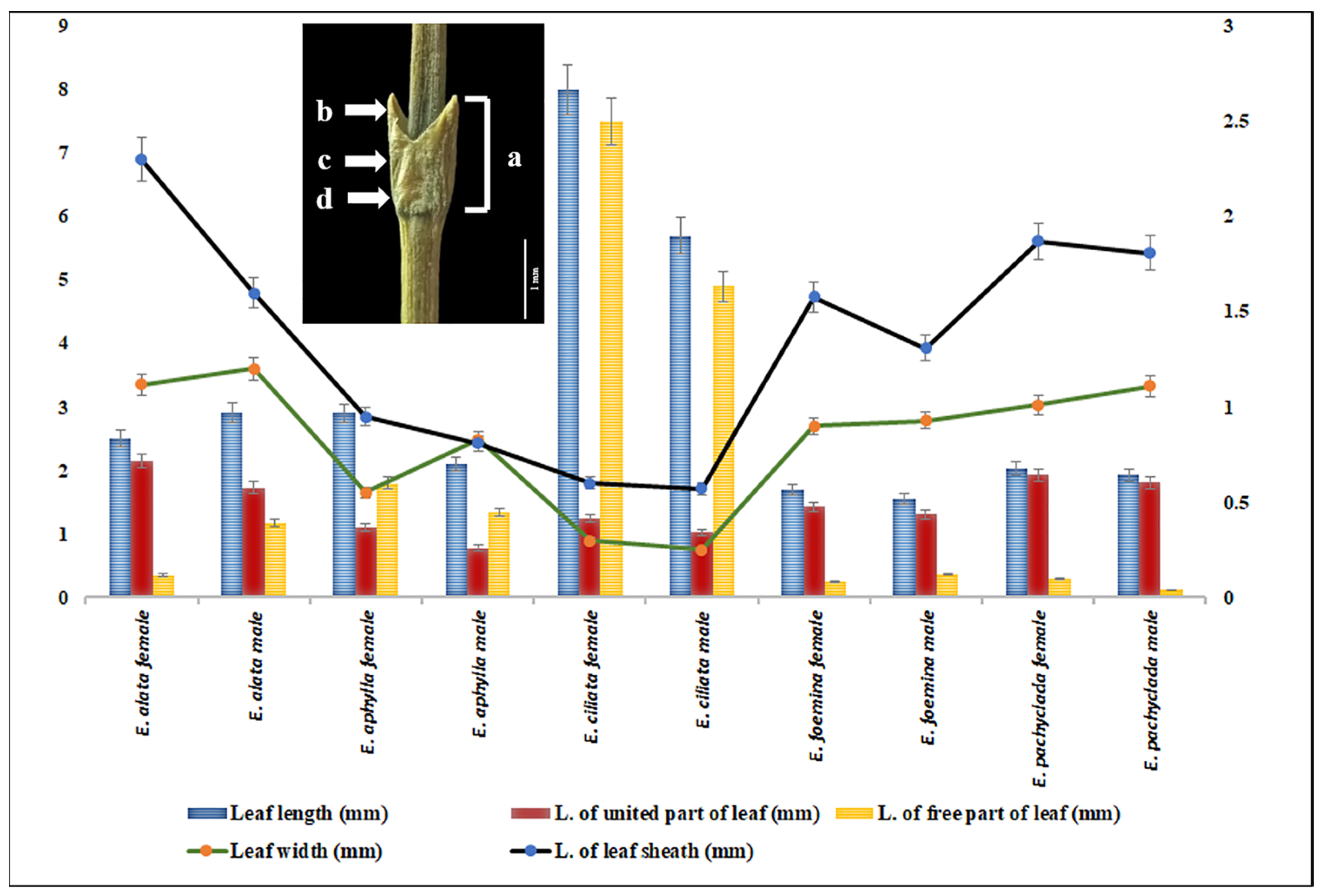
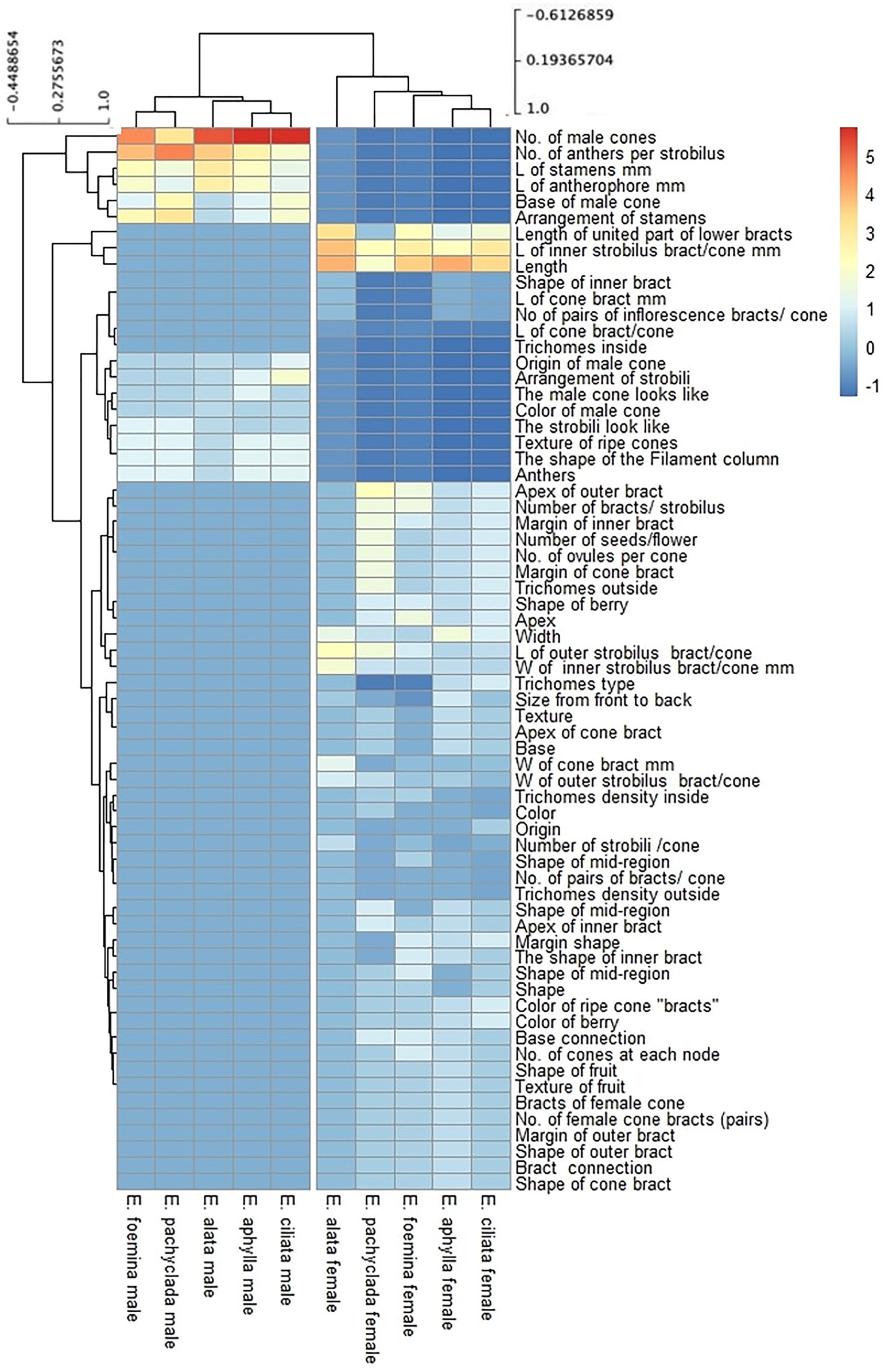
2.3. Morphometric Diversity at Interspecific and Intra-Generic Levels
2.4. Species Distribution and Habitats in Egypt
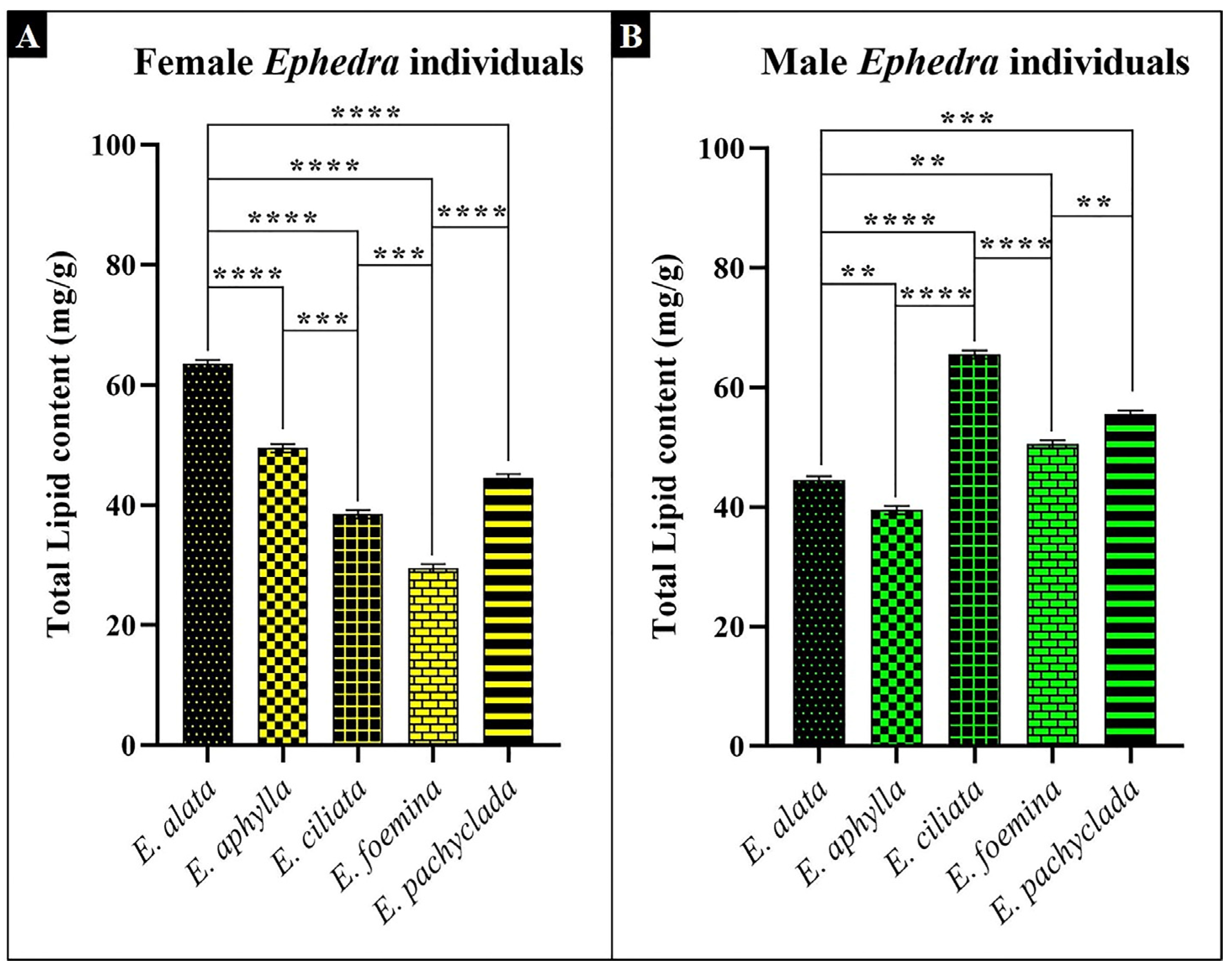
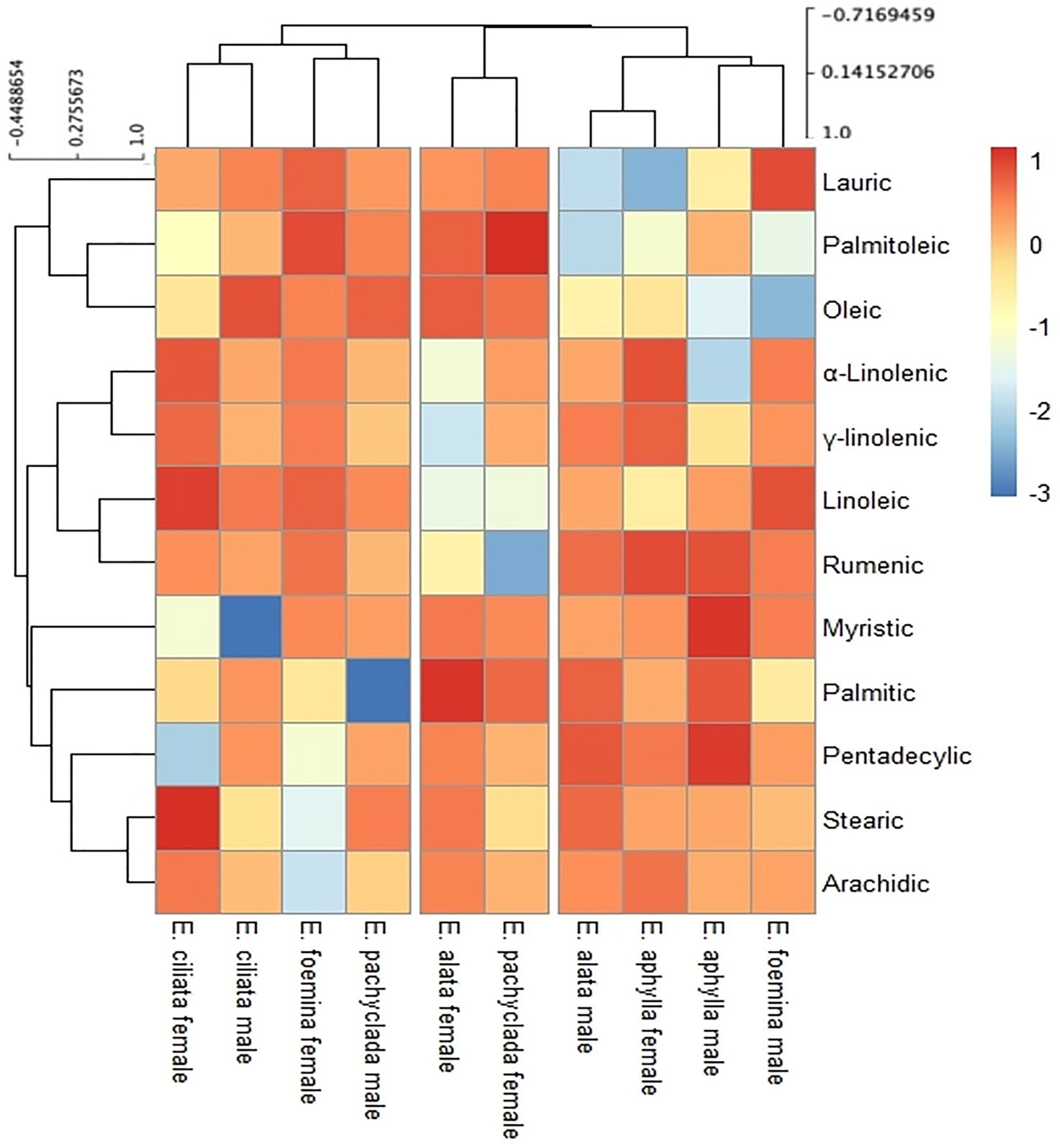
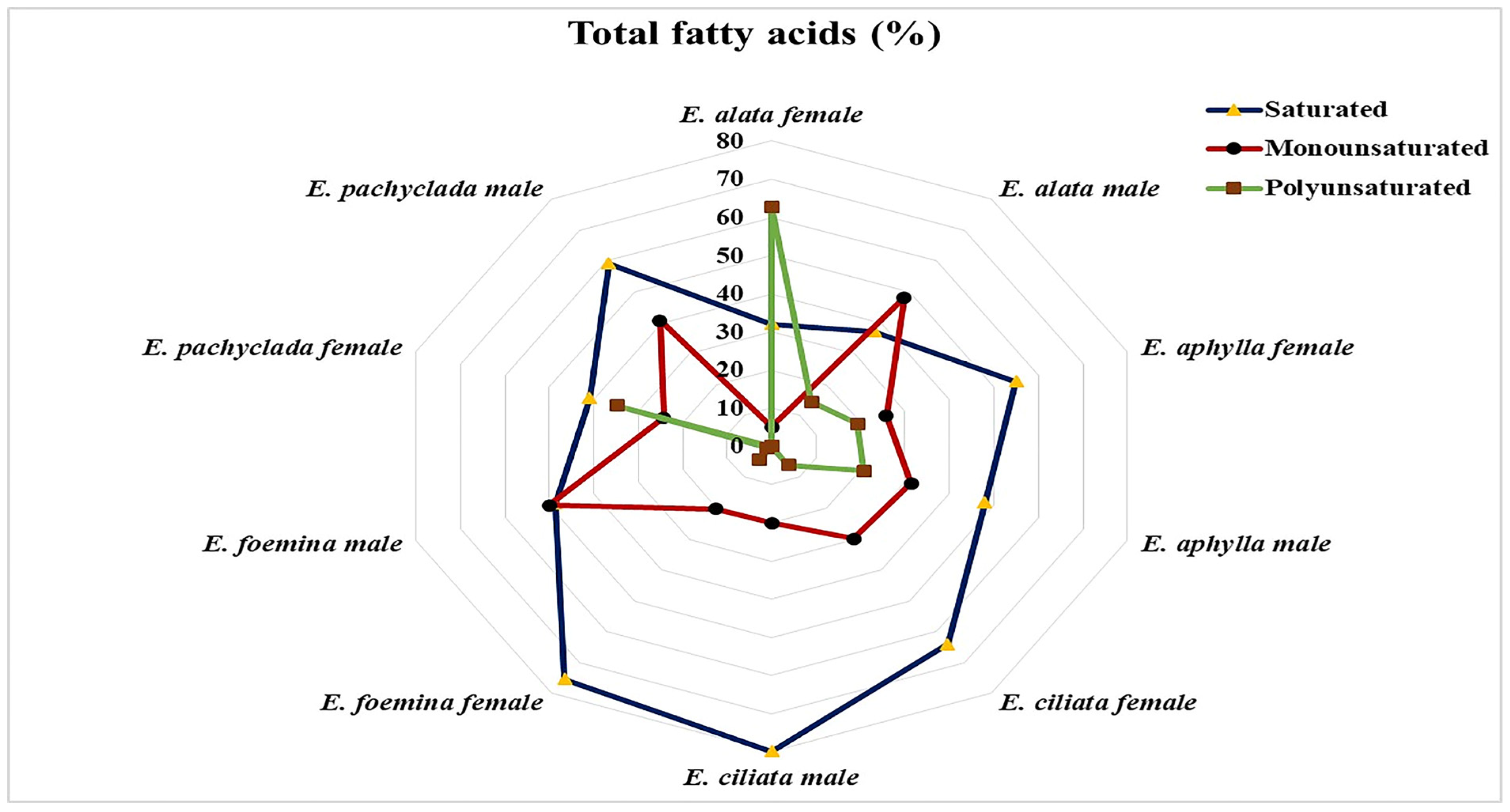
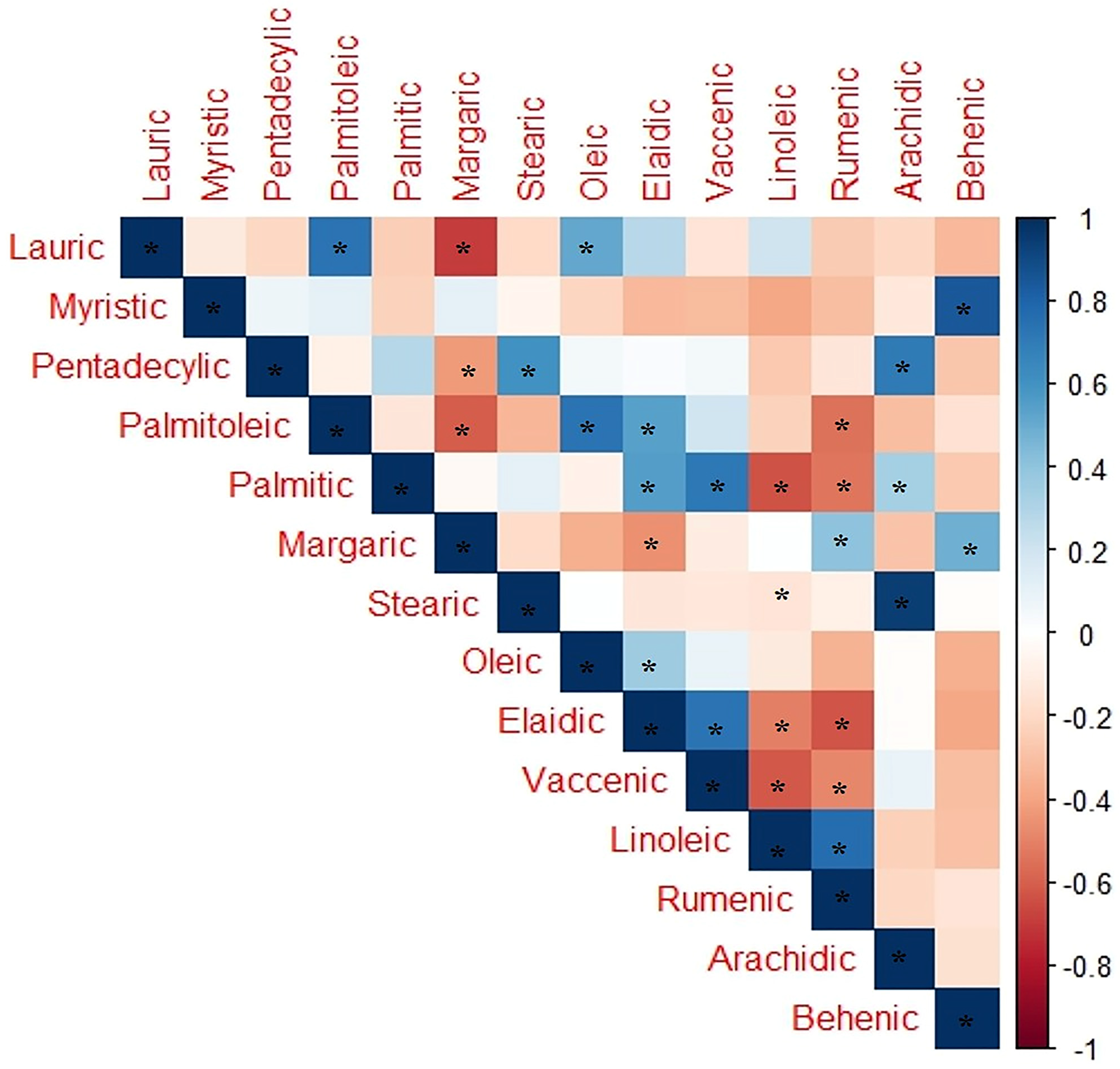
2.5. Lipid Content and Fatty Acid Composition at Interspecific and Intra-Generic Levels
2.6. Fatty Acid Composition at Interspecific and Intra-Gender Levels
3. Materials and Methods
3.1. Plant Material
3.2. Morphological Investigations
3.3. Determination of Crude Lipid
3.4. Identification of Fatty Acids Using Gas–Mass Chromatography (GC-Mass)
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mongrand, S.; Badoc, A.; Patouille, B.; Lacomblez, C.; Chavent, M.; Cassagne, C.; Bessoule, J.J. Taxonomy of Gymnospermae: Multivariate analyses of leaf fatty acid composition. Phytochemistry 2001, 58, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Boulos, L. Flora of Egypt Checklist.; Al-Hadara Publishing: Cairo, Egypt, 2009; pp. 198–201. [Google Scholar]
- Christenhusz, M.; Byng, J. The Number of Known Plants Species in the World Aand its Annual Increase. Phytotaxa 2016, 261, 21–217. [Google Scholar] [CrossRef]
- Faried, A.; EL-Banhawy, A.; Elqahtani, M. Taxonomic, DNA Barcoding and Phylogenetic Reassessment of the Egyptian Ephedra L. (Ephedraceae). Catrina 2018, 17, 1–13. [Google Scholar] [CrossRef]
