Changes in Soil Aggregate Carbon Components and Responses to Plant Input during Vegetation Restoration in the Loess Plateau, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Sample Site Setting
2.3. Screening of Soil Aggregates
2.4. Data Analysis
3. Results and Analysis
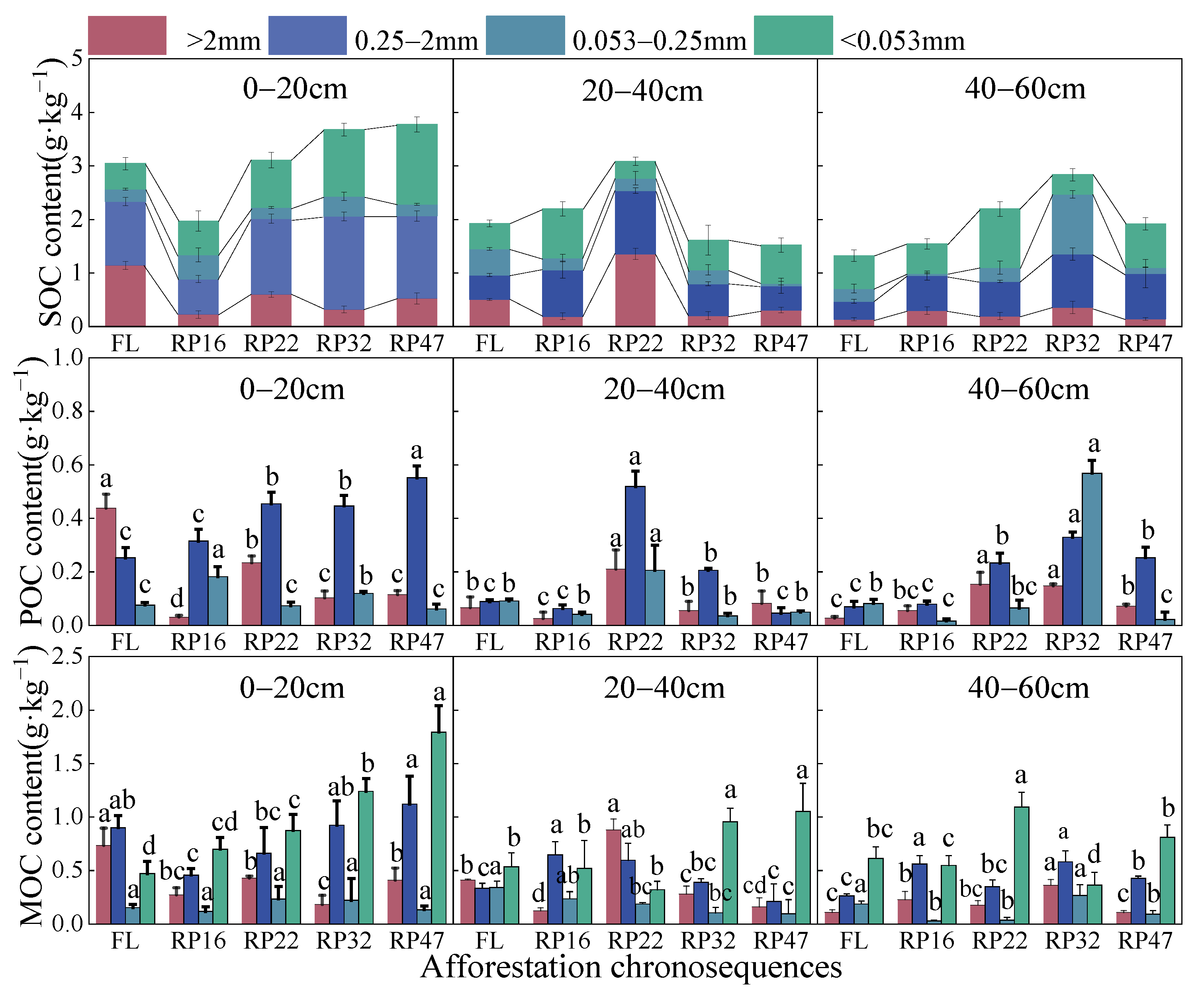
3.1. Change Characteristics of Soil Organic Carbon Components in Different Years of Tillage
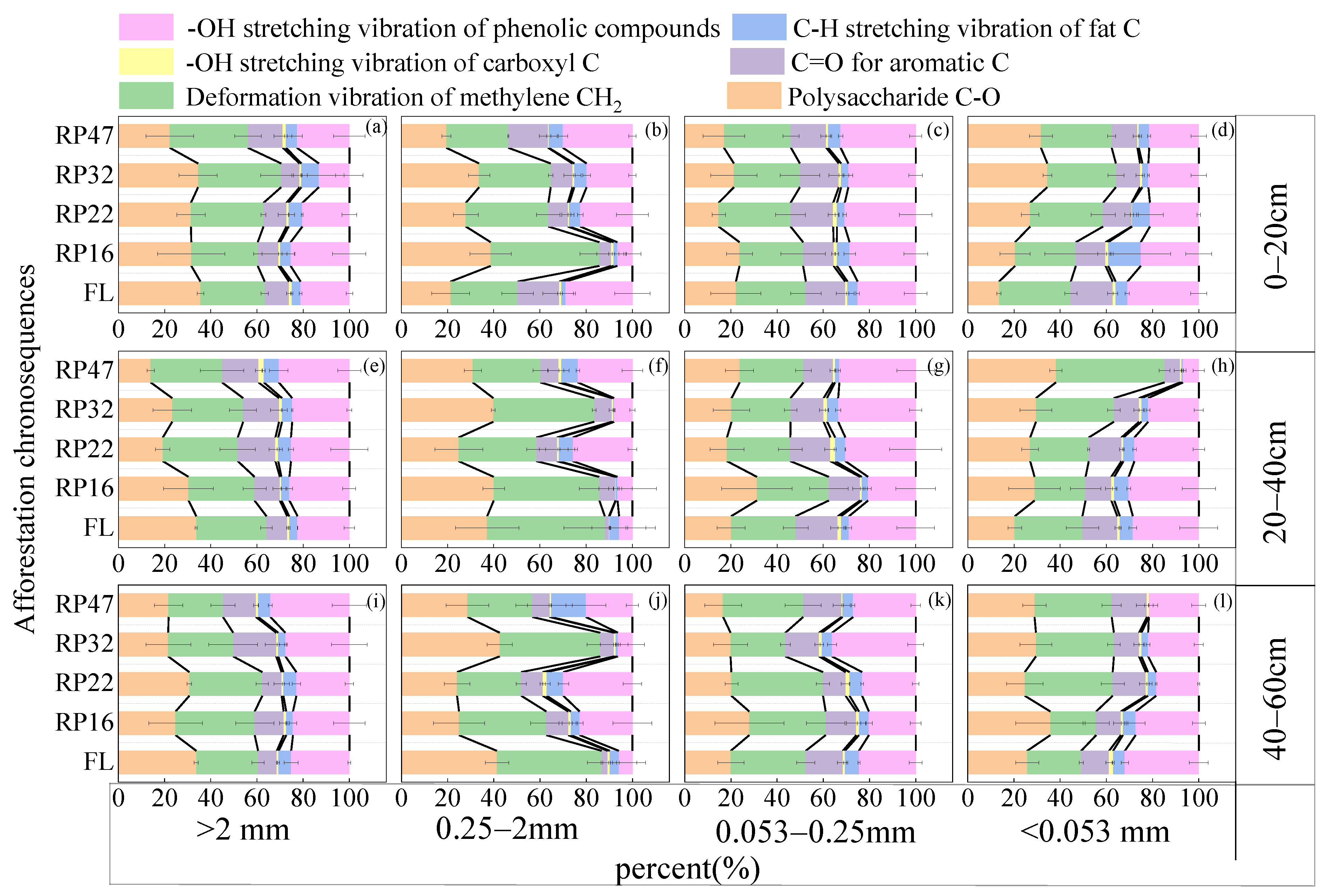
3.2. Modifications in the Properties of the Functional Groups of Organic Carbon in Soil Aggregates with Varying Tillage Years
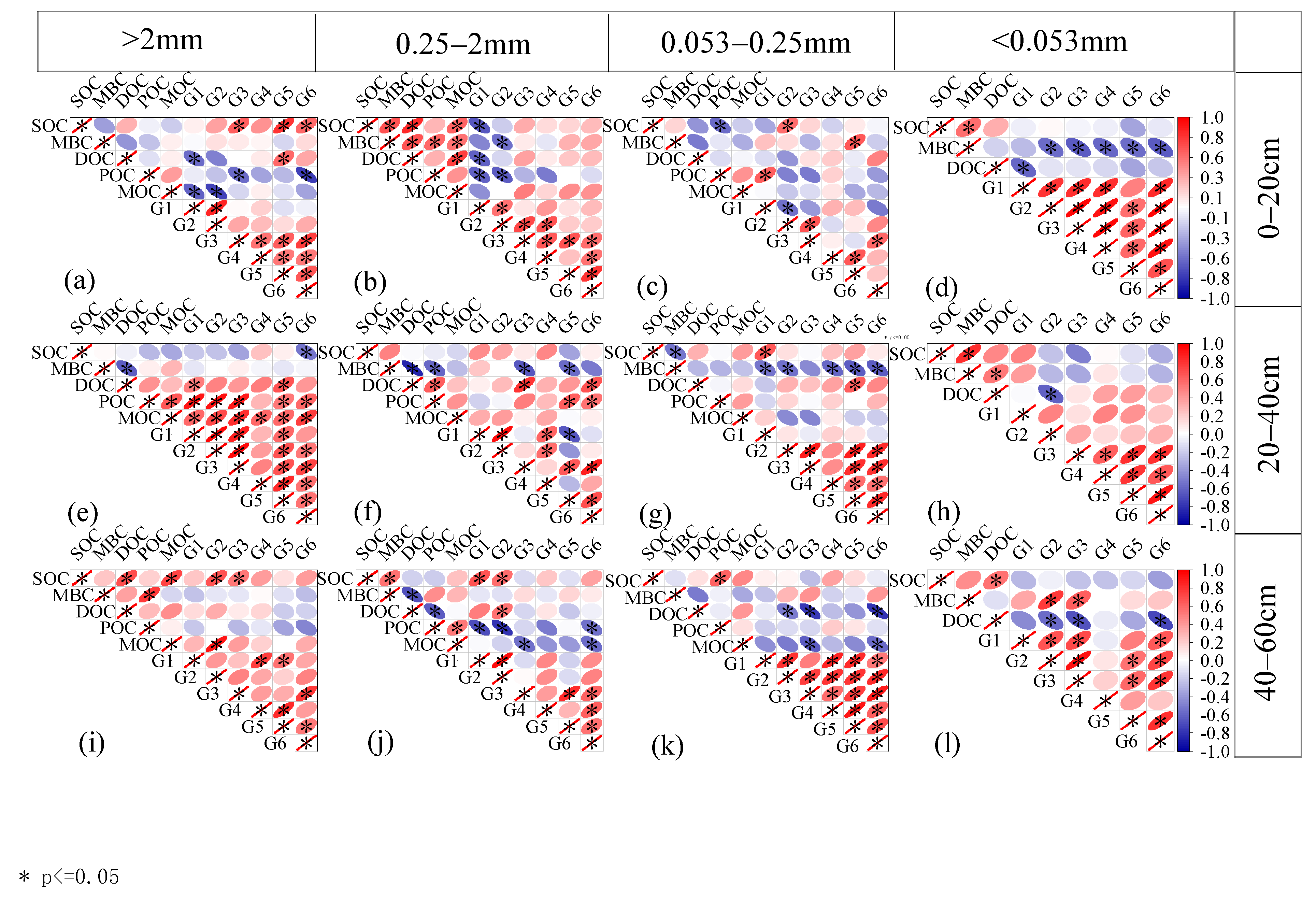
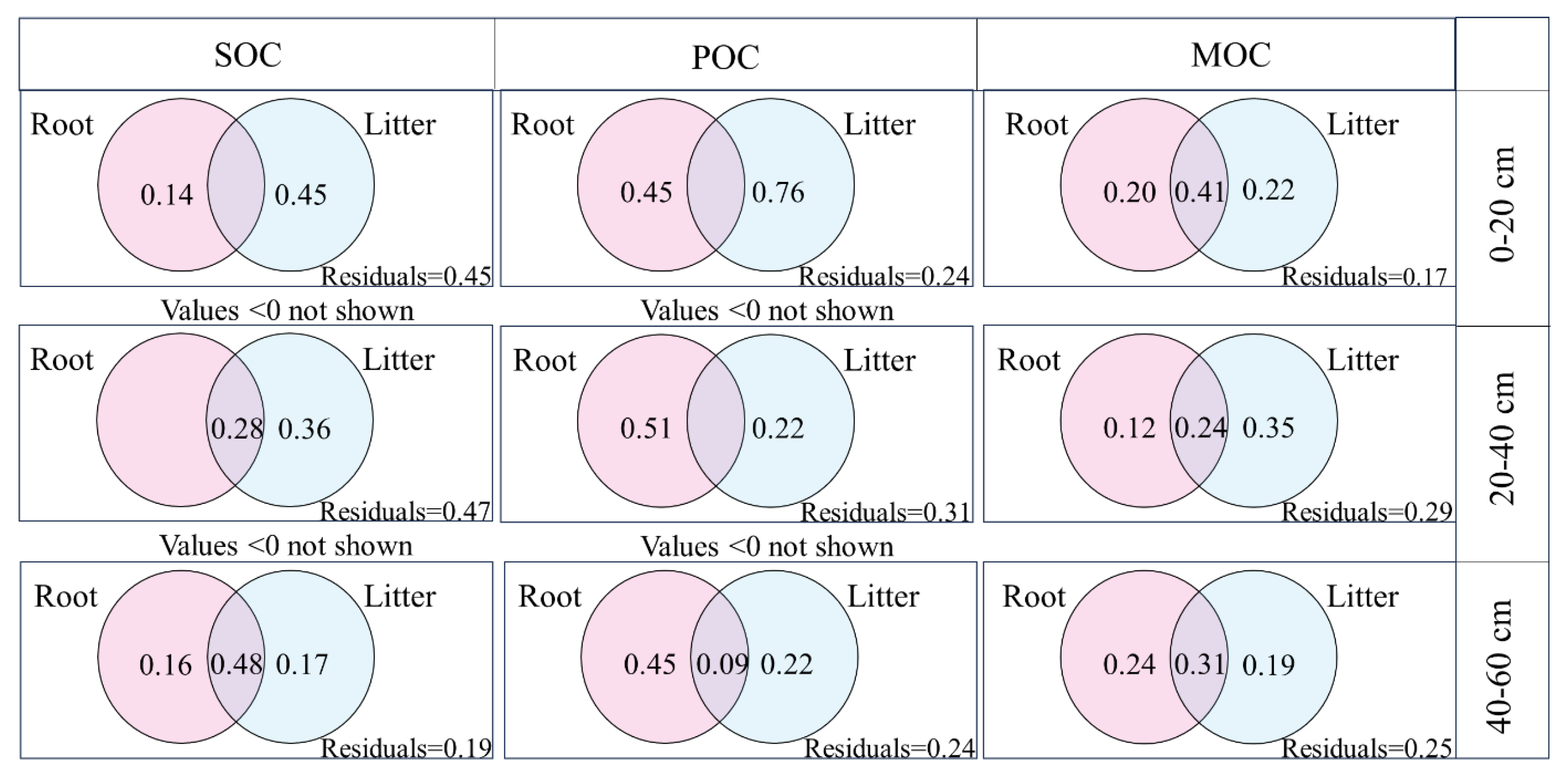
3.3. Response of Organic Carbon Components to Plant Input in the Aggregates of the Reverted Robinia pseudoacacia Forest
4. Discussion
4.1. Changing Characteristics of Soil Aggregate Components with Different Years of Tillage
4.2. Variations in the Properties of the Functional Groups of Organic Carbon in Soil Aggregates with Varying Tillage Years
4.3. Response of Organic Carbon Components to Plant Input in Aggregates of Reverted Robinia pseudoacacia Forest
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jozedaemi, E.; Golchin, A. Changes in aggregate-associated carbon and microbial respiration affected by aggregate size, soil depth, and altitude in a forest soil. Catena 2024, 234, 107567. [Google Scholar] [CrossRef]
