Bacterial Volatile Organic Compounds as a Strategy to Increase Drought Tolerance in Maize (Zea mays L.): Influence on Plant Biochemistry
Abstract
1. Introduction
2. Results
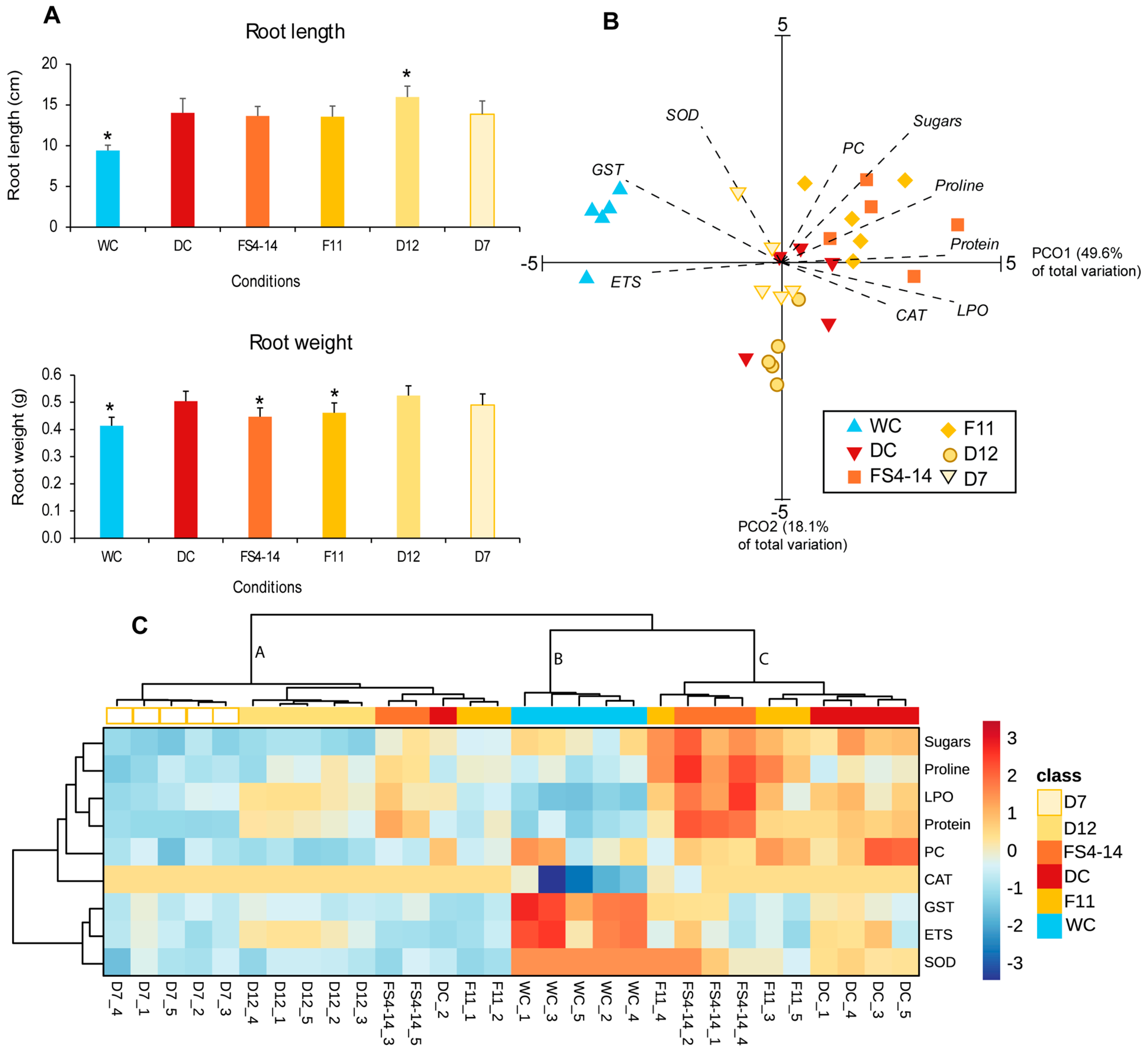
2.1. Growth and Biochemical Alterations in Roots
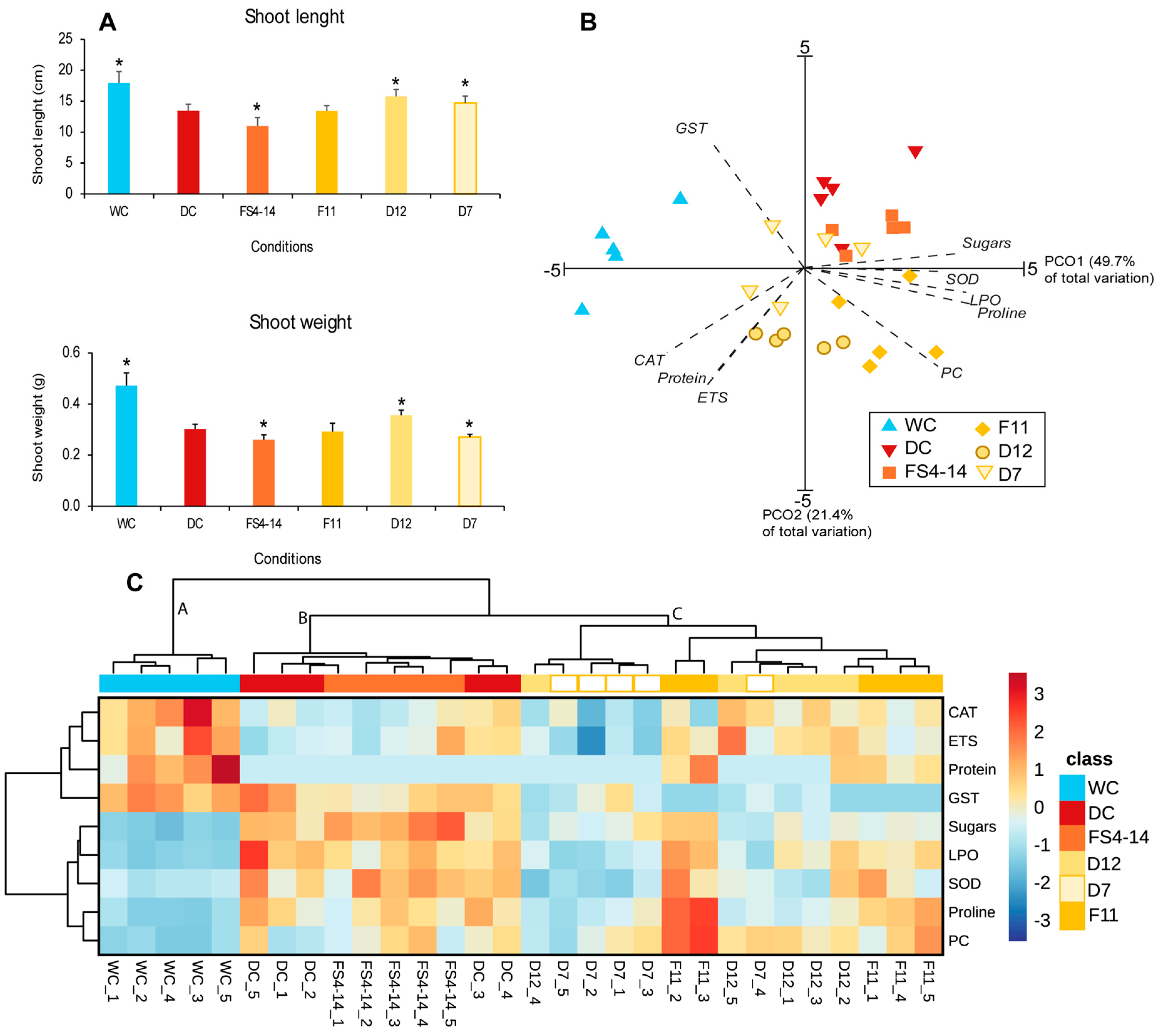
2.2. Growth and Biochemical Alterations in Shoots
3. Discussion
4. Material and Methods
4.1. Bacterial Strains
4.2. Plant Experiment
4.3. Biochemical Analysis
4.3.1. Extraction
4.3.2. Biochemical Assays
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, M.; Li, Y.; Biswas, A.; Chen, X.; Xie, L.; Liu, D.; Li, L.; Feng, H.; Wu, S.; Satoh, Y.; et al. Concurrent Drought Threatens Wheat and Maize Production and Will Widen Crop Yield Gaps in the Future. Agric. Syst. 2024, 220, 104056. [Google Scholar] [CrossRef]
- Nair, S.S.; Gupta, A.K.; Nathawat, M.S. Drought Disaster: Issues, Challenges and Risk Mitigation Strategies. In Disaster Risk and Management Under Climate Change; Gupta, A.K., Gupta, A., Acharya, P., Eds.; Springer Nature: Singapore, 2024; pp. 93–119. ISBN 978-981-9941-05-6. [Google Scholar]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Misra, S.; Ghosh, A. Chapter 6—Agriculture Paradigm Shift: A Journey from Traditional to Modern Agriculture. In Biodiversity and Bioeconomy; Singh, K., Ribeiro, M.C., Calicioglu, Ö., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 113–141. ISBN 978-0-323-95482-2. [Google Scholar]
- Kumar, P.; Raj, A.; Kumar, V.A. Approach to Reduce Agricultural Waste via Sustainable Agricultural Practices. In Valorization of Biomass Wastes for Environmental Sustainability: Green Practices for the Rural Circular Economy; Srivastav, A.L., Bhardwaj, A.K., Kumar, M., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 21–50. ISBN 978-3-031-52485-1. [Google Scholar]
- Schulz, S.; Dickschat, J.S. Bacterial Volatiles: The Smell of Small Organisms. Nat. Prod. Rep. 2007, 24, 814–842. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial Volatiles Promote Growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Haustein, M.; Molina, F.; Petri, A.; Scholz, B.; Piechulla, B. Bacterial Volatiles and Their Action Potential. Appl. Microbiol. Biotechnol. 2009, 81, 1001–1012. [Google Scholar] [CrossRef]
- Liu, X.-M.; Zhang, H. The Effects of Bacterial Volatile Emissions on Plant Abiotic Stress Tolerance. Front. Plant Sci. 2015, 6, 774. [Google Scholar] [CrossRef]
