Identification of Insertion and Deletion (InDel) Markers for Chickpea (Cicer arietinum L.) Based on Double-Digest Restriction Site-Associated DNA Sequencing
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. DNA Extraction
4.3. Library Preparation, Sequencing, and InDel Calling
4.4. Identification of InDel-Flanking Sequences and Primer Design
4.5. PCR Amplification
4.6. Genetic Diversity Analysis
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. FAO Statistical Databases (FAOSTAT). Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 17 November 2022).
- Elango, D.; Wang, W.; Thudi, M.; Sebastiar, S.; Ramadoss, B.R.; Varshney, R.K. Genome-wide association mapping of seed oligosaccharides in chickpea. Front. Plant Sci. 2022, 13, 1024543. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Song, C.; Saxena, R.K.; Azam, S.; Yu, S.; Sharpe, A.G.; Cannon, S.; Baek, J.; Rosen, B.D.; Tar’An, B.; et al. Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nat. Biotechnol. 2013, 31, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Deshmukh, R.K.; Singh, A.; Singh, A.K.; Gaikwad, K. Highly variable SSR markers suitable for rice genotyping using agarose gels. Mol. Breed. 2010, 25, 359–364. [Google Scholar] [CrossRef]
- Iruela, M.; Rubio, J.; Cubero, J.I.; Gil, J.; Milan, T. Phylogenetic analysis in the genus Cicer and cultivated chickpea using RAPD and ISSR markers. Theor. Appl. Genet. 2002, 104, 643–651. [Google Scholar] [CrossRef]
- Talebi, R.; Fayaz, F.; Mardi, M.; Pirsyedi, S.M.; Naji, A.M. Genetic relationships among chickpea (Cicer arietinum) elite lines based on RAPD and agronomic markers. Int. J. Agric. Biol. 2008, 10, 47. [Google Scholar]
- Nguyen, T.T.; Taylor, P.W.J.; Redden, R.J.; Ford, R. Genetic diversity estimates in Cicer using AFLP analysis. Plant Breed. 2004, 123, 173–179. [Google Scholar] [CrossRef]
- Shan, F.; Clarke, H.J.; Yan, G.; Plummer, J.A.; Siddique, K.H.M. Identification of duplicates and fingerprinting of primary and secondary wild annual Cicer gene pools using AFLP markers. Genet. Resour. Crop Evol. 2007, 54, 519–527. [Google Scholar] [CrossRef]
- Sudupak, M.A. Inter and intra-species Inter Simple Sequence Repeat (ISSR) variations in the genus Cicer. Euphytica 2004, 135, 229–238. [Google Scholar] [CrossRef]
- Aggarwal, H.; Rao, A.; Kumar, A.; Singh, J.; Rana, J.S.; Naik, P.K.; Chhokar, V. Assessment of genetic diversity among 125 cultivars of chickpea (Cicer arietinum L.) of Indian origin using ISSR markers. Turk. J. Bot. 2015, 39, 218–226. [Google Scholar] [CrossRef]