- Yu, P.; Liu, J.; Tang, H.; Ci, E.; Tang, X.; Liu, S.; Ding, Z.; Ma, M. The increased soil aggregate stability and aggregate-associated carbon by farmland use change in a karst region of Southwest China. Catena 2023, 231, 107284. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Liu, Y.; Liu, H.; Zhang, Z.; Gao, Q.; Wang, Y.; Feng, Y.; Yang, G.; Ren, C.; et al. The contributions of soil biochemical characteristics and soil organic carbon (soil organic carbon) structure to soil organic carbon mineralization rate during forest succession in the Loess Plateau, China. Land Degrad. Dev. 2022, 33, 3375–3386. [Google Scholar] [CrossRef]
- Tivet, F.; De Moraes Sá, J.C.; Lal, R.; Milori DM, B.P.; Briedis, C.; Letourmy, P.; Pinheiro, L.A.; Borszowskei, P.R.; Da Cruz Hartman, D. Assessing humification and organic C compounds by laser-induced fluorescence and FTIR spectroscopies under conventional and no-till management in Brazilian Oxisols. Geoderma 2013, 207–208, 71–81. [Google Scholar] [CrossRef]
- Chang, R.; Fu, B.; Liu, G.; Liu, S. Soil Carbon Sequestration Potential for “Grain for Green” Project in Loess Plateau, China. Environ. Manag. 2011, 48, 1158–1172. [Google Scholar] [CrossRef]
- Even, R.J.; Francesca Cotrufo, M. The ability of soils to aggregate, more than the state of aggregation, promotes protected soil organic matter formation. Geoderma 2024, 442, 116760. [Google Scholar]
- Brown, R.W.; Jones, D.L. Plasticity of microbial substrate carbon use efficiency in response to changes in plant carbon input and soil organic matter status. Soil Biol. Biochem. 2024, 188, 109230. [Google Scholar]
