Pleiotropic Quantitative Trait Loci (QTL) Mining for Regulating Wheat Processing Quality- and Yield-Related Traits
Abstract
1. Introduction
2. Results
2.1. Phenotype Assessments
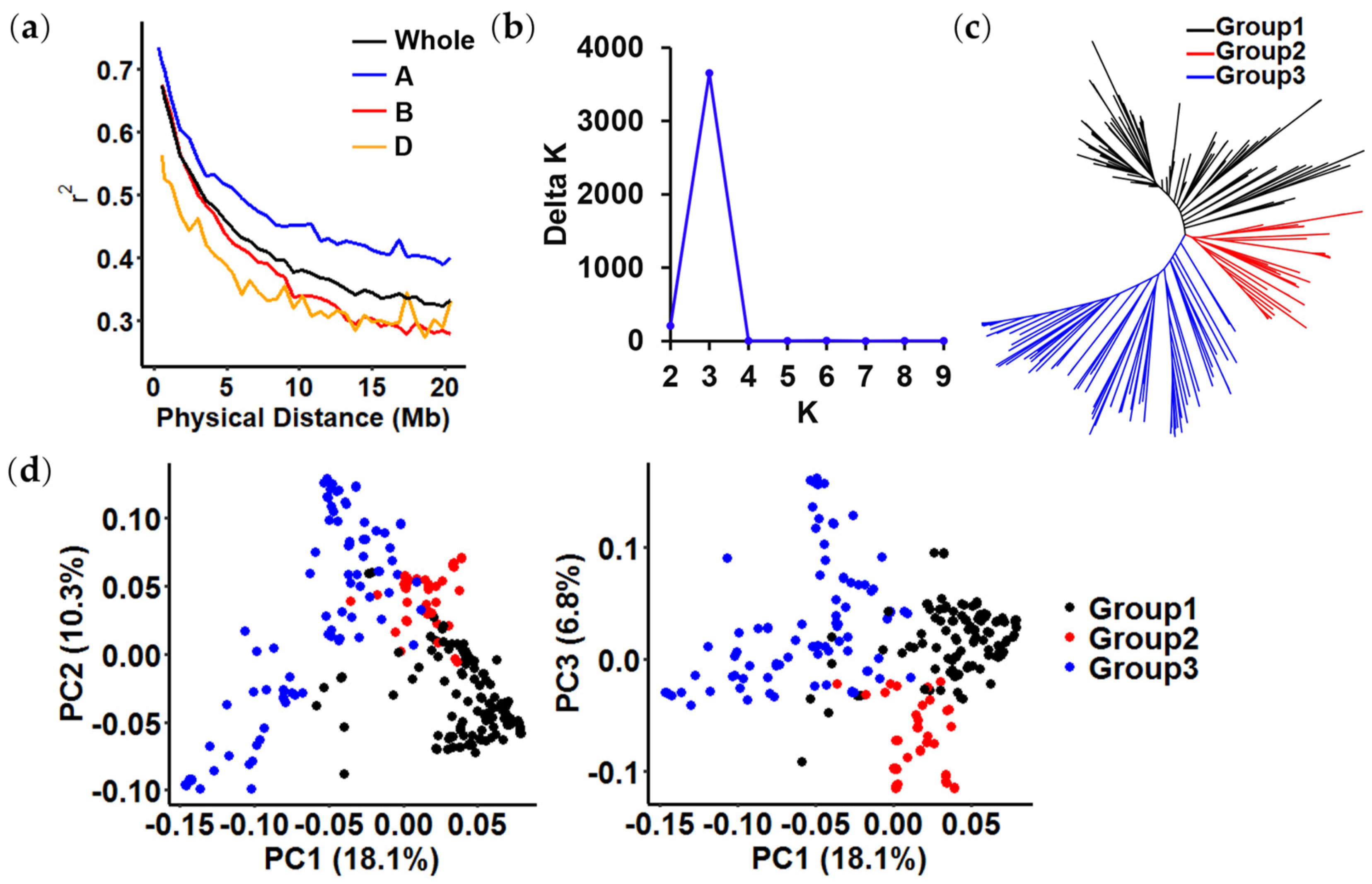
2.2. Linkage Disequilibrium (LD) Analysis and Population Structure
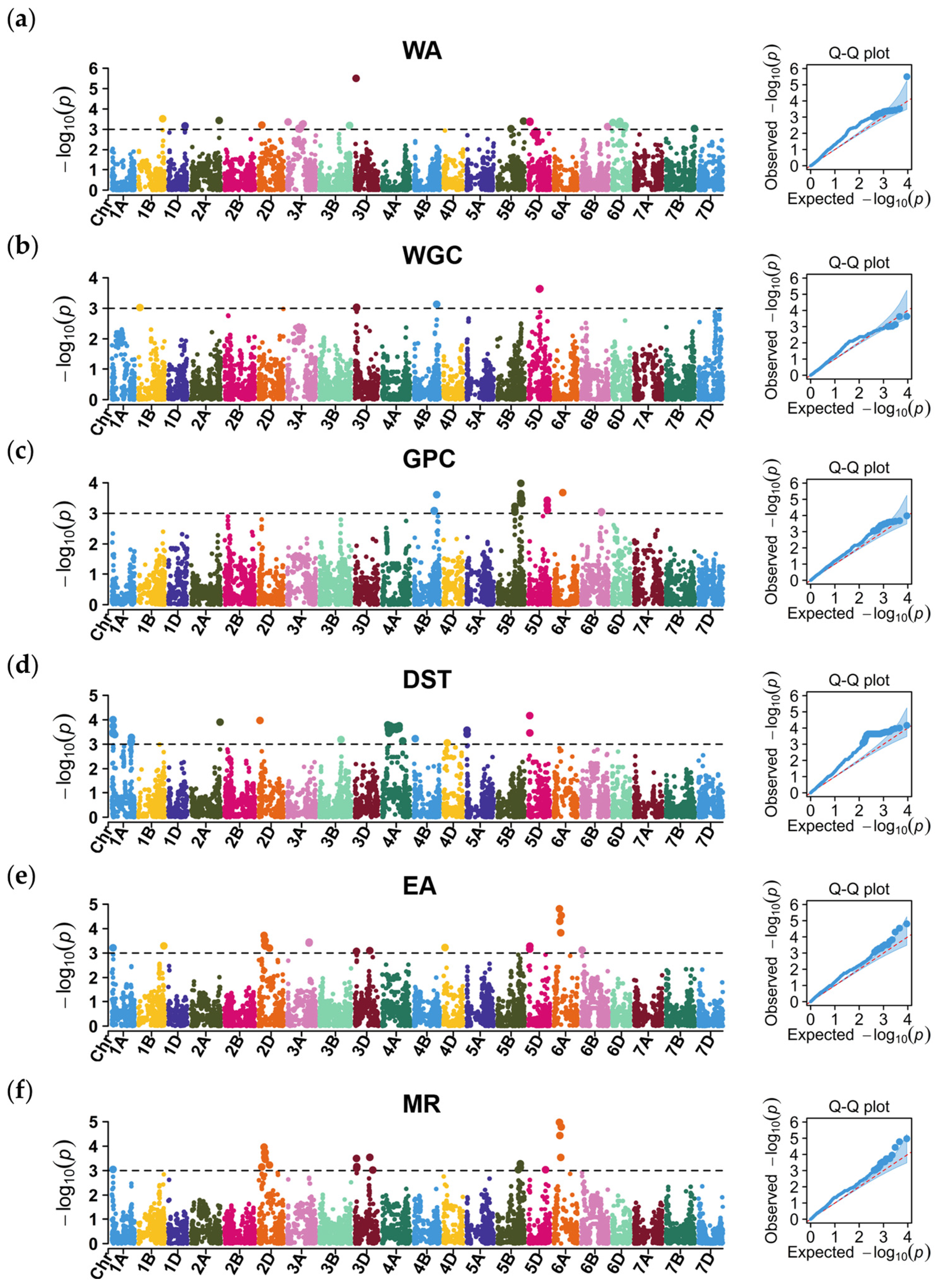
2.3. GWAS of the Grain Quality-Related Traits
2.3.1. Water Absorption
2.3.2. Wet Gluten Content
2.3.3. Grain Protein Content
2.3.4. Dough Stability Time
2.3.5. Extension Area
2.3.6. Maximum Resistance
2.4. GWAS of the Grain Yield-Related Traits
2.5. Analysis of Pleiotropic and Flour Quality-Related and Grain Yield-Related MTAs
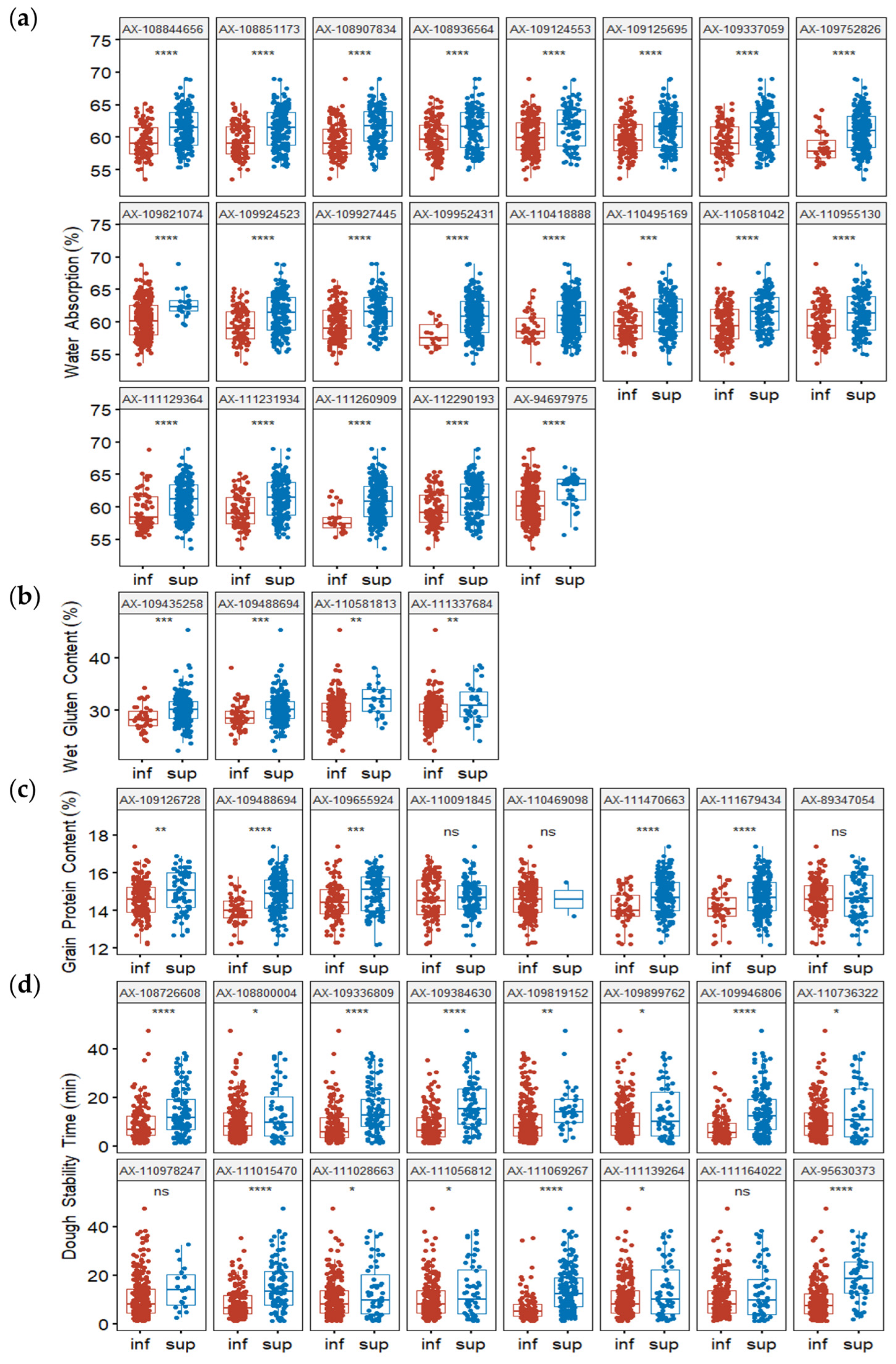
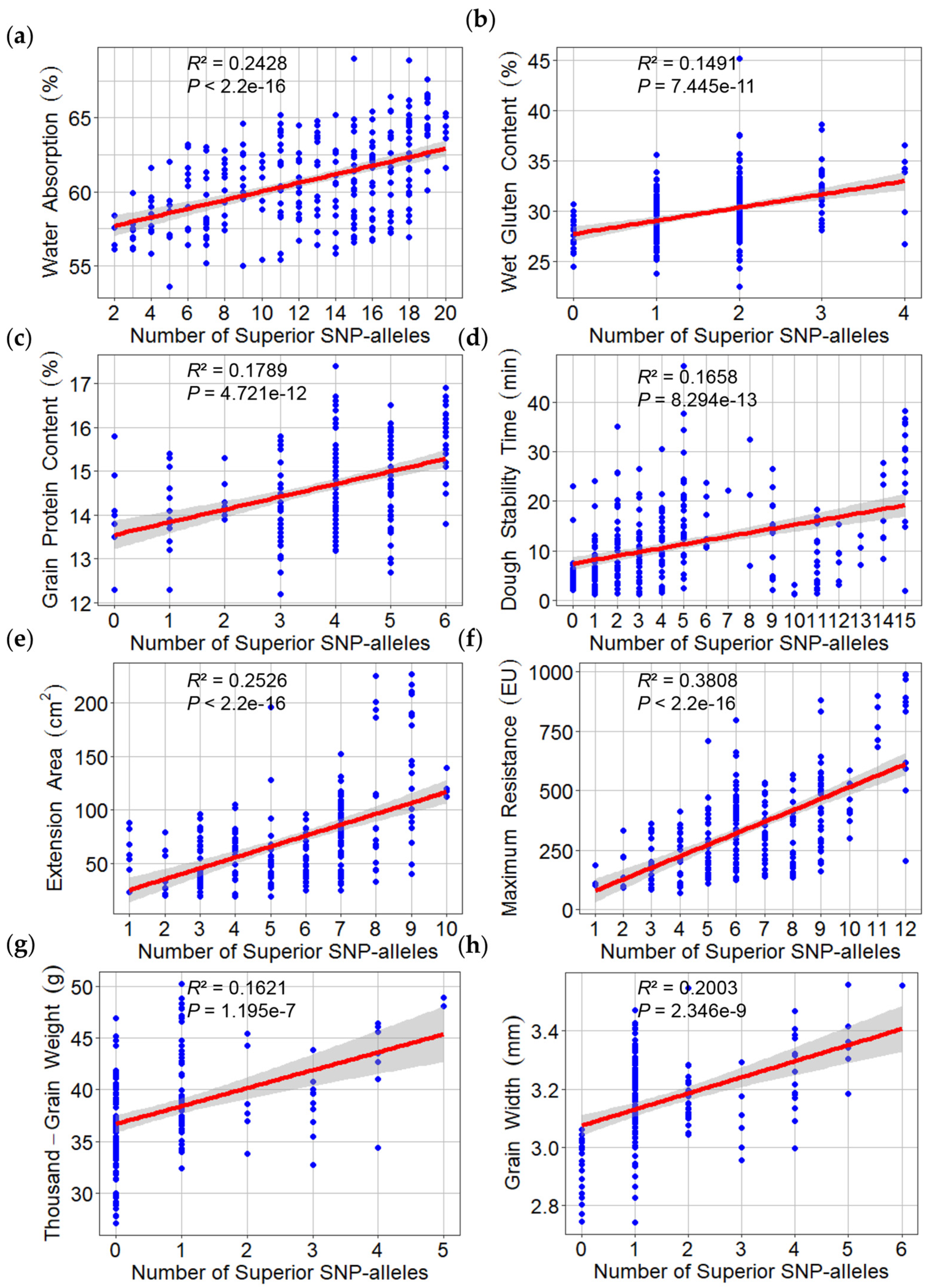
2.6. Effects of Superior Alleles on Phenotypes and Allelic SNPs on Wheat Quality and Yield
3. Discussion
3.1. Identification of MTAs and Comparisons with Previous Findings
3.2. The Validation of Rare and Multiple Phenotype-Related MTAs in the Association Population
4. Materials and Methods
4.1. Materials
4.2. Measurements of Flour Quality and Grain Traits
4.3. Phenotypic Data Analysis
4.4. SNP Genotyping
4.5. Linkage Disequilibrium
4.6. Population Structure and Kinship Analysis
4.7. Genome-Wide Association Mapping
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QTL | Quantitative trait loci |
| GWAS | Genome-wide association study |
| WA | Water absorption |
| WGC | Wet gluten content |
| GPC | Grain protein content |
| DST | Dough stability time |
| EA | Extension area |
| MR | Maximum resistance |
| TGW | Thousand-grain weight |
| GL | Grain length |
| GW | Grain width |
| SNP | Significant single-nucleotide polymorphism |
| MTA | Marker–trait association |
References