- Singh, A.; Devarumath, R.M.; Ramarao, S.; Singh, V.P.; Raina, S.N. Assessment of genetic diversity, and phylogenetic relationships based on ribosomal DNA repeat unit length variation and Internal Transcribed Spacer (ITS) sequences in chickpea (Cicer arietinum) cultivars and its wild species. Genet. Resour. Crop Evol. 2008, 55, 65–79. [Google Scholar] [CrossRef]
- Gaur, R.; Azam, S.; Jeena, G.; Khan, A.W.; Choudhary, S.; Jain, M.; Yadav, G.; Tyagi, A.K.; Chattopadhyay, D.; Bhatia, S. High-throughput SNP discovery and genotyping for constructing a saturated linkage map of chickpea (Cicer arietinum L.). DNA Res. 2012, 9, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Farahani, S.; Maleki, M.; Mehrabi, R.; Kanouni, H.; Scheben, A.; Batley, J.; Talebi, R. Whole genome diversity, population structure, and linkage disequilibrium analysis of chickpea (Cicer arietinum L.) genotypes using genome-wide DArTseq-based SNP markers. Genes 2019, 10, 676. [Google Scholar] [CrossRef]
- Asadi, A.; Ebrahimi, A.; Rashidi-Monfared, S.; Basiri, M.; Akbari-Afjani, J. Comprehensive functional analysis and mapping of SSR markers in the chickpea genome (Cicer arietinum L.). Comput. Biol. Chem. 2020, 84, 107169. [Google Scholar] [CrossRef] [PubMed]
- Sari, D.; Sari, H.; Ikten, C.; Toker, C. Genome-wide discovery of di-nucleotide SSR markers based on whole genome re-sequencing data of Cicer arietinum L. and Cicer reticulatum Ladiz. Sci Rep. 2023, 13, 10351. [Google Scholar] [CrossRef] [PubMed]
- Davey, J.W.; Hohenlohe, P.A.; Etter, P.D.; Boone, J.Q.; Catchen, J.M.; Blaxter, M.L. Genome-wide genetic marker discovery and genotyping using next-generation sequencing. Nat. Rev. Genet. 2011, 12, 499–510. [Google Scholar] [CrossRef]
- Zalapa, J.E.; Cuevas, H.; Zhu, H.; Steffan, S.; Senalik, D.; Zeldin, E.; McCown, B.; Harbut, R.; Simon, P. Using next-generation sequencing approaches to isolate simple sequence repeat (SSR) loci in the plant sciences. Am. J. Bot. 2012, 99, 193–208. [Google Scholar] [CrossRef]
- Sakiyama, N.S.; Ramos, H.C.C.; Caixeta, E.T.; Pereira, M.G. Plant breeding with marker-assisted selection in Brazil. Crop Breed. Appl. Biotechnol. 2014, 14, 54–60. [Google Scholar] [CrossRef]
- Ljungqvist, M.; Akesson, M.; Hansson, B. Do microsatellites reflect genome-wide genetic diversity in natural populations? A comment on Väli et al. (2008). Mol. Ecol. 2010, 19, 851–855. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Nawaz, M.A.; Shahid, M.Q.; Dogan, Y.; Comertpay, G.; Yıldız, M.; Hatipoglu, R.; Ahmad, F.; Alsaleh, A.; Labhane, N. DNA molecular markers in plant breeding: Current status and recent advancements in genomic selection and genome editing. Biotechnol. Biotechnol. Equip. 2017, 32, 261–285. [Google Scholar] [CrossRef]