- González-Juárez, D.E.; Escobedo-Moratilla, A.; Flores, J.; Hidalgo-Figueroa, S.; Martínez-Tagüeña, N.; Morales-Jiménez, J.; MuñizRamírez, A.; Pastor-Palacios, G.; Pérez-Miranda, S.; Ramírez-Hernández, A.; et al. A review of the Ephedra genus: Distribution, ecology, ethnobotany, phytochemistry and pharmacological properties. Molecules 2020, 25, 3283. [Google Scholar] [CrossRef] [PubMed]
- Ickert-Bond, S.M. Systematics of New World Ephedra L. (Ephedraceae): Integrating Morphological and Molecular Data; Arizona State University: Tempe, AZ, USA, 2003; p. 363. [Google Scholar]
- Ickert-Bond, S.M.; Rydin, C.; Renner, S.S. A fossil-calibrated relaxed clock for Ephedra indicates an Oligocene age for the divergence of Asian and New World clades and Miocene dispersal into South America. J. Syst. Evol. 2009, 47, 444–456. [Google Scholar] [CrossRef]
- El-Hadidi, M.N.; Hosni, H.A. Flora Aegyptiaca: Part 1; The Palm Press: Cairo, Egypt, 2000; Volume 1, p. 187. [Google Scholar]
- Di Stilio, V.S.; Ickert-Bond, S.M. Ephedra as a gymnosperm evo-devo model lineage. Evol. Dev. 2021, 23, 256–266. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q. The earliest fleshy cone of Ephedra from the Early Cretaceous Yixian Formation of Northeast China. PLoS ONE 2013, 8, e53652. [Google Scholar] [CrossRef]
- Yang, Y. Ontogenetic and metamorphic patterns of female reproductive organs of Ephedra sinica Stapf (Ephedraceae). Acta Bot. Sin. 2001, 43, 1011–1017. [Google Scholar]
