Dentatacid A: An Unprecedented 2, 3-Seco-arbor-2, 3-dioic Triterpenoid from the Invasive Plant Euphorbia dentata, with Cytotoxicity Effect on Colon Cancer
Abstract
:1. Introduction
2. Result and Discussion
2.1. Hypothetical Biosynthesis for Dentatacid A
2.2. Cytotoxicity Assay
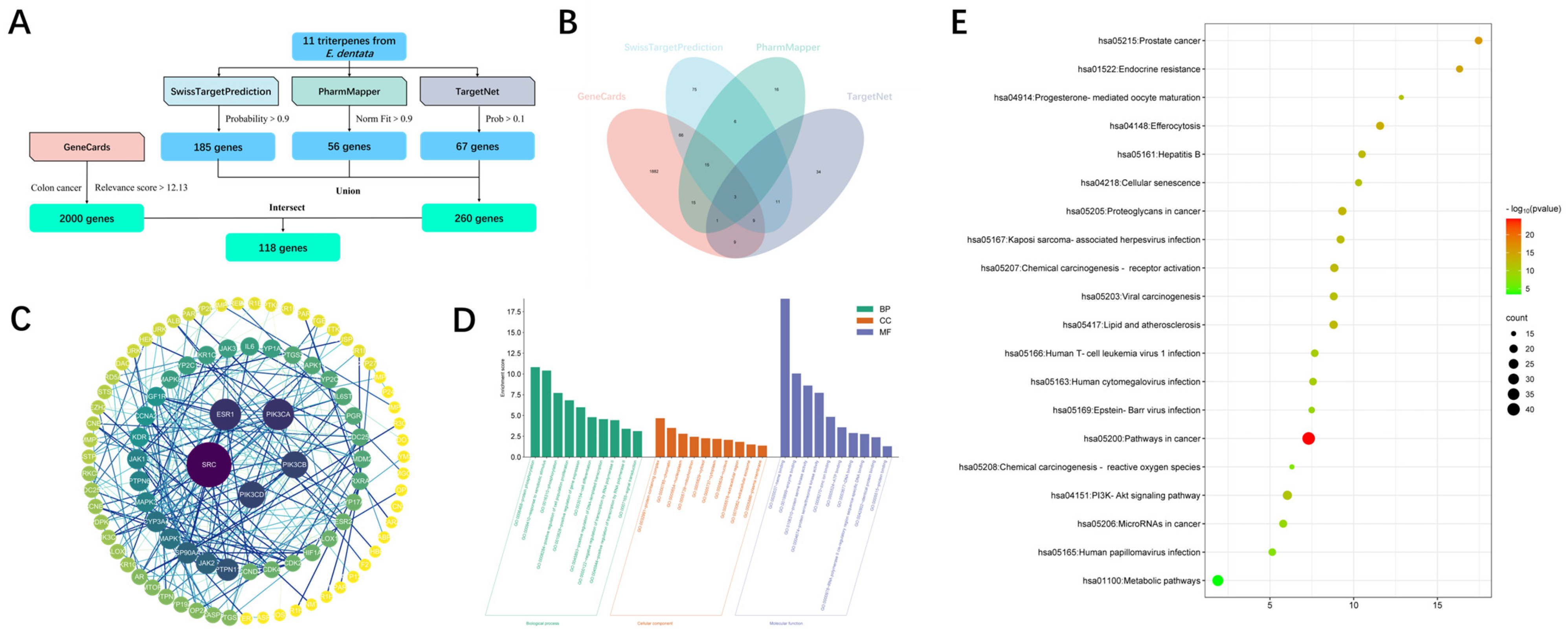
2.3. Bioinformatics Analysis
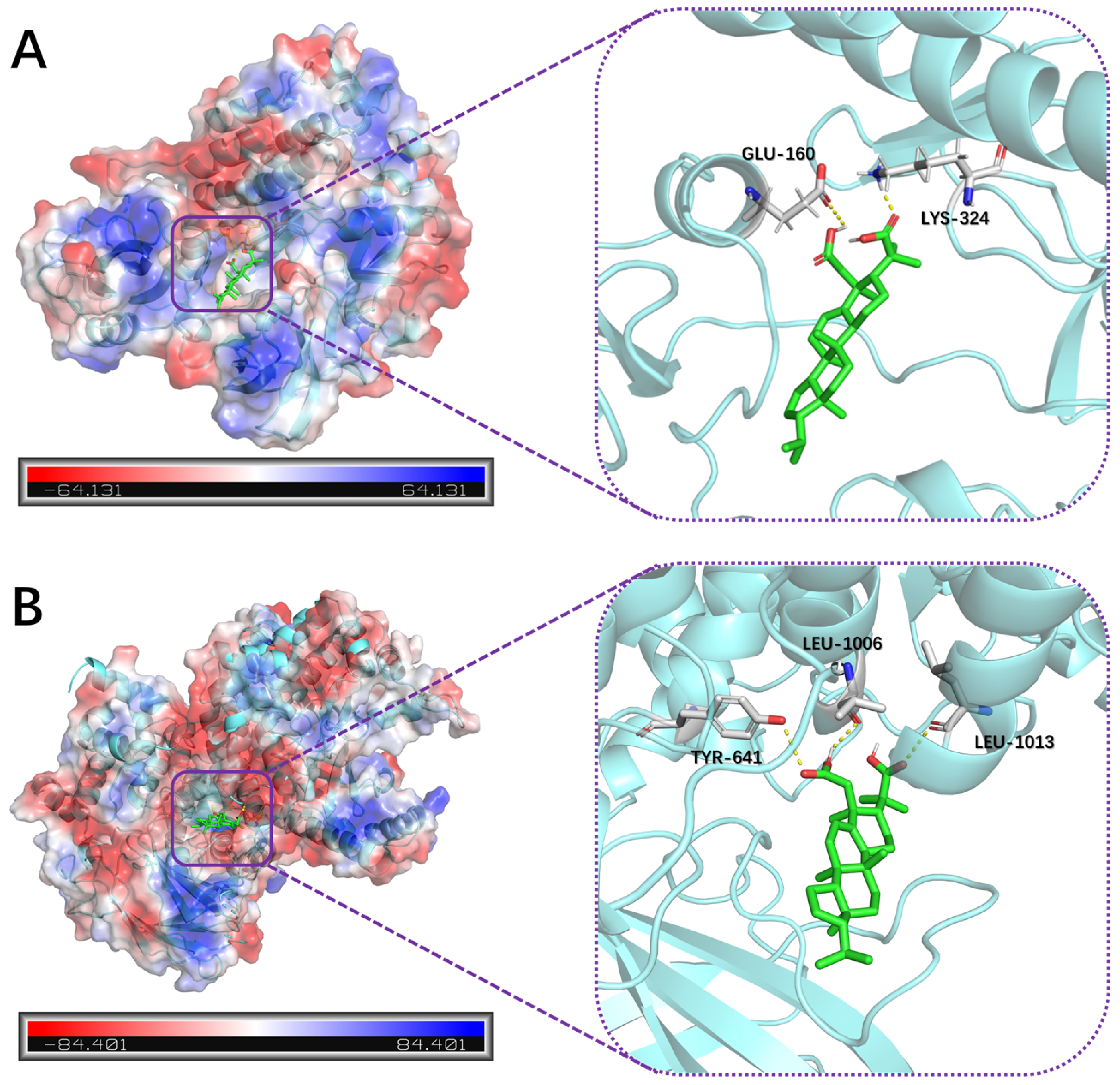
2.4. Molecular Docking Analysis
3. Experiment
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Compounds Characterization
3.5. ECD Calculations
3.6. Methods of Cytotoxicity Assay
3.6.1. Materials
3.6.2. Cell Culture
3.6.3. Cell Viability
3.7. Methods of Bioinformatics Analysis
3.7.1. Target Prediction of Triterpenoids from E. dentata
3.7.2. Screening of Potential Targets in Colon Cancer
3.7.3. Construction of the Target PPI Network between Triterpenoids from E. dentata and Colon Cancer
3.7.4. Enrichment Analysis of the Intersection Targets
3.8. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gioria, M.; Hulme, P.E.; Richardson, D.M.; Pyšek, P. Why Are Invasive Plants Successful? Annu. Rev. Plant Biol. 2023, 74, 635–670. [Google Scholar] [CrossRef] [PubMed]
- Augusthy, S.; Nizam, A.; Kumar, A. The diversity, drivers, consequences and management of plant invasions in the mangrove ecosystems. Sci. Total Environ. 2024, 945, 173851. [Google Scholar] [CrossRef]
- Marchessi, J.E.; Subils, R.; Scaramuzzino, R.L.; Crosta, H.N.; Eseiza, M.F.; Saint André, H.M.; Juan, V.F. Presencia de Euphorbia davidii Subils (Euphorbiaceae) en la Provincia de Buenos Aires: Morfología y anatomía de la especie. Kurtziana 2011, 36, 45–53. [Google Scholar]
- Liberato, J.R.; Wright, J.G.; Seth, M.; Shivas, R.G. Oidiopsi (Erysiphaceae) on Euphorbia spp. in Australia and Vanuatu. Australas. Plant Pathol. 2005, 34, 409–411. [Google Scholar] [CrossRef]