- Brumfield, R.T.; Beerli, P.; Nickerson, D.A.; Edwards, S.V. The utility of single nucleotide polymorphisms in inferences of population history. Trends Ecol. Evol. 2003, 18, 249–256. [Google Scholar] [CrossRef]
- Zhao, Z.; Fu, Y.X.; Hewett-Emmett, D.; Boerwinkle, E. Investigating single nucleotide polymorphism (SNP) density in the human genome and its implications for molecular evolution. Gene 2003, 312, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Sefera, T.; Abebie, B.; Gaur, P.M.; Assefa, K.; Varshney, R.K. Characterisation and genetic diversity analysis of selected chickpea cultivars of nine countries using simple sequence repeat (SSR) markers. Crop Pasture Sci. 2011, 62, 177–187. [Google Scholar] [CrossRef]
- Deokar, A.A.; Ramsay, L.; Sharpe, A.G.; Diapari, M.; Sindhu, A.; Bett, K.; Tar’an, B. Genome wide SNP identification in chickpea for use in development of a high density genetic map and improvement of chickpea reference genome assembly. BMC Genom. 2014, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Kujur, A.; Bajaj, D.; Upadhyaya, H.D.; Das, S.; Ranjan, R.; Shree, T.; Saxena, M.S.; Badoni, S.; Kumar, V.; Tripathi, S.; et al. Employing genome-wide SNP discovery and genotyping strategy to extrapolate the natural allelic diversity and domestication patterns in chickpea. Front. Plant Sci. 2015, 6, 162. [Google Scholar] [CrossRef]
- Sethy, N.K.; Shokeen, B.; Edwards, K.J.; Bhatia, S. Development of microsatellite markers and analysis of intraspecific genetic variability in chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2006, 112, 1416–1428. [Google Scholar] [CrossRef]
- Das, S.; Upadhyaya, H.D.; Srivastava, R.; Bajaj, D.; Gowda, C.L.L.; Sharma, S.; Parida, S.K. Genome-wide insertion–deletion (InDel) marker discovery and genotyping for genomics- assisted breeding applications in chickpea. DNA Res. 2015, 22, 377–386. [Google Scholar] [CrossRef]
- Britten, R.J.; Rowen, L.; Williams, J.; Cameron, R.A. Majority of divergence between closely related DNA samples is due to indels. Proc. Natl. Acad. Sci. USA 2003, 100, 4661–4665. [Google Scholar] [CrossRef]
- Moghaddam, M.S.; Song, Q.; Mamidi, S. Developing market class specific InDel markers from next generation sequence data in Phaseolus vulgaris L. Front. Plant Sci. 2014, 5, 185. [Google Scholar] [CrossRef]
- Wang, L.X.; Li, H.B.; Gu, T.C.; Liu, L.H.; Pang, B.S.; Qiu, J.; Zhao, C.P. Assessment of wheat variety stability using SSR markers. Euphytica 2014, 195, 435–452. [Google Scholar] [CrossRef]
