Agricultural Mitigation Strategies to Reduce the Impact of Romaine Lettuce Contamination
Abstract
1. Introduction
2. Results and Discussion
2.1. Screening for E. coli and Salmonella
2.2. Quantification of Heavy Metals
2.3. Influence of E. coli Contamination on Seed Germination
2.4. Effect of HMs on Bacterial Growth
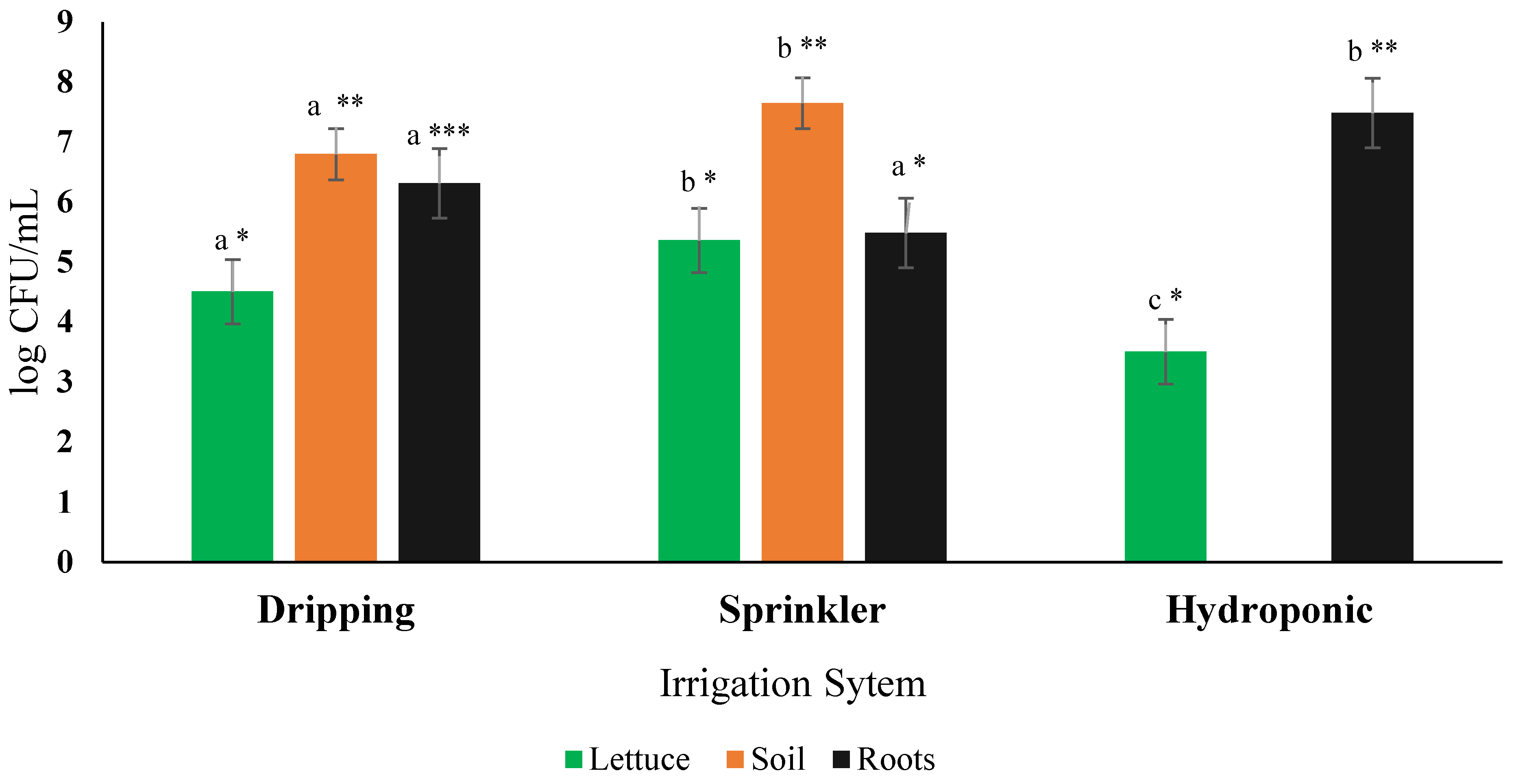
2.5. Testing the Effect of Irrigation Systems on E. coli Contamination
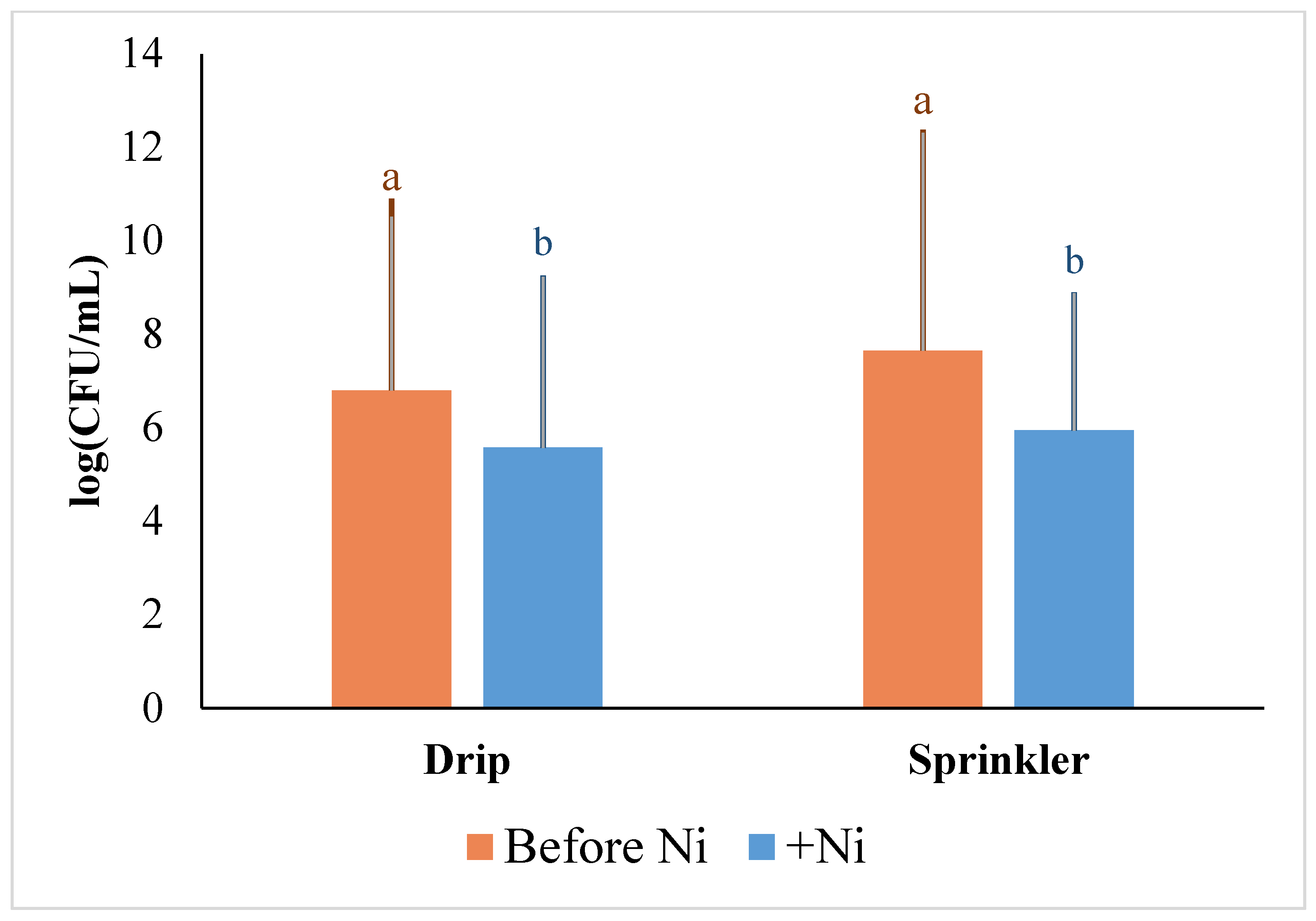
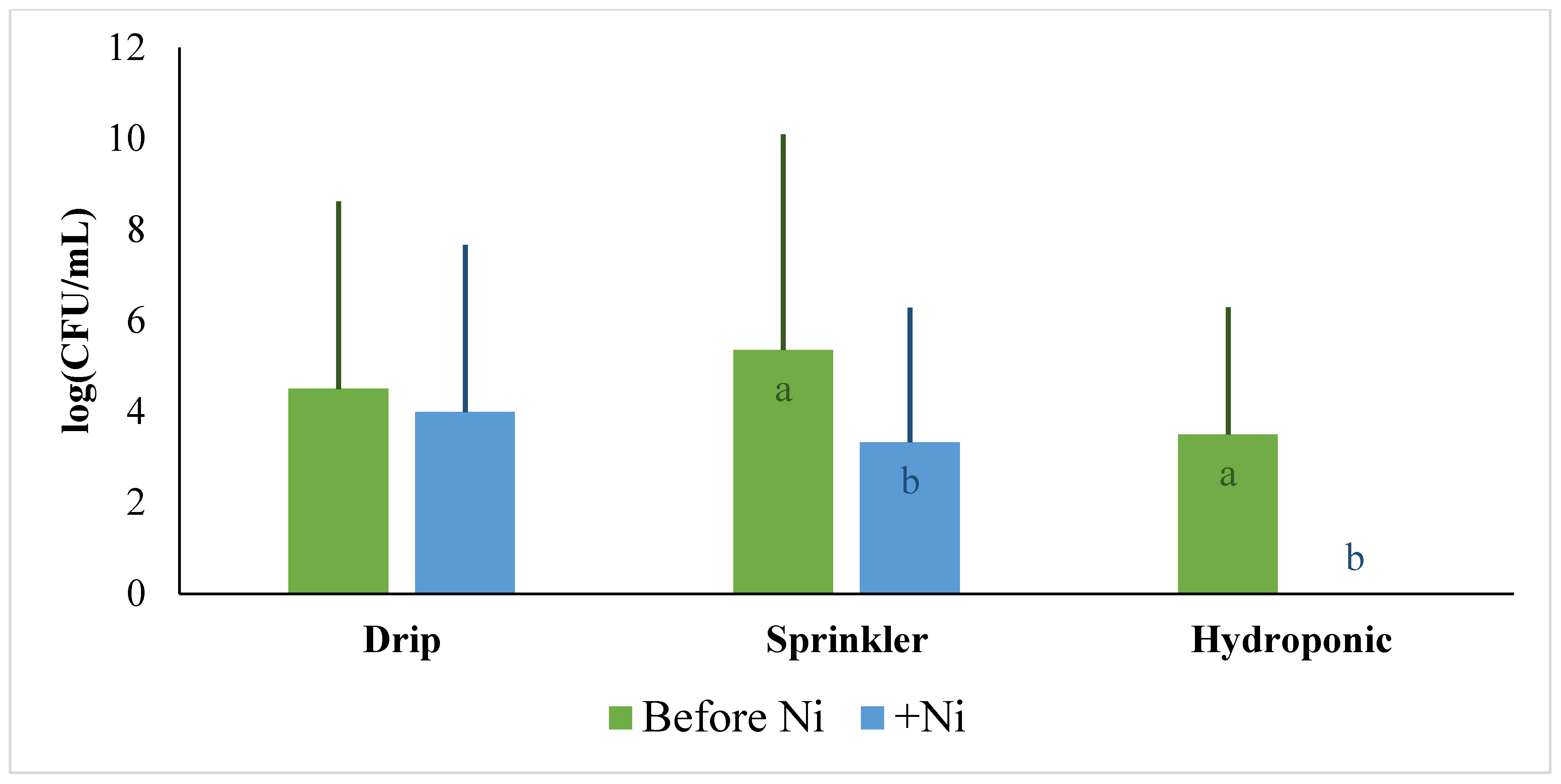
2.6. Effect of High Levels of Nickel on E. coli Survival in the Environment
3. Materials and Methods
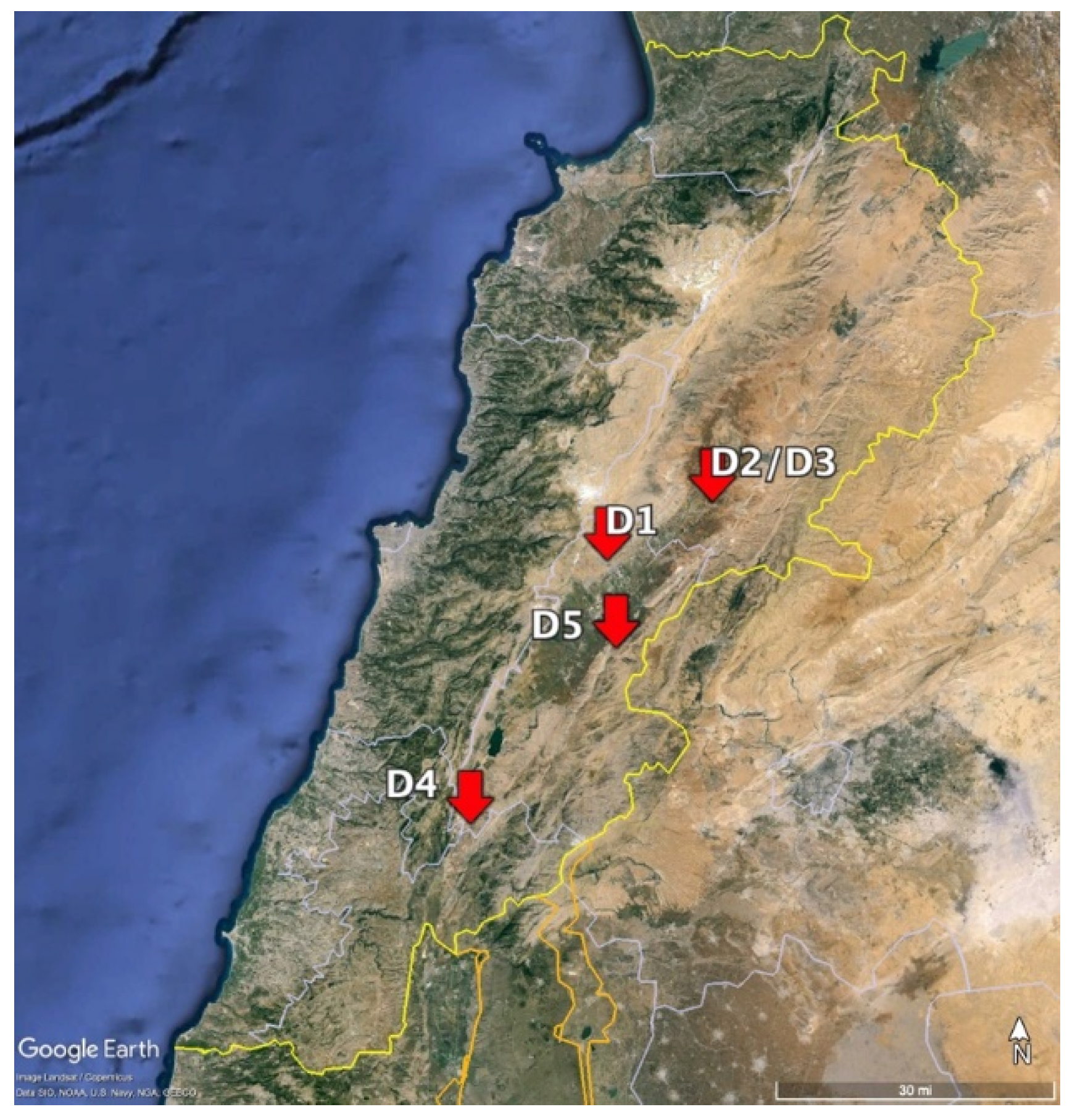
3.1. Demonstration Plot Selection and Sample Collection
3.2. Microbiological and Chemical Analysis
3.2.1. E. coli and Salmonella Detection
3.2.2. Heavy Metal Quantification
3.3. Effect of E. coli on Seed Germination
3.4. Effect of HMs on Bacterial Growth
3.4.1. Preparation of the Isolates
3.4.2. Preparation of the HM Solutions
3.4.3. Preparation of the Solutions Containing HMs and E. coli
3.5. Effect of the Irrigation Systems on Bacterial Contamination
3.5.1. Preparation of the Irrigation Water
3.5.2. Comparison between Irrigation Systems
3.5.3. Effect of Nickel on E. coli’s Presence
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Food Safety; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Castello, A.; Lo Cascio, G.; Ferraro, C.; Pantano, L.; Costa, A.; Butera, G.; Oliveri, G.; Rizzuto, M.L.; Alduina, R.; Cardamone, C. Food Risk Associated with Vegetable Consumption, Exposure to Antimicrobial-Resistant Strains and Pesticide Residues. Ital. J. Food Saf. 2023, 12, 11134. [Google Scholar] [CrossRef]
- FAO/WHO. Microbiological Hazards in Fresh Leafy Vegetables and Herbs—Meeting Report; Microbiological Risk Assessment Series; FAO/WHO: Rome, Italy, 2008; ISBN 978-92-4-156378-9. [Google Scholar]
- Kowalska, B.; Szczech, M. Differences in Microbiological Quality of Leafy Green Vegetables. Ann. Agric. Environ. Med. AAEM 2022, 29, 238–245. [Google Scholar] [CrossRef]
- Shehu, K.; Maishanu, A.M.; Salau, I.A. A Preliminary Study on Microbial Contamination of Leafy Vegetables in Sokoto Metropolis, Nigeria. Aceh Int. J. Sci. Technol. 2014, 3, 140–144. [Google Scholar] [CrossRef]
- Nousiainen, L.-L.; Joutsen, S.; Lunden, J.; Hänninen, M.-L.; Fredriksson-Ahomaa, M. Bacterial Quality and Safety of Packaged Fresh Leafy Vegetables at the Retail Level in Finland. Int. J. Food Microbiol. 2016, 232, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, M.; Ali, H.M.; Al-Omairi, M. The Safety of Leafy Vegetables in Oman. Am. J. Food Sci. Technol. 2024, 3, 42–50. [Google Scholar] [CrossRef]
