Application of Magnetized Ionized Water and Bacillus subtilis Improved Saline Soil Quality and Cotton Productivity
Abstract
:1. Introduction
2. Results
2.1. Soil Properties
2.1.1. Soil Physical Properties
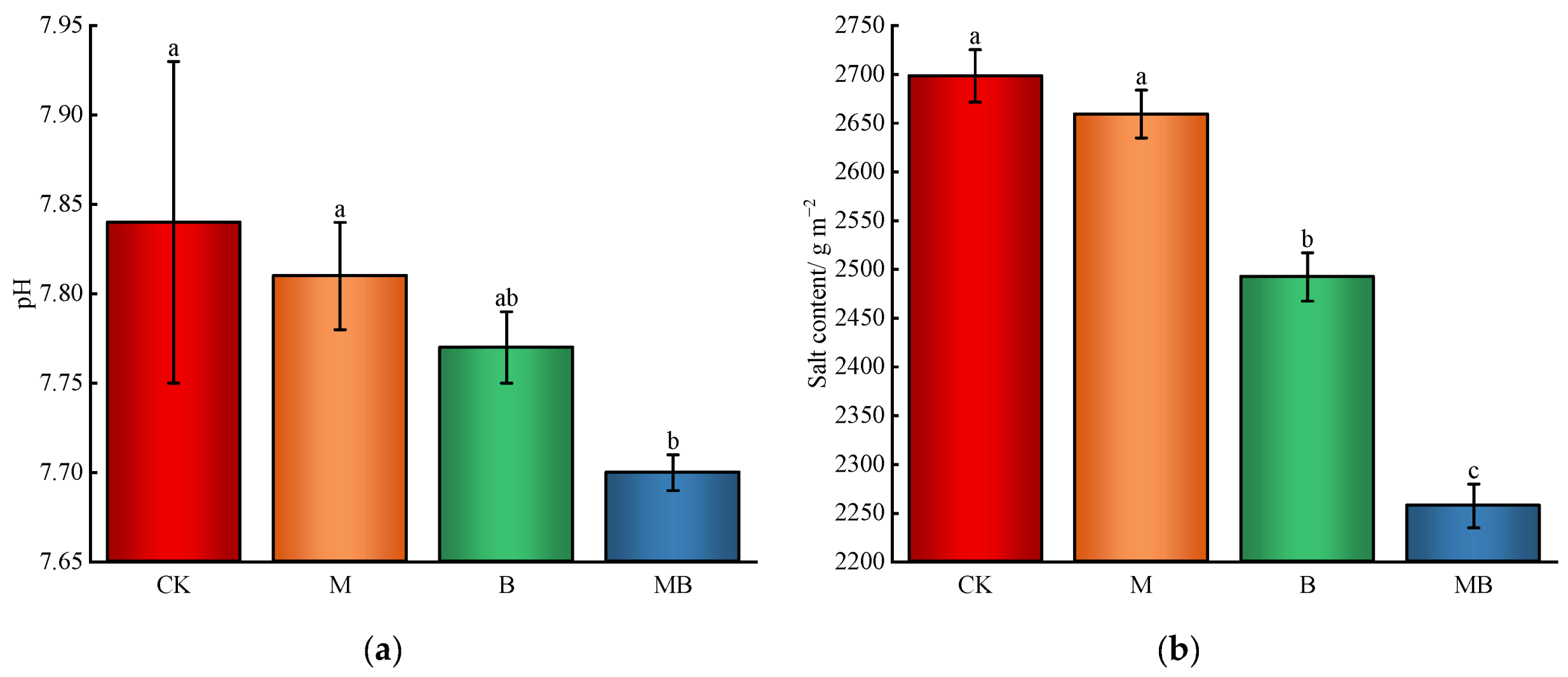
2.1.2. Soil Salinity and Alkalinity
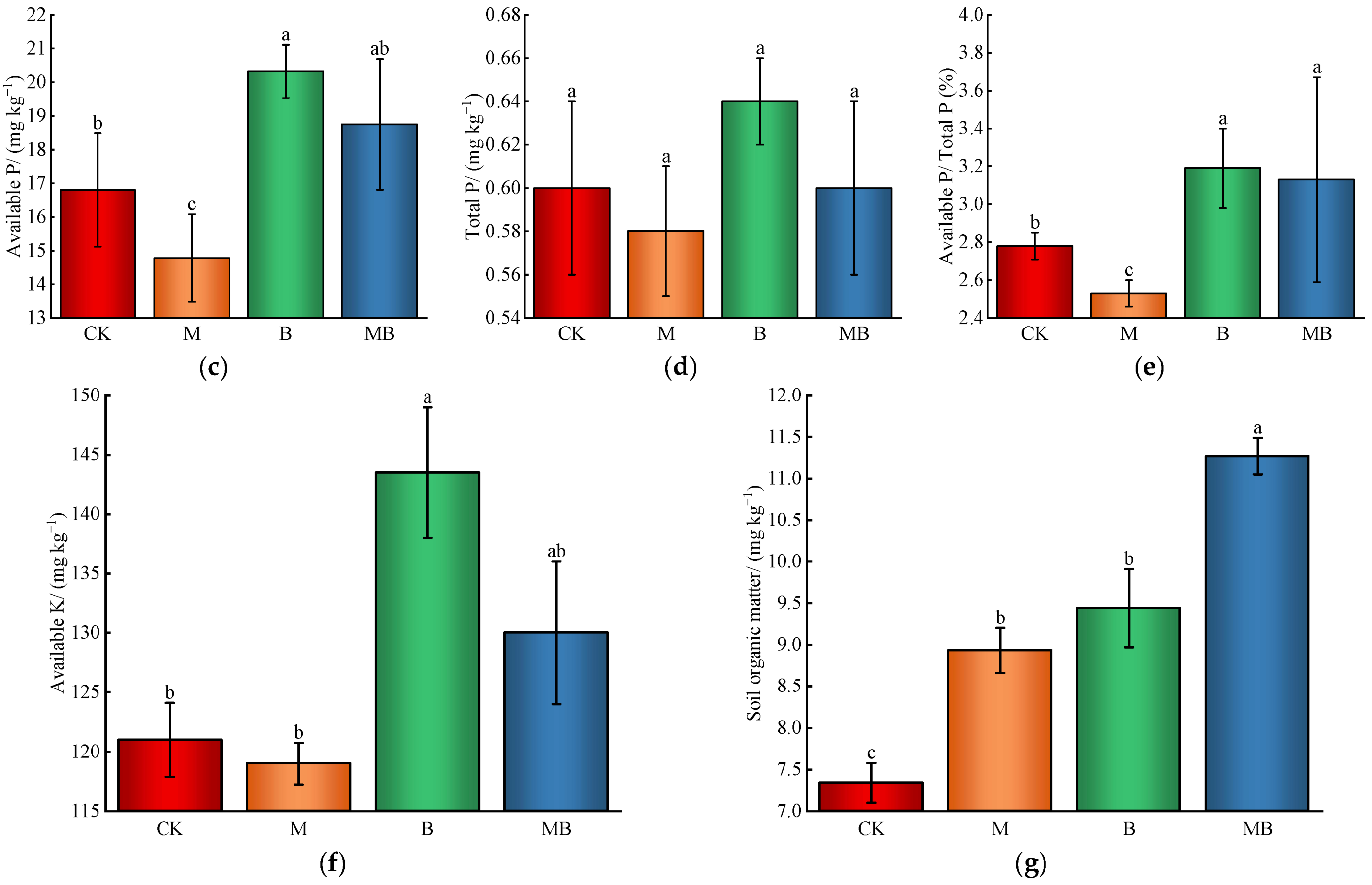
2.1.3. Soil Nutrient Content
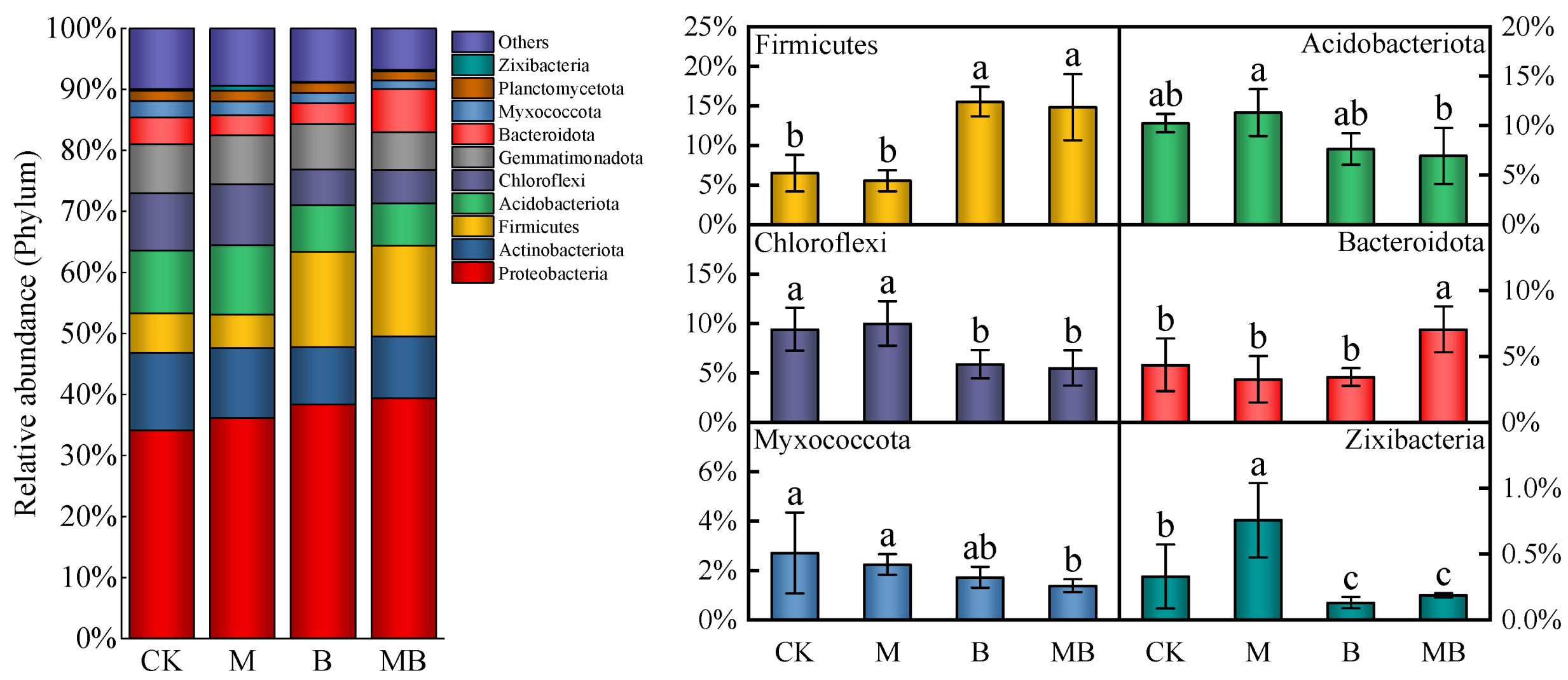
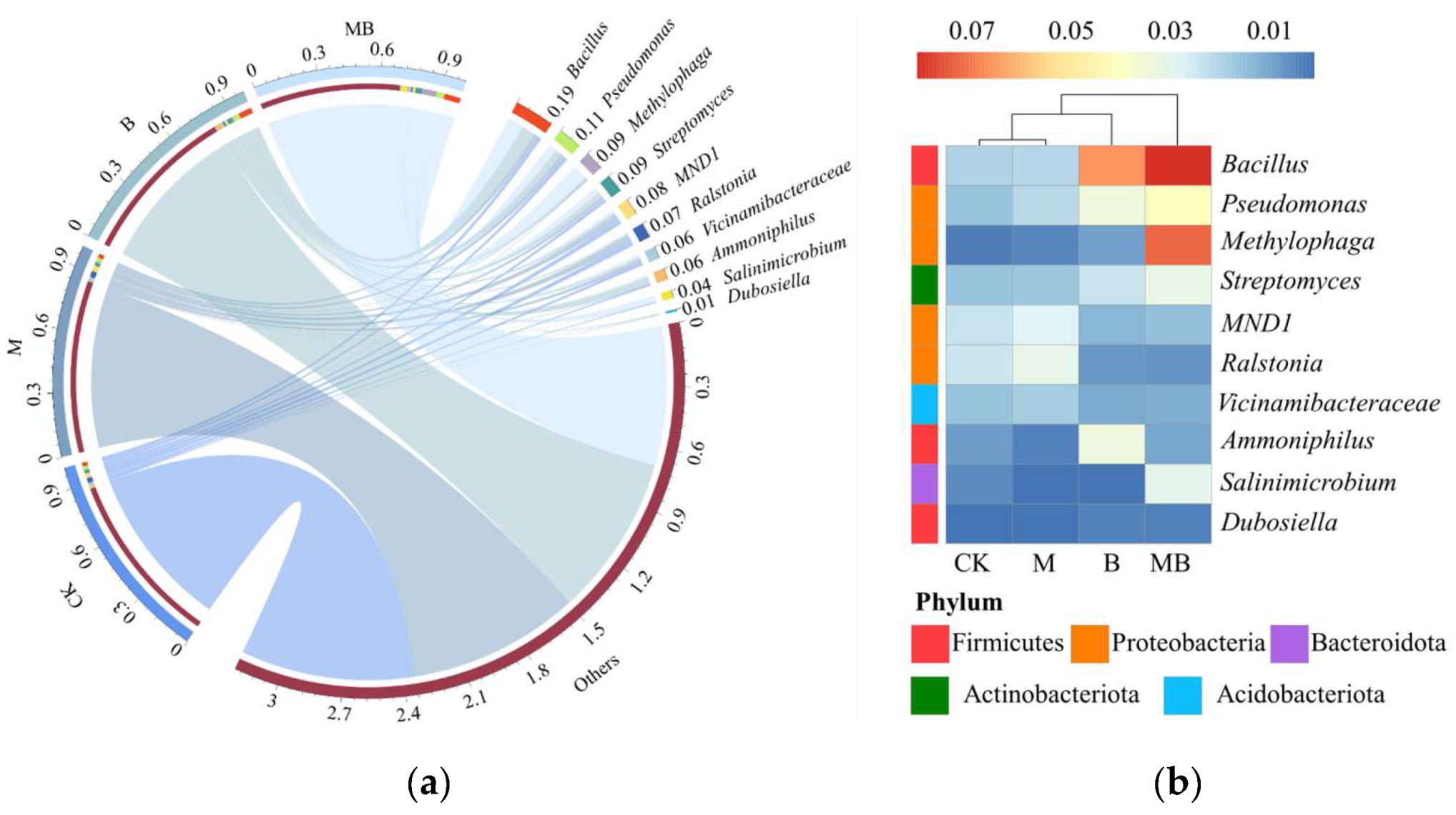