- Jain, A.; Roorkiwal, M.; Kale, S.; Garg, V.; Yadala, R.; Varshney, R.K. InDel markers: An extended marker resource for molecular breeding in chickpea. PLoS ONE 2019, 14, e0213999. [Google Scholar] [CrossRef]
- Li, H.; Ikram, M.; Xia, Y.; Li, R.; Yuan, Q.; Zhao, W.; Siddique, K.H.M.; Guo, P. Genome wide identification and development of InDel markers in tobacco (Nicotiana tabacum L.) using RAD-seq. Physiol. Mol. Biol. Plants 2022, 28, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, H.; Wang, C.; Tseng, H.; Hwu, K. Genome-wide InDel marker system for application in rice breeding and mapping studies. Euphytica 2013, 192, 131–143. [Google Scholar] [CrossRef]
- Shivaprasad, K.M.; Aski, M.; Mishra, G.P.; Sinha, S.K.; Gupta, S.; Mishra, D.C.; Singh, A.K.; Singh, A.; Tripathi, K.; Kumar, R.R.; et al. Genome-wide discovery of InDels and validation of PCR-Based InDel markers for earliness in a RIL population and genotypes of lentil (Lens culinaris Medik.). PLoS ONE 2024, 19, e0302870. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Dang, P.M.; Chen, C.Y. Development and Utilization of InDel markers to identify peanut (Arachis hypogaea) disease resistance. Front. Plant Sci. 2015, 13, lentil988. [Google Scholar] [CrossRef]
- Srivastava, R.; Singh, M.; Bajaj, D.; Parida, S.K. A High-Resolution inDel (Insertion–Deletion) markers-anchored consensus genetic map identifies major QTLs governing pod number and seed yield in chickpea. Front. Plant Sci. 2016, 7, 1362. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, L.; Chen, Z.; Fu, Y.; Sun, X.; Wang, B.; Xia, T. Climatic factors determine the yield and quality of Honghe flue-cured tobacco. Sci. Rep. 2020, 10, 19868. [Google Scholar] [CrossRef]
- Hasan, N.; Choudhary, S.; Naaz, N.; Sharma, N.; Laskar, R.A. Recent advancements in molecular marker-assisted selection and applications in plant breeding programmes. J. Genet. Eng. Biotechnol. 2021, 19, 1–26. [Google Scholar] [CrossRef]
- Gujaria, N.; Kumar, A.; Dauthal, P.; Dubey, A.; Hiremath, P.; Prakash, A.B.; Farmer, A.; Bhide, M.; Shah, T.; Gaur, P.M.; et al. Development and use of genic molecular markers (GMMs) for construction of a transcript map of chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2011, 122, 1577–1589. [Google Scholar] [CrossRef]