- Miller, A.G.; Cope, T.A. Flora of the Arabian Peninsula and Socotra; Edinburgh University Press: Edinburgh, UK, 1996; Volume 1, p. 438. [Google Scholar]
- Price, R.A. Systematics of the Gnetales: A review of morphological and molecular evidence. Int. J. Plant Sci. 1996, 157, S40–S49. [Google Scholar] [CrossRef]
- Villanueva-Almanza, L.; Fonseca, R.M. Taxonomic review and geographic distribution of Ephedra (Ephedraceae) in Mexico. Acta Bot. Mex. 2011, 96, 79–116. [Google Scholar] [CrossRef]
- Tackholm, V.; Boulos, L. Students’ Flora of Egypt; Cairo University: Cairo, Egypt, 1974; p. 888. [Google Scholar]
- Boulos, L. Flora of Egypt, Vol. 1. (Azollaceae-Oxalidaceae); Al Hadara Publishing: Cairo, Egypt, 1999; pp. 35–141. [Google Scholar]
- Ickert-Bond, S.M.; Rydin, C. Micromorphology of the seed envelope of Ephedra L. (Gnetales) and its relevance for the timing of evolutionary events. Int. J. Plant Sci. 2011, 172, 36–48. [Google Scholar] [CrossRef]
- Jamieson, G.R.; Reid, E.H. The leaf lipids of some conifer species. Phytochemistry 1972, 11, 269–275. [Google Scholar] [CrossRef]
- Vickery, W.L.; Nudds, T.D. Detection of density-dependent effects in annual duck censuses. Ecology 1984, 65, 96–104. [Google Scholar] [CrossRef]
- Itabashi, Y.; Takagi, T. Cis-5-olefinic nonmethylene-interrupted fatty acids in lipids of seeds, arils and leaves of Japanese Yew. JJOCS 1982, 31, 574–579. [Google Scholar] [CrossRef]
