Contrasting Retromer with a Newly Described Retriever in Arabidopsis thaliana
Abstract
1. Introduction
2. Fates of Endocytosed Cargo
3. Retromer in Yeast and Mammals
4. Plasma Membrane Protein Recycling by Retromer and Retriever
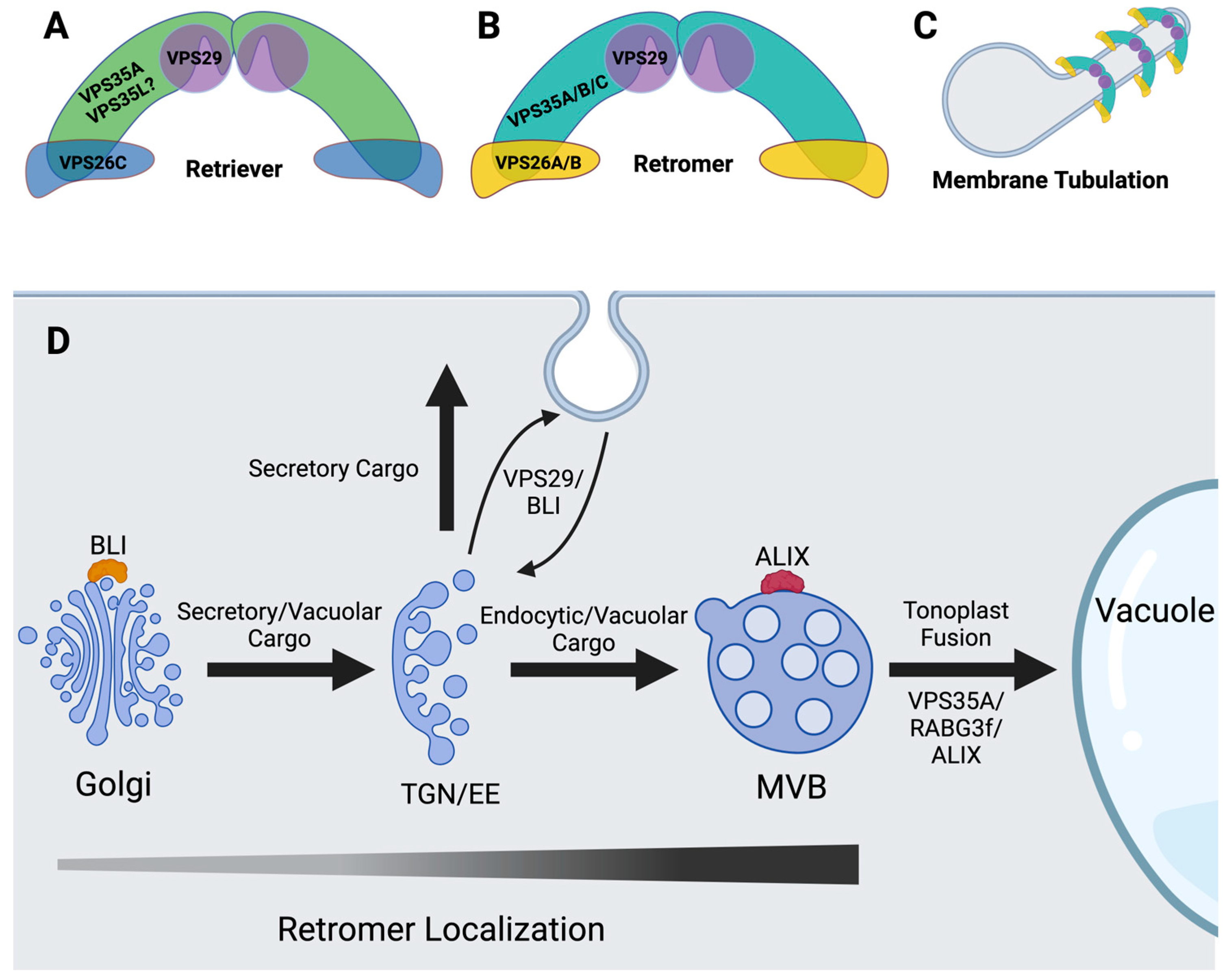
5. Plant Retromer and Retriever
6. Retromer-Binding Proteins
7. Is There a Retriever Complex in Plants?
8. Genetic Interactions between VTI11- and VTI13-Dependent Pathways to the Lytic Vacuole and Retromer/Retriever Function in Plants
9. Future Directions
- Is retriever involved in the recycling of plasma membrane proteins in plants and what are its cargoes?
- BLISTER and ALIX associate with retromer in Arabidopsis. What other proteins are associated with core retromer and retriever complexes in plants to modify/regulate their function?
- Do CCDC22 and CCDC93 associate with retriever alone or are they also involved in retromer function?
- Do CCDC22 and CCDC93 interact with other protein complexes in plants to compensate for the loss of COMMD proteins in seed plants?
- Are specific versions of the VPS35/29/26 complex required for cell-type-specific endosomal trafficking in plants?
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALIX | Apoptosis-linked gene-2-interacting protein X |
| BAR | Bin-Amphiphysin-Rvs |
| BLI | BLISTER |
| CCDC | Coiled-Coil Domain-Containing |
| CI-MPR | Cation-independent mannose 6-phosphate receptor |
| CLASP1 | Cytoplasmic linker-associated protein 1 |
| COMMD | Commander |
| CPY | Carboxypeptidase Y |
| DSCR3 | Down syndrome critical region 3 |
| EE | Early endosome |
| ER | Endoplasmic reticulum |
| ESCRT | Endosomal sorting complex required for transport |
| FREE1 | FYVE DOMAIN PROTEIN REQUIRED FOR ENDOSOMAL SORTING 1 |
| GEF | Guanine nucleotide exchange factor |
| GTPase | Guanosine triphosphate-activating protein |
| ILV | Intraluminal vesicles |
| LE | Late endosome |
| MBV | Multivesicular body |
| PI3P | Phosphatidylinositol 3-phosphate |
| PIN1 | Pin-formed |
| RAB | ras-associated binding |
| RFP | Red fluorescent protein |
| SCAR | Suppressor of cAMP receptor |
| SNX | Sorting nexin |
| TGN | trans-Golgi network |
| VPS | Vacuolar protein sorting |
| VSR | Vacuolar sorting receptor |
| VTI | VPS10 interacting |
| WASH | Wiskott–Aldrich syndrome |
References
- Fisher, T.J.; Flores-Sandoval, E.; Alvarez, J.P.; Bowman, J.L. PIN-FORMED is required for shoot phototropism/gravitropism and facilitates meristem formation in Marchantia polymorpha. New Phytol. 2023, 238, 1498–1515. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.G.; Larson, E.R.; Humble, J.; Domozych, D.S.; Barrington, D.S.; Tierney, M.L. Vacuolar Protein Sorting 26C encodes an evolutionarily conserved large retromer subunit in eukaryotes that is important for root hair growth in Arabidopsis thaliana. Plant J. 2018, 94, 595–611. [Google Scholar] [CrossRef]