- Halablab, M.A.; Sheet, I.H.; Holail, H.M. Microbiological Quality of Raw Vegetables Grown in Bekaa Valley, Lebanon. Am. J. Food Technol. 2010, 6, 129–139. [Google Scholar] [CrossRef]
- Al-Chaarani, N.; El-Nakat, J.; Obeid, P.; Aouad, S. Measurement of Levels of Heavy Metal Contamination in Vegetables Grown and Sold in Selected Areas in Lebanon. Jordan J. Chem. 2009, 4, 303–315. [Google Scholar]
- Turner, K.; Moua, C.N.; Hajmeer, M.; Barnes, A.; Needham, M. Overview of Leafy Greens–Related Food Safety Incidents with a California Link: 1996 to 2016. J. Food Prot. 2019, 82, 405–414. [Google Scholar] [CrossRef]
- FDA. Leafy Greens STEC Action Plan; FDA: Silver Spring, MD, USA, 2024. [Google Scholar]
- Yang, X.; Scharff, R. Foodborne Illnesses from Leafy Greens in the United States: Attribution, Burden, and Cost. J. Food Prot. 2024, 87, 100275. [Google Scholar] [CrossRef]
- Wright, K.M.; Chapman, S.; McGeachy, K.; Humphris, S.; Campbell, E.; Toth, I.K.; Holden, N.J. The Endophytic Lifestyle of Escherichia Coli O157:H7: Quantification and Internal Localization in Roots. Phytopathology® 2013, 103, 333–340. [Google Scholar] [CrossRef]
- Hou, Z.; Fink, R.C.; Radtke, C.; Sadowsky, M.J.; Diez-Gonzalez, F. Incidence of Naturally Internalized Bacteria in Lettuce Leaves. Int. J. Food Microbiol. 2013, 162, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Grivokostopoulos, N.C.; Makariti, I.P.; Tsadaris, S.; Skandamis, P.N. Impact of Population Density and Stress Adaptation on the Internalization of Salmonella in Leafy Greens. Food Microbiol. 2022, 106, 104053. [Google Scholar] [CrossRef]
- Akbar, A.; Anal, A.K. Food Safety Concerns and Food-Borne Pathogens, Slamonella, Escherichia coli and Campylobacter. FUUAST J. Biol. 2011, 1, 5–17. [Google Scholar]
- Chen, J.C.; Patel, K.; Smith, P.A.; Vidyaprakash, E.; Snyder, C.; Tagg, K.A.; Webb, H.E.; Schroeder, M.N.; Katz, L.S.; Rowe, L.A.; et al. Reoccurring Escherichia coli O157:H7 Strain Linked to Leafy Greens–Associated Outbreaks, 2016–2019. Emerg. Infect. Dis. J. 2023, 29, 1895–1899. [Google Scholar] [CrossRef]
- McClure, M.; Whitney, B.; Gardenhire, I.; Crosby, A.; Wellman, A.; Patel, K.; McCormic, Z.D.; Gieraltowski, L.; Gollarza, L.; Low, M.S.F.; et al. An Outbreak Investigation of Salmonella Typhimurium Illnesses in the United States Linked to Packaged Leafy Greens Produced at a Controlled Environment Agriculture Indoor Hydroponic Operation—2021. J. Food Prot. 2023, 86, 100079. [Google Scholar] [CrossRef]