- Hierro, M.T.G.; Robertson, G.; Christie, W.W.; Joh, Y.-G. The fatty acid composition of the seeds of Ginkgo biloba. J. Am. Oil Chem. Soc. 1996, 73, 575–579. [Google Scholar] [CrossRef]
- Nokhsorov, V.V.; Dudareva, L.V.; Semenova, N.V.; Sofronova, V.E. The Composition and the Content of ∆-5 Sterols, Fatty Acids, and the Activity of Acyl-Lipid Desaturases in the Shoots of Ephedra monosperma, Introduced in the Botanical Garden of the Cryolithozone of Yakutia. Horticulturae 2023, 9, 858. [Google Scholar] [CrossRef]
- Wannes, W.A.; Tounsi, M.S. Phytochemicals, Traditional Uses, Biological Effects, and Possible Molecular Mechanisms of Ephedra alata. Futur. Integr. Med. 2023, 2, 189–199. [Google Scholar] [CrossRef]
- Rydin, C.; Bolinder, K. Moonlight pollination in the gymnosperm Ephedra (Gnetales). Biol. Lett. 2015, 11, 10–13. [Google Scholar] [CrossRef]
- Danin, A.; Hedge, I.C. Contributions to the Flora of Sinai I: New and confused taxa. Notes Roy. Bot. Gard. 1973, 12, 5–15. [Google Scholar]
- Freitag, H.; Maier-Stolte, M. The Ephedra-species of P. Forsskål: Identity and typification. Taxon 1989, 38, 545–556. [Google Scholar] [CrossRef]
- Freitag, H.; Maier-Stolte, M. The genus Ephedra in NE tropical Africa. Kew Bull. 2003, 58, 415–426. [Google Scholar] [CrossRef]
- El-Hadidi, M.N.; Ghani, M.M.A.E.; Fahmy, A.G. The Plant Red Data Book of Egypt; Palm Press and Cairo University Herbarium: Cairo, Egypt, 1992. [Google Scholar]