- Hu, J.; Xiao, G.; Jiang, P.; Zhao, Y.; Zhang, G.; Ma, X.; Yao, J.; Xue, L.; Su, P.; Bao, Y. QTL detection for bread wheat processing quality in a nested association mapping population of semi-wild and domesticated wheat varieties. BMC Plant Biol. 2022, 22, 129. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Fan, X.; Chen, M.; Zhang, N.; Zhao, C.; Zhang, W.; Han, J.; Ji, J.; Zhao, X.; Yang, L.; et al. QTL detection for wheat kernel size and quality and the responses of these traits to low nitrogen stress. Theor. Appl. Genet. 2016, 129, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Singh, K.; Singh, B.; Grewal, S.; Dwivedi, N.; Alqarawi, A.A.; Abd Allah, E.F.; Ahmad, P.; Singh, N.K. Analysis of genetic control and QTL mapping of essential wheat grain quality traits in a recombinant inbred population. PLoS ONE 2019, 14, e0200669. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, G.; Guo, B.; Qu, C.; Zhang, M.; Kong, F.; Zhao, Y.; Li, S. QTL mapping for quality traits using a high-density genetic map of wheat. PLoS ONE 2020, 15, e0230601. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Tian, T.; Ma, J.; Liu, Y.; Zhang, P.; Chen, T.; Shahinnia, F.; Yang, D. Genome-Wide Association Study of Kernel Traits Using a 35K SNP Array in Bread Wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 905660. [Google Scholar] [CrossRef]
- Gasparis, S.; Miloszewski, M.M. Genetic basis of grain size and weight in rice, wheat, and barley. Int. J. Mol. Sci. 2023, 24, 16921. [Google Scholar] [CrossRef]
- Zanke, C.D.; Ling, J.; Plieske, J.; Kollers, S.; Ebmeyer, E.; Korzun, V.; Argillier, O.; Stiewe, G.; Hinze, M.; Neumann, F. Analysis of main effect QTL for thousand grain weight in European winter wheat (Triticum aestivum L.) by genome-wide association mapping. Front. Plant Sci. 2015, 6, 644. [Google Scholar] [CrossRef]