- Sun, T.; Mao, X.; Han, K.; Wang, X.; Cheng, Q.; Liu, X.; Zhou, J.; Ma, Q.; Ni, Z.; Wu, L. Nitrogen addition increased soil particulate organic carbon via plant carbon input whereas reduced mineral−associated organic carbon through attenuating mineral protection in agroecosystem. Sci. Total Environ. 2023, 899, 165705. [Google Scholar] [PubMed]
- Dorji, T.; Field, D.J.; Odeh IO, A.; Bhogal, A. Soil aggregate stability and aggregate-associated organic carbon under different land use or land cover types. Soil Use Manag. 2019, 36, 308–319. [Google Scholar] [CrossRef]
- Kang, L.; Wu, J.; Zhang, C.; Zhu, B.; Chu, G. Alterations of soil aggregates and intra-aggregate organic carbon fractions after soil conversion from paddy soils to upland soils: Distribution, mineralization and driving mechanism. Pedosphere 2024, 34, 121–135. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2017, 181, 104–136. [Google Scholar] [CrossRef]
- Jiménez-González, M.A.; Álvarez, A.M.; Carral, P.; Almendros, G. Chemometric assessment of soil organic matter storage and quality from humic acid infrared spectra. Sci. Total Environ. 2019, 685, 1160–1168. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.; Liu, J.; Wang, Z.; Zhang, Z.; Li, X.; Zhang, Q.; Liu, W.; Liu, H.; Zeng, J.; et al. Linking the soil carbon pool management index to ecoenzymatic stoichiometry and organic carbon functional groups in abandoned land under climate change. Catena 2024, 235, 107676. [Google Scholar] [CrossRef]
- Goydaragh, M.G.; Taghizadeh-Mehrjardi, R.; Jafarzadeh, A.A.; Triantafilis, J.; Lado, M. Using environmental variables and Fourier Transform Infrared Spectroscopy to predict soil organic carbon. Catena 2021, 202, 105280. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Fan, B.; Mao, X.; Luo, H.; Jiang, F.; Liang, C.; Chen, J.; Qin, H.; Xu, Q.; et al. Litter Inputs Control the Pattern of Soil Aggregate-Associated Organic Carbon and Enzyme Activities in Three Typical Subtropical Forests. Forests 2022, 13, 1210. [Google Scholar] [CrossRef]
- Li, S.; Gu, X.; Zhuang, J.; An, T.; Pei, J.; Xie, H.; Li, H.; Fu, S.; Wang, J. Distribution and storage of crop residue carbon in aggregates and its contribution to organic carbon of soil with low fertility. Soil Tillage Res. 2016, 155, 199–206. [Google Scholar] [CrossRef]
- Buchanan, S.-W.; Sauvadet, M.; Isaac, M.E. Decomposition of litter mixtures induces non-additive effects on soil priming across a riparian land use gradient. Soil Biol. Biochem. 2024, 190, 109285. [Google Scholar] [CrossRef]
- Wu, J.; Yang, G.; Han, X.Y.; Wen, Y.Q.; Yang, Y.W.; Li, W.L.; Liu, Y. Effects of different plantations on soil aggregates in the Nayman sand region. Arid Zone Res. 2022, 39, 1832–1841. [Google Scholar]
- Wang, J.; Wang, H.; Ding, Y.; Zhang, Y.; Cong, W.; Zang, R.; Liu, S. Shifting cultivation and logging change soil organic carbon functional groups in tropical lowland rainforests on Hainan Island in China. For. Ecol. Manag. 2023, 549, 121447. [Google Scholar] [CrossRef]
- Cheng, M.; Xiang, Y.; Xue, Z.; An, S.; Darboux, F. Soil aggregation and intra-aggregate carbon fractions in relation to vegetation succession in the Loess Plateau, China. Catena 2015, 124, 77–84. [Google Scholar] [CrossRef]