- Ickert-Bond, S.M.; Wojciechowski, M.F. Phylogenetic relationships in Ephedra (Gnetales): Evidence from nuclear and chloroplast DNA sequence data. Syst. Bot. 2004, 29, 834–849. [Google Scholar] [CrossRef]
- Rydin, C.; Pedersen, K.R.; Crane, P.R.; Friis, E.M. Former diversity of Ephedra (Gnetales): Evidence from Early Cretaceous seeds from Portugal and North America. Ann. Bot. 2006, 98, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Rydin, C.; Khodabandeh, A.; Endress, P.K. The female reproductive unit of Ephedra (Gnetales): Comparative morphology and evolutionary perspectives. Bot. J. Linn. Soc. 2010, 163, 387–430. [Google Scholar] [CrossRef] [PubMed]
- Freitag, H.; Maier-Stolte, M. A new species and a new combination in the genus Ephedra from Arabia. Edinb. J. Bot. 1992, 49, 89–93. [Google Scholar] [CrossRef]
- Hufford, L. The morphology and evolution of male reproductive structures of Gnetales. Int. J. Plant Sci. 1996, 157, S95–S112. [Google Scholar] [CrossRef]
- Rydin, C.; Källersjö, M.; Friis, E.M. Seed plant relationships and the systematic position of Gnetales based on nuclear and chloroplast DNA: Conflicting data, rooting problems, and the monophyly of conifers. Int. J. Plant Sci. 2002, 163, 197–214. [Google Scholar] [CrossRef]