- Sajjad, M.; Khan, S.; Ahmad, M.; Rasheed, A.; Mujeeb-Kazi, A.; Khan, I. Association mapping identifies QTLs on wheat chromosome 3A for yield related traits. Cereal Res. Commun. 2014, 42, 177–188. [Google Scholar] [CrossRef]
- Soriano, J.M.; Colasuonno, P.; Marcotuli, I.; Gadaleta, A. Meta-QTL analysis and identification of candidate genes for quality, abiotic and biotic stress in durum wheat. Sci. Rep. 2021, 11, 11877. [Google Scholar] [CrossRef]
- Semagn, K.; Iqbal, M.; Chen, H.; Perez-Lara, E.; Bemister, D.H.; Xiang, R.; Zou, J.; Asif, M.; Kamran, A.; N’Diaye, A.; et al. Physical mapping of QTL associated with agronomic and end-use quality traits in spring wheat under conventional and organic management systems. Theor. Appl. Genet. 2021, 134, 3699–3719. [Google Scholar] [CrossRef]
- Lou, H.; Zhang, R.; Liu, Y.; Guo, D.; Zhai, S.; Chen, A.; Zhang, Y.; Xie, C.; You, M.; Peng, H.; et al. Genome-wide association study of six quality-related traits in common wheat (Triticum aestivum L.) under two sowing conditions. Theor. Appl. Genet. 2021, 134, 399–418. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chai, Y.; Zhang, X.; Lu, S.; Zhao, Z.; Wei, D.; Chen, L.; Hu, Y.G. Multi-locus GWAS of quality traits in bread wheat: Mining more candidate genes and possible regulatory network. Front. Plant Sci. 2020, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, L.; Gao, H.; Hu, M.; Mu, L.; Cheng, X.; Wang, J.; Zhao, Y.; Li, Q.; Wang, P.; et al. Genome-wide association study of yield-related traits in common wheat (Triticum aestivum L.) under normal and drought treatment conditions. Front. Plant Sci. 2023, 13, 1098560. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cui, F.; Ding, A.M.; Zhao, C.H.; Wang, X.Q.; Wang, L.; Bao, Y.G.; Qi, X.L.; Li, X.F.; Gao, J.R.; et al. QTL detection of seven quality traits in wheat using two related recombinant inbred line populations. Euphytica 2012, 183, 207–226. [Google Scholar] [CrossRef]