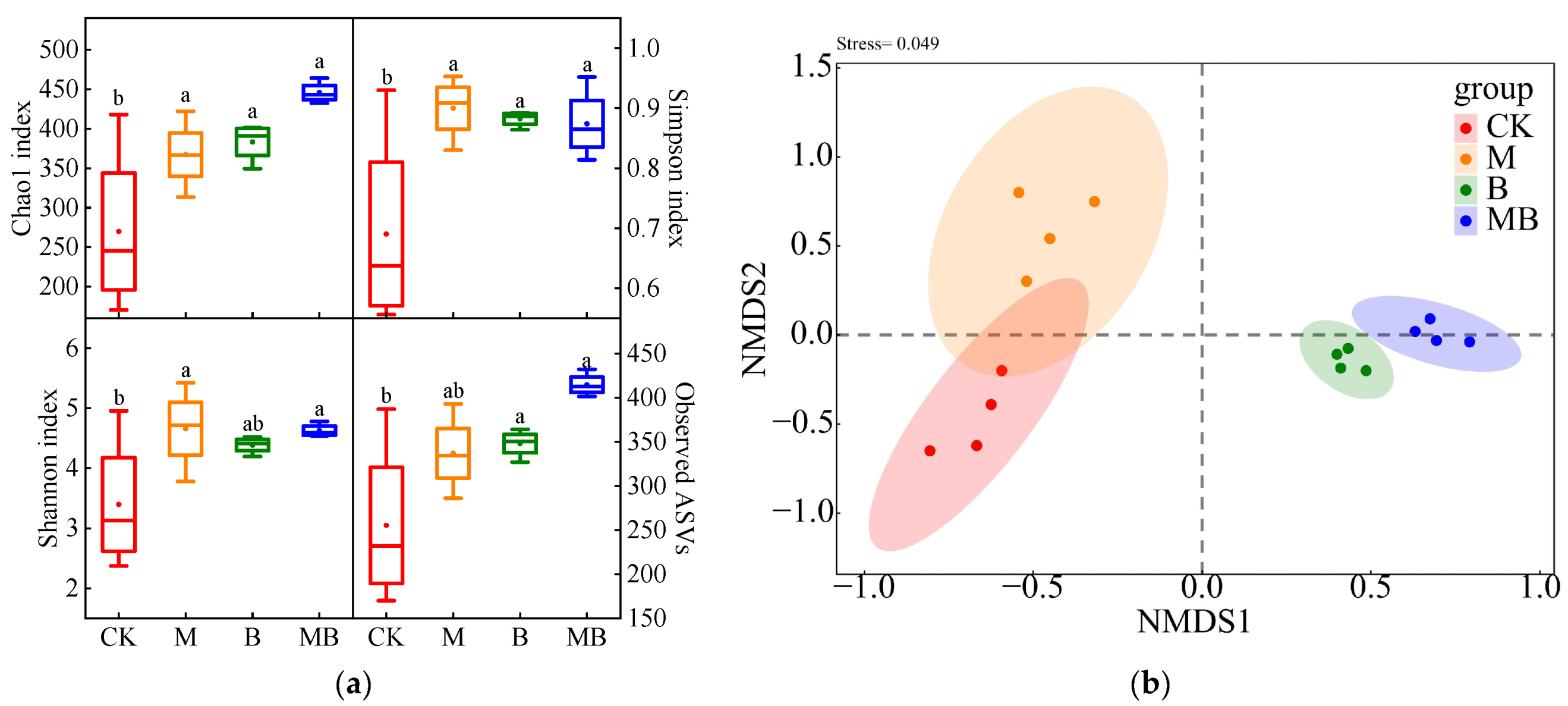
2.2. Diversities of Bacterial Communities
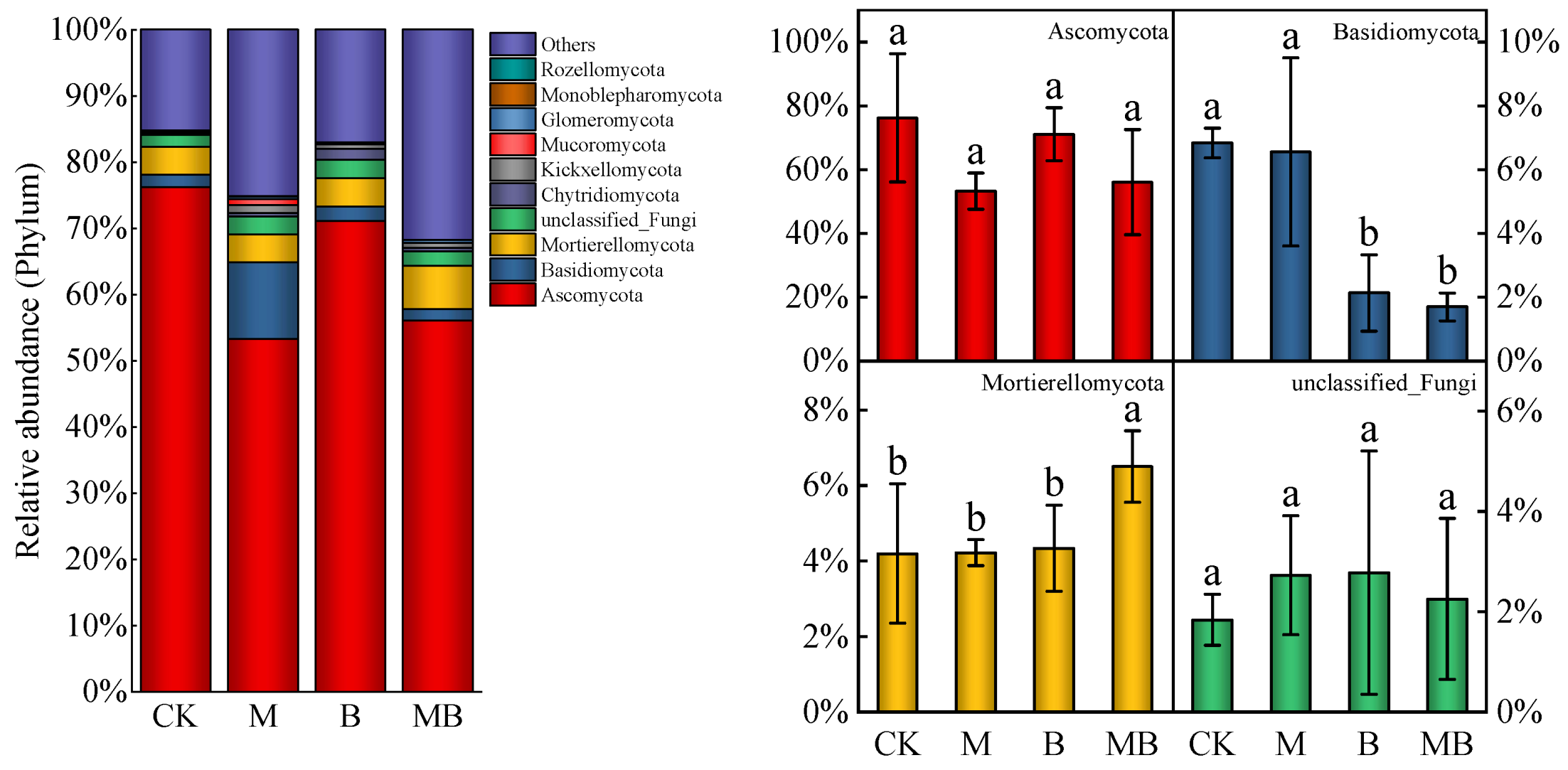
2.3. Diversities of Fungal Communities
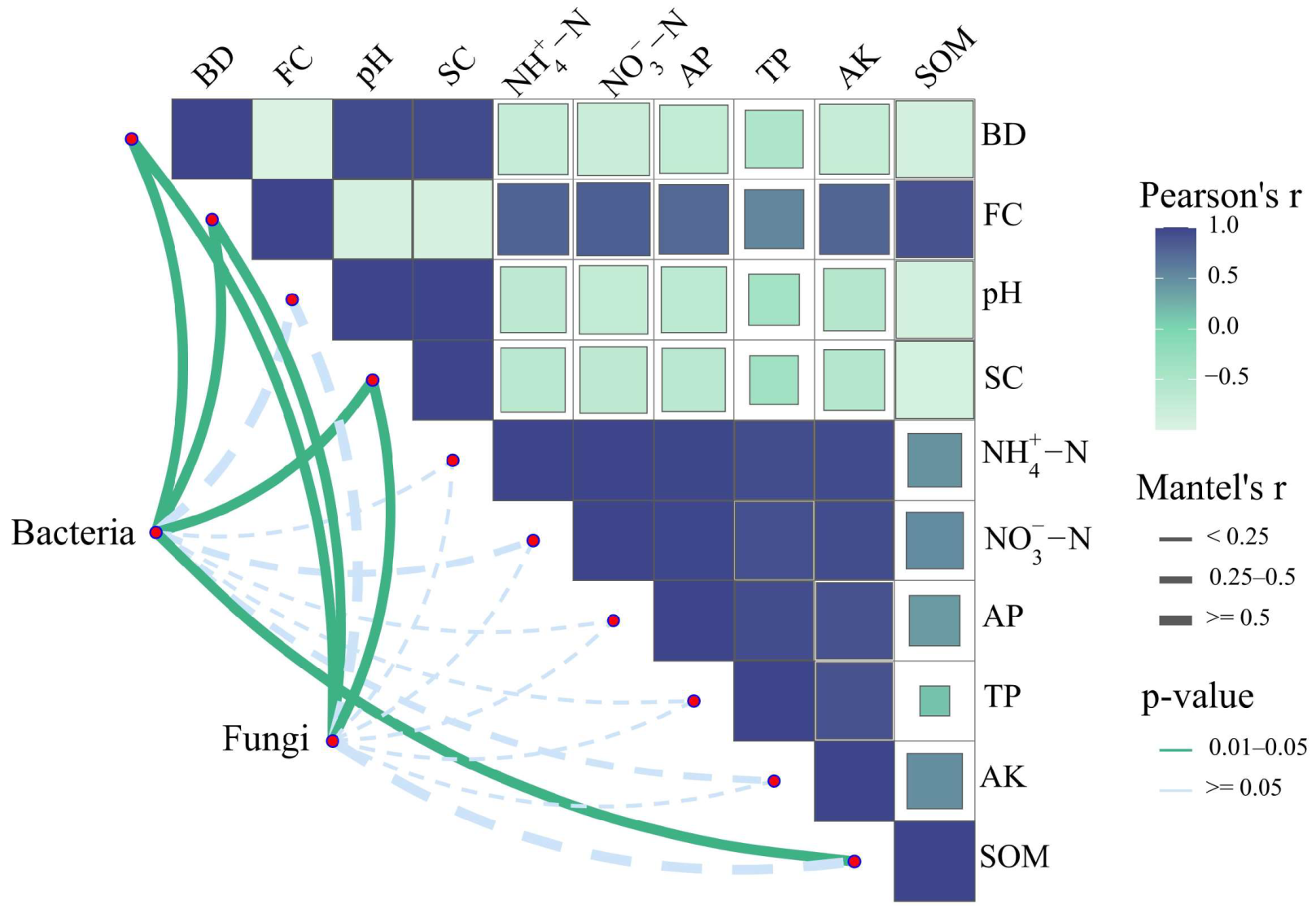
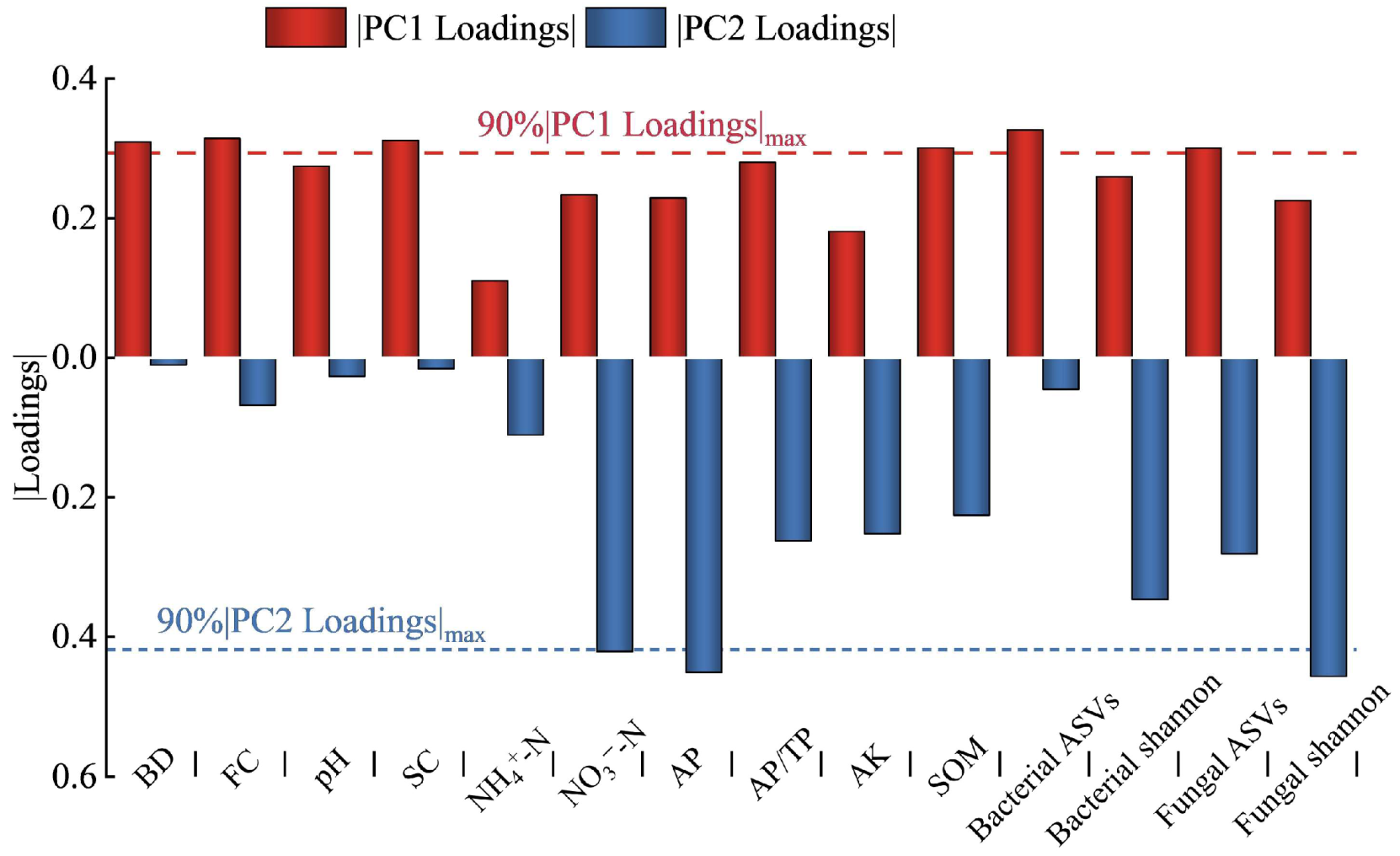
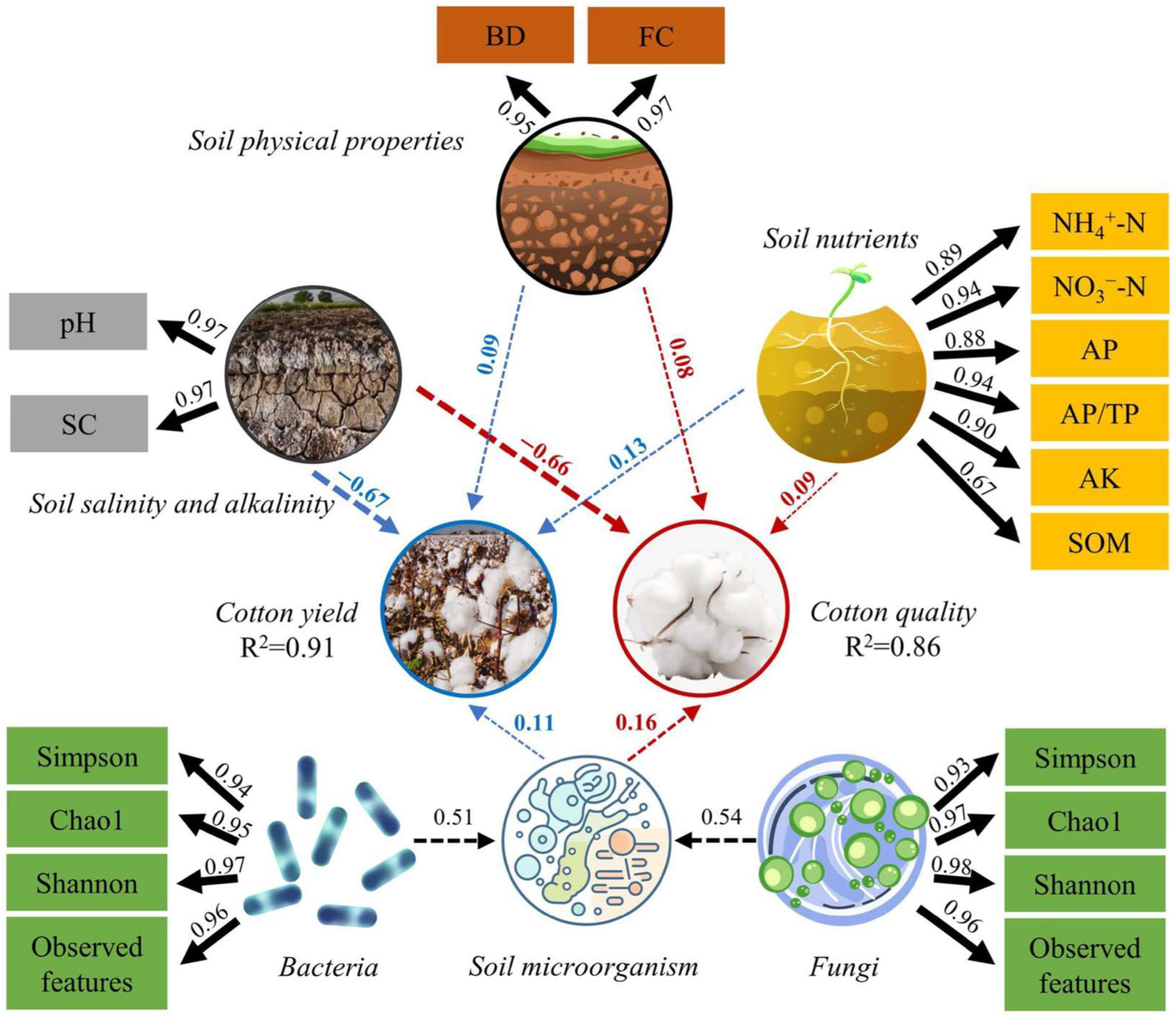
2.4. Relationship between Bacterial and Fungal Communities and Soil Properties
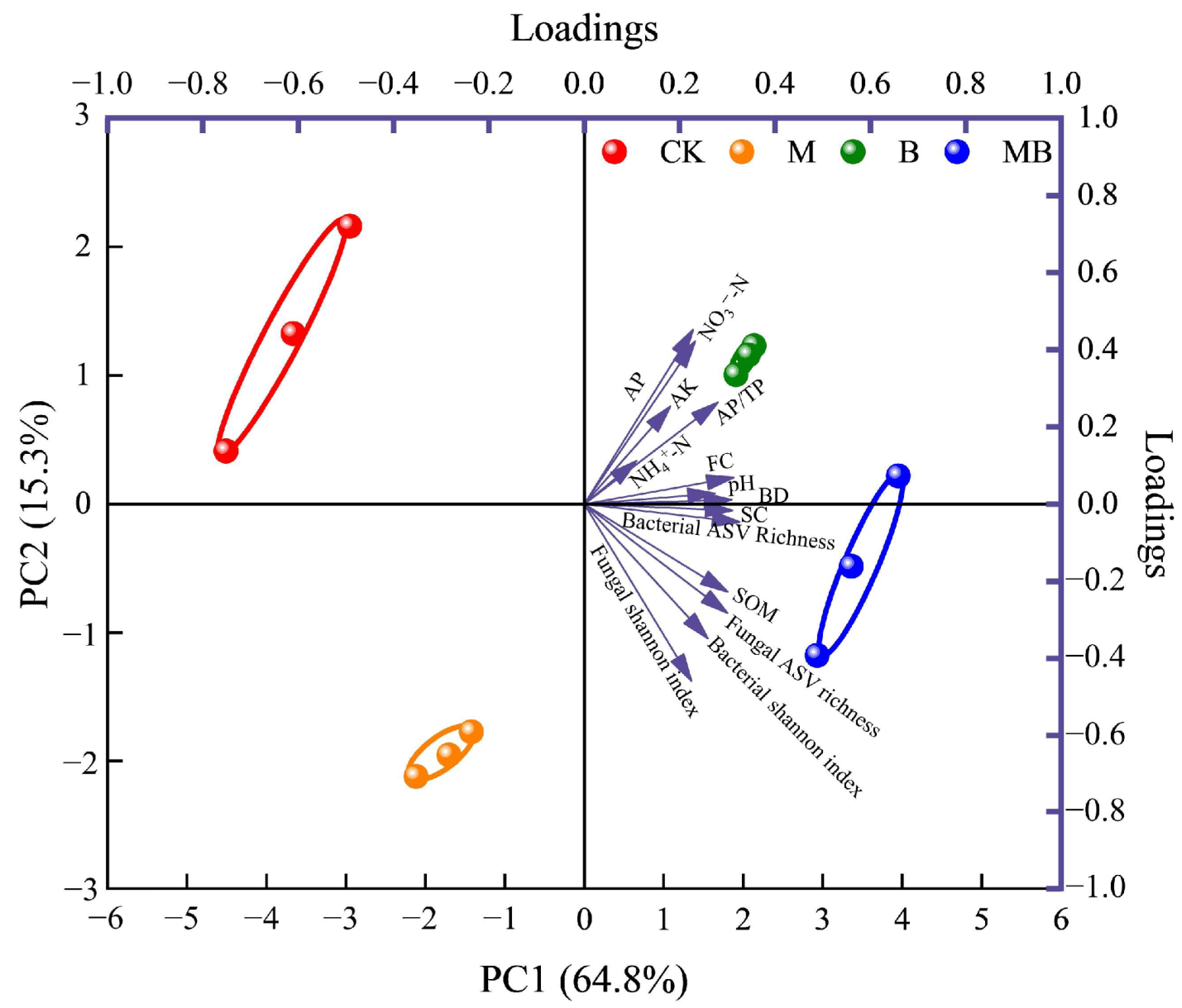
2.5. Soil Quality Index
2.6. Cotton Yield Component and Quality