- Wei, X.; Shao, M.; Gale, W.J.; Zhang, X.; Li, L. Dynamics of aggregate-associated organic carbon following conversion of forest to cropland. Soil Biol. Biochem. 2013, 57, 876–883. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, J.; Zhao, F. Soil stoichiometry influence C, N, and P distribution in soil aggregates after afforestation. Sci. For. 2021, 49, e3355. [Google Scholar] [CrossRef]
- Bicharanloo, B.; Bagheri Shirvan, M.; Cavagnaro, T.R.; Keitel, C.; Dijkstra, F.A. Nitrogen addition and defoliation alter belowground carbon allocation with consequences for plant nitrogen uptake and soil organic carbon decomposition. Sci. Total Environ. 2022, 846, 157430. [Google Scholar] [CrossRef]
- Jagadamma, S.; Mayes, M.A.; Zinn, Y.L.; Gísladóttir, G.; Russell, A.E. Sorption of organic carbon compounds to the fine fraction of surface and subsurface soils. Geoderma 2014, 213, 79–86. [Google Scholar] [CrossRef]
- Michel, K.; Matzner, E.; Dignac, M.-F.; Kögel-Knabner, I. Properties of dissolved organic matter related to soil organic matter quality and nitrogen additions in Norway spruce forest floors. Geoderma 2006, 130, 250–264. [Google Scholar] [CrossRef]
- Thevenot, M.; Dignac, M.-F.; Rumpel, C. Fate of lignins in soils: A review. Soil Biol. Biochem. 2010, 42, 1200–1211. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Gurmessa, B.; Ashworth, A.J.; Yang, Y.; Adhikari, K.; Savin, M.; Owens, P.; Sauer, T.; Pedretti, E.F.; Cocco, S.; Corti, G. Soil bacterial diversity based on management and topography in a silvopastoral system. Appl. Soil Ecol. 2021, 163, 103918. [Google Scholar] [CrossRef]