- Abi Saab, M.T.; Jomaa, I.; El Hage, R.; Skaf, S.; Fahed, S.; Rizk, Z.; Massaad, R.; Romanos, D.; Khairallah, Y.; Azzi, V.; et al. Are Fresh Water and Reclaimed Water Safe for Vegetable Irrigation? Empirical Evidence from Lebanon. Water 2022, 14, 1437. [Google Scholar] [CrossRef]
- Faour-Klingbeil, D.; Murtada, M.; Kuri, V.; Todd, E.C.D. Understanding the Routes of Contamination of Ready-to-Eat Vegetables in the Middle East. Food Control 2016, 62, 125–133. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Fatema-Tuj-Zohra; Mahdi, M.M.; Nurnabi, M.; Alam, M.Z.; Choudhury, T.R. Health Risk Assessment for Heavy Metal Accumulation in Leafy Vegetables Grown on Tannery Effluent Contaminated Soil. Toxicol. Rep. 2022, 9, 346–355. [Google Scholar] [CrossRef]
- Hassan, J.; Rajib, M.M.R.; Khan, M.N.-E.-A.; Khandaker, S.; Zubayer, M.; Ashab, K.R.; Kuba, T.; Marwani, H.M.; Asiri, A.M.; Hasan, M.M.; et al. Assessment of Heavy Metals Accumulation by Vegetables Irrigated with Different Stages of Textile Wastewater for Evaluation of Food and Health Risk. J. Environ. Manag. 2024, 353, 120206. [Google Scholar] [CrossRef]
- UNEP. A Snapshot of the World’s Water Quality: Towards a Global Assessment; UNEP: Nairobi, Kenya, 2016. [Google Scholar]
- Cui, Y.; Zhu, Y.-G.; Zhai, R.; Huang, Y.; Qiu, Y.; Liang, J. Exposure to Metal Mixtures and Human Health Impacts in a Contaminated Area in Nanning, China. Environ. Int. 2005, 31, 784–790. [Google Scholar] [CrossRef]
- Intawongse, M.; Dean, J.R. Uptake of Heavy Metals by Vegetable Plants Grown on Contaminated Soil and Their Bioavailability in the Human Gastrointestinal Tract. Food Addit. Contam. 2006, 23, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Ferri, R.; Donna, F.; Smith, D.R.; Guazzetti, S.; Zacco, A.; Rizzo, L.; Bontempi, E.; Zimmerman, N.J.; Lucchini, R.G. Heavy Metals in Soil and Salad in the Proximity of Historical Ferroalloy Emission. J. Environ. Prot. 2012, 3, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Begum, A.; Harikrishna, S. Pathogens and Heavy Metals Concentration in Green Leafy Vegetables. J. Chem. 2010, 7, 741815. [Google Scholar] [CrossRef]
- Mathur, J.; Chauhan, P. Mechanism of Toxic Metal Uptake and Transport in Plants. In Sustainable Solutions for Elemental Deficiency and Excess in Crop Plants; Mishra, K., Tandon, P.K., Srivastava, S., Eds.; Springer: Singapore, 2020; pp. 335–349. ISBN 9789811586361. [Google Scholar]