- Peterson, B.K.; Weber, J.N.; Kay, E.H.; Fisher, H.S.; Hoekstra, H.E. Double digest RADseq: An inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 2012, 7, e37135. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Shen, H.; Yang, W. In silico identification and experimental validation of insertion-deletion polymorphisms in tomato genome. DNA Res. 2014, 21, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R.; Verma, S.; Pradhan, S.; Ambreen, H.; Bhatia, S. A high-density SNP-based linkage map using genotyping-by-sequencing and its utilization for improved genome assembly of chickpea (Cicer arietinum L.). Funct. Integr. Genom. 2020, 20, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, Y.P.; Saxena, M.S.; Gaur, R.; Chattopadhyay, D.; Jain, M.; Parida, S.K.; Bhatia, S. Development and integration of genome-wide polymorphic microsatellite markers onto a reference linkage map for constructing a high-density genetic map of chickpea. PLoS ONE 2015, 10, e0125583. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, D.; Thudi, M.; Kale, S.; Azam, S.; Roorkiwal, M.; Gaur, P.M.; Kishor, P.B.; Nguyen, H.; Sutton, T.; Varshney, R.K. Genotyping-by-sequencing based intra-specific genetic map refines a ‘‘QTL-hotspot” region for drought tolerance in chickpea. Mol. Genet. Genom. 2015, 290, 559–571. [Google Scholar] [CrossRef]
- Thudi, M.; Khan, A.W.; Kumar, V.; Gaur, P.M.; Katta, K.; Garg, V.; Roorkiwal, M.; Samineni, S.; Varshney, R.K. Whole genome re-sequencing reveals genome-wide variations among parental lines of 16 mapping populations in chickpea (Cicer arietinum L.). BMC Plant Biol. 2016, 16, 53–64. [Google Scholar] [CrossRef]
- Varshney, R.K.; Thudi, M.; Nayak, S.N.; Gaur, P.M.; Kashiwagi, J.; Krishnamurthy, L.; Jaganathan, D.; Koppolu, J.; Bohra, A.; Tripathi, S.; et al. Genetic dissection of drought tolerance in chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2014, 127, 445–462. [Google Scholar] [CrossRef]
- Doddamani, D.; Khan, A.W.; Katta, M.A.K.; Agarwal, G.; Thudi, M.; Ruperao, P.; Edwards, D.; Varshney, R.K. CicArVarDB: SNP and InDel database for advancing genetics research and breeding applications in chickpea. Database 2015, 2015, bav078. [Google Scholar] [CrossRef]
- Hu, W.; Zhou, T.; Wang, P.; Wang, B.; Song, J.; Han, Z.; Chen, L.; Liu, K.; Xing, Y. Development of whole-genome agarose-resolvable LInDel markers in rice. Rice 2020, 13, 1. [Google Scholar] [CrossRef]
- Grover, C.E.; Kim, H.; Wing, R.A.; Paterson, A.H.; Wendel, J.F. Microcolinearity and genome evolution in the AdhA region of diploid and polyploid cotton (Gossypium). Plant J. 2007, 50, 995–1006. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Zhai, W.; Deng, J.; Wang, H.; Cui, Y.; Cheng, F.; Wang, X.; Wu, J. Development of InDel markers for Brassica rapa based on whole-genome re-sequencing. Theor. Appl. Genet. 2013, 126, 231–239. [Google Scholar] [CrossRef]