- Gao, F.; Cui, X.; Chen, M.; Sang, Y. Forest Conversion Changes Soil Particulate Organic Carbon and Mineral-Associated Organic Carbon via Plant Inputs and Microbial Processes. Forests 2023, 14, 1234. [Google Scholar] [CrossRef]
- Pausch, J.; Kuzyakov, Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Change Biol. 2017, 24, 1–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Fang, J.; Jia, W.; Wang, S.; Liu, H.; Liu, W.; Zhang, Q.; Yang, G.; Han, X.; Ren, G. Changes in Soil Aggregate Carbon Components and Responses to Plant Input during Vegetation Restoration in the Loess Plateau, China. Plants 2024, 13, 2455. https://doi.org/10.3390/plants13172455
Liang Y, Fang J, Jia W, Wang S, Liu H, Liu W, Zhang Q, Yang G, Han X, Ren G. Changes in Soil Aggregate Carbon Components and Responses to Plant Input during Vegetation Restoration in the Loess Plateau, China. Plants. 2024; 13(17):2455. https://doi.org/10.3390/plants13172455
Chicago/Turabian StyleLiang, Yaoyue, Jingbo Fang, Wenjing Jia, Shijie Wang, Hanyu Liu, Weichao Liu, Qi Zhang, Gaihe Yang, Xinhui Han, and Guangxin Ren. 2024. "Changes in Soil Aggregate Carbon Components and Responses to Plant Input during Vegetation Restoration in the Loess Plateau, China" Plants 13, no. 17: 2455. https://doi.org/10.3390/plants13172455
APA StyleLiang, Y., Fang, J., Jia, W., Wang, S., Liu, H., Liu, W., Zhang, Q., Yang, G., Han, X., & Ren, G. (2024). Changes in Soil Aggregate Carbon Components and Responses to Plant Input during Vegetation Restoration in the Loess Plateau, China. Plants, 13(17), 2455. https://doi.org/10.3390/plants13172455