- Szumlanski, A.L.; Nielsen, E. The Rab GTPase RabA4d regulates pollen tube tip growth in Arabidopsis thaliana. Plant Cell 2009, 2, 526–544. [Google Scholar] [CrossRef] [PubMed]
- Dubeaux, G.; Neveu, J.; Zelazny, E.; Vert, G. Metal Sensing by the IRT1 Transporter-Receptor Orchestrates Its Own Degradation and Plant Metal Nutrition. Mol. Cell 2018, 69, 953–964.e5. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Koumoto, Y.; Li, L.; Yamazaki, M.; Kondo, M.; Nishimura, M.; Hara-Nishimura, I. AtVPS29, a Putative Component of a Retromer Complex, is Required for the Efficient Sorting of Seed Storage Proteins. Plant Cell Physiol. 2006, 47, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Munch, D.; Teh, O.-K.; Malinovsky, F.G.; Liu, Q.; Vetukuri, R.R.; Kasmi, F.E.; Brodersen, P.; Hara-Nishimura, I.; Dangl, J.L.; Petersen, M.; et al. Retromer contributes to immunity-associated cell death in Arabidopsis. Plant Cell 2015, 27, 463–479. [Google Scholar] [CrossRef]
- Yamazaki, M.; Shimada, T.; Takahashi, H.; Tamura, K.; Kondo, M.; Nishimura, M.; Hara-Nishimura, I. Arabidopsis VPS35, a Retromer Component, is Required for Vacuolar Protein Sorting and Involved in Plant Growth and Leaf Senescence. Plant Cell Physiol. 2008, 49, 142–156. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, Z.; Teasdale, R.D. The functional roles of retromer in Parkinson’s disease. FEBS Lett. 2018, 7, 1096–1112. [Google Scholar] [CrossRef]
- Oliviusson, P.; Heinzerling, O.; Hillmer, S.; Hinz, G.; Tse, Y.C.; Jiang, L.; Robinson, D.G. Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell. 2006, 5, 1239–1252. [Google Scholar] [CrossRef]
- McNally, K.E.; Faulkner, R.; Steinberg, F.; Gallon, M.; Ghai, R.; Pim, D.; Langton, P.; Pearson, N.; Danson, C.M.; Nägele, H.; et al. Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling. Nat. Cell Biol. 2017, 19, 1214–1225. [Google Scholar] [CrossRef]
- Rodriguez-Furlan, C.; Minina, E.A.; Hicks, G.R. Remove, Recycle, Degrade: Regulating Plasma Membrane Protein Accumulation. Plant Cell 2019, 31, 2833–2854. [Google Scholar] [CrossRef]
- Cui, Y.; Shen, J.; Gao, C.; Zhuang, X.; Wang, J.; Jiang, L. Biogenesis of Plant Prevacuolar Multivesicular Bodies. Mol. Plant 2016, 9, 774–786. [Google Scholar] [CrossRef]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.M.; Palmquist, J.; Huang, S.D.; Jin, H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Li, B.; Huang, S.; Li, H.; Cao, W.; Chen, Y.; Liu, G.; Li, Z.; Yang, C.; Feng, L.; et al. The plant unique ESCRT component FREE1 regulates autophagosome closure. Nat. Commun. 