- Xue, H.-G.; Zhou, S.-D.; He, X.-J.; Yu, Y. Karyotype in fifteen populations belonging to thirteen species of Euphorbia (Euphorbiaceae) in China. Acta Phytotaxon. Sin. 2007, 45, 619–626. [Google Scholar] [CrossRef]
- Barina, Z.; Shevera, M.; Sirbu, C.; Pinke, G. Current distribution and spreading of Euphorbia davidii (E. dentata agg.) in Europe. Cent. Eur. J. Biol. 2013, 8, 87–95. [Google Scholar] [CrossRef]
- An, T.; Cao, D.; Zhang, Y.; Han, X.; Yu, Z.; Liu, Z. Norsesquiterpenes from the Latex of Euphorbia dentata and Their Chemical Defense Mechanisms against Helicoverpa armigera. Molecules 2023, 28, 7681. [Google Scholar] [CrossRef]
- Juan, V.F.; Saint-Andre, H.; Fernandez, R.R. Competencia de lecheron (Euphorbia dentata) en soja. Planta Daninha 2003, 21, 175–180. [Google Scholar] [CrossRef]
- Tanveer, A.; Khaliq, A.; Javaid, M.M.; Chaudhry, M.N.; Awan, I. Implications of weeds of genus euphorbia for crop production: A review. Planta Daninha 2013, 31, 723–731. [Google Scholar] [CrossRef]
- Rojas-Jiménez, S.; Valladares-Cisneros, M.G.; Salinas-Sánchez, D.O.; Pérez-Ramos, J.; Sánchez-Pérez, L.; Pérez-Gutiérrez, S.; Campos-Xolalpa, N. Anti-Inflammatory and Cytotoxic Compounds Isolated from Plants of Euphorbia Genus. Molecules 2024, 29, 1083. [Google Scholar] [CrossRef]
- Shi, Q.-W.; Su, X.-H.; Kiyota, H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yuan, C.; Chen, Y. New Advance on the Research of Chemical Constituents and Pharmacological Activities of Diterpenoids from the Plants of Genus Euphorbia. Chin. Wild Plant Resour. 2020, 39, 53–60. [Google Scholar]
- Zhao, H.; Sun, L.; Kong, C.; Mei, W.; Dai, H.; Xu, F.; Huang, S. Phytochemical and pharmacological review of diterpenoids from the genus Euphorbia Linn (2012–2021). J. Ethnopharmacol. 2022, 298, 115574. [Google Scholar] [CrossRef]