- Parida, S.K.; Dalal, V.; Singh, A.K.; Singh, N.K.; Mohapatra, T. Genic non-coding microsatellites in the rice genome: Characterization, marker design and use in assessing genetic and evolutionary relationships among domesticated groups. BMC Genom. 2009, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.K.; Verma, M.; Yadav, S.K.; Ambawat, S.; Das, S.; Garg, R.; Jain, M. Development of genome-wide informative simple sequence repeat markers for large-scale genotyping applications in chickpea and development of web resource. Front. Plant Sci. 2015, 6, 645. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.; Khanna, S.M.; Jain, P.K.; Bharadwaj, C.; Kumar, J.; Lakhera, P.C.; Srinivasan, R. Molecular characterization of primary gene pool of chickpea based on ISSR markers. Biochem. Genet. 2013, 51, 306–322. [Google Scholar] [CrossRef]
- Hiremath, P.J.; Kumar, A.; Penmetsa, R.V.; Farmer, A.; Schlueter, J.A.; Chamarthi, S.K.; Varshney, R.K. Large-scale development of cost- effective SNP marker assays for diversity assessment and genetic mapping in chickpea and comparative mapping in legumes. Plant Biotechnol. J. 2012, 10, 716–732. [Google Scholar] [CrossRef]
- Whankaew, S.; Kaewmanee, S.; Ruttajorn, K.; Phongdara, A. Indel marker analysis of putative stress-related genes reveals genetic diversity and differentiation of rice landraces in peninsular Thailand. Physiol. Mol. Biol. Plants 2020, 26, 1237–1247. [Google Scholar] [CrossRef]
- Li, Y.; Luo, X.; Peng, X.; Jin, Y.; Tan, H.; Wu, L.; Li, J.; Pei, Y.; Xu, X.; Zhang, W. Development of SNP and InDel markers by genome resequencing and transcriptome sequencing in radish (Raphanus sativus L.). BMC Genom. 2023, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Thudi, M.; Bohra, A.; Nayak, S.N.; Varghese, N.; Shah, T.M.; Penmetsa, R.V.; Thirunavukkarasu, N.; Gudipati, S.; Gaur, P.M.; Kulwal, P.L.; et al. Novel SSR markers from BAC-end sequences, DArT arrays and a comprehensive genetic map with 1,291 marker loci for chickpea (Cicer arietinum L.). PLoS ONE 2011, 6, e27275. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314. [Google Scholar] [PubMed]
- Doyle, J.J.; Doyle, J.L. A rapid total DNA preparation procedure for fresh plant tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Girardot, C.; Scholtalbers, J.; Sauer, S.; Su, S.Y.; Furlong, E.E. Je, a versatile suite to handle multiplexed NGS libraries with unique molecular identifiers. BMC Bioinform. 2016, 17, 419. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884i890. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Garrison, E.; Marth, G. Haplotype-based variant detection from short-read sequencing. arXiv 2012, arXiv:1207.3907. [Google Scholar]