- Bitas, V.; Kim, H.-S.; Bennett, J.W.; Kang, S. Sniffing on Microbes: Diverse Roles of Microbial Volatile Organic Compounds in Plant Health. Mol. Plant-Microbe Interact. 2013, 26, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Zhang, H.; Ryu, C.-M. Dynamic Chemical Communication between Plants and Bacteria through Airborne Signals: Induced Resistance by Bacterial Volatiles. J. Chem. Ecol. 2013, 39, 1007–1018. [Google Scholar] [CrossRef]
- Kanchiswamy, C.N.; Malnoy, M.; Maffei, M.E. Chemical Diversity of Microbial Volatiles and Their Potential for Plant Growth and Productivity. Front. Plant Sci. 2015, 6, 151. [Google Scholar] [CrossRef]
- Chaves, M.; Flexas, J.; Pinheiro, C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2008, 103, 551–560. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic Carbon Assimilation and Associated Metabolism in Relation to Water Deficits in Higher Plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.; Oliveira, M. Mechanisms Underlying Plant Resilience to Water Deficit: Prospects for Water-Saving Agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.-Y.; Lee, Y.-H.; Cho, B.H.; Yang, K.-Y.; Ryu, C.-M.; Kim, Y.C. 2R,3R-Butanediol, a Bacterial Volatile Produced by Pseudomonas chlororaphis O6, Is Involved in Induction of Systemic Tolerance to Drought in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Murzello, C.; Sun, Y.; Kim, M.-S.; Xie, X.; Jeter, R.M.; Zak, J.C.; Dowd, S.E.; Paré, P.W. Choline and Osmotic-Stress Tolerance Induced in Arabidopsis by the Soil Microbe Bacillus subtilis (GB03). Mol. Plant-Microbe Interact. 2010, 23, 1097. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, L.d.R.; Chiappero, J.; Palermo, T.B.; Giordano, W.; Banchio, E. Volatile Organic Compounds from Rhizobacteria Increase the Biosynthesis of Secondary Metabolites and Improve the Antioxidant Status in Mentha piperita L. Grown under Salt Stress. Agronomy 2020, 10, 1094. [Google Scholar] [CrossRef]
- Mi, N.; Cai, F.; Zhang, Y.; Ji, R.; Zhang, S.; Wang, Y. Differential Responses of Maize Yield to Drought at Vegetative and Reproductive Stages. Plant Soil Environ. 2018, 64, 260–267. [Google Scholar] [CrossRef]
- Saseendran, S.A.; Ahuja, L.R.; Ma, L.; Nielsen, D.C.; Trout, T.J.; Andales, A.A.; Chávez, J.L.; Ham, J. Enhancing the Water Stress Factors for Simulation of Corn in RZWQM2. Agron. J. 2014, 106, 81–94. [Google Scholar] [CrossRef]
- Aslam, M.; Maqbool, M.A.; Cengiz, R. Effects of Drought on Maize; Springer: Berlin/Heidelberg, Germany, 2015; pp. 5–17. ISBN 978-3-319-25440-1. [Google Scholar]
- Bailly, A.; Weisskopf, L. The Modulating Effect of Bacterial Volatiles on Plant Growth. Plant Signal. Behav. 2012, 7, 79–85. [Google Scholar] [CrossRef]
- Zou, C.; Li, Z.; Yu, D. Bacillus megaterium Strain XTBG34 Promotes Plant Growth by Producing 2-Pentylfuran. J. Microbiol. 2010, 48, 460–466. [Google Scholar] [CrossRef]