- Wei, X.-F.; Wang, Y.-K.; Liu, R.-T.; Wu, J.-P.; Xu, K.P. Review of natural plant-derived seco-triterpenoids and derived saponins from 2020 to 2023: New compounds, distributions, diverse activities and structure–activity relationships. Phytochem. Rev. 2024. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Tansakul, P.; Daengrot, C.; Ponglimanont, C.; Karalai, C. Anti-inflammatory principles from Heritiera littoralis bark. Phytomedicine 2010, 17, 851–855. [Google Scholar] [CrossRef]
- Xu, M.; Liu, J.; Sun, W.; Chen, Y.; Wang, M.; Zhou, Y. New dammarane-type triterpenoids from the whole plant of Euphorbia hypericifolia. Nat. Prod. Res. 2024, 1–6. [Google Scholar] [CrossRef]
- Fang, C.H.; Li, Y.P.; Li, Y.; Yue, J.M.; Bao, J.; Yu, J.H. Triterpenoids with multi-skeletons as 11 beta -HSD1 inhibitors from Euphorbia sikkimensis. Phytochemistry 2023, 211, 113684. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.G.; Vieira, I.G.; Mendes, F.N.; Albuquerque, I.L.; dos Santos, R.N.; Silva, F.O.; Morais, S.M. Variation of ursolic acid content in eight Ocimum species from northeastern Brazil. Molecules 2008, 13, 2482–2487. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Hao, J.J.; Tan, M.; Liao, C.C.; Liu, D.; Li, H.M.; Li, R.T. Iridoids and other constituents from the leaves and stems of Valeriana officinalis var. latifolia. Phytochemistry 2024, 218, 113934. [Google Scholar] [CrossRef]
- Sun, H.-B.; Zou, H.-F.; Xia, B.; Gu, Y.-C.; Wu, W.-L.; Zhou, Y. Targeted isolation of ursane-type triterpenoids from Tragopogon porrifolius based on Feature-Based Molecular Networking. Phytochem. Lett. 2023, 57, 67–72. [Google Scholar] [CrossRef]