- Freese, N.H.; Norris, D.C.; Loraine, A.E. Integrated genome browser: Visual analytics platform for genomics. Bioinformatics 2016, 32, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef]
- Kizil, S.; Basak, M.; Guden, B.; Tosun, H.S.; Uzun, B.; Yol, E. Genome-wide discovery of InDel markers in sesame (Sesamum indicum L.) using ddRADSeq. Plants 2020, 9, 1262. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
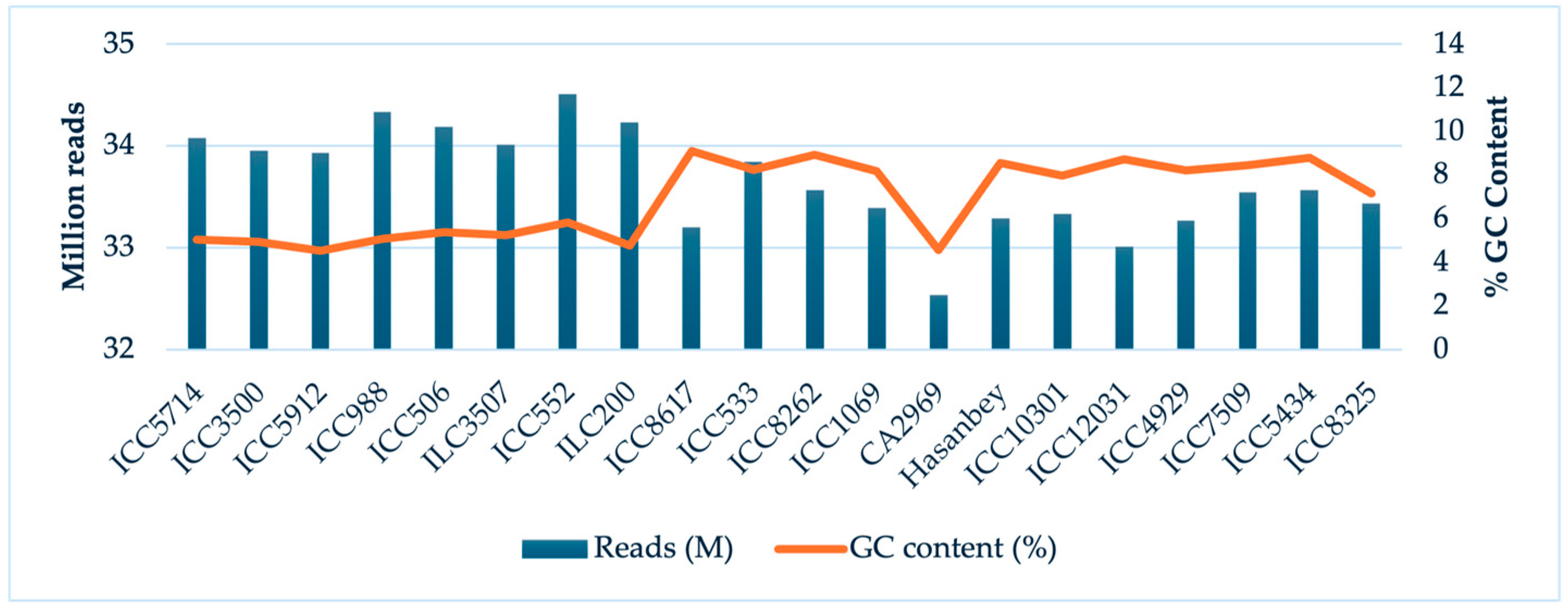
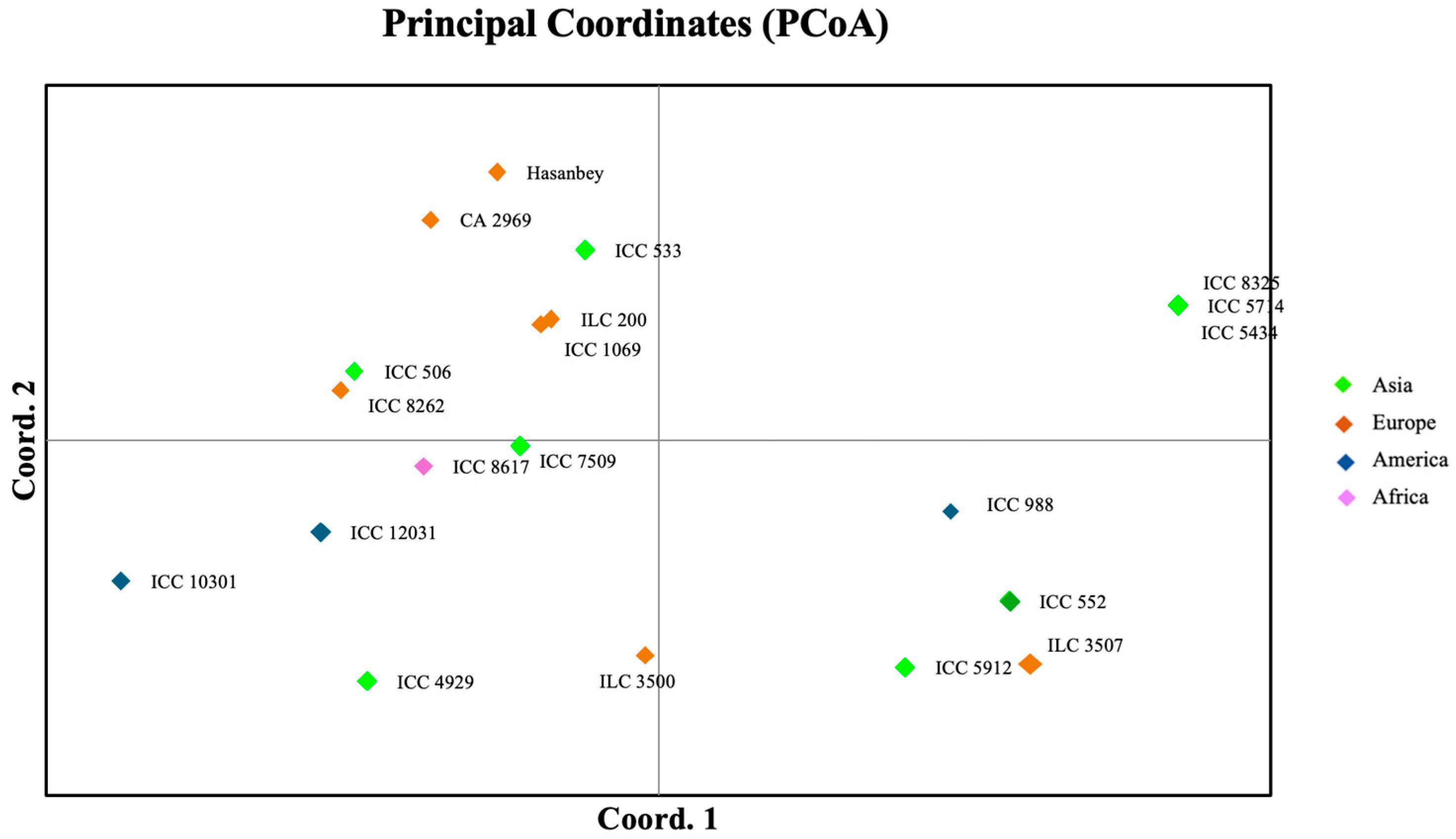
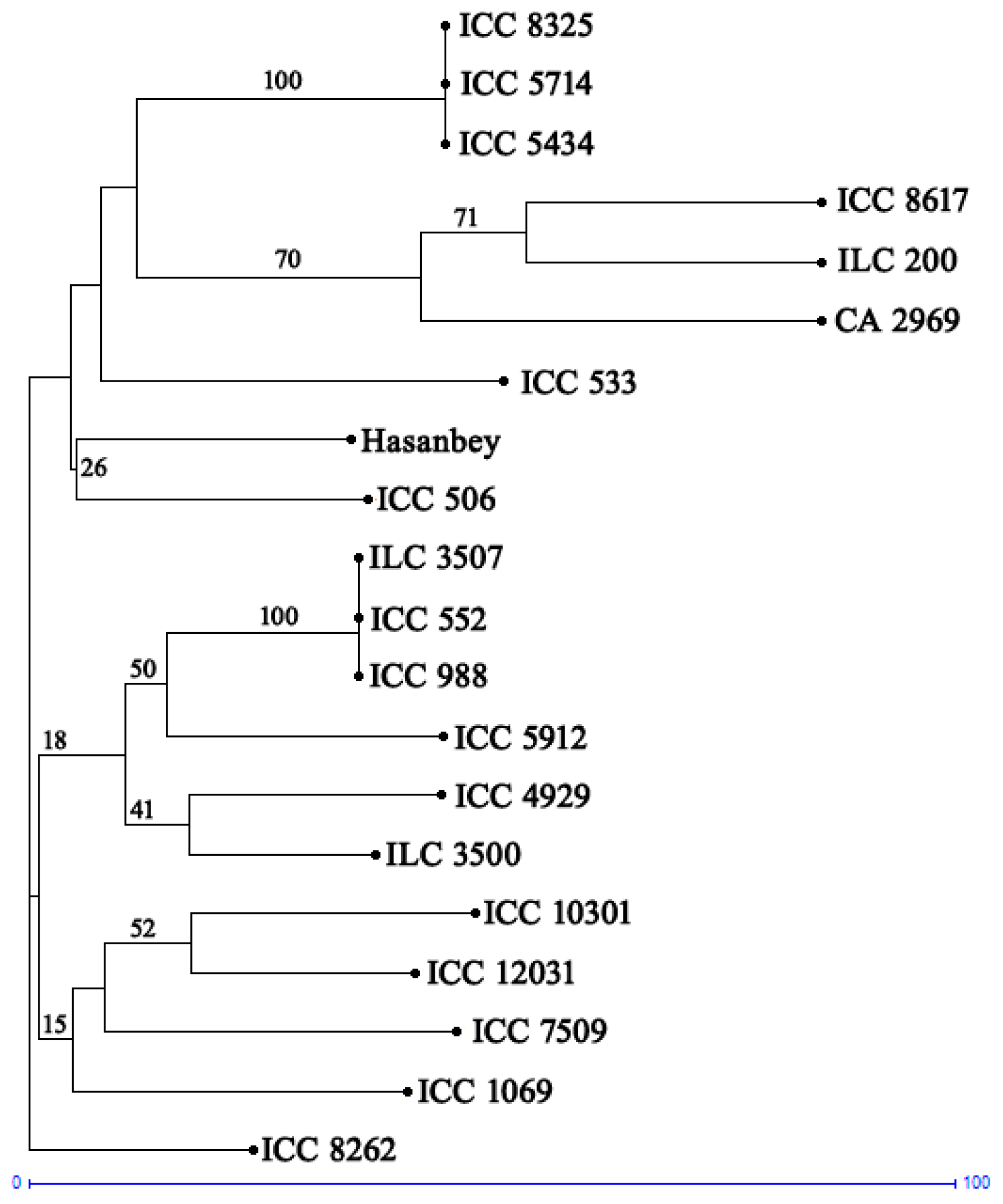
| No | Species | Kabuli/Desi | Gen Bank Number/Name | Origin | Continent |
|---|---|---|---|---|---|
| 1 | Cicer arietinum | Kabuli | ILC 200 | Russian Fed | Europe |
| 2 | Cicer arietinum | Desi | ICC 552 | India | Asia |
| 3 | Cicer arietinum | Kabuli | ILC 3507 | Spain | Europe |
| 4 | Cicer arietinum | Desi | ICC 506 | India | Asia |
| 5 | Cicer arietinum | Desi | ICC 988 | Mexico | America |
| 6 | Cicer arietinum | Desi | ICC 5912 | India | Asia |
| 7 | Cicer arietinum | Kabuli | ILC 3500 | Spain | Europe |
| 8 | Cicer arietinum | Desi | ICC 5714 | India | Asia |
| 9 | Cicer arietinum | Desi | ICC 8325 | India | Asia |
| 10 | Cicer arietinum | Desi | ICC 5434 | India | Asia |
| 11 | Cicer arietinum | Desi | ICC 7509 | Iran | Asia |
| 12 | Cicer arietinum | Desi | ICC 4929 | India | Asia |
| 13 | Cicer arietinum | Desi | ICC 12031 | Mexico | America |
| 14 | Cicer arietinum | Desi | ICC 10301 | USA | America |
| 15 | Cicer arietinum | Kabuli | Hasanbey | Turkey | Europe |
| 16 | Cicer arietinum | Kabuli | CA 2969 | Spain | Europe |
| 17 | Cicer arietinum | Desi | ICC 1069 | Russian Fed | Europe |
| 18 | Cicer arietinum | Desi | ICC 8262 | Turkey | Europe |
| 19 | Cicer arietinum | Desi | ICC 533 | India | Asia |
| 20 | Cicer arietinum | Desi | ICC 8617 | Ethiopia | Africa |
| InDel Type | InDel Size (bp) | Number | Frequency (%) |
|---|---|---|---|
| Insertion | 1 | 2843 | 50.15 |
| 2 | 655 | 11.55 | |
| 3 | 377 | 6.65 | |
| 4 | 294 | 5.19 | |
| 5 | 221 | 3.90 | |
| 6 | 192 | 3.39 | |
| 7 | 164 | 2.89 | |
| 8 | 134 | 2.36 | |
| 9 | 124 | 2.19 | |
| 10–20 | 622 | 11.00 | |
| >20 | 43 | 0.76 | |
| Total | 5669 | 100 | |
| Deletion | 1 | 13,589 | 90.41 |
| 2 | 681 | 4.53 | |
| 3 | 240 | 1.60 | |
| 4 | 135 | 0.90 | |
| 5 | 57 | 0.38 | |
| 6 | 76 | 0.51 | |
| 7 | 51 | 0.34 | |
| 8 | 35 | 0.23 | |
| 9 | 30 | 0.20 | |
| 10–20 | 124 | 0.82 | |
| >20 | 13 | 0.08 | |
| Total | 15,031 | 100 |
| Chromosome | Number of InDels | Number of Insertions | Number of Deletions | Frequency (InDels/Mb) |
|---|---|---|---|---|
| Chr1 | 2715 | 770 | 1945 | 13.11 |
| Chr2 | 1973 | 527 | 1446 | 9.53 |