3. Discussion
3.1. Effects of Magnetized Ionized Water on Soil Properties and Crop Production
3.2. Effects of B. subtilis on Characteristics of Soil and Yield of Crops
3.3. Soil Properties and Soil Quality
4. Materials and Methods
4.1. Description of the Experimental Site
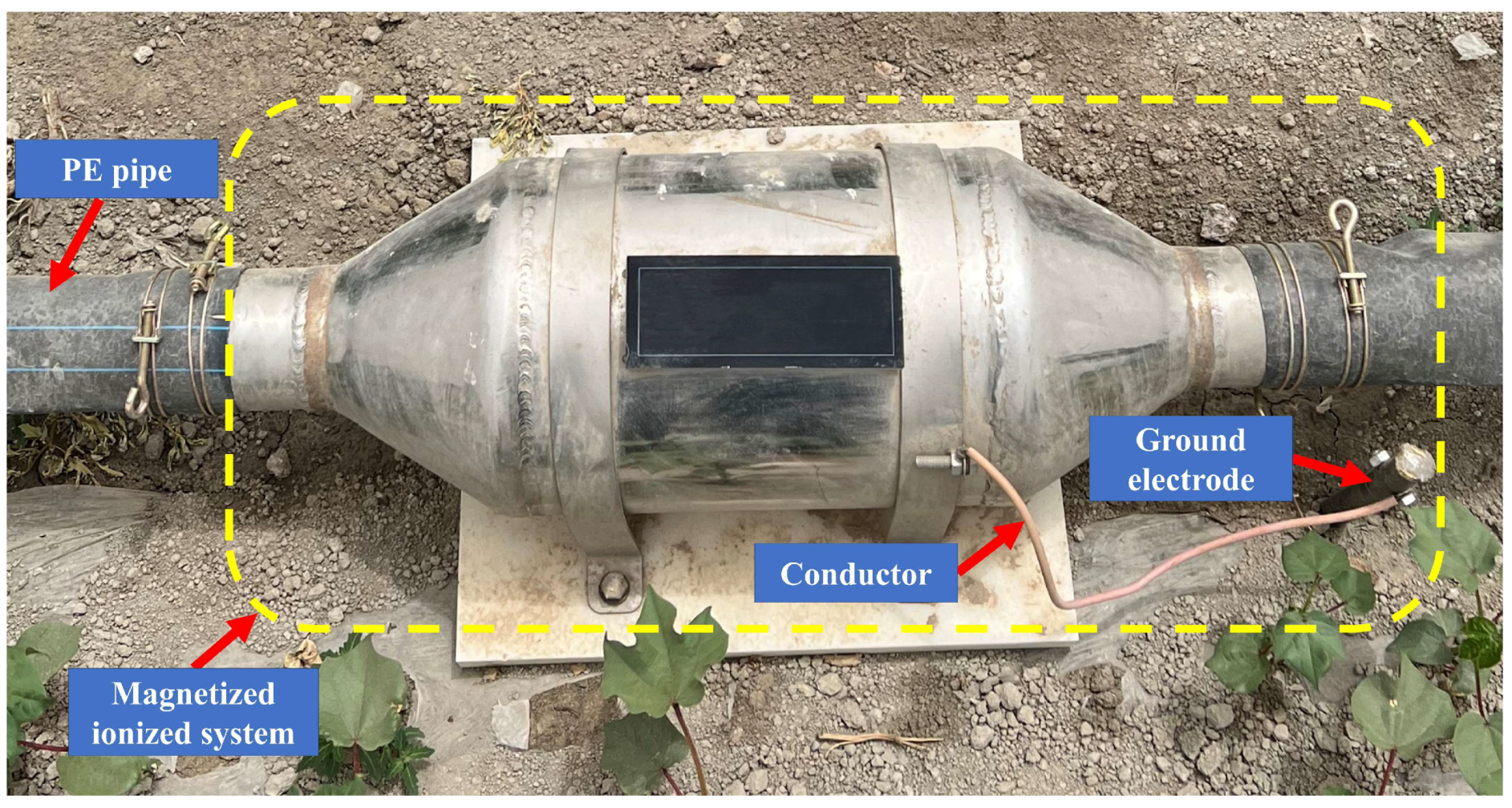
4.2. Magnetized Ionized System for Irrigation Water Treatment
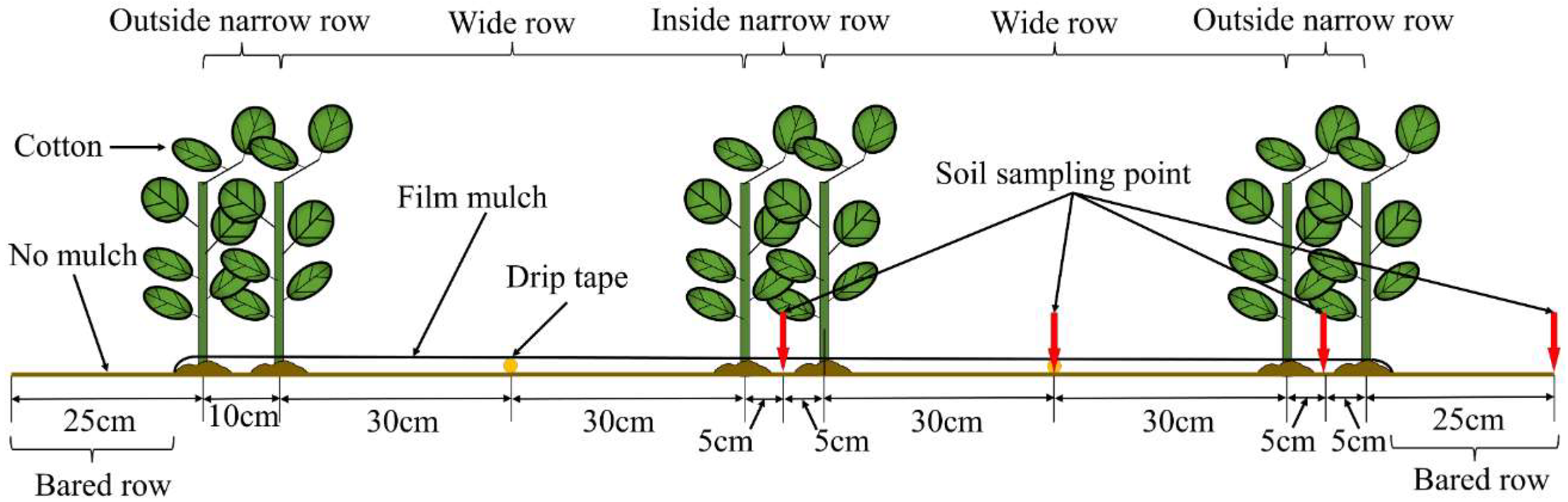
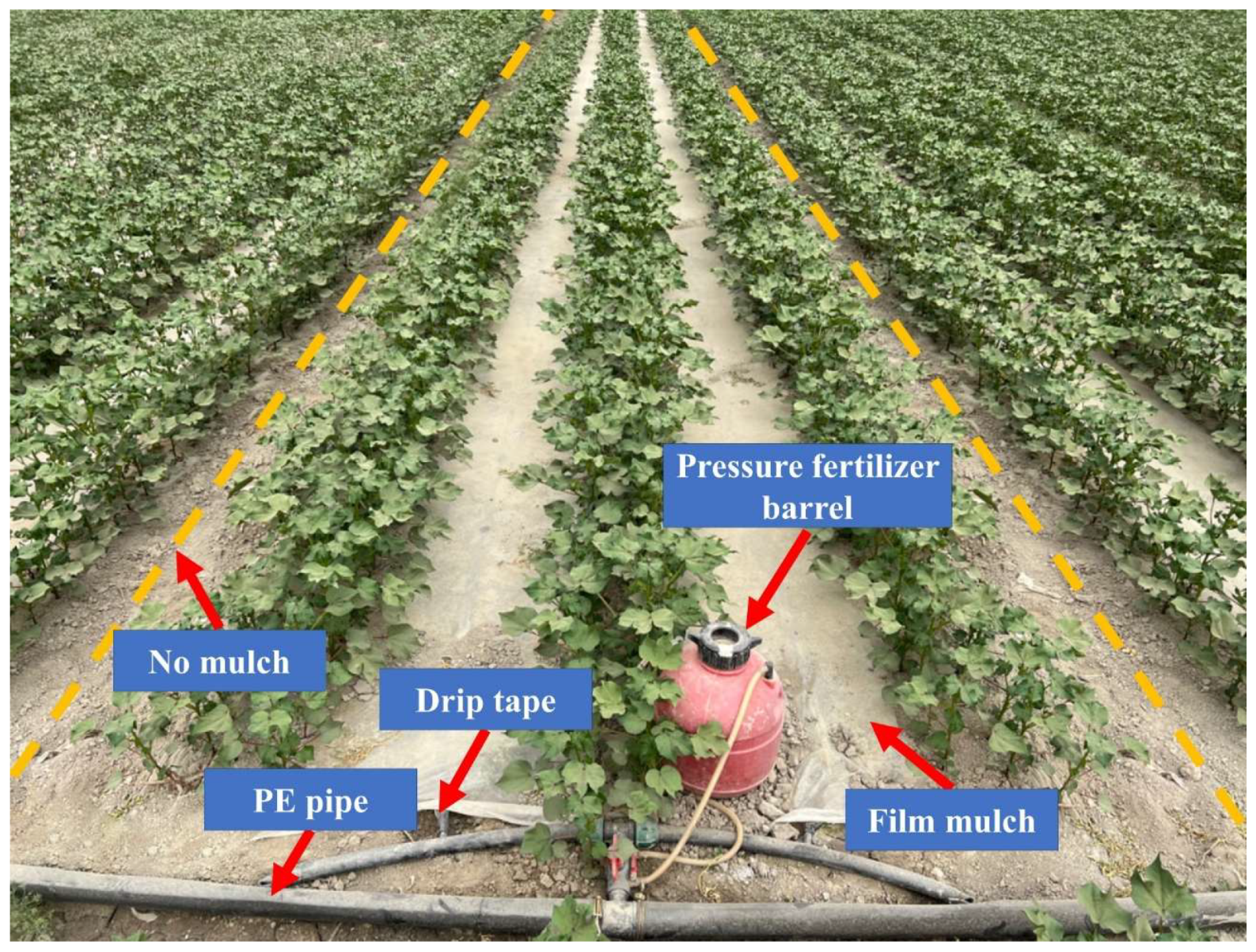