- Seki, H.; Ohyama, K.; Sawai, S.; Mizutani, M.; Ohnishi, T.; Sudo, H.; Akashi, T.; Aoki, T.; Saito, K.; Muranaka, T. Licorice β-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proc. Natl. Acad. Sci. USA 2008, 105, 14204–14209. [Google Scholar] [CrossRef] [PubMed]
- Kemertelidze, E.P.; Gvazava, L.N.; Alania, M.D.; Kikoladze, V.S.; Tsitsishvili, V.G. Digitoside, a novel triterpene glycoside from Digitalis ciliata. J. Nat. Prod. 1992, 55, 217–220. [Google Scholar] [CrossRef]
- Akihisa, T.; Watanabe, K.; Yoneima, R.; Suzuki, T.; Kimura, Y. Biotransformation of cycloartane-type triterpenes by the fungus Glomerella fusarioides. J. Nat. Prod. 2006, 69, 604–607. [Google Scholar] [CrossRef]
- Lizazman, M.A.; Jong, V.Y.M.; Chua, P.; Lim, W.K.; Karunakaran, T. Phytochemicals from Calophyllum canum Hook f. ex T. Anderson and their neuroprotective effects. Nat. Prod. Res. 2023, 37, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-M.; Peng, C.; Yang, H.; Liu, L.-S.; Yang, Y.-T.; Xie, X.-F.; Guo, L.; Liu, Z.-H.; Xiong, L. Steroids from the aerial parts of Leonurus japonicus. Phytochem. Lett. 2015, 12, 287–290. [Google Scholar] [CrossRef]
- Zhu, Y.; Soroka, D.; Sang, S. Oxyphytosterols as active ingredients in wheat bran suppress human colon cancer cell growth: Identification, chemical synthesis, and biological evaluation. J. Agric. Food Chem. 2015, 63, 2264–2276. [Google Scholar] [CrossRef]
- Ayyad, S.E.N.; Sowellim, S.Z.A.; El-Hosini, M.S.; Abo-Atia, A. The structural determination of a new steroidal metabolite from the brown alga Sargassum asperifolium. Z. Naturforschung C J. Biosci. 2003, 58, 333–336. [Google Scholar] [CrossRef]
- Foley, D.A.; O’Callaghan, Y.; O’Brien, N.M.; McCarthy, F.O.; Maguire, A.R. Synthesis and characterization of stigmasterol oxidation products. J. Agric. Food Chem. 2010, 58, 1165–1173. [Google Scholar] [CrossRef]
