Water Management Interventions, Organic Fertilization, and Harvest Time in Dry Land in the Biosaline Production of Cactus Pear
Abstract
1. Introduction
2. Materials and Methods
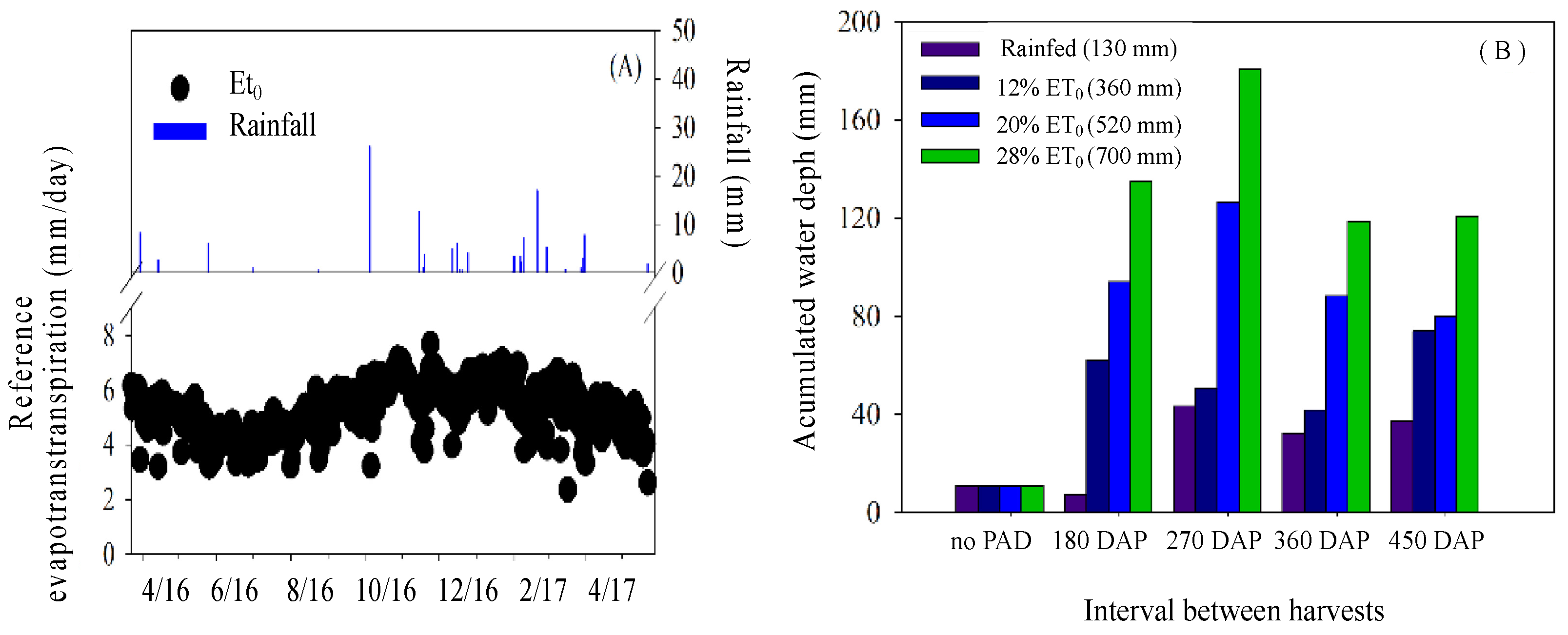
2.1. Experiment Location
2.2. Soil, Water, and Organic Matter
2.3. Experimental Design
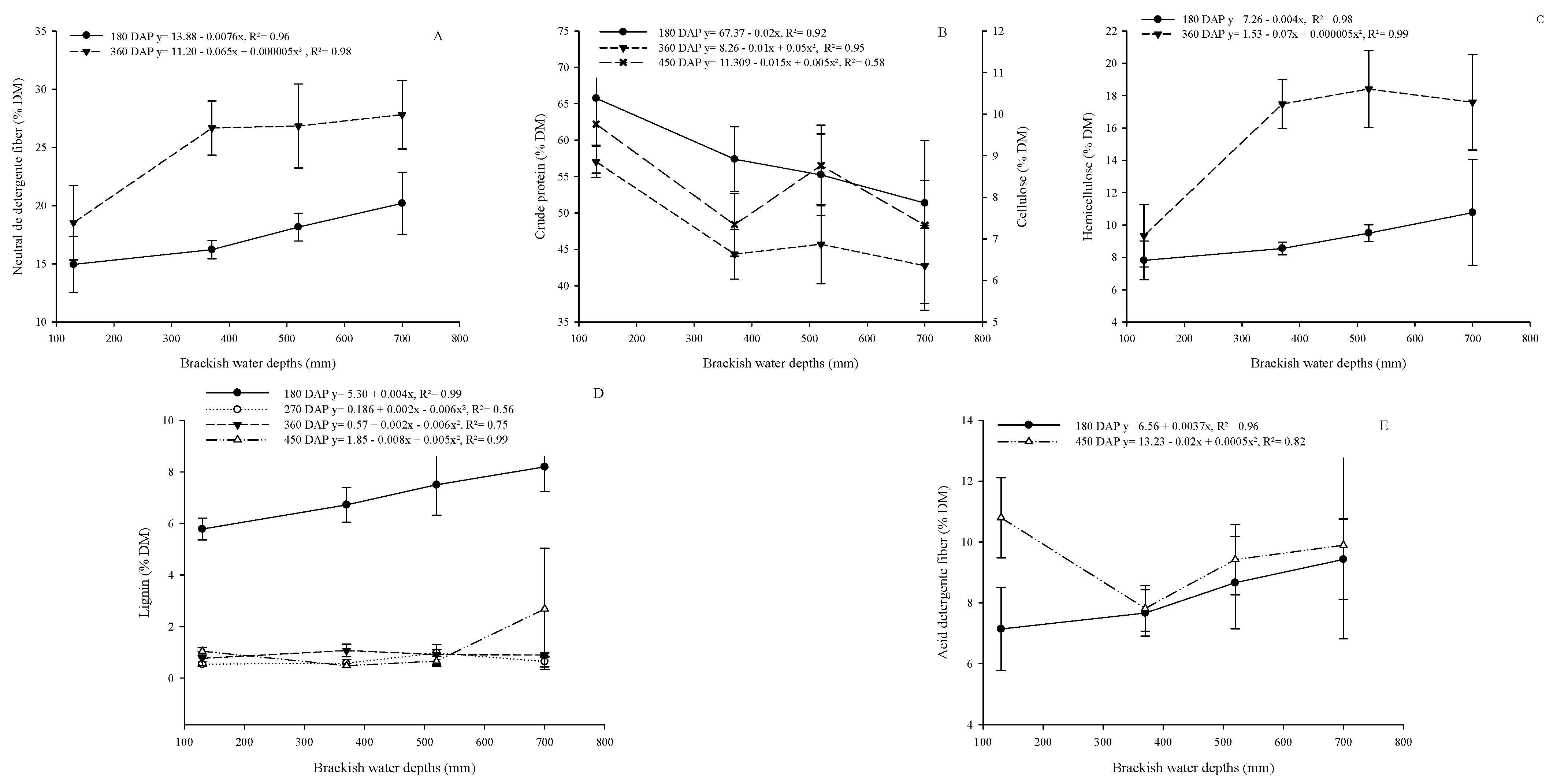
2.4. Growth, Productivity, and Chemical Composition of Cactus Pear
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Lira, J.B.; de Andrade, A.P.; Magalhães, A.L.R.; Campos, F.S.; Araújo, G.G.; Deon, D.S.; Gois, G.C.; Regitano Neto, A.; Cunha, D.S.; Tabosa, J.N.; et al. Production of pearl millet irrigated with different levels of brackish water and organic matter. Commun. Soil Sci. Plant Anal. 2020, 51, 701–709. [Google Scholar] [CrossRef]
- Cuevas, J.; Daliakopoulos, I.N.; del Moral, F.; Hueso, J.J.; Tsanis, I.K. A review of soil-improving cropping systems for soil salinization. Agronomy 2019, 9, 295. [Google Scholar] [CrossRef]
- Mansour, H.A.; Hongjouan, R.; Jiandong, H.; Feng, B.H.; Changmei, L. Performance of water desalination and modern irrigation systems for improving water productivity. In Irrigation—Water Productivity and Operation, Sustainability and Climate Change; Ricart, S., Rico, A.M., Olcina, J., Eds.; IntechOpen: London, UK, 2019; pp. 1–24. [Google Scholar] [CrossRef]
- Silva, J.O.N.; Araújo Júnior, G.N.; Jardim, A.M.; Alves, C.P.; Pinheiro, A.G.; Santos, J.P.A.S.; Souza, L.S.B.; Silva, T.G.F. Cultivation of forage cactus genotypes under biosalin agriculture as an alternative to increase forage input from the Brazilian semiarid region: A review. Res. Soc. Dev. 2021, 10, e16510514773. [Google Scholar] [CrossRef]