4.3. Field Management and Experimental Design
4.4. Measurements and Calculations
4.4.1. The Bulk Density and Field Capacity of Soil
4.4.2. The pH and Salinity of the Soil
4.4.3. Soil Nutrient
4.4.4. Soil Microbial Properties
4.4.5. Soil Quality
4.4.6. Cotton Quality
4.4.7. Calculation of Relative Yield and Relative Quality
4.4.8. Analysis of Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cui, Q.; Xia, J.; Yang, H.; Liu, J.; Shao, P. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ. 2021, 756, 143801. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Huang, J.; Kong, Y.L.; Xu, Q.S.; Zhu, C.Q.; Zhu, L.F.; Cao, X.C.; Hong, X.Z.; Zhang, J.H. Progresses for characteristics and amelioration measures of saline soil. Soil 2022, 54, 18–23, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Zhao, T.; Deng, X.; Xiao, Q.; Han, Y.; Zhu, S.; Chen, J. IAA priming improves the germination and seedling growth in cotton (Gossypium hirsutum L.) via regulating the endogenous phytohormones and enhancing the sucrose metabolism. Ind. Crop. Prod. 2020, 155, 112788. [Google Scholar] [CrossRef]
- China’s National Bureau of Statistics. Announcement on Cotton Yield in 2023 by the National Bureau of Statistics. Available online: https://www.stats.gov.cn/sj/zxfb/202312/t20231225_1945745.html (accessed on 22 July 2024).