- Schabdach, H.; Johne, S.; Steiner, U.; Seifert, K. Plant-disease resistance inducing activity of 7-oxosterols and 7-hydroxysterols. Z. Naturforschung C J. Biosci. 1995, 50, 257–262. [Google Scholar] [CrossRef]
- Brown, G.D.; Liang, G.Y.; Sy, L.K. Terpenoids from the seeds of Artemisia annua. Phytochemistry 2003, 64, 303–323. [Google Scholar] [CrossRef]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene biosynthesis in plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Fazio, G.C.; Matsuda, S.P. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004, 65, 261–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ren, F.C.; Liu, J.K. Alstonic acids A and B, unusual 2,3-secofernane triterpenoids from Alstonia scholaris. Phytochemistry 2009, 70, 650–654. [Google Scholar] [CrossRef]
- Vo, H.Q.; Viet Pham, T.; Le, A.T.; Hoang, H.N.T.; Nguyen, P.Q.D.; Doan, L.N.T.; Nguyen, H.T.; Ho, D.V. Two New Lupane-Type Triterpenes from the Stems and Leaves of Buxus latistyla Gagnep. and Their Cytotoxic Activity. Nat. Prod. Commun. 2024, 19, 1–5. [Google Scholar] [CrossRef]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [PubMed]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.W.; Wang, M.X.; Yu, H.L.; Chen, L.; Cai, Z.; Zhang, Y.; Gu, M.M.; Shao, Y.L.; Han, H.P.; et al. Brunonianines A-C, C(20)-diterpenoid alkaloids with cyano group from Delphinium brunonianum Royle. Phytochemistry 2024, 219, 113987. [Google Scholar] [CrossRef]
- Li, Q.; Gu, M.-M.; Wu, H.-W.; Xu, C.-S.; Yu, H.-L.; Zhang, Y.; Su, Y.-Y.; Han, H.-P.; Liao, Z.-X. Brunonianines D-F, three new C19-diterpenoid alkaloids from the Delphinium brunonianum, with therapeutic effect on ovarian cancer in vitro and in vivo. Bioorganic Chem. 2024, 148, 107478. [Google Scholar] [CrossRef]