- Darwish, T.; Shaban, A.; Masih, I.; Jaafar, H.; Jomaa, I.; Simaika, J.P. Sustaining the Ecological Functions of the Litani River Basin, Lebanon. Int. J. River Basin Manag. 2023, 21, 37–51. [Google Scholar] [CrossRef]
- Haydar, C.M.; Nehme, N.; Awad, S.; Koubaissy, B.; Fakih, M.; Yaacoub, A.; Toufaily, J.; Villeras, F.; Hamieh, T. Water Quality of the Upper Litani River Basin, Lebanon. Phys. Procedia 2014, 55, 279–284. [Google Scholar] [CrossRef]
- Mcheik, A.; Awad, A.; Fadel, A.; Mounzer, C.; Nasreddine, S. Effect of Irrigation Water Quality on the Microbial Contamination of Fresh Vegetables in the Bekaa Valley, Lebanon. Am. J. Agric. For. 2018, 6, 191–197. [Google Scholar] [CrossRef]
- Malaeb, M.; Bizri, A.R.; Ghosn, N.; Berry, A.; Musharrafieh, U. Salmonella Burden in Lebanon. Epidemiol. Infect. 2016, 144, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Faour-Klingbeil, D.; Kuri, V.; Fadlallah, S.; Matar, G. Prevalence of Antimicrobial-Resistant Escherichia coli from Raw Vegetables in Lebanon. J. Infect. Dev. Ctries. 2016, 10, 354–362. [Google Scholar] [CrossRef]
- FAO. Pesticides Contamination and Exposure Reduction; FAO: Rome, Italy, 2022. [Google Scholar]
- Sarré, F.B.; Dièye, Y.; Seck, A.M.; Fall, C.; Dieng, M. High Level of Salmonella Contamination of Leafy Vegetables Sold around the Niayes Zone of Senegal. Horticulturae 2023, 9, 97. [Google Scholar] [CrossRef]
- Aytaç, S.; Ben, U.; Cengiz, C.; Mercanoglu Taban, B. Evaluation of Salmonella and Listeria Monocytogenes Contamination on Leafy Green Vegetables. J. Food Agric. Environ. 2010, 8, 275–279. [Google Scholar]
- Priyanka; Meena, P.R.; Meghwanshi, K.K.; Rana, A.; Singh, A.P. Leafy Greens as a Potential Source of Multidrug-Resistant Diarrhoeagenic Escherichia coli and Salmonella. Microbiology 2021, 167, 001059. [Google Scholar] [CrossRef] [PubMed]
- Faour-Klingbeil, D. Food Safety in Beirut—Links between Microbiological Quality of Fresh Vegetables and Knowledge, Attitudes and Practices of Food Handlers; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Mohammed, H.; Tveten, A.-K.; Seidu, R. Modelling the Impact of Climate Change on Flow and E. coli Concentration in the Catchment of an Ungauged Drinking Water Source in Norway. J. Hydrol. 2019, 573, 676–687. [Google Scholar] [CrossRef]
- Petersen, F.; Hubbart, J.A. Physical Factors Impacting the Survival and Occurrence of Escherichia coli in Secondary Habitats. Water 2020, 12, 1796. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Erickstad, M.; Tadrist, L.; Ronan, E.; Gutierrez, E.; Wong-Ng, J.; Groisman, A. Aggregation Temperature of Escherichia coli Depends on Steepness of the Thermal Gradient. Biophys. J. 2020, 118, 2816–2828. [Google Scholar] [CrossRef]