- Tian, C.Y.; Mai, W.X.; Zhao, Z.Y. Study on key technologies of ecological management of saline alkali land in arid area of Xinjiang. Acta Ecol. Sin. 2016, 36, 7064–7068, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Gong, Z.; Gao, K.; Ou, Y.; Zhang, J. The effect of a static magnetic field on the hydrogen bonding in water using frictional experiments. J. Mol. Struct. 2013, 1052, 102–104. [Google Scholar] [CrossRef]
- Amiri, M.; Dadkhah, A.A. On reduction in the surface tension of water due to magnetic treatment. Colloids Surf. A Physicochem. Eng. Asp. 2006, 278, 252–255. [Google Scholar] [CrossRef]
- Mostafazadeh-Fard, B.; Khoshravesh, M.; Mousavi, S.F.; Kiani, A.R. Effects of magnetized water on soil chemical components underneath trickle irrigation. J. Irrig. Drain. Eng. 2012, 138, 1075–1081. [Google Scholar] [CrossRef]
- Zhang, R.X.; Chu, G.X.; Wang, W.B.; Ye, J. Effects of magnetized water on salt leaching under drip irrigation condition. Xinjiang Agric. Sci. 2013, 50, 1656–1661, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Wei, K.; Zhang, J.; Wang, Q.; Guo, Y.; Mu, W. Irrigation with ionized brackish water affects cotton yield and water use efficiency. Ind. Crop. Prod. 2022, 175, 114244–114256. [Google Scholar] [CrossRef]
- Hill, G.; Mitkowski, N.; Aldrich-Wolfe, L.; Emele, L.; Jurkonie, D.; Ficke, A.; Maldonado-Ramirez, S.; Lynch, S.; Nelson, E. Methods for assessing the composition and diversity of soil microbial communities. Appl. Soil Ecol. 2000, 15, 25–36. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, X.; Qi, X.; Zeng, Y.; Guo, X.; Zhuang, G.; Ma, A. Soil bacterial communities associated with multi-nutrient cycling under long-term warming in the alpine meadow. Front. Microbiol. 2023, 14, 1136187. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Zhu, L.S.; Li, D.Q.; Liu, H.; Gong, Z.W.; Jiang, N.; Liu, Y.Y. Effect of different amount of Bacillus subtilis on nutrients of tobacco planting soils. Southwest China J. Agric. Sci. 2018, 31, 779–785, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Li, W.; Wang, J.T. Effects of Bacillus subtilis and phosphobacteria on soil properties and fruit quality in apple orchards. Jiangsu Agric. Sci. 2018, 46, 140–144, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Wang, T.; Liu, M.-Q.; Li, H.-X. Inoculation of phosphate solubilizing bacteria Bacillus thuringiensis B1 increases available phosphorus and growth of peanut in acidic soil. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2014, 64, 252–259. [Google Scholar] [CrossRef]
- Zhou, B.; Liang, C.; Chen, X.; Ye, S.; Peng, Y.; Yang, L.; Duan, M.; Wang, X. Magnetically-treated brackish water affects soil water-salt distribution and the growth of cotton with film mulch drip irrigation in Xinjiang, China. Agric. Water Manag. 2022, 263, 107487. [Google Scholar] [CrossRef]
- Alattar, E.; Radwan, E.; Elwasife, K. Improvement in growth of plants under the effect of magnetized water. AIMS Biophys. 2022, 9, 346–387. [Google Scholar] [CrossRef]
- Mahapatra, S.; Yadav, R.; Ramakrishna, W. Bacillus subtilis impact on plant growth, soil health and environment: Dr. Jekyll and Mr. Hyde. J. Appl. Microbiol. 2022, 132, 3543–3562. [Google Scholar] [CrossRef]
- Blake, C.; Christensen, M.N.; Kovács, Á.T. Molecular aspects of plant growth promotion and protection by Bacillus subtilis. Mol. Plant-Microbe Interact. 2021, 34, 15–25. [Google Scholar] [CrossRef]
- Lin, S.; Wang, Q.; Wei, K.; Sun, Y.; Shao, F.; Lei, Q.; Deng, M. Enhancing pakchoi (Brassica chinensis L.) agriculture with magnetized-ionized brackish water and organic fertilizers: A sustainable approach to soil quality and crop yield optimization. J. Clean. Prod. 2024, 450, 141935. [Google Scholar] [CrossRef]
- Zhen-Rui, D.; Ya-Jun, Z.; Feng-Ling, C.; Jin-Zhong, C.; Shu-Xing, D. Magnetization mechanism of magnetized water. Acta Phys. Sin. 2011, 60, 432–439. [Google Scholar] [CrossRef]
- Liu, J.; Cao, Y. Experimental study on the surface tension of magnetized water. Int. Commun. Heat Mass Transf. 2020, 121, 105091. [Google Scholar] [CrossRef]
- Al-Talib, A.A.; Mahmood, M.T.; Ghani, O.M.A. The effect of magnetizing irrigation water on the infiltration rate in soil. Tikrit J. Eng. Sci. 2013, 20, 18–23. [Google Scholar] [CrossRef]
- Khoshravesh-Miangoleh, M.; Kiani, A. Effect of magnetized water on infiltration capacity of different soil textures. Soil Use Manag. 2014, 30, 588–594. [Google Scholar] [CrossRef]
- Hamza, J.N. Investigation on using magnetic water technology for leaching high saline-sodic soils. Environ. Monit. Assess. 2019, 191, 519. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Raheem, L.H.; Azzubaidi, R.Z. Evaluation of using magnetized water in leaching salts in sandy loam soil. J. Eng. 2021, 27, 35–46. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Kumari, B.D.R. Influence of pulsed magnetic field on soybean (Glycine max L.) seed germinate seedling growth and soil microbial population. Indian J. Biochem. Biophys. 2013, 50, 312–317. [Google Scholar] [CrossRef]
- Cui, H.R.; Liu, X.M.; Jing, R.Y.; Zhang, M.Z.; Wang, L.; Zheng, L.; Kong, L.G.; Wang, H.T.; Ma, F.Y. Irrigation with magnetized water affects the soil microenvironment and fruit quality of eggplants in a covered vegetable production system in Shouguang city, China. J. Soil Sci. Plant Nutr. 2020, 20, 2684–2697. [Google Scholar] [CrossRef]
- Zhou, B.B.; Yang, L.; Chen, X.P.; Ye, S.T.; Peng, Y.; Liang, C.F. Effect of magnetic water irrigation on the improvement of salinized soil and cotton growth in Xinjiang. Agric. Water Manag. 2021, 248, 106784. [Google Scholar] [CrossRef]
- Zhang, J.H. Effect Mechanism Research of Drip Irrigation under Mulch with Magnetized Water on Soil Fertility and Cotton Growth. Ph.D. Thesis, Xi’an University of Technology, Xi’an, China, 2022. [Google Scholar]
- Benoit, I.; Esker, M.H.; Patyshakuliyeva, A.; Mattern, D.J.; Blei, F.; Zhou, M.M.; Dijksterhuis, J.; Brakhage, A.A.; Kuipers, O.P.; Vries, R.P.; et al. Bacillus subtilis attachment to Aspergillus niger hyphae results in mutually altered metabolism. Environ. Microbiol. 2015, 17, 2099–2113. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Meena, S.K.; Meena, R.K.; Kumar, A.; Verma, J.P.; Singh, N.P. Can Bacillus species enhance nutrient availability in agricultural soils. In Bacilli and Agrobiotechnology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 367–395. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd-Allah, E.F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Radhakrishnan, R.; Lee, K.E.; You, Y.H.; Ko, J.H.; Kim, J.H.; Lee, I.J. Mechanism of plant growth promotion elicited by Bacillus sp. LKE15 in oriental melon. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 637–647. [Google Scholar] [CrossRef]
- Kang, S.M.; Radhakrishnan, R.; You, Y.H.; Joo, G.J.; Lee, I.J.; Lee, K.E.; Kim, J.H. Phosphate solubilizing Bacillus megaterium mj1212 regulates endogenous plant carbohydrates and amino acids contents to promote mustard plant growth. Indian J. Microbiol. 2014, 54, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.J.; Zeng, S.Q.; Bais, H.; LaManna, J.M.; Hussey, D.S.; Jacobson, D.L. Plant growth-promoting rhizobacteria (PGPR) reduce evaporation and increase soil water retention. Water Resour. Res. 2018, 54, 3673–3687. [Google Scholar] [CrossRef]
- Zhou, B.B.; Hou, Y.L.; Wang, Q.J. Characteristics of water and salt migration in process of improving saline alkali soil with Bacillus subtilis. Trans. Chin. Soc. Agric. Eng. 2018, 34, 104–110, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Nagorska, K.; Bikowski, M.; Obuchowski, M. Multicellular behaviour and production of a wide variety of toxic substances support usage of Bacillus subtilis as a powerful biocontrol agent. Acta Biochim. Pol. 2007, 54, 495–508. [Google Scholar] [CrossRef]
- Selvakumar, G.; Panneerselvam, P.; Bindu, G.H.; Ganeshamurthy, A.N. Pseudomonads: Plant growth promotion and beyond. In Plant Microbes Symbiosis: Applied Facets; Springer: Berlin/Heidelberg, Germany, 2015; pp. 193–208. [Google Scholar] [CrossRef]