- Carter, A.H.; Garland-Campbell, K.; Morris, C.F.; Kidwell, K.K. Chromosomes 3B and 4D are associated with several milling and baking quality traits in a soft white spring wheat (Triticum aestivum L.) population. Theor. Appl. Genet. 2012, 124, 1079–1096. [Google Scholar] [CrossRef]
- Krystkowiak, K.; Langner, M.; Adamski, T.; Salmanowicz, B.P.; Kaczmarek, Z.; Krajewski, P.; Surma, M. Interactions between Glu-1 and Glu-3 loci and associations of selected molecular markers with quality traits in winter wheat (Triticum aestivum L.) DH lines. J. Appl. Genet. 2017, 58, 37–48. [Google Scholar] [CrossRef]
- Nordborg, M.; Weigel, D. Next-generation genetics in plants. Nature 2008, 456, 720–723. [Google Scholar] [CrossRef]
- Hayes, B. Overview of statistical methods for genome-wide association studies (GWAS). Methods Mol. Biol. 2013, 1019, 149–169. [Google Scholar]
- Gao, L.; Meng, C.; Yi, T.; Xu, K.; Cao, H.; Zhang, S.; Yang, X.; Zhao, Y. Genome-wide association study reveals the genetic basis of yield- and quality-related traits in wheat. BMC Plant Biol. 2021, 21, 144. [Google Scholar] [CrossRef]
- Fan, X.; Liu, X.; Feng, B.; Zhou, Q.; Deng, G.; Long, H.; Cao, J.; Guo, S.; Ji, G.; Xu, Z.; et al. Construction of a novel wheat 55 K SNP array-derived genetic map and its utilization in QTL mapping for grain yield and quality related traits. Front. Genet. 2022, 13, 978880. [Google Scholar] [CrossRef]