- Percival, S.L.; Williams, D.W. Chapter Six—Escherichia coli. In Microbiology of Waterborne Diseases, 2nd ed.; Percival, S.L., Yates, M.V., Williams, D.W., Chalmers, R.M., Gray, N.F., Eds.; Academic Press: London, UK, 2014; pp. 89–117. ISBN 978-0-12-415846-7. [Google Scholar]
- Geara-Matta, D.; Moilleron, R.; El Samarani, A.; Lorgeoux, C.; Chebbo, G. State of Art about Water Uses and Wastewater Management in Lebanon. In World Wide Workshop for Young Environmental Scientists: 2010; Thevenot, D., Ed.; HAL: Arcueil, France, 2010; Volume WWW-YES-2010. [Google Scholar]
- Huang, G.-Z.; Hsu, T.-C.; Yu, C.-K.; Huang, J.-C.; Lin, T.-C. Dilution and Precipitation Dominated Regulation of Stream Water Chemistry of a Volcanic Watershed. J. Hydrol. 2020, 583, 124564. [Google Scholar] [CrossRef]
- Maphanga, T.; Madonsela, B.S.; Chidi, B.S.; Shale, K.; Munjonji, L.; Lekata, S. The Effect of Rainfall on Escherichia coli and Chemical Oxygen Demand in the Effluent Discharge from the Crocodile River Wastewater Treatment; South Africa. Water 2022, 14, 2802. [Google Scholar] [CrossRef]
- Ramamurthy, T.; Ghosh, A.; Pazhani, G.P.; Shinoda, S. Current Perspectives on Viable but Non-Culturable (VBNC) Pathogenic Bacteria. Front. Public Health 2014, 2, 103. [Google Scholar] [CrossRef]
- Klein, T.M.; Alexander, M. Bacterial Inhibitors in Lake Water. Appl. Environ. Microbiol. 1986, 52, 114–118. [Google Scholar] [CrossRef]
- Tien, Y.-C.; Li, B.; Zhang, T.; Scott, A.; Murray, R.; Sabourin, L.; Marti, R.; Topp, E. Impact of Dairy Manure Pre-Application Treatment on Manure Composition, Soil Dynamics of Antibiotic Resistance Genes, and Abundance of Antibiotic-Resistance Genes on Vegetables at Harvest. Sci. Total Environ. 2017, 581–582, 32–39. [Google Scholar] [CrossRef]
- Bushen, A.; Tekalign, E.; Abayneh, M. Drug- and Multidrug-Resistance Pattern of Enterobacteriaceae Isolated from Droppings of Healthy Chickens on a Poultry Farm in Southwest Ethiopia. Infect. Drug Resist. 2021, 14, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Mounzer, C.K.; Baydoun, S.A.; Amer, R.A.; Borjac, J.M. Occurrence of Antibiotics and Antibiotic-Resistant Bacteria in the Lebanese Polluted Litani River. Environ. Monit. Assess. 2023, 196, 90. [Google Scholar] [CrossRef] [PubMed]
- Wolters, Claire Why Do Lettuce and Spinach Keep Getting Contaminated with E. coli? Available online: https://www.verywellhealth.com/lettuce-e-coli-contamination-6542307 (accessed on 6 November 2023).