- Porto, E.R.; Hermes, L.C.; Ferreira, R.S.; Veiga, H.P.; Saia, A. Agricultura Biossalina: Desafios e Alternativas para o Uso de Águas Salobras e Salinas no Semiárido Brasileiro, 1st ed.; Documentos 121; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2019. [Google Scholar]
- Reda, T.H.; Atsbha, M.K. Nutritional composition, antinutritional factors, antioxidant activities, functional properties, and sensory evaluation of cactus pear (Opuntia ficus-indica) seeds grown in Tigray Region, Ethiopia. J. Exp. Bot. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Davis, S.C.; Simpson, J.; Gil-Veja, K.C.; Niechayev, N.A.; Van Tongerlo, E.; Castano, N.H.; Dever, L.V.; Búrquez, A. Undervalued potential of crassulacean acid metabolism for current and future agricultural production. J. Exp. Bot. 2019, 70, 6521–6537. [Google Scholar] [CrossRef]
- Oliveira, J.P.F.; Ferreira, M.A.F.; Alves, A.M.S.V.; Melo, A.C.C.; Andrade, I.B.; Urbano, A.S.; Suassuna, J.M.A.; Barros, L.J.A.; Melo, T.T.B. Carcass characteristics of lambs fed spineless cactus as a replacement for sugarcane. Asian-Australas. J. Anim. Sci. 2018, 31, 529–536. [Google Scholar] [CrossRef]
- Mayer, J.A.; Cushman, J.C. Nutritional and mineral content of prickly pear cactus: A highly water-use efficient forage, fodder and food species. J. Agron. Crop Sci. 2019, 205, 625–634. [Google Scholar] [CrossRef]
- Fonseca, V.A.; Santos, M.R.; Silva, J.A.; Donato, S.L.R.; Rodrigues, C.S.; Brito, C.F.B. Morpho-physiology, yield, and water-use efficiency of Opuntia ficus-indica irrigated with saline water. Acta Sci. Agron. 2019, 41, 1–11. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Jardim, A.M.R.F.; Silva, T.G.F.; Souza, L.S.B.; Souza, M.S. Interaction of agroecosystem intercropped with forage cactus-sorghum in the semi-arid environment: A review. J. Env. Anal. Progr. 2020, 5, 69–87. [Google Scholar] [CrossRef]