- Costa, R.; Salles, J.F.; Berg, G.; Smalla, K. Cultivation-independent analysis of Pseudomonas species in soil and in the rhizosphere of field-grown Verticillium dahliae host plants. Environ. Microbiol. 2006, 8, 2136–2149. [Google Scholar] [CrossRef]
- Weller, D.M. Pseudomonas biocontrol agents of soilborne pathogens: Looking back over 30 years. Phytopathology 2007, 97, 250–256. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, R.S.; Lade, H.; Paul, D. Methylotrophic bacteria in sustainable agriculture. World J. Microbiol. Biotechnol. 2016, 32, 120. [Google Scholar] [CrossRef]
- Huang, K.; Chen, C.; Zhang, J.; Tang, Z.; Shen, Q.R.; Rosen, B.P.; Zhao, F.J. Efficient arsenic methylation and volatilization mediated by a novel bacterium from an arsenic-contaminated paddy soil. Environ. Sci. Technol. 2016, 50, 6389–6396. [Google Scholar] [CrossRef]
- Sharavin, D.; Kovalevskaya, N. Effect of halotolerant methylotrophs isolated from saline soil rhizosphere on diminution of salt stress in wheat (Triticum Aestivum L.). J. Agric. Environ. 2017, 3, 4. [Google Scholar] [CrossRef]
- Oskay, M. Comparison of Streptomyces diversity between agricultural and non-agricultural soils by using various culture media. Sci. Res. Essays 2009, 4, 997–1005. [Google Scholar]
- Vergnes, S.; Gayrard, D.; Veyssière, M.; Toulotte, J.; Martinez, Y.; Dumont, V.; Bouchez, O.; Rey, T.; Dumas, B. Phyllosphere colonisation by a soil Streptomyces sp. promotes plant defense responses against fungal infection. Mol. Plant-Microbe Interact. 2020, 33, 233–234. [Google Scholar] [CrossRef]
- Mahesh, A.; Sangwoo, K.; Raj, Y.; Hyun, U.; Seung, K.; Burm, L.; Su, L. A new record of Pseudeurotium bakeri from crop field soil in Korea. Korean J. Mycol. 2016, 44, 145–149. [Google Scholar] [CrossRef]
- Sogonov, M.V.; Schroers, H.J.; Gams, W.; Dijksterhuis, J.; Summerbell, R.C. The hyphomycete Teberdinia hygrophila gen. nov., sp. nov. and related anamorphs of Pseudeurotium species. Mycologia 2005, 97, 695–709. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, Q.; Li, S.; Lu, X.; Dong, L.; Wang, P.; Zhang, X.; Su, Z.; Ma, P. Application of Bacillus subtilis NCD-2 can suppress cotton verticillium wilt and its effect on abundant and rare microbial communities in rhizosphere. Biol. Control. 2022, 165, 104812–104822. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Zhang, S.L.; Guo, J.J.; Ding, F.J.; Fu, N.F.; Zhou, B.; Wang, M.H.; Li, L.Z. Effects of Bacillus subtilis AMCC 100001 on the promotion of cotton growth. China Cotton 2013, 40, 16–18, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Andersson, K.O.; Tighe, M.K.; Guppy, C.N.; Milham, P.J.; McLaren, T.I. Incremental acidification reveals phosphorus release dynamics in alkaline vertic soils. Geoderma 2015, 259, 35–44. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Reischke, S.; Rousk, J.; Bååth, E. The effects of glucose loading rates on bacterial and fungal growth in soil. Soil Biol. Biochem. 2014, 70, 88–95. [Google Scholar] [CrossRef]
- Rath, K.M.; Maheshwari, A.; Bengtson, P.; Rousk, J. Comparative toxicities of salts on microbial processes in soil. Appl. Environ. Microbiol. 2016, 82, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Magdoff, F.; Weil, R.R. Soil Organic Matter in Sustainable Agriculture; CRC Press: Boca Raton, FL, USA, 2007; 412p. [Google Scholar] [CrossRef]
- Sun, R.B.; Dsouza, M.; Gilbert, J.A.; Guo, X.S.; Wang, D.Z.; Guo, Z.B.; Ni, Y.Y.; Chu, H.Y. Fungal community composition in soils subjected to long-term chemical fertilization is most influenced by the type of organic matter. Environ. Microbiol. 2016, 18, 5137–5150. [Google Scholar] [CrossRef]
- Esmaeilzadeh, J.; Ahangar, A.G. Influence of soil organic matter content on soil physical, chemical and biological properties. Int. J. Plant Anim. Environ. Sci. 2014, 2014, 244–252. [Google Scholar]
- Lin, S.D.; Lei, Q.Y.; Liu, Y.; Zhao, Y.M.; Su, L.J.; Wang, Q.J.; Tao, W.H.; Deng, M.J. Quantifying the impact of organic fertilizers on soil quality under varied irrigation water sources. Water 2023, 15, 3618. [Google Scholar] [CrossRef]
- Mohammad, S.A.; Nicholas, M.H. Quantitative soil quality indexing of temperate arable management systems. Soil Tillage Res. 2015, 150, 57–67. [Google Scholar] [CrossRef]
- Karlen, D.L.; Ditzler, C.A.; Andrews, S.S. Soil quality: Why and how? Geoderma 2003, 114, 145–156. [Google Scholar] [CrossRef]
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Cotar, A.I.; Andronescu, E.; Ficai, A.; Ghitulica, C.D.; Grumezescu, V.; Vasile, B.S.; Chifiriuc, M.C. In vitro activity of the new water-dispersible Fe3O4@usnic acid nanostructure against planktonic and sessile bacterial cells. J. Nanoparticle Res. 2013, 15, 1766. [Google Scholar] [CrossRef]
- Abrol, I.P.; Yadav, J.S.P.; Massoud, F.I. Salt-Affected Soils and Their Management. FAO Soils Bulletin 39. 1988. Available online: https://www.fao.org/3/x5871e/x5871e04.htm#3.%20SALINE%20SOILS%20AND%20THEIR%20MANAGEMENT (accessed on 1 April 2024).
- Jiang, Z.B.; Wang, Q.J.; Ning, S.R.; Hu, X.Q.; Yuan, S. Growth and yield response and water use efficiency of cotton under film-mulched drip irrigation to magnetized ionized water and Bacillus subtilis in saline soil in Xinjiang. Agronomy 2023, 13, 1644. [Google Scholar] [CrossRef]
- Chen, W.L.; Jin, M.G.; Ferré, T.P.A.; Liu, Y.F.; Xian, Y.; Shan, T.R.; Ping, X. Spatial distribution of soil moisture, soil salinity, and root density beneath a cotton field under mulched drip irrigation with brackish and fresh water. Field Crops Res. 2018, 215, 207–221. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods for Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Bao, S.D. Soil Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]








| Index | CK | M | B | MB |
|---|---|---|---|---|
| Seed cotton yield (kg ha−1) | 6045.77 c ± 36.77 | 6390.49 bc ± 503.15 | 6941.02 ab ± 235.97 | 7260.63 a ± 516.86 |
| Boll number per plant | 3.91 b ± 0.20 | 4.25 b ± 0.25 | 4.17 b ± 0.11 | 5.19 a ± 0.41 |
| Upper half mean length (mm) | 27.16 c ± 0.53 | 27.19 c ± 0.39 | 29.27 b ± 0.51 | 31.92 a ± 0.43 |
| Uniformity index (%) | 77.80 d ± 0.59 | 80.44 c ± 0.66 | 82.12 b ± 0.84 | 85.69 a ± 0.69 |
| Specific breaking strength (cN tex−1) | 29.09 b ± 1.20 | 30.73 b ± 0.72 | 30.41 ab ± 1.84 | 32.54 a ± 1.02 |
| Micronaire value | 5.08 a ± 0.08 | 4.92 ab ± 0.17 | 4.90 ab ± 0.16 | 4.76 b ± 0.10 |
| Cotton Production Index | Linear Function Model | Average Slope | R2 | RMSE | |
|---|---|---|---|---|---|
| Yield | Seed cotton yield | yY = 0.24SQI + 0.80 | 0.26 | 0.99 | 0.01 |
| Boll number per plant | yB = 0.28SQI + 0.70 | 0.66 | 0.08 | ||
| Quality | Upper half mean length | yL = 0.21SQI + 0.86 | 0.13 | 0.83 | 0.03 |
| Uniformity index | yU = 0.12SQI + 0.89 | 0.94 | 0.01 | ||
| Specific breaking strength | yS = 0.12SQI + 0.88 | 0.74 | 0.03 | ||
| Micronaire value | yM = 0.08SQI + 0.93 | 0.88 | 0.01 | ||
| Soil Layer (cm) | Particle Composition | Soil texture | Bulk Density (g cm−3) | Soil Water Content (cm3 cm−3) | ||||
|---|---|---|---|---|---|---|---|---|
| Clay (%) | Silt (%) | Sand (%) | θWP | θFC | θS | |||
| 0–20 | 12.18 | 79.11 | 8.60 | Silty loam | 1.46 | 0.042 | 0.31 | 0.41 |
| 20–40 | 12.66 | 81.93 | 5.42 | Silty loam | 1.52 | 0.042 | 0.33 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Wang, Q.; Ning, S.; Lin, S.; Hu, X.; Song, Z. Application of Magnetized Ionized Water and Bacillus subtilis Improved Saline Soil Quality and Cotton Productivity. Plants 2024, 13, 2458. https://doi.org/10.3390/plants13172458
Jiang Z, Wang Q, Ning S, Lin S, Hu X, Song Z. Application of Magnetized Ionized Water and Bacillus subtilis Improved Saline Soil Quality and Cotton Productivity. Plants. 2024; 13(17):2458. https://doi.org/10.3390/plants13172458
Chicago/Turabian StyleJiang, Zhanbo, Quanjiu Wang, Songrui Ning, Shudong Lin, Xiaoqin Hu, and Zhaoxin Song. 2024. "Application of Magnetized Ionized Water and Bacillus subtilis Improved Saline Soil Quality and Cotton Productivity" Plants 13, no. 17: 2458. https://doi.org/10.3390/plants13172458