| Chr3 | 2278 | 544 | 1734 | 11.00 |
| Chr4 | 3710 | 1250 | 2460 | 17.92 |
| Chr5 | 2695 | 677 | 2018 | 13.02 |
| Chr6 | 3408 | 905 | 2503 | 16.46 |
| Chr7 | 2785 | 704 | 2081 | 13.45 |
| Chr8 | 1136 | 292 | 844 | 5.49 |
| Chromosome | Number of Insertions | Number of Deletions |
|---|---|---|
| Chr1 | 33 | 24 |
| Chr2 | 60 | 12 |
| Chr3 | 67 | 9 |
| Chr4 | 109 | 25 |
| Chr5 | 84 | 16 |
| Chr6 | 90 | 14 |
| Chr7 | 91 | 16 |
| Chr8 | 36 | 3 |
| Total | 570 | 119 |
| Marker/Locus | Na * | Ne | I | He | uHe | PIC |
|---|---|---|---|---|---|---|
| CA-I-1-345 | 2.00 | 1.10 | 0.20 | 0.09 | 0.10 | 0.09 |
| CA-D-2-792 | 2.00 | 1.10 | 0.20 | 0.09 | 0.10 | 0.09 |
| CA-D-2-357 | 2.00 | 2.00 | 0.69 | 0.50 | 0.51 | 0.37 |
| CA-I-2-359 | 2.00 | 1.11 | 0.21 | 0.10 | 0.10 | 0.09 |
| CA-I-2-326 | 2.00 | 1.10 | 0.20 | 0.09 | 0.10 | 0.09 |
| CA-D-3-394 | 2.00 | 1.83 | 0.65 | 0.45 | 0.47 | 0.35 |
| CA-D-3-327 | 2.00 | 1.10 | 0.20 | 0.09 | 0.10 | 0.09 |
| CA-I-3-371 | 2.00 | 1.47 | 0.50 | 0.32 | 0.33 | 0.27 |
| CA-I-3-142 | 2.00 | 1.10 | 0.20 | 0.09 | 0.10 | 0.09 |
| CA-D-4-414 | 2.00 | 2.00 | 0.69 | 0.50 | 0.51 | 0.37 |
| CA-D-4-604 | 2.00 | 1.10 | 0.20 | 0.09 | 0.10 | 0.09 |
| CA-I-4-408 | 2.00 | 1.95 | 0.68 | 0.49 | 0.50 | 0.37 |
| CA-I-4-727 | 2.00 | 1.10 | 0.20 | 0.09 | 0.10 | 0.09 |
| CA-D-5-283 | 2.00 | 1.10 | 0.20 | 0.09 | 0.10 | 0.09 |
| CA-D-5-385 | 2.00 | 1.34 | 0.42 | 0.25 | 0.26 | 0.22 |
| CA-I-5-413 | 2.00 | 1.98 | 0.69 | 0.49 | 0.51 | 0.37 |
| CA-I-5-445 | 2.00 | 1.22 | 0.32 | 0.18 | 0.18 | 0.16 |
| CA-D-6-397 | 2.00 | 1.36 | 0.44 | 0.27 | 0.27 | 0.23 |
| CA-I-6-765 | 2.00 | 1.22 | 0.32 | 0.18 | 0.18 | 0.16 |
| CA-I-6-531 | 2.00 | 1.92 | 0.67 | 0.48 | 0.49 | 0.36 |
| CA-D-7-330 | 2.00 | 1.34 | 0.42 | 0.25 | 0.26 | 0.22 |
| Mean | 2.00 ± 0.00 | 1.40 ± 0.08 | 0.39 ± 0.04 | 0.25 ± 0.04 | 0.26 ± 0.04 | 0.20 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sari, D. Identification of Insertion and Deletion (InDel) Markers for Chickpea (Cicer arietinum L.) Based on Double-Digest Restriction Site-Associated DNA Sequencing. Plants 2024, 13, 2530. https://doi.org/10.3390/plants13172530
Sari D. Identification of Insertion and Deletion (InDel) Markers for Chickpea (Cicer arietinum L.) Based on Double-Digest Restriction Site-Associated DNA Sequencing. Plants. 2024; 13(17):2530. https://doi.org/10.3390/plants13172530
Chicago/Turabian StyleSari, Duygu. 2024. "Identification of Insertion and Deletion (InDel) Markers for Chickpea (Cicer arietinum L.) Based on Double-Digest Restriction Site-Associated DNA Sequencing" Plants 13, no. 17: 2530. https://doi.org/10.3390/plants13172530