- Muqaddasi, Q.H.; Brassac, J.; Ebmeyer, E.; Kollers, S.; Korzun, V.; Argillier, O.; Stiewe, G.; Plieske, J.; Ganal, M.W.; Roder, M.S. Prospects of GWAS and predictive breeding for European winter wheat’s grain protein content, grain starch content, and grain hardness. Sci. Rep. 2020, 10, 12541. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wen, W.; Liu, J.; Zhai, S.; Zhang, Y.; Yan, J.; Liu, Z.; Xia, X.; He, Z. Genome-wide QTL mapping for wheat processing quality parameters in a Gaocheng 8901/Zhoumai 16 recombinant inbred line population. Front. Plant Sci. 2016, 7, 1032. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, G.; Guo, X.; Chi, S.; Yu, H.; Jin, K.; Huang, H.; Wang, D.; Wu, C.; Tian, J.; et al. Genetic dissection of protein and starch during wheat grain development using QTL mapping and GWAS. Front. Plant Sci. 2023, 14, 1189887. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Miao, Y.; Ma, J.; Zhang, P.; Chen, T.; Liu, Y.; Che, Z.; Shahinnia, F.; Yang, D. Consensus genomic regions for grain quality traits in wheat revealed by Meta-QTL analysis and in silico transcriptome integration. Plant Genome 2023, 16, e20336. [Google Scholar] [CrossRef]
- Gao, Y.; An, K.; Guo, W.; Chen, Y.; Zhang, R.; Zhang, X.; Chang, S.; Rossi, V.; Jin, F.; Cao, X.; et al. The endosperm-specific transcription factor TaNAC019 regulates glutenin and starch accumulation and its elite allele improves wheat grain quality. Plant Cell 2021, 33, 603–622. [Google Scholar] [CrossRef]
- Yi, L.; Song, Y.; Zhou, R.; Granlard, G.; Jia, J. Detection of QTLs for bread-making quality in wheat using a recombinant inbred line population. Plant Breed. 2009, 128, 235–243. [Google Scholar]
- Payne, P.I.; Holt, L.M.; Worland, A.J.; Law, C.N. Structural and genetical studies on the high-molecular-weight subunits of wheat glutenin: Part 3. Telocentric mapping of the subunit genes on the long arms of the homoeologous group 1 chromosomes. Theor. Appl. Genet. 1982, 63, 129–138. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G.; Lafiandra, D. Genetics of wheat gluten proteins. Adv. Genet. 2003, 49, 111–184. [Google Scholar]