- CDC Outbreak of E. coli Infections Linked to Leafy Greens|CDC. Available online: https://www.cdc.gov/ecoli/2020/o157h7-10-20b/index.html (accessed on 6 November 2023).
- Haydar, C.M.; Tarawneh, K.; Nehme, N.; Amaireh, M.; Yaacoub, A.; Diab, W. Heavy Metals Content in Water and Sediments in the upper Litani River Basin, Lebanon. J. Geosci. Environ. Prot. 2022, 10, 139–158. [Google Scholar] [CrossRef]
- Ahmad, M.S.A.; Ashraf, M. Essential Roles and Hazardous Effects of Nickel in Plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2011; pp. 125–167. ISBN 978-1-4614-0668-6. [Google Scholar]
- Ali, U.; Bashir, S.; Shaaban, M.; Zhou, X.; Gao, R.; Zhu, J.; Fu, Q.; Hu, H. Influence of Various Passivators for Nickel Immobilization in Contaminated Soil of China. Environ. Eng. Sci. 2019, 36, 1396–1403. [Google Scholar] [CrossRef]
- Lopez, S.; Goux, X.; van der Ent, A.; Erskine, P.D.; Echevarria, G.; Calusinska, M.; Morel, J.L.; Benizri, E. Bacterial Community Diversity in the Rhizosphere of Nickel Hyperaccumulator Species of Halmahera Island (Indonesia). Appl. Soil Ecol. 2019, 133, 70–80. [Google Scholar] [CrossRef]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in Soils, Water and Food Crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. Enrichment of Cereal Grains with Zinc: Agronomic or Genetic Biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Dirani, L. Heavy Metal Contamination in Food Crops in Lebanon: Comparative Assessment of Pollution Using Atomic Absorption and Hyperspectral Imaging. Ph.D. Thesis, American University of Beirut, Beirut, Lebanon, 2023. [Google Scholar]
- FAO/WHO. Joint FAO/WHO Standards Programme. Codex Committee on Contaminants in Foods; FAO/WHO: Rome, Italy, 2021. [Google Scholar]
- Dala-Paula, B.M.; Custódio, F.B.; Knupp, E.A.N.; Palmieri, H.E.L.; Silva, J.B.B.; Glória, M.B.A. Cadmium, Copper and Lead Levels in Different Cultivars of Lettuce and Soil from Urban Agriculture. Environ. Pollut. 2018, 242, 383–389. [Google Scholar] [CrossRef]
- Gharbi, F.; Rejeb, S.; Ghorbal, M.H.; Morel, J.L. Plant Response to Copper Toxicity as Affected by Plant Species and Soil Type. J. Plant Nutr. 2005, 28, 379–392. [Google Scholar] [CrossRef]
- Najmi, A.; Albratty, M.; Al-Rajab, A.J.; Alhazmi, H.A.; Javed, S.A.; Ahsan, W.; Rehman, Z.U.; Hassani, R.; Alqahtani, S.S. Heavy Metal Contamination in Leafy Vegetables Grown in Jazan Region of Saudi Arabia: Assessment of Possible Human Health Hazards. Int. J. Environ. Res. Public. Health 2023, 20, 2984. [Google Scholar] [CrossRef]
- Fadel, D.; Kelepertzis, E.; Argyraki, A.; Bakir, A. Potentially Harmful Elements in Lebanese Fattoush Salad; ResearchGate: Berlin, Germany, 2019. [Google Scholar]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Darwish, T.; Jooma, I.; Awad, M.; AbouDaher, M.; Msann, J. Inventory and Management of Lebanese Soils Integrating the Soil Geographical Database of Euro-Mediterranean Countries. Leban. Sci. J. 2005, 6, 57–70. [Google Scholar]
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and their Bioavailability; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 11–50. ISBN 978-94-007-4470-7. [Google Scholar]
- Stevenson, F.J.; Cole, M.A. Cycles of Soils: Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients; John Wiley & Sons: Hoboken, NJ, USA, 1999; ISBN 978-0-471-32071-5. [Google Scholar]
- Dixit, A.R.; Khodadad, C.L.M.; Hummerick, M.E. Persistence of Escherichia coli in the Microbiomes of Red Romaine Lettuce (Lactuca sativa cv. ‘outredgeous’) and Mizuna Mustard (Brassica rapa var. japonica)—Does Seed Sanitization Matter? BMC Microbiol. 2021, 21, 289. [Google Scholar] [CrossRef]
- Wright, K.M.; Wright, P.J.; Holden, N.J. Plant Species-Dependent Transmission of Escherichia coli O157:H7 from the Spermosphere to Cotyledons and First Leaves. Environ. Microbiol. Rep. 2022, 14, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Čapek, J.; Večerek, B. Why Is Manganese so Valuable to Bacterial Pathogens? Front. Cell. Infect. Microbiol. 2023, 13, 943390. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Waters, L.S.; Storz, G.; Imlay, J.A. The Escherichia coli Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese. PLoS Genet. 2015, 11, e1004977. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.K.; Byrd, I.W.; Boedeker, E.C. Virulence Inhibition by Zinc in Shiga-Toxigenic Escherichia coli. Infect. Immun. 2011, 79, 1696–1705. [Google Scholar] [CrossRef][Green Version]