2023, 14, 1768. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhuang, X.; Shen, J.; Jiang, L. Plant ESCRT Complexes: Moving Beyond Endosomal Sorting. Trends Plant Sci. 2017, 22, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Viotti, C.; Bubeck, J.; Stierhof, Y.D.; Krebs, M.; Langhans, M.; van den Berg, W.; van Dongen, W.; Richter, S.; Geldner, N.; Takano, J.; et al. Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell 2010, 22, 1344–1357. [Google Scholar] [CrossRef]
- Grant, B.; Donaldson, J. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2009, 10, 597–608. [Google Scholar] [CrossRef]
- Kleine-Vehn, J.; Wabnik, K.; Martinière, A.; Langowski, L.; Willig, K.; Naramoto, S.; Leitner, J.; Tanaka, H.; Jakobs, S.; Robert, S.; et al. Recycling, clustering, and endocytosis jointly maintain PIN auxin carrier polarity at the plasma membrane. Mol. Syst. Biol. 2011, 7, 540. [Google Scholar] [CrossRef]
- Geldner, N.; Hyman, D.L.; Wang, X.; Schumacher, K.; Chory, J. Endosomal signaling of plant steroid receptor kinase BRI1. Genes. Dev. 2007, 21, 1598–1602. [Google Scholar] [CrossRef]
- Chinchilla, D.; Bauer, Z.; Regenass, M.; Boller, T.; Felix, G. The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 2006, 18, 465–476. [Google Scholar] [CrossRef]
- Takano, J.; Tanaka, M.; Toyoda, A.; Miwa, K.; Kasai, K.; Fuji, K.; Onouchi, H.; Naito, S.; Fujiwara, T. Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 5220–5225. [Google Scholar] [CrossRef] [PubMed]
- Barberon, M.; Dubeaux, G.; Kolb, C.; Isono, E.; Zelazny, E.; Vert, G. Polarization of IRON-REGULATED TRANSPORTER 1 (IRT1) to the plant-soil interface plays crucial role in metal homeostasis. Proc. Natl. Acad. Sci USA 2014, 111, 8293–8298. [Google Scholar] [CrossRef] [PubMed]
- Jaillais, Y.; Santambrogio, M.; Rozier, F.; Fobis-Loisy, I.; Miège, C.; Gaude, T. The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell 2007, 130, 1057–1070. [Google Scholar] [CrossRef]
- Seaman, M.N.; Marcusson, E.G.; Cereghino, J.L.; Emr, S.D. Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J. Cell Biol. 1997, 137, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Seaman, M.N.; McCaffery, J.M.; Emr, S.D. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 1998, 142, 665–681. [Google Scholar] [CrossRef]
- Seaman, M.N.; Williams, H.P. Identification of the functional domains of yeast sorting nexins Vps5p and Vps17p. Mol. Biol. Cell. 2002, 13, 2826–2840. [Google Scholar] [CrossRef]
- van Weering, J.R.; Verkade, P.; Cullen, P.J. SNX-BAR proteins in phosphoinositide-mediated, tubular-based endosomal sorting. Semin. Cell Dev. Biol. 2010, 21, 371–380. [Google Scholar] [CrossRef]