- Mekki, B.B. High salinity stress tolerant halophytic plant species for sustainable agriculture in desert regions: A review. World Appl. Sci. J. 2016, 34, 1603–1611. [Google Scholar] [CrossRef]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef] [PubMed]
- Farrag, K.; Abdelhakim, S.G.; El-Tawab, A.R.A.; Abdelrahman, H. Growth response of blue panic grass (Panicum antidotale) to saline water irrigation and compost applications. Water Sci. 2021, 35, 31–38. [Google Scholar] [CrossRef]
- Cavalcante, E.S.; Lacerda, C.F.; Costa, R.N.T.; Gheyi, H.R.; Pinho, L.L.; Bezerra, F.M.S.; Oliveira, A.C.; Canjá, J.F. Supplemental irrigation using brackish water on maize in tropical semi-arid regions of Brazil: Yield and economic analysis. Sci. Agric. 2021, 78, e20200151. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Melo Filho, J.S.; Véras, M.L.M.; Silva, T.I.; Alves, L.S.; Dias, T.J. Organic fertilizers as mitigating effects of water salinity on Passiflora cincinnata seedlings. Acta Agron. 2018, 67, 501–511. [Google Scholar] [CrossRef]
- Ding, Z.; Kheir, A.M.S.; Ali, M.G.M.; Abdelaal, A.I.N.; Lin, X.; Zhou, Z.; Wang, B.; Liu, B.; He, Z. The integrated effect of salinity, organic amendments, phosphorus fertilizers, and deficit irrigation on soil properties, phosphorus fractionation and wheat productivity. Sci. Rep. 2020, 10, e2736. [Google Scholar] [CrossRef]