- Bertrand, A.; Castonguay, Y. Plant adaptations to overwintering stresses and implications of climate change. Rev. Can. Bot. 2004, 81, 1145–1152. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Hashem, A.; Abd-Allah, E.F. Lipid metabolism and oxidation in plants subjected to abiotic stresses. In Plant-Environment Interaction: Responses and Approaches to Mitigate Stress; Azooz, M.M., Ahmad, P., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 205–213. [Google Scholar] [CrossRef]
- Roughan, P.G.; Slack, C.R. Cellular organization of glycerolipid metabolism. Plant Physiol. 1982, 33, 97–132. [Google Scholar] [CrossRef]
- Khilari, V.J.; Sharma, P. studies on total lipid content of some wildedible fruits using conventional and ultrasound. J. Adv. Sci. Res. 2016, 7, 20–24. [Google Scholar]
- Krone, N.; Hughes, B.A.; Lavery, G.G.; Stewart, P.M.; Arlt, W.; Shackleton, C.H.L. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J. Steroid Biochem. Mol. Biol. 2010, 121, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Barbas, C. Gas Chromatography-Mass Spectrometry (GC-MS)-Based Metabolomics. In Metabolic Profiling; Humana Press: Totowa, NJ, USA, 2010; pp. 191–204. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 23 April 2021).
- Kassambara, A.; Mundt, F. Package “Factoextra” for R: Extract and Visualize the Results of Multivariate Data Analyses. Available online: https://rpkgs.datanovia.com/factoextra/index.html (accessed on 15 April 2020).
- Soetewey, A. Correlation Coefficient and Correlation Test in R. Available online: https://www.statsandr.com/blog/correlation-coefficient-and-correlation-test-in-r/ (accessed on 20 February 2021).
- Mäechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K.M.S.; Roudier, P.; Gonzalez, J. “Finding Groups in Data”: Cluster Analysis Extended; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]


| Species | Locality | GPS Coordinates | Gender | Date | |
|---|---|---|---|---|---|
| N | E | ||||
| E. alata | Gebal El-Shaieb, Red sea | 26°58′47″ | 33°29′11″ | Male | 11 February 2021 |
| Wadi Feiran, Sinai * | 28°43′06″ | 33°37′06″ | Female | 15 May 2024 | |
| S. Galala near Ras Zafarana | 28°44′33″ | 32°23′17″ | Male | 6 February 1960 | |
| Wadi Hamamet faraon, Sinai near red sea * | 28°53′50″ | 33°22′22″ | Female | 17 June 2023 | |
| Sinai: Abo Zeinema | 29°02′31″ | 33°06′30″ | Female and Male | 14 June 2021 | |
| Wadi Abu Khodirate, 85 Km from Zafarana | 29°06′42″ | 32°39′35″ | Female | 14 March 1997 | |
| Sinai: Wadi Fereeh | 29°07′15″ | 33°10′47″ | Female and Male | 20 May 2021 | |
| El Saff desert, Wadi Warag | 29°20′54″ | 33°14′31″ | Female | 23 March 1982 | |
| Hills of South Galala | 29°21′29″ | 32°13′04″ | Female | 4 March 2021 | |
| Wadi Araba, Eastern Desert * | 29°34′39″ | 34°59′59″ | Female | 28 April 2023 | |
| Wadi Houimela | 29°36′27″ | 30°46′35″ | Female | 4 March 1973 | |
| Wadi Hoff, Helwan desert * | 29°52′51″ | 31°18′30″ | Female and Male | 14 March 2023 | |
| Wadi At fihi, El Saff desert | 29°56′54″ | 31°22′38″ | Male | 24 March 1982 | |
| Gebl Katania, Suez road | 29°58′00″ | 32°33′02″ | Female | 20 November 1998 | |
| Wadi Katamiya, Suez road | 30°00′25″ | 31°25′23″ | Female and Male | 11 March 1960 | |
| Mitla Pass, Sinai * | 30°00′41″ | 32°53′30″ | Male | 15 August 2023 | |
| Cairo: Wadi Degla * | 30°01′32″ | 31°19′09″ | Female and Male | 29 May 2023 | |
| Wadi Gondall, Cairo, Suez Road | 30°02′39″ | 31°14′08″ | Male | 9 April 1990 | |
| Cairo, Suez, desert road | 30°05′07″ | 31°57′20″ | Male | 15 March 2020 | |
| Suez Road, kilo 20 | 30°05′09″ | 31°56′27″ | Male | 15 April 2008 | |
| Suez Road, kilo 22 | 30°05′11″ | 31°56′14″ | Female | 15 April 2008 | |
| Plateau between Qara and Marsa Matrouh | 31°16′37″ | 27°11′37″ | Male | 5 April 1998 | |
| E. aphylla | W of Marsa Halaib, in the wadies | 22°13′36″ | 36°38′44″ | Female | 22 January 1933 |
| Wadi Lethi, Saint Catherine, Sinai * | 28°33′07″ | 33°58′24″ | Male | 15 July 2023 | |
| Deir El-Rahba garden, Saint Catherin * | 28°33′37″ | 33°56′52″ | Female and Male | 5 May 2024 | |
| Sinai: Wadi El-Kid | 28°34′47″ | 34°17′16″ | Female and Male | 28 March 2004 | |
| Tabouk, Saint Catherine, Sinai at 1813 altitude * | 28°54′08″ | 33°93′12″ | Male | 16 August 2023 | |
| Wadi Al-Arbaeen Saint Catherine, Sinai at 1782 altitude * | 28°55′88″ | 33°94′98″ | Female and Male | 17 August 2023 | |
| W Qiseib, N. Galala | 29°24′36″ | 32°28′38″ | Male | 9 February 1956 | |
| Wadi Hoff, Helwan desert * | 29°52′51″ | 31°18′30″ | Female and Male | 14 March 2023 | |
| Wadi Bad of Red Sea Coast | 29°41′39″ | 32°16′58″ | Female | 9 June 2021 | |
| Cairo: Wadi Degla * | 30°01′32″ | 31°19′09″ | Female and Male | 29 May 2023 | |
| Cairo: Zohria garden | 30°02′45″ | 31°13′35″ | Female | 15 November 1928 | |
| Wadi El-Habes, before Agiba | 30°02′48″ | 31°19′57″ | Female and Male | 23 March 1974 | |
| Medicinal garden, Barrage | 30°03′56″ | 31°08′38″ | Female and Male | 21 May 1979 | |
| Gebel Elba | 30°06′00″ | 31°20′05″ | Female | 21 February 1998 | |
| Between Khanka and Abu Zaabal | 30°13′53″ | 31°21′41″ | Female | 29 February 1960 | |
| Burg El Arab: Bramly′s grotto * | 30°52′25″ | 29°33′36″ | Female and Male | 15 July 2023 | |
| Burg El Arab (Roman Cistern) | 30°54′03″ | 29°35′05″ | Male | 11 March 2022 | |
| On the coastal road 46 Km before Marsa Matrouh | 30°59′24″ | 29°39′40″ | Male | 3 May 1998 | |
| Mariout | 31°09′04″ | 29°54′06″ | Female | 25 May 2008 | |
| Ras El Hekma | 31°10′17″ | 27°49′43″ | Female | 25 May 1997 | |