- Han, S.H.; Lee, S.J.; Moon, J.H.; Park, K.H.; Yang, K.Y.; Cho, B.H.; Kim, K.Y.; Kim, Y.W.; Lee, M.C.; Anderson, A.J.; et al. GacS-Dependent Production of 2R, 3R-Butanediol by Pseudomonas chlororaphis O6 Is a Major Determinant for Eliciting Systemic Resistance Against Erwinia carotovora but Not Against Pseudomonas syringae pv. tabaci in Tobacco. Mol. Plant-Microbe Interact. 2006, 19, 924–930. [Google Scholar] [CrossRef]
- Wenke, K.; Kai, M.; Piechulla, B. Belowground Volatiles Facilitate Interactions between Plant Roots and Soil Organisms. Planta 2010, 231, 499–506. [Google Scholar] [CrossRef]
- Ping, L.; Boland, W. Signals from the Underground: Bacterial Volatiles Promote Growth in Arabidopsis. Trends Plant Sci. 2004, 9, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Tan, H.; Liao, Y.; Jian, G.; Kang, M.; Dong, F.; Watanabe, N.; Yang, Z. Increasing Temperature Changes Flux into Multiple Biosynthetic Pathways for 2-Phenylethanol in Model Systems of Tea (Camellia sinensis) and Other Plants. J. Agric. Food Chem. 2019, 67, 10145–10154. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Volatile 1-Octen-3-Ol Induces a Defensive Response in Arabidopsis thaliana. J. Gen. Plant Pathol. 2007, 73, 35–37. [Google Scholar] [CrossRef]
- Fincheira, P.; Quiroz, A. Microbial Volatiles as Plant Growth Inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Blom, D.; Fabbri, C.; Eberl, L.; Weisskopf, L. Volatile-Mediated Killing of Arabidopsis thaliana by Bacteria Is Mainly Due to Hydrogen Cyanide. Appl. Environ. Microbiol. 2011, 77, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, H.; Tadayon, M.R.; Nadeem, M.; Cheema, M.; Razmjoo, J. Proline-Mediated Changes in Antioxidant Enzymatic Activities and the Physiology of Sugar Beet under Drought Stress. Acta Physiol. Plant. 2019, 41, 23. [Google Scholar] [CrossRef]
- El Sabagh, A.; Hossain, A.; Barutçular, C.; Gormus, O.; Ahmad, Z.; Hussain, S.; Islam, M.S.; Alharby, S.; Bamagoos, A.; Kumar, N.; et al. Effects of Drought Stress on the Quality of Major Oilseed Crops: Implications and Possible Mitigation Strategies—A Review. Appl. Ecol. Environ. Res. 2019, 17, 4019–4043. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Regulation in Photosynthetic Organisms: Signaling, Acclimation, and Practical Implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Cruz, C.; Cardoso, P.; Santos, J.; Matos, D.; Figueira, E. Bioprospecting Soil Bacteria from Arid Zones to Increase Plant Tolerance to Drought: Growth and Biochemical Status of Maize Inoculated with Plant Growth-Promoting Bacteria Isolated from Sal Island, Cape Verde. Plants 2022, 11, 2912. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Sensing and Signalling Associated with Reactive Oxygen in Chloroplasts, Peroxisomes and Mitochondria. Physiol. Plant. 2003, 119, 355–364. [Google Scholar] [CrossRef]