- Freire, J.L.; Santos, M.V.F.; Dubeux Júnior, J.C.B.; Bezerra Neto, E.; Lira, M.A.; Cunha, M.V.; Santos, D.C.; Amorim, S.O.; Mello, A.C.L. Growth of cactus pear cv. Miúda under different salinity levels and irrigation frequencies. An. Acad. Bras. Ciênc. 2018, 90, 3893–3900. [Google Scholar] [CrossRef]
- Freire, J.L.; Santos, M.V.F.; Dubeux Júnior, J.C.B.; Bezerra Neto, E.; Lira, M.A.; Cunha, M.V.; Santos, D.C.; Mello, A.C.L.; Oliveira, C.G.S. Evaluation of cactus pear clones subjected to salt stress. Trop. Grassl.-Forrajes Trop. 2021, 9, 235–242. [Google Scholar] [CrossRef]
- Embrapa. Empresa Brasileira de Pesquisa Agropecuária. Sistema Brasileiro de Classificação de Solos, 3rd ed.; Embrapa: Brasília, Brazil, 1954. [Google Scholar]
- AOAC. Official Methods of Analysis, 20th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2016; p. 3100. [Google Scholar]
- Bataglia, O.; Teixeira, J.; Furlani, P.; Furlani, A. Métodos de Análise Química de Plantas; Instituto Agronômico de Campinas: Campinas, Brazil, 1983.
- Holanda Filho, R.S.; Santos, D.B.D.; Azevedo, C.A.; Coelho, E.F.; Lima, V.L. Agua salina nos atributos químicos do solo e no estado nutricional da mandioqueira. Bras. Eng. Agri. Ambient. 2011, 15, 60–66. [Google Scholar] [CrossRef]
- Coldebella, N.; Lorenzetti, E.; Tartaro, J.; Treib, E.L.; Pinto, R.E.; Fontana, A.; Alves, A.B. Desempenho do milho a elevação da participação do cálcio na CTC. Sci. Agrar. Paraná 2018, 17, 443–450. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements, 1st ed.; FAO: Rome, Italy, 1998. [Google Scholar]
- Inmet. Dados Agrometeorológicos. 2017. Available online: http://www.inmet.gov.br/portal/ (accessed on 1 June 2020).
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; USDA Agricultural Handbook 60; Department of Agriculture: Washington, DC, USA, 1954.