- Crane, J.K.; Broome, J.E.; Reddinger, R.M.; Werth, B.B. Zinc Protects against Shiga-Toxigenic Escherichia coli by Acting on Host Tissues as Well as on Bacteria. BMC Microbiol. 2014, 14, 145. [Google Scholar] [CrossRef]
- Uemura, R.; Katsuge, T.; Sasaki, Y.; Goto, S.; Sueyoshi, M. Effects of Zinc Supplementation on Shiga Toxin 2e-Producing Escherichia coli in Vitro. J. Vet. Med. Sci. 2017, 79, 1637–1643. [Google Scholar] [CrossRef]
- Velasco, E.; Wang, S.; Sanet, M.; Fernández-Vázquez, J.; Jové, D.; Glaría, E.; Valledor, A.F.; O’Halloran, T.V.; Balsalobre, C. A New Role for Zinc Limitation in Bacterial Pathogenicity: Modulation of α-Hemolysin from Uropathogenic Escherichia coli. Sci. Rep. 2018, 8, 6535. [Google Scholar] [CrossRef]
- Chang, Y.; Mei, J.; Yang, T.; Zhang, Z.; Liu, G.; Zhao, H.; Chen, X.; Tian, G.; Cai, J.; Wu, B.; et al. Effect of Dietary Zinc Methionine Supplementation on Growth Performance, Immune Function and Intestinal Health of Cherry Valley Ducks Challenged with Avian Pathogenic Escherichia coli. Front. Microbiol. 2022, 13, 849067. [Google Scholar] [CrossRef] [PubMed]
- Samanovic, M.I.; Ding, C.; Thiele, D.J.; Darwin, K.H. Copper in Microbial Pathogenesis: Meddling with the Metal. Cell Host Microbe 2012, 11, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Jara, A.; Cordero, N.; Aguirre, J.; Troncoso, M.; Figueroa, G. Antibacterial Effect of Copper on Microorganisms Isolated from Bovine Mastitis. Front. Microbiol. 2016, 7, 626. [Google Scholar] [CrossRef] [PubMed]
- Moreira Martins, P.M.; Gong, T.; de Souza, A.A.; Wood, T.K. Copper Kills Escherichia coli Persister Cells. Antibiotics 2020, 9, 506. [Google Scholar] [CrossRef]
- Ford, G. Iron Uptake and Accumulation Is a Target of Nickel Toxicity during the Lag Phase in Escherichia coli. Ph.D. Thesis, University of South Carolina, Columbia, SC, USA, 2016. [Google Scholar]
- Washington-Hughes, C.L.; Ford, G.T.; Jones, A.D.; McRae, K.; Outten, F.W. Nickel Exposure Reduces Enterobactin Production in Escherichia coli. MicrobiologyOpen 2018, 8, e00691. [Google Scholar] [CrossRef]
- Mahalakshmi, T.; Ilamathi, M.; Siva, R.; Sridharan, T.B. Effect of Nickel Stress on Escherichia coli and Saccharomyces cerevisiae. J. Ind. Pollut. Control 2010, 26, 5–13. [Google Scholar]
- EFSA. Scientific Opinion on the Risk Posed by Pathogens in Food of Non-Animal Origin. Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/j.efsa.2014.3600 (accessed on 7 November 2023).
- Esteves, R.G.R.; Gerba, C.P.; Slack, D.C. Evaluation of Subsurface Irrigation on Microorganism Contamination of Romaine Lettuce. E3S Web Conf. 2020, 187, 04009. [Google Scholar] [CrossRef]
- Pérez-Lavalle, L.; Carrasco, E.; Vallesquino-Laguna, P.; Cejudo-Gómez, M.; Posada-Izquierdo, G.D.; Valero, A. Internalization Capacity of Salmonella enterica Sv Thompson in Strawberry Plants via Root. Food Control 2021, 126, 108080. [Google Scholar] [CrossRef]
- Gomes, C.; Da Silva, P.; Moreira, R.G.; Castell-Perez, E.; Ellis, E.A.; Pendleton, M. Understanding E. coli Internalization in Lettuce Leaves for Optimization of Irradiation Treatment. Int. J. Food Microbiol. 2009, 135, 238–247. [Google Scholar] [CrossRef]
- Saldaña, Z.; Sánchez, E.; Xicohtencatl-Cortes, J.; Puente, J.L.; Giron, J.A. Surface Structures Involved in Plant Stomata and Leaf Colonization by Shiga-Toxigenic Escherichia coli O157:H7. Front. Microbiol. 2011, 2, 7506. [Google Scholar] [CrossRef]
- Hirneisen, K.A.; Sharma, M.; Kniel, K.E. Human Enteric Pathogen Internalization by Root Uptake into Food Crops. Foodborne Pathog. Dis. 2012, 9, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.M.; Crozier, L.; Marshall, J.; Merget, B.; Holmes, A.; Holden, N.J. Differences in Internalization and Growth of Escherichia coli O157:H7 within the Apoplast of Edible Plants, Spinach and Lettuce, Compared with the Model Species Nicotiana benthamiana. Microb. Biotechnol. 2017, 10, 555–569. [Google Scholar] [CrossRef] [PubMed]