- Guo, W.; Xing, Y.; Luo, X.; Li, F.; Ren, M.; Liang, Y. Reactive Oxygen Species: A Crosslink between Plant and Human Eukaryotic Cell Systems. Int. J. Mol. Sci. 2023, 24, 13052. [Google Scholar] [CrossRef]
- Palma, J.M.; Sandalio, L.M.; Javier Corpas, F.; Romero-Puertas, M.C.; McCarthy, I.; del Río, L.A. Plant Proteases, Protein Degradation, and Oxidative Stress: Role of Peroxisomes. Plant Physiol. Biochem. 2002, 40, 521–530. [Google Scholar] [CrossRef]
- Lopes, T.; Cruz, C.; Cardoso, P.; Pinto, R.; Marques, P.A.A.P.; Figueira, E. A Multifactorial Approach to Untangle Graphene Oxide (GO) Nanosheets Effects on Plants: Plant Growth-Promoting Bacteria Inoculation, Bacterial Survival, and Drought. Nanomaterials 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Fujita, M. Drought Stress Responses in Plants, Oxidative Stress, and Antioxidant Defense. In Climate Change and Plant Abiotic Stress Tolerance; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 209–250. ISBN 978-3-527-67526-5. [Google Scholar]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-Induced Responses of Photosynthesis and Antioxidant Metabolism in Higher Plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.-P.; Sui, F.-G.; Ge, T.-D.; Sun, Z.-H.; Lu, Y.-Y.; Zhou, G.-S. Effect of Soil Drought Stress on Leaf Water Status, Membrane Permeability and Enzymatic Antioxidant System of Maize. Pedosphere 2006, 16, 326–332. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Y.; Wang, X.; Yan, C.; Ma, C.; Liu, J.; Dong, S. Effects of Different Drought Degrees on Physiological Characteristics and Endogenous Hormones of Soybean. Plants 2022, 11, 2282. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Cordovez, V.; de Boer, W.; Raaijmakers, J.; Garbeva, P. Volatile Affairs in Microbial Interactions. ISME J. 2015, 9, 2329–2335. [Google Scholar] [CrossRef]
- Cardoso, P.; Santos, M.; Freitas, R.; Rocha, S.M.; Figueira, E. Response of Rhizobium to Cd Exposure: A Volatile Perspective. Environ. Pollut. 2017, 231, 802–811. [Google Scholar] [CrossRef]
- Matos, D.; Sá, C.; Cardoso, P.; Pires, A.; Rocha, S.M.; Figueira, E. The Role of Volatiles in Rhizobium Tolerance to Cadmium: Effects of Aldehydes and Alcohols on Growth and Biochemical Endpoints. Ecotoxicol. Environ. Saf. 2019, 186, 109759. [Google Scholar] [CrossRef]