- Silva, T.G.F.; Araújo Primo, J.T.; Silva, S.M.S.; Moura, M.S.B.; Santos, D.C.; Silva, M.C.; Araújo, J.E.M. Indicadores de eficiência do uso da água e de nutrientes de clones de palma forrageira em condições de sequeiro no Semiárido brasileiro. Bragantia 2014, 73, 184–191. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Silva, D.J.; Queiroz, A.C. Análise de Alimentos: Métodos Químicos e Biológicos, 2nd ed.; Editora UFV: Viçosa, Brazil, 2002. [Google Scholar]
- Hall, M.B. Challenges with nonfibre carbohydrate methods. J. Anim. Sci. 2003, 81, 3226–3232. [Google Scholar] [CrossRef] [PubMed]
- Sniffen, C.J.; O’Connor, J.D.; Van Soest, P.J.; Fox, D.G.; Russell, J.B. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. J. Anim. Sci. 1992, 70, 3562–3577. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. SAS/STAT®14.1 User’s Guide: High-Performance Procedures; University Edition; SAS Institute Inc: Cary, NC, USA, 2015. [Google Scholar]
- Silva, T.G.F.; Morais, J.E.F.; Diniz, W.J.S.; Souza, C.A.A.; Silva, M.C. Crescimento e produtividade de clones de palma forrageira no semiárido e relações com variáveis meteorológicas. Rev. Caatinga 2015, 28, 10–18. [Google Scholar]
- Lima, L.R.; Silva, T.G.F.; Pereira, P.C.; Morais, J.E.F.; Assis, M.C.S. Productive-economic benefit of forage cactus-sorghum intercropping systems irrigated with saline water. Rev. Caatinga 2018, 31, 191–201. [Google Scholar] [CrossRef]
- Lima, G.F.C.; Rego, M.M.T.; Dantas, F.D.G.; Lôbo, R.N.B.; Silva, J.G.M.; Aguiar, E.M. Morphological characteristics and forage productivity of irrigated cactus pear under different cutting intensities. Rev. Caatinga 2016, 29, 481–488. [Google Scholar] [CrossRef][Green Version]
- Nadaf, S.K.; Al-Farsi, S.M.; Al-Hinai, S.A.; Al-Hinai, A.S.; Al-Harthy, A.A.S.; Al-Khamisi, S.A.; Al-Bakri, A.N. Potential of forage cactus pear accessions under saline water irrigation in arid areas. J. Prof. Assoc. Cactus Dev. 2018, 20, 68–81. [Google Scholar] [CrossRef]
- Rocha, R.S.; Voltolini, T.V.; Gava, C.A.T. Características produtivas e estruturais de genótipos de palma forrageira irrigada em diferentes intervalos de corte. Ar. Zootec. 2017, 66, 363–371. [Google Scholar] [CrossRef]
- Arba, M.; Falisse, A.; Choukr-Allah, R.; Sindicd, M. Effect of irrigation at critical stages on the phenology of flowering and fruiting of the cactus Opuntia spp. Braz. J. Biol. 2018, 78, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Lemos, M.; Ferreira-Neto, M.; Santos Fernandes, C.; Lima, Y.B.; Dias, N.D.S.; Medeiros, J.F.; Sá, F.V.S. The effect of domestic sewage effluent and planting density on growth and yield of prickly pear cactus in the semiarid region of Brazil. J. Arid. Environ. 2021, 185, e104372. [Google Scholar] [CrossRef]
- Pereira, P.C.; Silva, T.G.F.; Zolnier, S.; Morais, J.E.F.; Santos, D.C. Growth evolution of cactus forage drip irrigated. Rev. Caatinga 2015, 28, 184–195. [Google Scholar] [CrossRef]
- Ramos, J.P.F.; Leite, M.L.M.V.; Oliveira Junior, S.; Nascimento, J.P.; Santos, E.M. Crescimento vegetativo de Opuntia fícus-indica em diferentes espaçamentos de plantio. Rev. Caatinga 2011, 24, 41–48. [Google Scholar]