- Yang, Y.; Amo, A.; Wei, D.; Chai, Y.; Zheng, J.; Qiao, P.; Cui, C.; Lu, S.; Chen, L.; Hu, Y.G. Large-scale integration of meta-QTL and genome-wide association study discovers the genomic regions and candidate genes for yield and yield-related traits in bread wheat. Theor. Appl. Genet. 2021, 134, 3083–3109. [Google Scholar] [CrossRef]
- Cui, F.; Zhao, C.; Ding, A.; Li, J.; Wang, L.; Li, X.; Bao, Y.; Li, J.; Wang, H. Construction of an integrative linkage map and QTL mapping of grain yield-related traits using three related wheat RIL populations. Theor. Appl. Genet. 2014, 127, 659–675. [Google Scholar] [CrossRef]
- Huang, X.Q.; Coster, H.; Ganal, M.W.; Roder, M.S. Advanced backcross QTL analysis for the identification of quantitative trait loci alleles from wild relatives of wheat (Triticum aestivum L.). Theor. Appl. Genet. 2003, 106, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Zhang, X.; Zhang, W.; Zhang, N.; Song, L.; Liu, L.; Xue, X.; Liu, G.; Liu, J.; Meng, D.; et al. QTL detection for kernel size and weight in bread wheat (Triticum aestivum L.) using a high-density SNP and SSR-based linkage map. Front. Plant Sci. 2018, 9, 1484. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Feng, Z.; Du, X.; Song, Y.; Liu, X.; Qi, Z.; Song, L.; Li, J.; Li, L.; Peng, H.; et al. A novel allele of TaGW2-A1 is located in a finely mapped QTL that increases grain weight but decreases grain number in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2018, 131, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, D.; Meng, Z.; Xu, K.; Yan, J.; Xia, X.; Cao, S.; Tian, Y.; He, Z.; Zhang, Y. QTL mapping for grain yield-related traits in bread wheat via SNP-based selective genotyping. Theor. Appl. Genet. 2020, 133, 857–872. [Google Scholar] [CrossRef]