- Shao, Y.; Li, Q.; Wang, M.; Wang, C.; Zhang, Y.; Xu, C.; Liao, Z.; Han, H. Triterpenoid acids characterized by the oxidation of the C-27-methy from the roots of Astilbe grandis Stapf ex Wils. Fitoterapia 2023, 168, 105556. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, C.; Li, Q.; Zhang, Y.; Cai, Z.; Yu, H.; Gu, M.; Su, Y.; Han, H.; Liao, Z. Structures and Tumor Cell Lines Proliferation Activities of Triterpenes Isolated from Astilbe grandis. Chem. Biodivers. 2024, 21, e202400100. [Google Scholar] [CrossRef]
- Wu, H.; Li, Q.; Yu, H.; Gu, M.; Wang, Y.; Xu, C.; Liao, Z. Comprehensive utilization of Hainan cashew nut shell: Process optimization of cashew nut shell liquid extraction and cardanol refinement by catalytic transfer hydrogenation. Ind. Crops Prod. 2023, 203, 117168. [Google Scholar] [CrossRef]
- Li, X.; Cao, Q.; Xu, N.; Hu, Y.; Zhu, J.; Liu, M.; Li, L. The proliferation mechanism of colon cancer cell lines HT-29 and Colo-205. J. Toxicol. 2022, 36, 130–136. [Google Scholar]
- Zhang, X.; Xu, H.; Bi, X.; Hou, G.; Liu, A.; Zhao, Y.; Wang, G.; Cao, X. Src acts as the target of matrine to inhibit the proliferation of cancer cells by regulating phosphorylation signaling pathways. Cell Death Dis. 2021, 12, 931. [Google Scholar] [CrossRef] [PubMed]
- Wavefunction, Inc. Spartan ’14; Wavefunction, Inc.: Irvine, CA, USA, 2014. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the Comparison of Calculated and Experimental Electronic Circular Dichroism Spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Pescitelli, G.; Bruhn, T. Good Computational Practice in the Assignment of Absolute Configurations by TDDFT Calculations of ECD Spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef]