- NandaKafle, G.; Christie, A.A.; Vilain, S.; Brözel, V.S. Growth and Extended Survival of Escherichia coli O157:H7 in Soil Organic Matter. Front. Microbiol. 2018, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Zulfiqar, U.; Mumtaz, M.Z.; Radziemska, M.; Haider, F.U.; Holatko, J.; Hammershmiedt, T.; Naveed, M.; Ali, H.; Kintl, A.; et al. Nickel (Ni) Phytotoxicity and Detoxification Mechanisms: A Review. Chemosphere 2023, 328, 138574. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Chen, Q.; White, J.F. Evaluation of Colonization and Mutualistic Endophytic Symbiosis of Escherichia coli with Tomato and Bermuda Grass Seedlings. PeerJ 2022, 10, e13879. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]

| Water | Soil | Lettuce | |||
|---|---|---|---|---|---|
| Demo Plot | Pre-Planting | Harvest | Pre-Planting | Harvest | Harvest |
| D1 | + | − | − | − | − |
| D2 | + | + | − | − | − |
| D3 | + | − | − | + | + |
| D4 | − | + | − (G1/G3) | − (G1/G3) | − (G1/G3) |
| + (G2/G4) | + (G2/G4) | + (G2/G4) | |||
| D5 | − | + | + | + | + |
| Time | Sample | Location | Ni | Zn | Cu | Mn |
|---|---|---|---|---|---|---|
| Pre-planting | Soil | D1 | 0.89 ± 0.02 a | 2.05 ± 0.02 a | 0.94 ± 0.06 b | 12.4 ± 0.4 b |
| D2 | 1.26 ± 0.09 a | 2.82 ± 0.98 a | 1.66 ± 0.22 ab | 144 ± 15 a | ||
| D3 | 1.29 ± 0.08 a | 2.90 ± 0.59 a | 1.62 ± 0.15 ab | 135.6 ± 9.5 a | ||
| D4 | 0.42 ± 0.04 b | 0.80 ± 0.08 b | 1.33 ± 0.10 b | 7.5 ± 0.7 c | ||
| D5 | 0.32 ± 0.03 b | 2.32 ± 0.41 a | 1.93 ± 0.14 a | 11.6 ± 0.8 b | ||
| Final | Soil | D1 | 0.98 ± 0.03 a | 1.99 ± 0.55 b | 1.05 ± 0.04 c | 12.7 ± 0.7 b |
| D2 | 1.14 ± 0.20 a | 3.99 ± 0.68 a | 1.90 ± 0.16 a | 100 ± 18 a | ||
| D3 | 0.96 ± 0.17 a | 3.49 ± 0.36 a | 1.88 ± 0.25 a | 116 ± 8 a | ||
| D4 | 0.40 ± 0.04 b | 0.78 ± 0.05 c | 1.36 ± 0.08 bc | 7.8 ± 1.0 c | ||
| D5 | 0.27 ± 0.02 b | 1.81 ± 0.33 b | 1.74 ± 0.16 ab | 5.3 ± 0.5 c | ||
| Lettuce | D1 | 1.16 ± 0.07 b | 16.7 ± 1.6 d | 12.1 ± 2.4 a | 61.7 ± 7.2 c | |
| D2 | 0.54 ± 0.03 c | 170 ± 11 a | 10.4 ± 2.6 b | 118.0 ± 4.9 b | ||
| D3 | 1.08 ± 0.20 b | 23.7 ± 5.9 c | 8.36 ± 0.66 b | 107.5 ± 7.9 b | ||
| D4 | 1.33 ± 0.27 ab | 45.2 ± 2.9 b | 10.3 ± 0.36 a | 163 ± 10 a | ||
| D5 | 1.73 ± 0.34 a | 25.5 ± 2.2 c | 7.57 ± 0.55 b | 112.6 ± 9.9 b |
| Plot ID | Location | GPS Location | Setup | Irrigation Type | Growing Season | Lettuce Planted: Seeds or Seedlings |
|---|---|---|---|---|---|---|
| D1 | Zahle | 33.84675, 35.90203 | Open field | Drip | October 2022–January 2023 | Seedlings |
| D2 | Talya | 33.9366930, 36.0968463 | Greenhouse | Drip | October 2022–January 2023 | Seedlings |
| D3 | Talya | 33.9366930, 36.0968463 | Greenhouse | Drip | December 2022–March 2023 | Seedlings |
| D4 | Lousse | 33.441124, 35.6493756 | Greenhouse | Drip | July–September 2023 | Seedlings |
| D5 | Majdal Anjar | 33.7123210, 35.9182159 | Open field | Sprinkler | August–November 2023 | Seeds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Kayal, W.; Darwiche, L.; Farhat, Y.A.; Hdeib, M.; AlJardaly, R.; Shbaro, M.; Iskandar, C.F. Agricultural Mitigation Strategies to Reduce the Impact of Romaine Lettuce Contamination. Plants 2024, 13, 2460. https://doi.org/10.3390/plants13172460
El Kayal W, Darwiche L, Farhat YA, Hdeib M, AlJardaly R, Shbaro M, Iskandar CF. Agricultural Mitigation Strategies to Reduce the Impact of Romaine Lettuce Contamination. Plants. 2024; 13(17):2460. https://doi.org/10.3390/plants13172460
Chicago/Turabian StyleEl Kayal, Walid, Linda Darwiche, Yasmine A. Farhat, Mariane Hdeib, Roaa AlJardaly, Mostapha Shbaro, and Christelle F. Iskandar. 2024. "Agricultural Mitigation Strategies to Reduce the Impact of Romaine Lettuce Contamination" Plants 13, no. 17: 2460. https://doi.org/10.3390/plants13172460
APA StyleEl Kayal, W., Darwiche, L., Farhat, Y. A., Hdeib, M., AlJardaly, R., Shbaro, M., & Iskandar, C. F. (2024). Agricultural Mitigation Strategies to Reduce the Impact of Romaine Lettuce Contamination. Plants, 13(17), 2460. https://doi.org/10.3390/plants13172460