- Chen, K.E.; Healy, M.D.; Collins, B.M. Towards a molecular understanding of endosomal trafficking by Retromer and Retriever. Traffic 2019, 20, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Koumandou, V.L.; Klute, M.J.; Herman, E.K.; Nunez-Miguel, R.; Dacks, J.B.; Field, M.C. Evolutionary reconstruction of the retromer complex and its function in Trypanosoma brucei. J. Cell Sci. 2011, 124, 1496–1509. [Google Scholar] [CrossRef]
- Simonetti, B.; Danson, C.M.; Heesom, K.J.; Cullen, P.J. Sequence-dependent cargo recognition by SNX-BARs mediates retromer-independent transport of CI-MPR. J. Cell Biol. 2017, 216, 3695–3712. [Google Scholar] [CrossRef]
- Meyer, C.; Zizioli, D.; Lausmann, S.; Eskelinen, E.L.; Hamann, J.; Saftig, P.; von Figura, K.; Schu, P. mu1A-adaptin-deficient mice: Lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000, 19, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Kvainickas, A.; Jimenez-Orgaz, A.; Nagele, H.; Hu, Z.; Dengjel, J.; Steinberg, F. Cargo-selective SNX-BAR proteins mediate retromer trimer independent retrograde transport. J. Cell Biol. 2017, 216, 3677–3693. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Carosi, J.M.; Yang, Z.; Ariotti, N.; Kerr, M.C.; Parton, R.G.; Sargeant, T.J.; Teasdale, R.D. Retromer has a selective function in cargo sorting via endosome transport carriers. J. Cell Biol. 2019, 218, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Temkin, P.; Lauffer, B.; Jäger, S.; Cimermancic, P.; Krogan, N.J.; von Zastrow, M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat. Cell Biol. 2011, 13, 715–721. [Google Scholar] [CrossRef]
- Gallon, M.; Cullen, P.J. Retromer and sorting nexins in endosomal sorting. Biochem. Soc. Trans. 2015, 43, 33–47. [Google Scholar] [CrossRef]
- Harrison, M.S.; Hung, C.S.; Liu, T.T.; Christiano, R.; Walther, T.C.; Burd, C.G. A mechanism for retromer endosomal coat complex assembly with cargo. Proc. Natl. Acad. Sci. USA 2014, 111, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Gallon, M.; Clairfeuille, T.; Steinberg, F.; Mas, C.; Ghai, R.; Sessions, R.B.; Teasdale, R.D.; Collins, B.M.; Cullen, P.J. A unique PDZ domain and arrestin-like fold interaction reveals mechanistic details of endocytic recycling by SNX27-retromer. Proc. Natl. Acad. Sci. USA 2014, 111, E3604–E3613. [Google Scholar] [CrossRef]
- Yong, X.; Zhao, L.; Hu, W.; Sun, Q.; Ham, H.; Liu, Z.; Ren, J.; Zhang, Z.; Zhou, Y.; Yang, Q.; et al. SNX27-FERM-SNX1 complex structure rationalizes divergent trafficking pathways by SNX17 and SNX27. Proc. Natl. Acad. Sci. USA 2021, 118, e2105510118. [Google Scholar] [CrossRef]