- Yao, Z.-J.; Dong, J.; Che, Y.-J.; Zhu, M.-F.; Wen, M.; Wang, N.-N.; Wang, S.; Lu, A.-P.; Cao, D.-S. TargetNet: A web service for predicting potential drug–target interaction profiling via multi-target SAR models. J. Comput. -Aided Mol. Des. 2016, 30, 413–424. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]


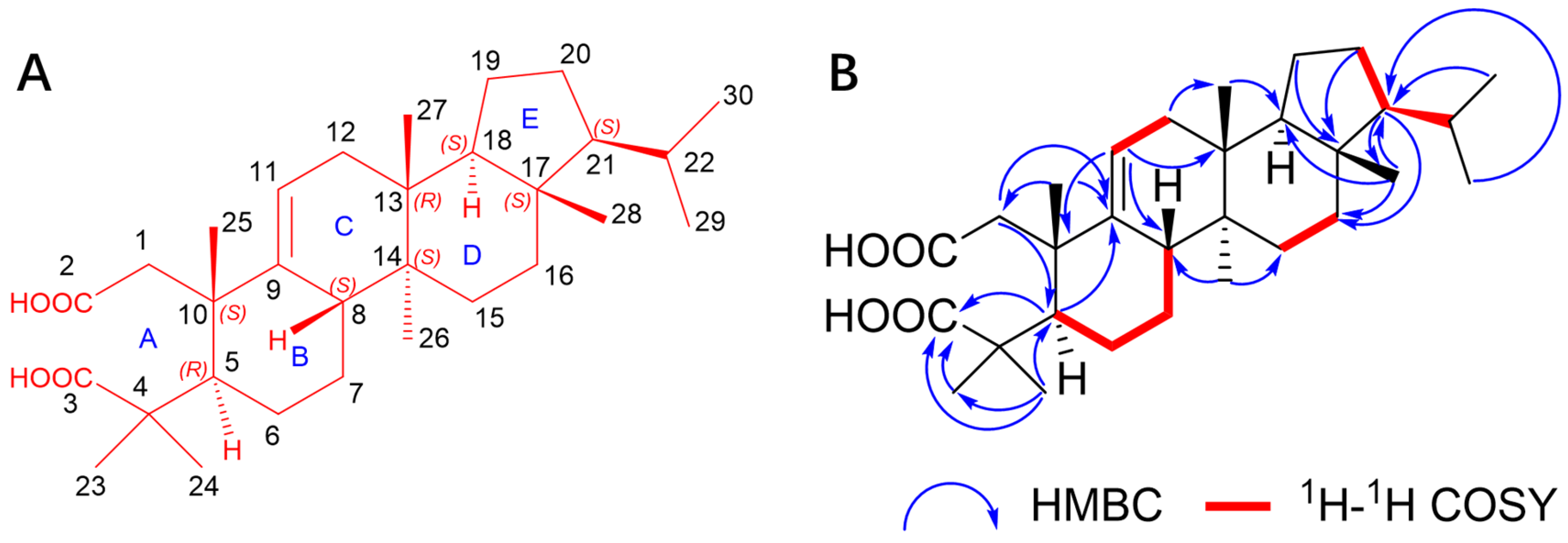
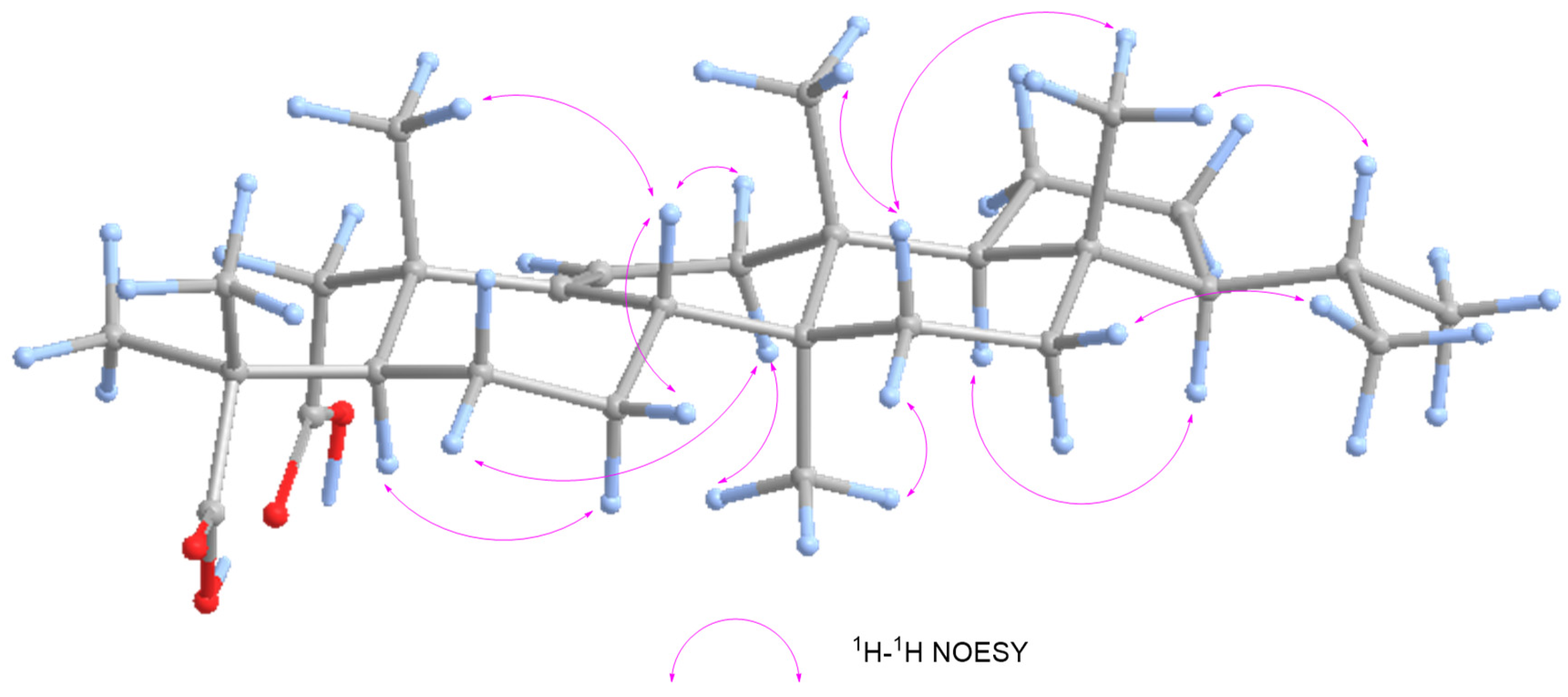
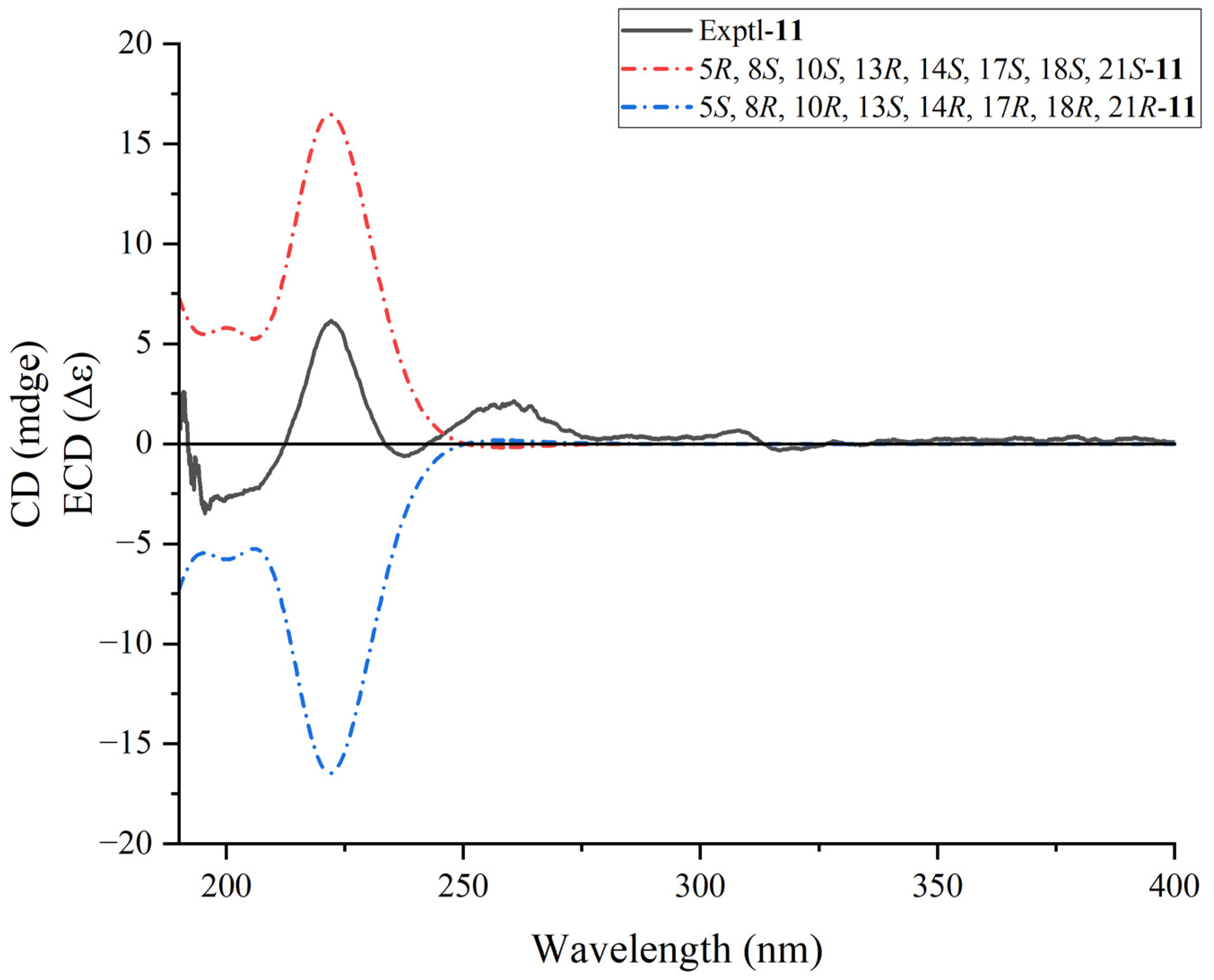
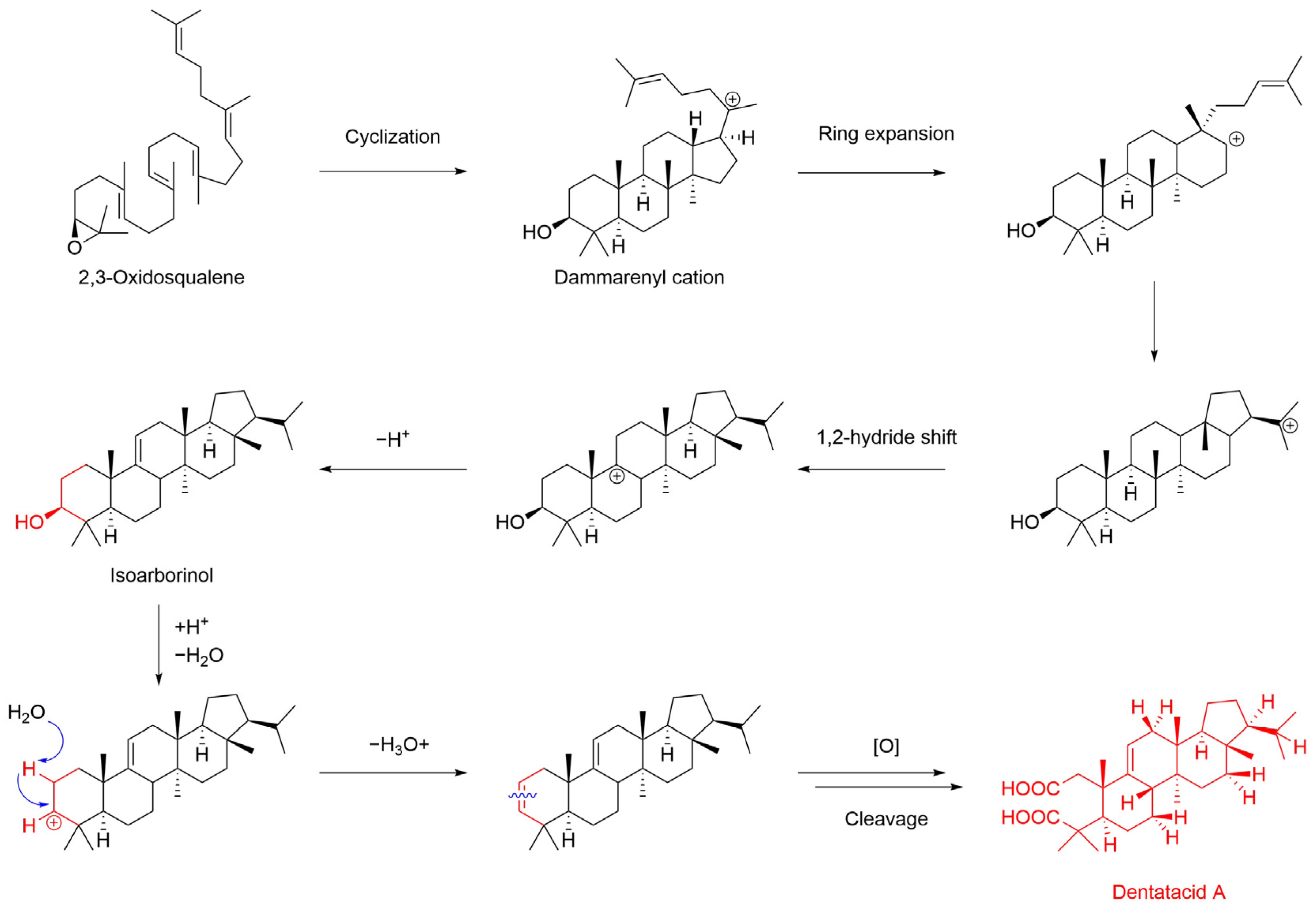
| No. | δC | δC, Type | δH, (mult, J) |
|---|---|---|---|
| 1 | 42.0 | CH2 | 2.67 (d, J = 18.0 Hz), 2.56 (d, J = 18.0 Hz) |
| 2 | 172.4 | C | - |
| 3 | 180.6 | C | - |
| 4 | 45.6 | C | - |
| 5 | 46.7 | CH | 2.52 (dd, J = 11.9, 3.2 Hz) |
| 6 | 23.6 | CH2 | α 1.65 a (m), β 1.50 a (m) |
| 7 | 24.5 | CH2 | β 1.68 a (m), α 1.17 a (m) |
| 8 | 41.8 | CH | 1.91 (d, J = 13.1 Hz) |
| 9 | 145.9 | C | - |
| 10 | 43.4 | C | - |
| 11 | 114.5 | CH | 5.23 (d, J = 5.7 Hz) |
| 12 | 36.2 | CH2 | α 1.59 (d, J = 2.9 Hz), β 1.39 a (m) |
| 13 | 35.9 | C | - |
| 14 | 37.9 | C | - |
| 15 | 29.0 | CH2 | β 1.21 (s), α 1.27 a (m) |
| 16 | 35.5 | CH2 | α 1.34 (dd, J = 13.3, 2.9 Hz), β 1.59 (dd, J = 13.3, 2.9 Hz) |
| 17 | 42.4 | C | - |
| 18 | 51.5 | CH | 1.52 a (m) |
| 19 | 19.7 | CH2 | 1.28 a (m) |
| 20 | 27.8 | CH2 | 1.15 a (m), 1.76 (m) |
| 21 | 59.0 | CH | 0.94 (q, J = 9.4 Hz) |
| 22 | 30.3 | CH | 1.39 a (m) |
| 23 | 27.3 | CH3 | 1.09 a (s) |
| 24 | 23.5 | CH3 | 1.10 (s) |
| 25 | 25.3 | CH3 | 1.09 a (s) |
| 26 | 16.5 | CH3 | 0.74 (s) |
| 27 | 15.3 | CH3 | 0.71 (s) |
| 28 | 13.8 | CH3 | 0.70 (s) |
| 29 | 22.9 | CH3 | 0.79 (d, J = 6.5 Hz) |
| 30 | 22.0 | CH3 | 0.85 (d, J = 6.5 Hz) |
| Compounds | A549 (μM) a | HepG2 (μM) a | HT-29 (μM) a | 143B (μM) a |