- Reis Filho, R.J.C.; Carneiro, M.S.S.; Pereira, E.S.; Furtado, R.N.; Morais Neto, L.B.; Magalhães, J.A.; Lopes, M.N. Biomass components and water use efficiency in cactus pear under different irrigation systems and harvest frequencies. Rev. Bras. Zootec. 2022, 51, 1–15. [Google Scholar] [CrossRef]
- Lédo, A.A.; Donato, S.L.R.; Aspiazu, I.; Silva, J.A.; Donato, P.E.R.; Carvalho, A.J. Yield and water use efficiency of cactus pear under arrangements, spacings and fertilizations. Rev. Bras. Eng. Agríc. Ambient. 2019, 23, 413–418. [Google Scholar] [CrossRef]
- Araújo Júnior, G.N.; Silva, T.G.F.; Souza, L.S.B.; Souza, M.S.; Araújo, G.G.L.; Moura, M.S.B.; Alves, H.K.M.N. Productivity, bromatological composition and economic benefits of using irrigation in the forage cactus under regulated deficit irrigation in a semiarid environment. Bragantia 2021, 80, 1–12. [Google Scholar] [CrossRef]
- Dubeux JR, J.C.B.; Santos, M.V.; Mello, A.C.L.; Cunha, M.V.; Ferreira, M.A.; Santos, D.C.; Lira, M.A.; Silva, M.C. Forage potential of cacti on drylands. Acta Hort. 2015, 1067, 181–186. [Google Scholar] [CrossRef]
- Rego, M.M.T.; Lima, G.F.C.; Silva, J.G.M.; Guedes, F.X.; Dantas, F.D.G.; Lobo, R.N.B. Morfologia e rendimento de biomassa da palma miúda irrigada sob doses de adubação orgânica e intensidades de corte. Rev. Cient. Prod. Anim. 2014, 16, 118–130. [Google Scholar] [CrossRef]
- Ramos, J.P.F.; Santos, E.M.; Pinho, R.M.A.; Bezerra, H.F.C.; Pereira, G.A.; Beltrão, G.R.; Oliveira, J.S. Crescimento da palma forrageira em função da adubação orgânica. Rev. Electrón. Vet. 2015, 16, 1–11. [Google Scholar]
- Donato, P.E.R.; Pires, A.J.V.; Donato, S.L.; Bonomo, P.; Silva, J.A.; Aquino, A.A. Morfometria e rendimento da palma forrageira “Gigante” sob diferentes espaçamentos e doses de adubação orgânica. Rev. Bras. Ciênc. Agr. 2014, 9, 151–158. [Google Scholar] [CrossRef]
- Donato, P.E.R.; Donato, S.L.R.; Silva, J.A.; Pires, A.J.V.; Rosa, R.C.; Aquino, A.A. Valor nutritivo da palma forrageira “Gigante” cultivada sob diferentes espaçamentos e doses de esterco bovino. Rev. Caatinga 2014, 27, 163–172. [Google Scholar]





| Layer 0–20 cm | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC | pH | C | N | P | K | Na | Ca | Mg | H + Al | SB | CEC | V |
| mS/cm | - | g/kg | mg/dm3 | cmol/dm3 | % | |||||||
| 1.90 | 6.0 | 6.8 | 0.4 | 11.4 | 0.3 | 0.9 | 2.6 | 2.8 | 2.6 | 3.2 | 5.8 | 54 |
| Organic Matter | Brackish Water Depths (mm) | SEM | p-Valor | |||
|---|---|---|---|---|---|---|
| 130 | 370 | 520 | 700 | |||
| Plant height (cm) | ||||||
| 0 | 34.18 ± 5.06 | 37.31 ± 5.43 | 39.40 ± 5.11 | 42.87 ± 4.01 | 3.81 | 0.434 |
| 15 | 42.62 ± 7.83 | 46.18 ± 6.84 | 50.00 ± 6.36 | 53.00 ± 11.88 | 3.81 | 0.243 |
| 30 | 41.62 ± 8.66 b | 48.31 ± 11.07 b | 62.43 ± 15.62 a | 69.81 ± 17.94 a | 3.81 | <0.001 |
| 45 | 41.31 ± 4.82 b | 56.93 ± 9.82 a | 58.50 ± 12.55 a | 59.37 ± 13.44 a | 3.81 | 0.002 |
| Total number of cladodes (n°) | ||||||
| 0 | 7.75 ± 3.00 | 8.75 ± 1.92 | 6.18 ± 0.78 | 7.25 ± 2.00 | 1.15 | 0.463 |
| 15 | 9.87 ± 4.13 | 11.18 ± 2.14 | 10.37 ± 2.25 | 10.93 ± 4.53 | 1.15 | 0.854 |