- Hu, S.; Li, B.; Wu, F.; Zhu, D.; Zouhar, J.; Gao, C.; Shimada, T.; Rojo, E.; Hara-Nishimura, I.; Jiang, L.; et al. Plant ESCRT protein ALIX coordinates with retromer complex in regulating receptor-mediated sorting of soluble vacuolar proteins. Proc. Natl. Acad. Sci. USA 2022, 119, e2200492119. [Google Scholar] [CrossRef]
- Nodzynski, T.; Feraru, M.I.; Hirsch, S.; De Rycke, R.; Niculaes, C.; Boerjan, W.; Van Leene, J.; De Jaeger, G.; Vanneste, S.; Friml, J. Retromer subunits VPS35A and VPS29 mediate prevacuolar compartment (PVC) function in Arabidopsis. Mol. Plant 2013, 6, 1849–1862. [Google Scholar] [CrossRef]
- Niemes, S.; Labs, M.; Scheuring, D.; Krueger, F.; Longhand, M.; Jesenofsky, B.; Robinson, D.G.; Pimpl, P.N. Sorting of plant vacuolar proteins is initiated in the ER. Plant J. 2010, 62, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Niemes, S.; Longhans, M.; Viotti, C.; Scheuring, D.; Yan, M.S.W.; Jiang, L.; Hillmer, S.; Robinson, D.G.; Pimpl, P. Retromer recycles vacuolar sorting receptors from the trans-Golgi network. Plant J. 2010, 61, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Xu, H.; Pimpl, P. Nanobody-based VSR7 tracing shows clattering-dependent TGN to Golgi recycling. Nat. Commun. 2023, 14, 6926. [Google Scholar] [CrossRef]
- Salanenka, Y.; Verstraeten, I.; Löfke, C.; Tabata, K.; Naramoto, S.; Glanc, M.; Friml, J. Gibberellin DELLA signaling targets the retromer complex to redirect protein trafficking to the plasma membrane. Proc. Natl. Acad. Sci. USA 2018, 115, 3716–3721. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.X.; Li, C.; Shi, Y.; Song, Y.; Zhang, D.; Wang, H.; Li, Y.; Wang, T. The retromer protein ZmVPS29 regulates maize kernel morphology likely through an auxin-dependent process(es). Plant Biotechnol. J. 2020, 18, 1004–1014. [Google Scholar] [CrossRef]
- Zelazny, E.; Santambrogio, M.; Pourcher, M.; Chambrier, P.; Berne-Dedieu, A.; Fobis-Loisy, I.; Miège, C.; Jaillais, Y.; Gaude, T. Mechanisms governing the endosomal membrane recruitment of the core retromer in Arabidopsis. J. Biol. Chem. 2013, 288, 8815–8825. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, R.; Liao, Y.; Yang, S.; Feng, B.; Qin, H.; Zhou, J.; Zeng, Y.; Shen, J.; Zhuang, X.; et al. A plant-unique protein BLISTER coordinates with core retromer to modulate endosomal sorting of plasma membrane and vacuolar proteins. Proc. Natl. Acad. Sci. USA 2023, 120, e2211258120. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Niihama, M.; Takahashi, T.; Saito, C.; Nakano, A.; Tasaka, M.; Morita, M.T. Loss-of-function mutations of retromer large subunit genes suppress the phenotype of an Arabidopsis zig mutant that lacks Qb-SNARE VTI11. Plant Cell 2010, 22, 159–172. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Q.; Gao, C.; Ding, Y.; Zeng, Y.; Ueda, T.; Nakano, A.; Jiang, L. Activation of the Rab7 GTPase by the MON1-CCZ1 Complex Is Essential for PVC-to-Vacuole Trafficking and Plant Growth in Arabidopsis. Plant Cell 2014, 26, 2080–2097. [Google Scholar] [CrossRef]