- Prashant, R.; Kadoo, N.; Desale, C.; Kore, P.; Dhaliwal, H.S.; Chhuneja, P.; Gupta, V. Kernel morphometric traits in hexaploid wheat (Triticum aestivum L.) are modulated by intricate QTL× QTL and genotype× environment interactions. J. Cereal Sci. 2012, 56, 432–439. [Google Scholar] [CrossRef]
- Jia, M.; Li, Y.; Wang, Z.; Tao, S.; Sun, G.; Kong, X.; Wang, K.; Ye, X.; Liu, S.; Geng, S.; et al. TaIAA21 represses TaARF25-mediated expression of TaERFs required for grain size and weight development in wheat. Plant J. 2021, 108, 1754–1767. [Google Scholar] [CrossRef]
- Ramya, P.; Chaubal, A.; Kulkarni, K.; Gupta, L.; Kadoo, N.; Dhaliwal, H.S.; Chhuneja, P.; Lagu, M.; Gupta, V. QTL mapping of 1000-kernel weight, kernel length, and kernel width in bread wheat (Triticum aestivum L.). J. Appl. Genet. 2010, 51, 421–429. [Google Scholar] [CrossRef]
- Sharp, P.J.; Kreis, M.; Shewry, P.R.; Gale, M.D. Location of β-amylase sequences in wheat and its relatives. Theor. Appl. Genet. 1988, 75, 286–290. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, X.; Gebrewahid, T.W.; Zhou, Y.; Yang, E.; Xia, X.; He, Z.; Li, Z.; Liu, D. Genome-wide association mapping of leaf rust and stripe rust resistance in wheat accessions using the 90K SNP array. Theor. Appl. Genet. 2021, 134, 1233–1251. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, Y.; Zhang, Z. Performing genome-wide association studies with multiple models using GAPIT. Methods Mol. Biol. 2022, 2481, 199–217. [Google Scholar] [PubMed]
- Wang, X.; Guan, P.; Xin, M.; Wang, Y.; Chen, X.; Zhao, A.; Liu, M.; Li, H.; Zhang, M.; Lu, L.; et al. Genome-wide association study identifies QTL for thousand grain weight in winter wheat under normal- and late-sown stressed environments. Theor. Appl. Genet. 2021, 134, 143–157. [Google Scholar] [CrossRef] [PubMed]