- Cardoso, P.; Alves, A.; Silveira, P.; Sá, C.; Fidalgo, C.; Freitas, R.; Figueira, E. Bacteria from Nodules of Wild Legume Species: Phylogenetic Diversity, Plant Growth Promotion Abilities and Osmotolerance. Sci. Total Environ. 2018, 645, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- King, F.D.; Packard, T.T. Respiration and the Activity of the Respiratory Electron Transport System in Marine Zooplankton. Limnol. Oceanogr. 1975, 20, 849–854. [Google Scholar] [CrossRef]
- De Coen, W.M.; Janssen, C.R. The Use of Biomarkers in Daphnia Magna Toxicity Testing. IV. Cellular Energy Allocation: A New Methodology to Assess the Energy Budget of Toxicant-Stressed Daphnia Populations. J. Aquat. Ecosyst. Stress Recovery 1997, 6, 43–55. [Google Scholar] [CrossRef]
- Robinson, H.W.; Hogden, C.G. The Biuret Reaction in the Determination of Serum Proteins: I. A Study of the Conditions Necessary for the Production of a Stable Color Which Bears a Quantitative Relationship to the Protein Concentration. J. Biol. Chem. 1940, 135, 707–725. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Johansson, L.H.; Borg, L.A. A Spectrophotometric Method for Determination of Catalase Activity in Small Tissue Samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-Dinitrophenylhydrazine Spectrophotometric Assay for Quantification of Carbonyls in Oxidized Proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Anderson, M. Permanova+ for Primer: Guide to Software and Statistical Methods; Primer-E Ltd.: Auckland, New Zealand, 2008. [Google Scholar]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, T.; Costa, P.; Cardoso, P.; Figueira, E. Bacterial Volatile Organic Compounds as a Strategy to Increase Drought Tolerance in Maize (Zea mays L.): Influence on Plant Biochemistry. Plants 2024, 13, 2456. https://doi.org/10.3390/plants13172456
Lopes T, Costa P, Cardoso P, Figueira E. Bacterial Volatile Organic Compounds as a Strategy to Increase Drought Tolerance in Maize (Zea mays L.): Influence on Plant Biochemistry. Plants. 2024; 13(17):2456. https://doi.org/10.3390/plants13172456
Chicago/Turabian StyleLopes, Tiago, Pedro Costa, Paulo Cardoso, and Etelvina Figueira. 2024. "Bacterial Volatile Organic Compounds as a Strategy to Increase Drought Tolerance in Maize (Zea mays L.): Influence on Plant Biochemistry" Plants 13, no. 17: 2456. https://doi.org/10.3390/plants13172456
APA StyleLopes, T., Costa, P., Cardoso, P., & Figueira, E. (2024). Bacterial Volatile Organic Compounds as a Strategy to Increase Drought Tolerance in Maize (Zea mays L.): Influence on Plant Biochemistry. Plants, 13(17), 2456. https://doi.org/10.3390/plants13172456