- Takemoto, K.; Ebine, K.; Askani, J.C.; Krüger, F.; Gonzalez, Z.A.; Ito, E.; Goh, T.; Schumacher, K.; Nakano, A.; Ueda, T. Distinct sets of tethering complexes, SNARE complexes, and Rab GTPases mediate membrane fusion at the vacuole in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E2457–E2466. [Google Scholar] [CrossRef]
- Rodriguez-Furlan, C.; Domozych, D.; Qian, W.; Enquist, P.A.; Li, X.; Zhang, C.; Schenk, R.; Winbigler, H.S.; Jackson, W.; Raikhel, N.V.; et al. Interaction between VPS35 and RABG3f is necessary as a checkpoint to control fusion of late compartments with the vacuole. Proc. Natl. Acad. Sci. USA 2019, 116, 21291–21301. [Google Scholar] [CrossRef] [PubMed]
- Laulumaa, S.; Kampala, E.-P.; Huskonen, J.T.; Varjosalo, M. Structure and interactions of the endogenous human Commander complex. Nat. Struct. Mol. Biol. 2024, 31, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.D.; Preston, J.C.; Tierney, M.L. CCDC22 and CCDC93, two potential retriever-interacting proteins, are required for root and root hair growth in Arabidopsis. Front. Plant Sci. 2022, 13, 1051503. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.R.; Domozych, D.S.; Tierney, M.L. SNARE VTI13 plays a unique role in endosomal trafficking pathways associated with the vacuole and is essential for cell wall organization and root hair growth in Arabidopsis. Ann. Bot. 2014, 114, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Yano, D.; Sato, M.; Saito, C.; Sato, M.H.; Morita, M.T.; Tasaka, M. A SNARE complex containing SGR3/AtVAM3 and ZIG/VTI11 in gravity-sensing cells is important for Arabidopsis shoot gravitropism. Proc. Natl. Acad. Sci. USA 2003, 100, 8589–8594. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, C.D.; Tierney, M.L. Contrasting Retromer with a Newly Described Retriever in Arabidopsis thaliana. Plants 2024, 13, 2470. https://doi.org/10.3390/plants13172470
Lewis CD, Tierney ML. Contrasting Retromer with a Newly Described Retriever in Arabidopsis thaliana. Plants. 2024; 13(17):2470. https://doi.org/10.3390/plants13172470
Chicago/Turabian StyleLewis, Connor D., and Mary L. Tierney. 2024. "Contrasting Retromer with a Newly Described Retriever in Arabidopsis thaliana" Plants 13, no. 17: 2470. https://doi.org/10.3390/plants13172470
APA StyleLewis, C. D., & Tierney, M. L. (2024). Contrasting Retromer with a Newly Described Retriever in Arabidopsis thaliana. Plants, 13(17), 2470. https://doi.org/10.3390/plants13172470