| 30 | 11.06 ± 2.95 b | 12.31 ± 1.73 b | 13.62 ± 4.17 b | 18.37 ± 5.70 a | 1.15 | <0.001 |
| 45 | 9.31 ± 2.06 b | 11.06 ± 1.38 ab | 13.27 ± 3.69 ab | 14.56 ± 4.11 a | 1.15 | 0.007 |
| Fresh matter production (t/ha) | ||||||
| 0 | 19.32 ± 6.72 | 18.75 ± 3.69 | 17.70 ± 6.65 | 16.58 ± 5.41 | 12.48 | 1.002 |
| 15 | 20.92 ± 14.28 | 58.15 ± 25.81 | 48.63 ± 25.89 | 65.23 ± 36.48 | 12.48 | 0.066 |
| 30 | 18.27 ± 12.44 b | 68.31 ± 29.39 a | 81.91 ± 44.37 a | 110.95 ± 53.34 a | 12.48 | <0.001 |
| 45 | 48.47 ± 14.95 b | 111.87 ± 57.84 a | 72.49 ± 39.47 ab | 113.13 ± 45.18 a | 12.48 | <0.001 |
| Dry matter productivity (t/ha) | ||||||
| 0 | 2.16 ± 0.94 | 1.75 ± 0.28 | 1.69 ± 0.64 | 1.46 ± 0.49 | 0.93 | 0.963 |
| 15 | 1.97 ± 1.15 b | 4.64 ± 1.89 ab | 4.19 ± 2.42 ab | 5.86 ± 2.89 a | 0.93 | 0.030 |
| 30 | 1.69 ± 1.06 b | 5.38 ± 2.54 b | 6.66 ± 3.87 ab | 9.08 ± 3.69 a | 0.93 | <0.001 |
| 45 | 4.14 ± 1.02 b | 7.99 ± 3.52 a | 5.80 ± 2.78 ab | 8.53 ± 3.34 a | 0.93 | <0.001 |
| Water productivity (ton/ha) | ||||||
| 0 | 17.16 ± 5.78 | 16.99 ± 3.42 | 16.00 ± 6.02 | 15.11 ± 4.92 | 11.57 | 1.003 |
| 15 | 18.95 ± 13.22 | 53.50 ± 24.12 | 44.44 ± 23.47 | 59.37 ± 33.60 | 11.57 | 0.070 |
| 30 | 16.57 ± 11.38 b | 62.93 ± 26.85 a | 75.25 ± 40.50 a | 101.86 ± 49.68 a | 11.57 | <0.001 |
| 45 | 44.33 ± 14.01 b | 103.87 ± 54.34 a | 66.69 ± 36.70 ab | 104.60 ± 42.23 a | 11.57 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, T.C.M.D.; Araújo, G.G.L.d.; Silva, T.G.F.d.; Voltolini, T.V.; Gois, G.C.; Araújo, C.d.A.; Zanine, A.d.M.; Ferreira, D.d.J.; Pereira, D.M.; Santos, F.N.d.S.; et al. Water Management Interventions, Organic Fertilization, and Harvest Time in Dry Land in the Biosaline Production of Cactus Pear. Plants 2024, 13, 2540. https://doi.org/10.3390/plants13182540
Nunes TCMD, Araújo GGLd, Silva TGFd, Voltolini TV, Gois GC, Araújo CdA, Zanine AdM, Ferreira DdJ, Pereira DM, Santos FNdS, et al. Water Management Interventions, Organic Fertilization, and Harvest Time in Dry Land in the Biosaline Production of Cactus Pear. Plants. 2024; 13(18):2540. https://doi.org/10.3390/plants13182540
Chicago/Turabian StyleNunes, Tarcia Carielle Miranda Dantas, Gherman Garcia Leal de Araújo, Thieres George Freire da Silva, Tadeu Vinhas Voltolini, Glayciane Costa Gois, Cleyton de Almeida Araújo, Anderson de Moura Zanine, Daniele de Jesus Ferreira, Danillo Marte Pereira, Francisco Naysson de Sousa Santos, and et al. 2024. "Water Management Interventions, Organic Fertilization, and Harvest Time in Dry Land in the Biosaline Production of Cactus Pear" Plants 13, no. 18: 2540. https://doi.org/10.3390/plants13182540
APA StyleNunes, T. C. M. D., Araújo, G. G. L. d., Silva, T. G. F. d., Voltolini, T. V., Gois, G. C., Araújo, C. d. A., Zanine, A. d. M., Ferreira, D. d. J., Pereira, D. M., Santos, F. N. d. S., Parente, H. N., Turco, S. H. N., Parente, M. d. O. M., & Campos, F. S. (2024). Water Management Interventions, Organic Fertilization, and Harvest Time in Dry Land in the Biosaline Production of Cactus Pear. Plants, 13(18), 2540. https://doi.org/10.3390/plants13182540