| Alexandria: Vectoria | 31°14′11″ | 29°58′10″ | Female and Male | 25 August 1921 | |
| Khan Younis | 31°20′46″ | 34°18′14″ | Female | 16 September 1955 | |
| E. ciliata | Marsa Halaib | 22°14′00″ | 36°39′00″ | Female | 22 January 1929 |
| Gebl Hamata, Red Sea coast | 24°20′21″ | 35°12′06″ | Female | 7 June 2009 | |
| Wadi Marsa Kwan | 26°33′16″ | 34°02′04″ | Female | 10 February 1962 | |
| Sinai: Wadi Alletehi | 28°09′73″ | 34°04′54″ | Female and Male | 11 April 2004 | |
| Sinai: Wadi Al Ratam | 28°23′90″ | 34°23′85″ | Female and Male | 28 March 2004 | |
| Sinai: Wadi Gebal | 28°32′28″ | 33°52′53″ | Female and Male | 28 April 2004 | |
| Wadi Lethi, Saint Catherine, Sinai * | 28°33′07″ | 33°58′24″ | Male | 15 July 2023 | |
| Sinia, the garden of. St, Catherine′s Monastery * | ″28°33′25″ | 33°58′23 | Male | 25 August 2023 | |
| The Garden of Deir el Arba′ in Sinai * | ″28°47′25″ | 33°35′23 | Male | 17 July 2023 | |
| Al-Rassis Cathrine, Sinia at 1580 altitude * | 28°55′88″ | 33°94′98″ | Female and Male | 15 May 2023 | |
| Sinai: Wadi Reem | 28°66′80″ | 33°66′74″ | Female and Male | 23 April 2004 | |
| Sinai: Wadi Aleyaat | 28°66′86″ | 33°65′37″ | Female and Male | 22 April 2004 | |
| Al fred Bircher′s garden, El Saff | 29°34′34″ | 31°17′28″ | Female | 22 August 2005 | |
| Wadi Alkwamtra | 29°38′12″ | 32°35′02″ | Male | 27 February 1967 | |
| Wadi Yahameib | 29°50′19″ | 31°08′59″ | Female | 5 April 2020 | |
| Wadi Degla | 30°01′32″ | 31°19′09″ | Female and Male | 29 May 2022 | |
| Cairo: Zohria garden | 30°02′45″ | 31°13′35″ | Female | 15 November 1928 | |
| Gebel Elba | 30°06′00″ | 31°20′05″ | Female | 21 February 1998 | |
| Zaafaran Garden, Abbassia, Cairo | 30°17′59″ | 31°29′39″ | Male | 16 June 2022 | |
| Wadi El Habs, between Marsa Matruh and Agiba * | 30°39′36″ | 29°21′57″ | Male | 7 August 2023 | |
| El Mansouryeh | 31°46′48″ | 35°14′42″ | Male | 23 May 1997 | |
| E. foemina | Gabal Mousa, Sinai * | 28°54′13″ | 33°97′55″ | Female and Male | 16 July 2023 |
| Wadi Halazeen, 45 Km west of Marsa Matruh | 31°11′27″ | 27°38′01″ | Female and Male | 25 April 2022 | |
| Busaili, old Rashid Road, and 15 km before Rashid, Alexandria * | 31°11′39″ | 29°56′40″ | Female and Male | 19 March 2023 | |
| Brance, Marsa Matruh * | 31°16′37″ | 27°15′49″ | Female and Male | 28 May 2023 | |
| E. pachyclada | Sinai: Wadi Al-Talaa, Al-Kabera | 28°23′45″ | 33°52′45″ | Female and Male | 27 March 2004 |
| Sinai: Ain Al-Tofaha | 28°32′54″ | 33°56′26″ | Female and Male | 28 March 2004 | |
| Sinai: Wadi El-Kid | 28°34′47″ | 34°17′16″ | Female and Male | 14 March 2004 | |
| Abu Walia, Saint Catherine, Sinai at 1905 altitude * | 28°53′55″ | 33°91′39″ | Female | 16 August 2023 | |
| Abu Walia, Saint Catherine, Sinai at 1891 altitude * | 28°53′60″ | 33°90′92″ | Male | 16 August 2023 | |
| Gabal Mousa, Sinai at 2275 altitude * | 28°53′83″ | 33°97′50″ | Female and Male | 27 August 2023 | |
| Gabal Mousa, Sinai at 2260 altitude * | 28°53′92″ | 33°97′53″ | Female and Male | 27 May 2024 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalaf, M.H.; Amer, W.M.; Shaye, N.A.A.; Hassan, M.O.; Gomaa, N.H. Taxonomic Revision of Genus Ephedra Tourn. ex L. in Egypt with Intra-Gender Diversity in Morphometric Traits and Fatty Acid Composition. Plants 2024, 13, 2442. https://doi.org/10.3390/plants13172442
Khalaf MH, Amer WM, Shaye NAA, Hassan MO, Gomaa NH. Taxonomic Revision of Genus Ephedra Tourn. ex L. in Egypt with Intra-Gender Diversity in Morphometric Traits and Fatty Acid Composition. Plants. 2024; 13(17):2442. https://doi.org/10.3390/plants13172442
Chicago/Turabian StyleKhalaf, Maha H., Wafaa M. Amer, Najla A. Al Shaye, Mahmoud O. Hassan, and Nasr H. Gomaa. 2024. "Taxonomic Revision of Genus Ephedra Tourn. ex L. in Egypt with Intra-Gender Diversity in Morphometric Traits and Fatty Acid Composition" Plants 13, no. 17: 2442. https://doi.org/10.3390/plants13172442