| Trait | WA (%) | WGC (%) | GPC (%) | DST (min) | EA (cm2) | MR (EU) | TGW (g) | GL (mm) | GW (mm) |
|---|---|---|---|---|---|---|---|---|---|
| Mean | 60.68 | 29.99 | 14.68 | 11.04 | 72.34 | 351.10 | 38.08 | 6.35 | 3.16 |
| Min | 53.60 | 22.50 | 12.20 | 1.20 | 19.00 | 72.00 | 27.11 | 5.49 | 2.74 |
| Max | 69.00 | 45.20 | 17.40 | 47.40 | 227.00 | 989.00 | 50.23 | 7.00 | 3.56 |
| SD a | 2.93 | 2.77 | 0.99 | 8.62 | 43.85 | 195.18 | 5.04 | 0.29 | 0.16 |
| CV b (%) | 4.82 | 9.23 | 6.78 | 78.05 | 60.62 | 55.59 | 13.25 | 4.59 | 5.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Sun, L.; Hu, M.; Liu, Q.; Xu, J.; Mu, L.; Wang, J.; Yang, J.; Wang, P.; Li, Q.; et al. Pleiotropic Quantitative Trait Loci (QTL) Mining for Regulating Wheat Processing Quality- and Yield-Related Traits. Plants 2024, 13, 2545. https://doi.org/10.3390/plants13182545
Zhao J, Sun L, Hu M, Liu Q, Xu J, Mu L, Wang J, Yang J, Wang P, Li Q, et al. Pleiotropic Quantitative Trait Loci (QTL) Mining for Regulating Wheat Processing Quality- and Yield-Related Traits. Plants. 2024; 13(18):2545. https://doi.org/10.3390/plants13182545
Chicago/Turabian StyleZhao, Jie, Lijing Sun, Mengyun Hu, Qian Liu, Junjie Xu, Liming Mu, Jianbing Wang, Jing Yang, Peinan Wang, Qianying Li, and et al. 2024. "Pleiotropic Quantitative Trait Loci (QTL) Mining for Regulating Wheat Processing Quality- and Yield-Related Traits" Plants 13, no. 18: 2545. https://doi.org/10.3390/plants13182545
APA StyleZhao, J., Sun, L., Hu, M., Liu, Q., Xu, J., Mu, L., Wang, J., Yang, J., Wang, P., Li, Q., Li, H., & Zhang, Y. (2024). Pleiotropic Quantitative Trait Loci (QTL) Mining for Regulating Wheat Processing Quality- and Yield-Related Traits. Plants, 13(18), 2545. https://doi.org/10.3390/plants13182545