|---|---|---|---|---|
| 1 | 22.79 ± 2.86 | >50 | 25.10 ± 1.87 | 19.98 ± 3.16 |
| 2 | 22.69 ± 1.46 | 24.32 ± 1.40 | 15.66 ± 0.38 | >50 |
| 3 | >50 | 12.29 ± 0.11 | 11.87 ± 2.73 | 19.11 ± 5.53 |
| 4 | 4.74 ± 2.61 | 10.10 ± 1.04 | 8.92 ± 2.82 | 26.34 ± 1.06 |
| 5 | 31.56 ± 6.57 | 26.68 ± 4.00 | 5.94 ± 2.13 | >50 |
| 6 | >50 | 24.82 ± 1.13 | 22.77 ± 3.66 | 26.98 ± 0.65 |
| 7 | 10.04 ± 2.22 | >50 | 18.90 ± 1.56 | >50 |
| 8 | 9.63 ± 2.25 | >50 | 21.48 ± 4.15 | 19.27 ± 1.95 |
| 9 | 29.68 ± 3.69 | 23.68 ± 1.72 | 15.07 ± 13.56 | 17.01 ± 2.73 |
| 10 | >50 | 31.52 ± 4.42 | 13.01 ± 1.14 | >50 |
| 11 | 4.85 ± 1.14 | 10.65 ± 2.17 | 2.64 ± 0.78 | 18.82 ± 0.60 |
| HCPT b | 2.89 ± 1.47 | 3.19 ± 0.46 | 2.70 ± 0.16 | 2.56 ± 1.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.-S.; Shao, Y.-L.; Li, Q.; Zhang, Y.; Wu, H.-W.; Yu, H.-L.; Su, Y.-Y.; Zhang, J.; Wang, C.; Liao, Z.-X. Dentatacid A: An Unprecedented 2, 3-Seco-arbor-2, 3-dioic Triterpenoid from the Invasive Plant Euphorbia dentata, with Cytotoxicity Effect on Colon Cancer. Plants 2024, 13, 2533. https://doi.org/10.3390/plants13172533
Xu C-S, Shao Y-L, Li Q, Zhang Y, Wu H-W, Yu H-L, Su Y-Y, Zhang J, Wang C, Liao Z-X. Dentatacid A: An Unprecedented 2, 3-Seco-arbor-2, 3-dioic Triterpenoid from the Invasive Plant Euphorbia dentata, with Cytotoxicity Effect on Colon Cancer. Plants. 2024; 13(17):2533. https://doi.org/10.3390/plants13172533
Chicago/Turabian StyleXu, Chen-Sen, Yuan-Ling Shao, Qing Li, Yu Zhang, Hong-Wei Wu, Hao-Lin Yu, Yun-Yun Su, Jing Zhang, Chao Wang, and Zhi-Xin Liao. 2024. "Dentatacid A: An Unprecedented 2, 3-Seco-arbor-2, 3-dioic Triterpenoid from the Invasive Plant Euphorbia dentata, with Cytotoxicity Effect on Colon Cancer" Plants 13, no. 17: 2533. https://doi.org/10.3390/plants13172533





